Abstract
Patients with pulmonary neoplasms have an increased risk for developing a second tumor of the lung, either at the same time or different times. It is important to determine if the second tumor represents an independent primary tumor or recurrence/metastasis, because it will significantly change the management and prognosis. Microsatellite instability (MSI) and loss of heterozygosity (LOH) represents molecular disorders acquired by the cell during neoplastic transformation. Both are associated with genetic instability. Functional silencing of tumour suppressor genes may be the consequence of genomic instability, particularly of the globally occurring LOH phenomenon. Numerous studies have confirmed the role of MSI/LOH at both the early and the late stages of multiple primary lung cancer. This paper reviews the published literatures focused on the role of MSI/LOH significance in multiple primary lung cancer. Additionally, a new method based on the allelic variations at polymorphic microsatellite markers was offered that it does not rely on collection of normal tissue, performed with minimal tumor sample, and will complement clinical criteria for diagnostic discrimination between multiple primary cancers versus solitary metastatic diseases.
Keywords: Microsatellite instability (MSI), loss of heterozygosity (LOH), multiple primary lung cancer
Introduction
Lung cancer is the most common cause of cancer death in both males and females in the world. In clinical practice, it is not common to encounter patients with multiple anatomically isolate but histologically similar lung tumors. The incidence of this condition has been reported to range from 0.2% to 2.0% in patients with primary lung cancer (1-3). Coexisting lung cancers in a patient can be characterized as either synchronous or metachronous. The incidence of synchronous lung carcinomas is variably reported between 1% and 16% (4). Metachronous primary lung cancers are likely more common, representing 40-60% of all patients with multiple lung cancers (5). Two different theories have been proposed to explain the multifocality of lung tumors by Slaughter and his colleges. The first is that multifocal tumors arise separately from anatomically distinct malignant progenitor cells that independently undergo different genetic alterations, leading to neoplastic transformation. The second is that these tumors are of monoclonal origin, arising from a single malignant cell that forms a neoplasm, which metastasizes to other regions of the lung parenchyma (6). This phenomenon has been related to the chronic exposure of the bronchial tree to carcinogens through a so-called “field cancerization” process (7). Distinguishing between these two possible mechanisms for the development of multifocal lesions has important surgical, therapeutic, and prognostic implications.
Molecular analysis of microsatellite markers is a method that has been used to assess clonality (8). Microsatellite markers, or tandem simple sequence repeats (SSR), are abundant across genomes and show high levels of polymorphism. Genomic microsatellites, iterations of 1-6 bp nucleotide motifs, have been detected in the genomes of every organism analyzed so far, and are often found at frequencies much higher than would be predicted purely on the grounds of base composition (9). Microsatellite alterations include microsatellite instability (MSI) and loss of heterozygosity (LOH). The relevance of genomic imbalance is further underscored by the association between aneuploidy with disease aggressiveness in cancers of many tissues.
This review summarizes molecular analysis of allelic variations at microsatellite markers with the MSI or LOH can be used to determine lineage relationships between multiple tumors. At the same time, a new method based on the allelic variations at polymorphic microsatellite markers was offered that it does not rely on collection of normal tissue, performed with minimal tumor sample, and will complement clinical criteria for diagnostic discrimination between multiple primary cancers versus solitary metastatic disease.
MSI in multiple primary lung cancer
MSI describes a genomic imbalance occurring because of alterations in the length of microsatellites due to small deletions or expansions, which consists of long, tandem repeats of between one and six nucleotides. The number of nucleotide repeats varies, ranging from 5 to 100 segments, with a total length of repetitive DNA of 100-600 bp. Defective DNA mismatch repair (MMR) leading to MSI has been implicated in tumorigenesis (10). The primary function of the MMR is to eliminate base–base mismatches and insertion/deletion loops that arise as a consequence of DNA polymerase slippage during DNA synthesis (11,12). MSI was initially noted in colon cancers of patients with the hereditary nonpolyposis colon cancer (HNPCC) which is the most common hereditary colorectal cancer syndrome and is associated with a spectrum of extracolonic malignancies (13-15). The most common MMR gene germline mutations identified in HNPCC involve MSH2, MLH1, PMS2 and MSH6 (16,17).
A total of 52 sporadic primary non-small-cell lung cancers (NSCLC) were examined for MSI by Lawes and his colleagues (18). Six different microsatellite markers localized on chromosomes 2, 5, 8, 10, 11 and 17 were used. In their research, a total of 27 primary squamous-cell carcinomas, 15 adenocarcinomas (ADC), 7 bronchiolo-alveolar carcinomas and 3 anaplastic carcinomas were investigated for occurrence of MSI with 6 anonymous microsatellite markers from 6 different chromosomes. The instability was evidenced by the appearance of additional bands in tumor DNA compared with normal DNA and consisted of either expansion or compression of a single band or a ‘‘ladder’’ of bands. Genomic instability was observed in 35% (18/52) of NSCLC at single or multiple loci. At the same time, they collected the histopathological data regarding size, histotype, stage, grade and lymph-node metastasis were available for each tumors. Statistical evaluation of the possible correlation between MSI and pathological feature of each tumor showed no kind of correlation. As their report showed that the genomic instability observed as MSI is probably due to a mechanism other than mismatch-repair defect, even if it cannot be excluded that inactivation of one of the DNA-repair genes may occur as a later event in the progression of a sub-set of NSCLC, as demonstrated by Wieland et al. (19). Canney reported a case of a 59-year-old man who presented mucinous ADC of his ascending colon. Four years later the patient presented with a biopsy proven metachronous ADC in his descending colon. While undergoing clinical staging, two additional lesions were identified in the right lung: one in the upper lobe and a second in the middle lobe. CT-guided biopsy of one of the lesions confirmed an invasive moderately differentiated ADC and the patient proceeded to have wedge resections of both lesions (20). In their research, immunohistochemical studies of both lung lesions showed positivity for thyroid transcription factor 1, with negative staining for cytokeratin 20 and CDX2; this immunoprofile supported a primary lung origin for both tumours. MMR protein immunocytochemistry showed loss of MSH2 and MSH6 proteins in both colonic carcinomas and the right middle lobe lung tumour, with retention of MLH1 and PMS2. Expression of all four MMR proteins was retained in the second lung tumour.
Little is known about the role of MMR genes in lung cancer and the literature is conflicting regarding the pattern and frequency of MSI in lung neoplasms. There is evidence to suggest that MLH1 and MSH2 gene inactivation occurs in NSCLC, and that it is likely to be related to DNA promoter methylation (21). Hsu et al. demonstrated significantly lower overall survival and cancer-specific survival rates in a population of non-smoking female patients with NSCLC showing promoter hypermethylation of the HMSH2 gene (22). Xinarianos et al. showed a greater frequency of reduced MSH2 expression in lung ADC; however, no significant association was identified between MSH2 expression and prognostic factors such as T stage and nodal metastasis (23).
LOH in multiple primary lung cancer
LOH is a phenomenon contrary to MSI and is frequently seen in cancer cells and is thought to occur through genetic instability at a chromosomal or similar level. In this genetic disorder, one of the gene alleles is lost in a neoplastic cell (24). The mechanism of LOH is chromosome-specific, and it may concern the entire chromosome. However, loss of genetic material is more often associated with deletion of a fragment of chromosome, leading to LOH regarded as a generalised form of allelic imbalance. Loss of function of suppressor as well as mutator genes may be a functional consequence of the globally occurring LOH (25).
Two conventional methods are widely used for LOH analysis: polymerase chain reaction (PCR)-based assays and fluorescence in situ hybridization (FISH). PCR-based LOH assays with polymorphic markers are commonly used to detect LOH regions. Tumor genotypes at certain polymorphic regions are compared with normal cells from the patient. A disadvantage of this assay is the need for normal control DNA and multiple markers, as not every marker is always informative. However, we can easily select and design primers as appropriate polymorphic markers for specific regions. Furthermore, experiments with single nucleotide polymorphisms (SNPs) instead of microsatellite markers allow more quantitative and detailed analysis for LOH (26,27). These assays detect ‘‘allelic imbalance’’ without detailed information on the gene copy number, thus including LOH as well as trisomy or local gene amplifications. Snuderl et al. recently reported that polysomy at 1p/19q may predict the clinical course of gliomas (28).
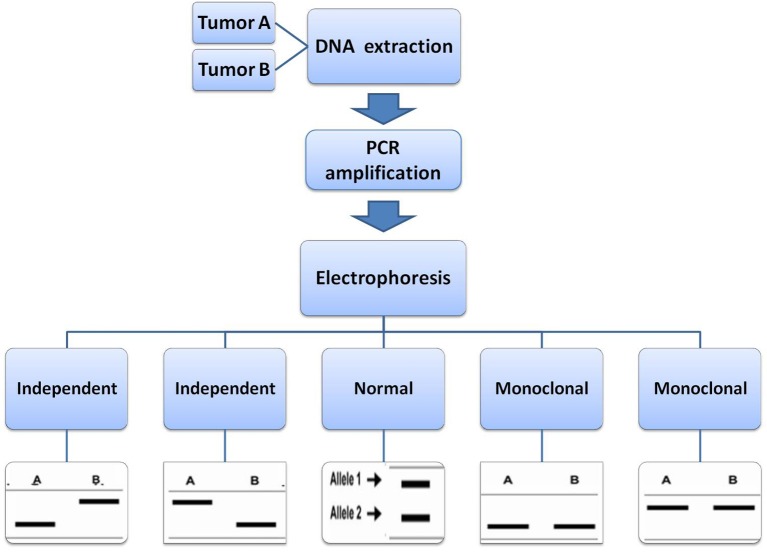
In 2001, using microdissection and LOH analysis, Huang et al. studied specimens from patients with multiple pulmonary neoplasms to determine if modern techniques can help us distinguish true independent primary from recurrence or metastasis (29). Among the four synchronous pulmonary tumors, the paired tumors in two cases appeared to be genetically different because they had very different profiles of LOH and the procedures for how to detect LOH was showed in Figure 1. Therefore, they probably represent true synchronous tumors. The two tumors in one case appeared to have identical patterns of LOH and may actually represent the same clone. A total of 25 patients in whom there was a suspicion of solitary metastasis or second primary tumor were evaluated by van der Sijp and his colleagues (30). In all 25 patients, LOH was detected in all tumors with at least three of the markers used. LOH in different tumors from one patient was regarded as identical when the same markers demonstrated loss of the same allele. LOH was regarded as different when in different tumors from one patient different markers showed LOH or when the same marker had loss of different alleles. In the 15 patients with a second primary tumor, clinical decision making was influenced in 10 patients.
Figure 1.
The procedure for determination of allele loss patterns in LOH. Tumors with different allele loss patterns would be grouped in independent clonal origin, whereas tumors with same allele loss patterns are grouped in a monoclonal origin. LOH, loss of heterozygosity.
To distinguish a metastasis from a second primary tumor in patients with a history of head and neck squamous cell carcinoma and subsequent pulmonary squamous cell carcinoma, in another research, 44 patients were analysed with clinical data, histology, and LOH (31). Using these clinical criteria, 6 patients were considered to be likely to have a second primary lung tumor, whereas 38 patients were thought to have lung metastasis. With the analysis of LOH, 11 tumor pairs were classified as metastasis and 8 as probable metastases. A total of 22 cases were classified as certain and 3 as probable second primary tumors. In one case, the LOH analysis was inconclusive. In this case, there was not enough concordant or discordant LOH to draw any conclusions. Then, a case of three different synchronous primary lung tumours that two nodules were located in the upper lobe and consisted of an ADC, an endobronchial poorly differentiated squamous cell carcinoma (SCC) and a third nodule of the lower lobe corresponded to a small cell neuroendocrine carcinoma (SCLC) was reported by Froio et al. (32). They examined all three independent lung neoplasms for LOH by the analysis of 40 microsatellite markers. Of these, 29 out of 38 were informative for LOH analysis (76.3%). No MSI was observed in any tumour component. Distinction of multiple primary lung carcinomas from intrapulmonary metastases using empiric clinical and histopathologic criteria can be difficult. Recent advances have provided several molecular markers that can be used for clonal analysis of separate tumor nodules and enhance tumor staging and subsequent treatment and prognosis. To address this issue, Dacic and his workmates performed a microdissection based allelotyping of 20 cases of histologically similar ADC (33). By observation, the patients could be discriminated in survivors and nonsurvivors if the percentage of discordant microsatellite markers within primary tumor was equal or less than 40% or above 40%, respectively. Using 40% as a cutoff in a validation cohort of pathologic stage T4 cases, each tumor was identified as having a number of discordances above or below this percentage. If the percentage of discordances for all microsatellite markers was equal or less than 40%, the pattern of LOH for this particular tumor was defined as “homogenous”. On the other hand, when the percentage of discordances was greater than 40%, the pattern of LOH status was defined as “heterogenous”. In their study, molecularly homogenous tumors were more frequently associated with angiolymphatic invasion, although that difference did not reach statistical significance. This suggests that molecularly homogenous tumors may actually represent intrapulmonary metastases, rather than independent lung cancers. Furthermore, visceral pleural invasion, which is a known adverse prognostic factor in non-small cell carcinoma, was more commonly seen in the molecularly homogenous group. These two features, which are clearly associated with biologically more aggressive lung tumors, support an idea of possible monoclonal origin of molecularly homogenous synchronous lung tumors.
The results of all the studies above concerning the role of LOH in the process of neoplastic transformation of multiple primary lung cancer have demonstrated that inactivation of various genes by deletions in their loci is already present in preneoplastic lesions, being associated with the process of carcinogenesis initiation in the lung. However, the role of that phenomenon varies, depending on chromosomal region and the significance of the LOH-affected loci in the physiology of the lung.
“Different trend” in multiple primary lung cancer
An interesting hypothesis as to the molecular sequence of genetic events in carcinogenesis in the multiple primary lung cancer was proposed by Mercer et al. (34). Since genomic instability is a common feature of cancer, they hypothesized that independently arising neoplasm in an individual patient would exhibit measurable genomic variation, enabling discrimination of tumor lineage and relatedness. Allelic variation between neoplasms often reflects accumulation of differential chromosomal deletion events. These chromosomal deletions are tolerated (non-lethal), but distinct from the molecular alterations that drive tumorigenesis, which will be common to most/all tumors of a specific type. Comparison of molecular signatures between two (or more) tumors from a single individual facilitates identification of common and unique genetic alterations. In the study, they described a molecular approach for analysis of genetic variation among multiple tumors from a single patient that does not rely on collection of normal tissue, and which can be performed with minimal tumor samples. A total of 25 paraffin-embedded human tissues corresponding to 14 squamous cell carcinomas of the H/N, 10 squamous cell carcinomas of the lung were selected for inclusion in this study. In addition to these 10 patients, tumor samples from a patient presenting with squamous cell carcinoma of the larynx that was metastatic to the lymph nodes of the neck was also included. The tumor samples corresponding to this patient have a known lineage relationship: primary squamous cell carcinoma of the H/N and metastatic squamous cell carcinoma of a proximal lymph node and its consequence was regarded as the contrast standard. Of the 20 microsatellite markers evaluated, 18 (90%) detected allelic imbalance or allelic variation among multiple tumors from at least one patient. D20S171 and D21S1432 detected no differences between tumors for any of the patients examined. The lack of detectable allelic variation at these loci may be related to their specific chromosomal locations and/or their proximity to genes that are essential for cell survival. The other 18 microsatellite markers examined identified allelic variation in 10-80% of patients, and the majority of microsatellite markers (14/20, 70%) detected allelic differences in tumors from 10-50% of patients examined. The results presented in this study demonstrate that molecular analysis of allelic variations at polymorphic microsatellite markers can be used to determine lineage relationships between multiple tumors, facilitating the discrimination of second primary cancer versus metastatic disease.
In 2013, Shen et al. offered a new concept that the “unique trend” and the “contradictory trend” (35). The “unique trend” that represents metastasis cancers and the “contradictory trend” that represents primary multiple tumors are useful in the diagnosis between tumors found at the same time in the pulmonary even diagnosed with the histopathological evaluation. This study enrolled 13 patients, with multiple primary lung cancers demonstrating with the histology and 10 patients who were diagnosed as metastasis disease during the same period for comparison purposes. Genomic DNA from lung cancers from individual patients was analyzed by six microsatellites (D2S1363, D6S1056, D7S1824, D10S1239, D15S822, and D22S689) with PCR to identify discordant allelic variation. In the report, all of the 10 patients with distant metastasis showed a consistent consequence that was called “unique trend” between primary tumor and distant metastasis. The “trend” means that all alleles corresponding to six microsatellite markers were detected in DNA from primary tumors but were reduced or not observed in DNA from metastatic tumors. In the group of synchronous lung tumor with different histological types, the result showed a “contradictory trend”. Some alleles were detected in DNA from primary tumors but were reduced or not observed in DNA from metastatic tumors and other alleles corresponding to six microsatellite markers were detected in DNA from metastatic tumors but were reduced or not observed in DNA from primary tumors. This approach is rapid and sensitive. It is paramount that such a test be amenable to the utilization of DNA samples from formalin-fixed paraffin-embedded tissues since these may be the only available source of DNA for the prior cancer.
Conclusions
LOH seems to be a more frequent phenomenon than MSI, inducing genetic instability in lung neoplasms in many chromosomal regions. MSI may be causatively associated with the initiation of molecular changes only, which may later lead to neoplasia, whereas the incidence of LOH overtly increases with tumour progression. This phenomenon increases with the degree of neoplastic progression, which indicates a successive accumulation of molecular disorders in cells and a coincidence of LOH/mutations in multiple primary lung cancer. The new methods of Mercer et al. and Shen et al. studies, which were ideal for clinical use given that the methodology is straightforward, rapid, and inexpensive, were to refine a molecular method to analyze multiple tumors that does not rely on collection of normal tissue, can be performed with minimal tumor sample, and will complement clinical criteria for diagnostic discrimination between multiple primary cancer versus solitary metastatic disease.
Acknowledgements
We greatly appreciate the assistance of the staff of the Department of Thoracic Surgery, West-China Hospital, Sichuan University, and thank them for their efforts.
Funding: This work supported by the National Science Foundation (No: 81071929, to Guowei Che) and National Science Foundation (No: 81272595, to Guowei Che).
Disclosure: The authors declare no conflict of interest.
References
- 1.Wu SC, Lin ZQ, Xu CW, et al. Multiple primary lung cancers. Chest 1987;92:892-6. [DOI] [PubMed] [Google Scholar]
- 2.Ferguson MK, DeMeester TR, DesLauriers J, et al. Diagnosis and management of synchronous lung cancers. J Thorac Cardiovasc Surg 1985;89:378-85. [PubMed] [Google Scholar]
- 3.Carey FA, Donnelly SC, Walker WS, et al. Synchronous primary lung cancers: prevalence in surgical material and clinical implications. Thorax 1993;48:344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flynn MJ, Rassl D, El Shahira A, et al. Metachronous and synchronous lung tumors: five malignant lung pathologies in 1 patient during 7 years. Ann Thorac Surg 2004;78:2154-5. [DOI] [PubMed] [Google Scholar]
- 5.Okada M, Tsubota N, Yoshimura M, et al. Operative approach for multiple primary lung carcinomas. J Thorac Cardiovasc Surg 1998;115:836-40. [DOI] [PubMed] [Google Scholar]
- 6.Ninomiya H, Nomura K, Satoh Y, et al. Genetic instability in lung cancer: concurrent analysis of chromosomal, mini- and microsatellite instability and loss of heterozygosity. Br J Cancer 2006;94:1485-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sikkink SK, Liloglou T, Maloney P, et al. In-depth analysis of molecular alterations within normal and tumour tissue from an entire bronchial tree. Int J Oncol 2003;22:589-95. [PubMed] [Google Scholar]
- 8.Silk AD, Zasadil LM, Holland AJ, et al. Chromosome missegregation rate predicts whether aneuploidy will promote or suppress tumors. Proc Natl Acad Sci U S A 2013;110:E4134-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Epplen C, Melmer G, Siedlaczck I, et al. On the essence of “meaningless” simple repetitive DNA in eukaryote genomes. EXS 1993;67:29-45. [DOI] [PubMed] [Google Scholar]
- 10.Chang IY, Kim SH, Cho HJ, et al. Human AP endonuclease suppresses DNA mismatch repair activity leading to microsatellite instability. Nucleic Acids Res 2005;33:5073-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buermeyer AB, Deschênes SM, Baker SM, et al. Mammalian DNA mismatch repair. Annu Rev Genet 1999;33:533-64. [DOI] [PubMed] [Google Scholar]
- 12.Jiricny J, Nyström-Lahti M.Mismatch repair defects in cancer. Curr Opin Genet Dev 2000;10:157-61. [DOI] [PubMed] [Google Scholar]
- 13.Söreide K, Janssen EA, Söiland H, et al. Microsatellite instability in colorectal cancer. Br J Surg 2006;93:395-406. [DOI] [PubMed] [Google Scholar]
- 14.Ionov Y, Peinado MA, Malkhosyan S, et al. Ubiquitous somatic mutations in simple repeated sequences reveal a new mechanism for colonic carcinogenesis. Nature 1993;363:558-61. [DOI] [PubMed] [Google Scholar]
- 15.Subramanian S, Mishra RK, Singh L. Genome-wide analysis of microsatellite repeats in humans: their abundance and density in specific genomic regions. Genome Biol 2003;4:R13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rouprêt M, Yates DR, Comperat E, et al. Upper urinary tract urothelial cell carcinomas and other urological malignancies involved in the hereditary nonpolyposis colorectal cancer (lynch syndrome) tumor spectrum. Eur Urol 2008;54:1226-36. [DOI] [PubMed] [Google Scholar]
- 17.Hendriks YM, de Jong AE, Morreau H, et al. Diagnostic approach and management of Lynch syndrome (hereditary nonpolyposis colorectal carcinoma): a guide for clinicians. CA Cancer J Clin 2006;56:213-25. [DOI] [PubMed] [Google Scholar]
- 18.Lawes DA, SenGupta S, Boulos PB. The clinical importance and prognostic implications of microsatellite instability in sporadic cancer. Eur J Surg Oncol 2003;29:201-12. [DOI] [PubMed] [Google Scholar]
- 19.Wieland I, Ammermüller T, Böhm M, et al. Microsatellite instability and loss of heterozygosity at the hMLH1 locus on chromosome 3p21 occur in a subset of nonsmall cell lung carcinomas. Oncol Res 1996;8:1-5. [PubMed] [Google Scholar]
- 20.Canney A, Sheahan K, Keegan D, et al. Synchronous lung tumours in a patient with metachronous colorectal carcinoma and a germline MSH2 mutation. J Clin Pathol 2009;62:471-3. [DOI] [PubMed] [Google Scholar]
- 21.Fong KM, Zimmerman PV, Smith PJ. Microsatellite instability and other molecular abnormalities in non-small cell lung cancer. Cancer Res 1995;55:28-30. [PubMed] [Google Scholar]
- 22.Hsu HS, Wen CK, Tang YA, et al. Promoter hypermethylation is the predominat mechanism in hMLH1 and hMSH2 deregulation and is a poor prognostic factor in nonsmoking lung cancer. Clin Cancer Res 2005;11:5410-16. [DOI] [PubMed] [Google Scholar]
- 23.Xinarianos G, Liloglou T, Prime W, et al. hMLH1 and hMSH2 expression correlates with allelic imbalance on chromosome 3p in non-small cell lung carcinomas. Cancer Res 2000;60:4216-21. [PubMed] [Google Scholar]
- 24.Boland CR, Thibodeau SN, Hamilton SR, et al. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res 1998;58:5248-57. [PubMed] [Google Scholar]
- 25.Devilee P, Cleton-Jansen AM, Cornelisse CJ. Ever since Knudson. Trends Genet 2001;17:569-73. [DOI] [PubMed] [Google Scholar]
- 26.Hata N, Yoshimoto K, Yokoyama N, et al. Allelic losses of chromosome 10 in glioma tissues detected by quantitative single-strand conformation polymorphism analysis. Clin Chem 2006;52:370-8. [DOI] [PubMed] [Google Scholar]
- 27.Guan Y, Hata N, Kuga D, et al. Narrowing of the regions of allelic losses of chromosome 1p36 in meningioma tissues by an improved SSCP analysis. Int J Cancer 2008;122:1820-6. [DOI] [PubMed] [Google Scholar]
- 28.Snuderl M, Eichler AF, Ligon KL, et al. Polysomy for chromosomes 1 and 19 predicts earlier recurrence in anaplastic oligodendrogliomas with concurrent 1p/19q loss. Clin Cancer Res 2009;15:6430-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang J, Behrens C, Wistuba I, et al. Molecular analysis of synchronous and metachronous tumors of the lung: impact on management and prognosis. Ann Diagn Pathol 2001;5:321-9. [DOI] [PubMed] [Google Scholar]
- 30.van der Sijp JR, van Meerbeeck JP, Maat AP, et al. Determination of the molecular relationship between multiple tumors within one patient is of clinical importance. J Clin Oncol 2002;20:1105-14. [DOI] [PubMed] [Google Scholar]
- 31.Geurts TW, Nederlof PM, van den Brekel MW, et al. Pulmonary squamous cell carcinoma following head and neck squamous cell carcinoma: metastasis or second primary? Clin Cancer Res 2005;11:6608-14. [DOI] [PubMed] [Google Scholar]
- 32.Froio E, D’Adda T, Fellegara G, et al. Three different synchronous primary lung tumours: a case report with extensive genetic analysis and review of the literature. Lung Cancer 2008;59:395-402. [DOI] [PubMed] [Google Scholar]
- 33.Dacic S, Ionescu DN, Finkelstein S, et al. Patterns of allelic loss of synchronous adenocarcinomas of the lung. Am J Surg Pathol 2005;29:897-902. [DOI] [PubMed] [Google Scholar]
- 34.Mercer RR, Lucas NC, Simmons AN, et al. Molecular discrimination of multiple primary versus metastatic squamous cell cancers of the head/neck and lung. Exp Mol Pathol 2009;86:1-9. [DOI] [PubMed] [Google Scholar]
- 35.Shen C, Xu H, Liu L, et al. “Unique trend” and “contradictory trend” in discrimination of primary synchronous lung cancer and metastatic lung cancer. BMC Cancer 2013;13:467. [DOI] [PMC free article] [PubMed] [Google Scholar]