Abstract
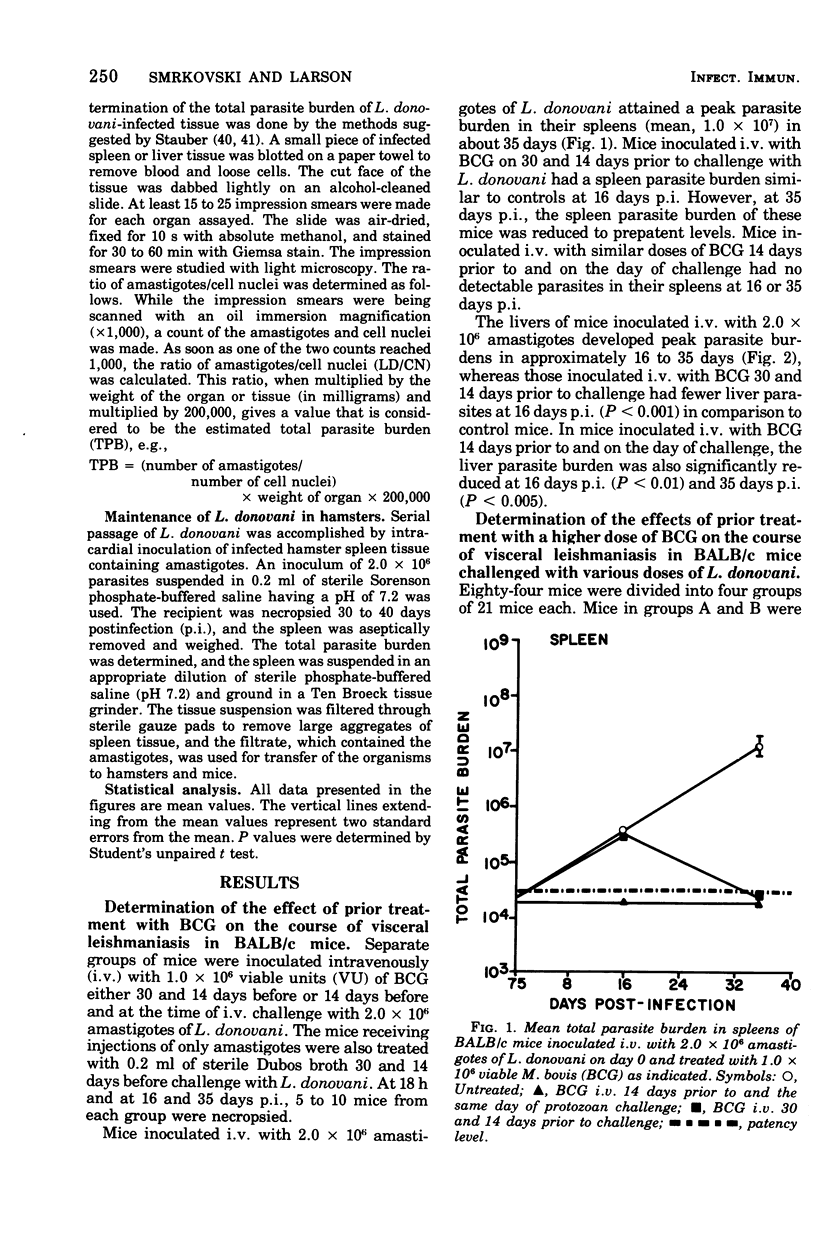
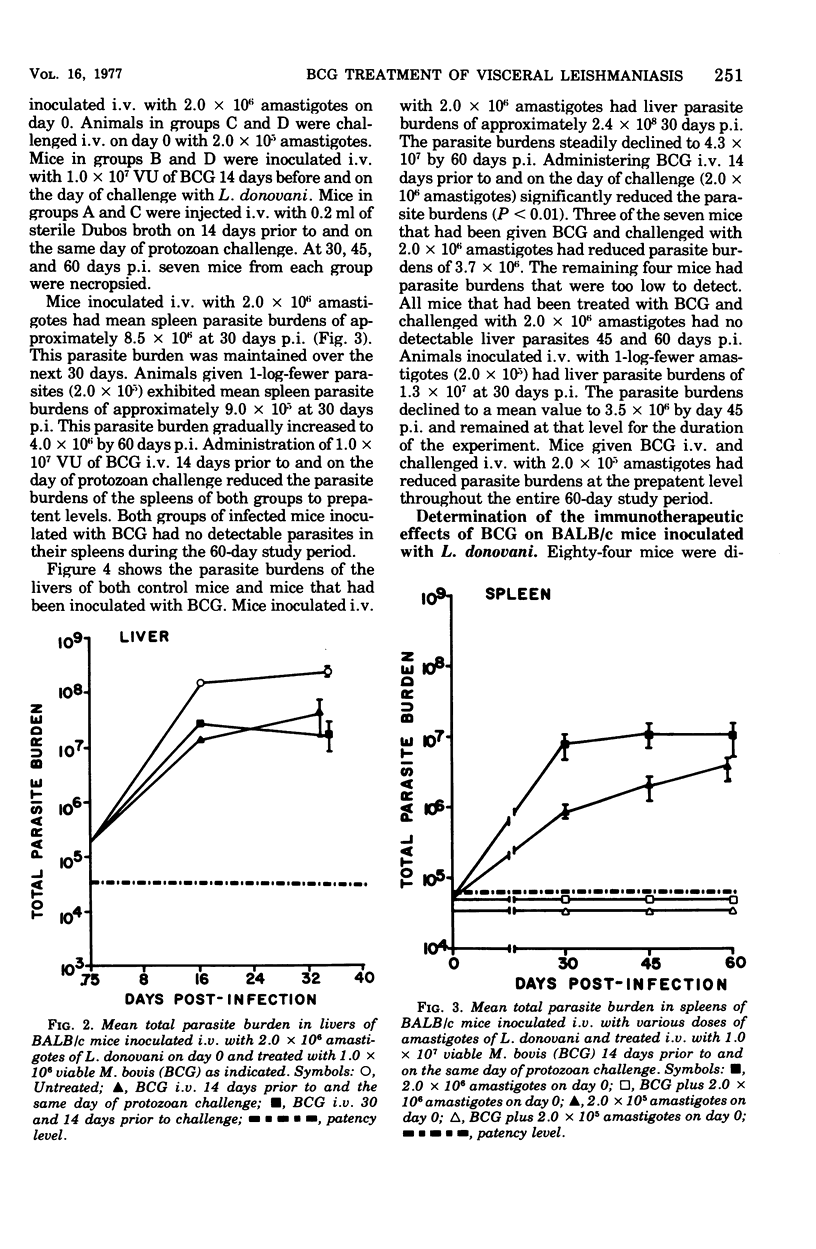
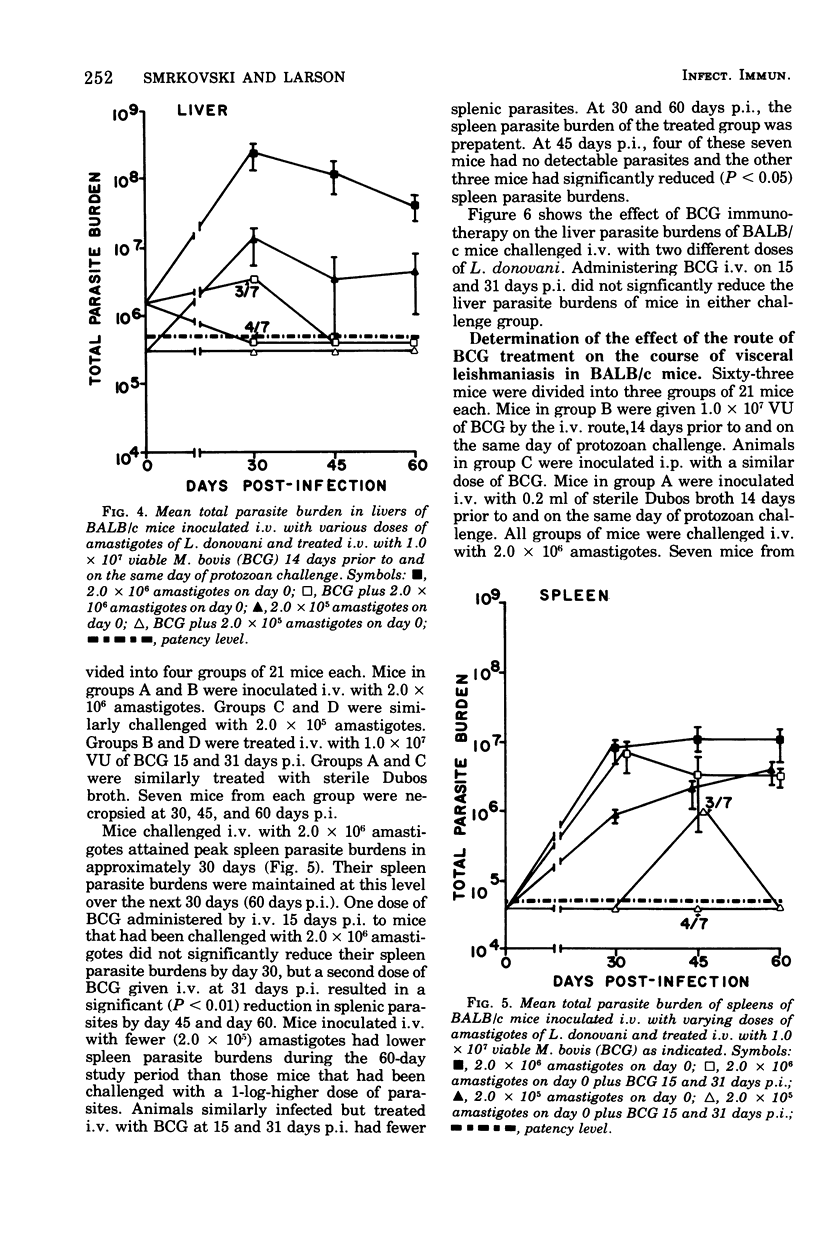
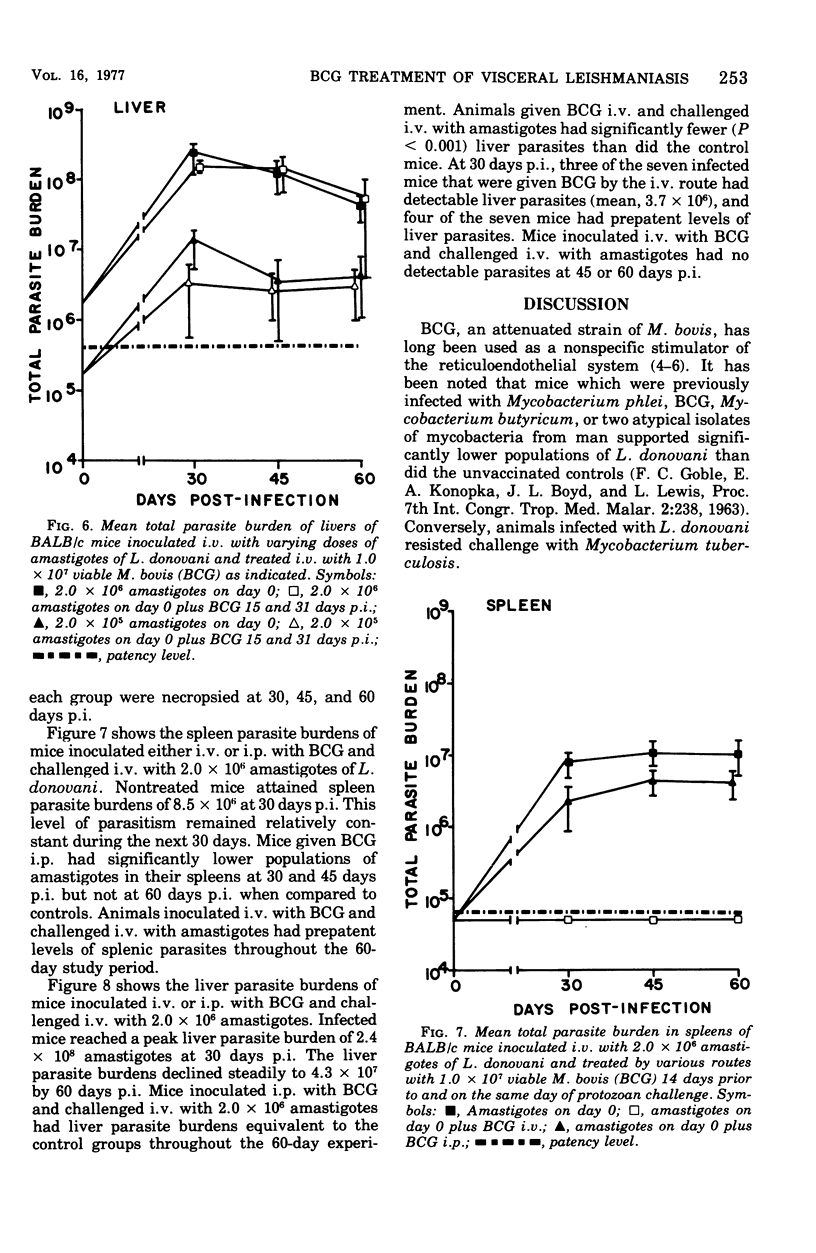
Intravenous inoculation of BCG was found to be both prophylactic and therapeutic in BALB/c mice against challenge with amastigotes of Leishmania donovani. Spleens and livers of mice inoculated with BCG maintained total parasite burdens at significantly lower levels when compared to controls. BCG administered intravenously 14 days prior to and on the same day of protozoan challenge was more protective than vaccine given 30 and 14 days prior to challenge. A level of 10(7) viable units of BCG provided more protection against challenge with parasites than did 10(6) viable units. BCG given the same route as the challenge dose of amastigotes provided more protection than if administered via some other route. BCG given to mice with an already established infection was shown to significantly reduce their parasite burdens.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADLER S. IMMUNOLOGY OF LEISHMANIASIS. Isr J Med Sci. 1965 Jan;1:9–13. [PubMed] [Google Scholar]
- Allwood G. G., Asherson G. L. Depression of delayed hypersensitivity by pretreatment with Freund-type adjuvants. 3. Depressed arrival of lymphoid cells at recently immunized lymph nodes in mice pretreated with adjuvants. Clin Exp Immunol. 1972 Aug;11(4):579–584. [PMC free article] [PubMed] [Google Scholar]
- Anacker R. L., Matsumoto J., Ribi E., Smith R. F., Yamamoto K. Enhancement of resistance of mice to tuberculosis by purified components of mycobacterial lipid fractions. J Infect Dis. 1973 Apr;127(4):357–364. doi: 10.1093/infdis/127.4.357. [DOI] [PubMed] [Google Scholar]
- BIOZZI G., BENACERRAF B., GRUMBACH F., HALPERN B. N., LEVADITI J., RIST N. Etude de l'activité granulopexique du système réticulo-endothélial au cours de l'infection tuberculeuse expérimentale de la souris. Ann Inst Pasteur (Paris) 1954 Sep;87(3):291–300. [PubMed] [Google Scholar]
- Blanden R. V. Modification of macrophage function. J Reticuloendothel Soc. 1968 Jun;5(3):179–202. [PubMed] [Google Scholar]
- Blewett T. M., Kadivar D. M., Soulsby E. J. Cutaneous leishmaniasis in the guinea pig. Delayed-type hypersensitivity, lymphocyte stimulation, and inhibition of macrophage migration. Am J Trop Med Hyg. 1971 Jul;20(4):546–551. [PubMed] [Google Scholar]
- Bloom B. R., Bennett B. Migration inhibitory factor associated with delayed-type hypersensitivity. Fed Proc. 1968 Jan-Feb;27(1):13–15. [PubMed] [Google Scholar]
- Bryceson A. D., Bray R. S., Dumonde D. C. Experimental cutaneous leishmaniasis. IV. Selective suppression of cell-mediated immunity during the response of guinea-pigs to infection with Leishmania enriettii. Clin Exp Immunol. 1974 Feb;16(2):189–202. [PMC free article] [PubMed] [Google Scholar]
- Bryceson A. D. Diffuse cutaneous leishmaniasis in Ethiopia. 3. Immunological studies. IV. Pathogenesis of diffuse cutaneous leishmaniasis. Trans R Soc Trop Med Hyg. 1970;64(3):380–393. doi: 10.1016/0035-9203(70)90174-4. [DOI] [PubMed] [Google Scholar]
- Bryceson A. D. Immunological aspects of clinical leishmaniasis. Proc R Soc Med. 1970 Oct;63(10):1056–1060. [PMC free article] [PubMed] [Google Scholar]
- Bryceson A. D., Preston P. M., Bray R. S., Dumonde D. C. Experimental cutaneous leishmaniasis. II. Effects of immunosuppression and antigenic competition on the course of infection with Leishmania enriettii in the guinea-pig. Clin Exp Immunol. 1972 Feb;10(2):305–335. [PMC free article] [PubMed] [Google Scholar]
- Bryceson A. D., Turk J. L. The effect of prolonged treatment with antilymphocyte serum on the course of infections with BCG and Leishmania enriettii in the guinea-pig. J Pathol. 1971 Jul;104(3):153–165. doi: 10.1002/path.1711040302. [DOI] [PubMed] [Google Scholar]
- Clinton B. A., Stauber L. A., Palczuk N. C. Leishmania donovani: antibody response to chicken ovalbumin by infected golden hamsters. Exp Parasitol. 1969 Aug;25(1):171–180. doi: 10.1016/0014-4894(69)90063-0. [DOI] [PubMed] [Google Scholar]
- DAVID J. R., LAWRENCE H. S., THOMAL L. DELAYED HYPERSENSITIVITY IN VITRO. II. EFFECT OF SENSITIVE CELLS ON NORMAL CELLS IN THE PRESENCE OF ANTIGEN. J Immunol. 1964 Aug;93:274–278. [PubMed] [Google Scholar]
- Evans R., Alexander P. Cooperation of immune lymphoid cells with macrophages in tumour immunity. Nature. 1970 Nov 14;228(5272):620–622. doi: 10.1038/228620a0. [DOI] [PubMed] [Google Scholar]
- Guirges S. Y. Natural and experimental re-infection of man with Oriental sore. Ann Trop Med Parasitol. 1971 Jun;65(2):197–205. doi: 10.1080/00034983.1971.11686746. [DOI] [PubMed] [Google Scholar]
- Hanna M. G., Jr, Zbar B., Rapp H. J. Histopathology of tumor regression after intralesional injection of Mycobacterium bovis. I. Tumor growth and metastasis. J Natl Cancer Inst. 1972 May;48(5):1441–1455. [PubMed] [Google Scholar]
- Heyneman D. Immunology of leishmaniasis. Bull World Health Organ. 1971;44(4):499–514. [PMC free article] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Lambert L. H., Jr, Remington J. S. Adjuvant induced resistance to tumor development in mice. Proc Soc Exp Biol Med. 1972 Mar;139(3):1053–1056. doi: 10.3181/00379727-139-36296. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Lambert L. H., Jr, Remington J. S. Control of carcinogenesis: a possible role for the activated macrophage. Science. 1972 Sep 15;177(4053):998–1000. doi: 10.1126/science.177.4053.998. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Lambert L. H., Jr, Remington J. S. In vitro nonimmunologic destruction of cells with abnormal growth characteristics by adjuvant activated macrophages. Proc Soc Exp Biol Med. 1972 Mar;139(3):1049–1052. doi: 10.3181/00379727-139-36295. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Lambert L. H., Jr, Remington J. S. Possible role of macrophage mediated nonspecific cytotoxicity in tumour resistance. Nat New Biol. 1972 Jan 12;235(54):48–50. doi: 10.1038/newbio235048a0. [DOI] [PubMed] [Google Scholar]
- KHALEQUE K. A. A new method for preparing an antigen from Kedrowsky's bacillus for the complement fixation test for kala-azar. J Pathol Bacteriol. 1962 Jan;83:284–287. [PubMed] [Google Scholar]
- KOJEVNIKOV P. V. [Some results of the works of Soviet scholars in the study of cutaneous leishmaniasis]. Dermatologica. 1961 Dec;123:341–356. [PubMed] [Google Scholar]
- Krahenbuhl J. L., Remington J. S. In vitro induction of nonspecific resistance in macrophages by specifically sensitized lymphocytes. Infect Immun. 1971 Oct;4(4):337–343. doi: 10.1128/iai.4.4.337-343.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lainson R., Bray R. S. Studies on the immunology and serology of leishmaniasis. II. Cross-immunity experiments among different forms of American cutaneous leishmaniasis in monkeys. Trans R Soc Trop Med Hyg. 1966;60(4):526–532. doi: 10.1016/0035-9203(66)90278-1. [DOI] [PubMed] [Google Scholar]
- Larson C. L., Baker R. E., Ushijima R. N., Baker M. B., Gillespie C. Immunotherapy of Friend disease in mice employing viable BCG vaccine. Proc Soc Exp Biol Med. 1972 Jun;140(2):700–702. doi: 10.3181/00379727-140-36534. [DOI] [PubMed] [Google Scholar]
- Lodmell D. L., Niwa A., Hayashi K., Notkins A. L. Prevention of cell-to-cell spread of herpes simplex virus by leukocytes. J Exp Med. 1973 Mar 1;137(3):706–720. doi: 10.1084/jem.137.3.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANSON-BAHR P. E. VARIATIONS IN THE CLINICAL MANIFESTATIONS OF LEISHMANIASIS CAUSED BY L. TROPICA. J Trop Med Hyg. 1964 Apr;67:85–87. [PubMed] [Google Scholar]
- Mackaness G. B., Blanden R. V. Cellular immunity. Prog Allergy. 1967;11:89–140. [PubMed] [Google Scholar]
- Majumdar T. D. Post-kala-azar dermal leishmaniasis. Dermatol Int. 1967 Jul-Sep;6(3):174–177. doi: 10.1111/j.1365-4362.1967.tb05261.x. [DOI] [PubMed] [Google Scholar]
- Neal R. A., Garnham P. C., Cohen S. Immunization against protozoal diseases. Br Med Bull. 1969 May;25(2):194–201. doi: 10.1093/oxfordjournals.bmb.a070692. [DOI] [PubMed] [Google Scholar]
- North R. J. Cellular kinetics associated with the development of acquired cellular resistance. J Exp Med. 1969 Aug 1;130(2):299–314. doi: 10.1084/jem.130.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAGHER F., VERBI S., ZUCKERMAN A. Immunity to reinfection following recovery from cutaneous leishmaniasis (oriental sore). J Invest Dermatol. 1955 Apr;24(4):417–421. doi: 10.1038/jid.1955.56. [DOI] [PubMed] [Google Scholar]
- TORREALBA J. W., CHAVES-TORREALBA J. EMPLEO DE ANTIGENO DE B.C.G. EN LA REACCION DE FIJACION DEL COMPLEMENTO PARA EL DIAGNOSTICO DE LA LEISHMANIASIS VISCERAL. (NOTA PREVIA) Rev Inst Med Trop Sao Paulo. 1964 Sep-Oct;6:252–253. [PubMed] [Google Scholar]
- Tremonti L., Walton B. C. Blast transformation and migration-inhibition in toxoplasmosis and leishmaniasis. Am J Trop Med Hyg. 1970 Jan;19(1):49–56. doi: 10.4269/ajtmh.1970.19.49. [DOI] [PubMed] [Google Scholar]


