Abstract
Background
Individuals with a high risk of stroke are also more prone to cognitive impairment perhaps due to concomitant vascular risk factors. In addition, clinical stroke increases the risk of subsequent dementia. Nevertheless, the relationship between clinical stroke and subsequent cognitive function in initially non-demented individuals remains less clear as most prior studies examined case series without controls.
Aims
To specify among non-demented individuals the cognitive domains affected by clinical stroke, independently of vascular risk factors and pre-stroke cognition.
Methods
One hundred-thirty-two Framingham Study participants (mean age=77±9 years, 54% women) with prospectively validated initial strokes, as well as age- and sex-matched controls, underwent identical cognitive evaluations ~6 months after the stroke. Linear regression models were used to assess the differences in cognitive scores between stroke cases and controls adjusting for pre-stroke cognitive function as assessed by Mini-Mental State Examination scores, and with and without adjustment for vascular risk factors.
Results
Adjusting for pre-stroke cognition and vascular risk factors, persons with stroke had poorer cognitive function in the domains of immediate recall of logical and visual memories (β=−1.27±0.60; P=0.035, β=−1.03±0.47; P=0.028, respectively), verbal learning (paired associate test; β=−1.31±0.57; P=0.023), language (Boston naming test; β=−0.27±0.08; P=0.002), executive function (Digit span backwards; β=−0.53±0.21; P=0.015) and visuo-spatial and motor skills (block design; β=−3.02±1.06; P=0.005).
Conclusions
Clinical stroke is associated with subsequent poorer performance in multiple cognitive domains. This association cannot be entirely explained by the individual’s cognitive function prior to stroke or by concomitant vascular risk factor levels.
Keywords: cerebrovascular disease, stroke related outcomes, cognitive function, vascular risk factors, neuropsychology matched cohort study
Introduction
Vascular contributions to cognitive impairment and dementia are important.1 Individuals with a high risk of stroke are also more prone to future lower cognitive performance and dementia.2,3 However, clinical stroke itself, independent of cardiovascular risk factors or pre-stroke cognitive status, is associated with a marked increase in dementia risk, and the effect of a stroke may be much larger than the cumulative effect of the underlying risk factors leading to stroke.4-6 To date, the effect of stroke on subsequent cognitive performance in individuals without dementia has not been adequately studied. In order to do so, one must adjust for pre-stroke cognitive function, as poor cognitive performance may precede the occurrence of stroke.7-9 In a previous nested case-control study within the Framingham Heart Study (FHS) cohort, a greater decline in Mini-Mental State Examination (MMSE) score between 6 months post-stroke and pre-stroke evaluations was demonstrated in stroke cases compared with controls.10 In the present nested matched cohort study we used a comprehensive neuropsychological battery to evaluate cognitive performance 6 months after stroke in individuals who were free of dementia at the time of the stroke, and compared their cognitive abilities with that of age- and sex-matched dementia- and stroke-free controls. We tested whether these associations were independent of concomitant vascular risk factors, and adjusted for pre-stroke cognitive function.
Methods
Study population
The FHS is an ongoing longitudinal cohort study that began in 1948 with the recruitment of 5,209 participants into the Original Cohort to prospectively investigate risk factors for cardiovascular disease.11 In 1971, the Offspring cohort was enrolled, including 5,124 offspring of the original cohort and their spouses.12 Our study sample was derived from both generations. Between January 1, 1982 and December 31, 2008, 691 participants sustained a stroke, 481 of them survived at least until the cognitive evaluation 6 months after the stroke, and 190 were available for this evaluation. They were selected such that their cognitive evaluation was at least 100 days after the acute event in order to avoid misclassification due to delirium and allow for initial recovery and stabilization of deficits in the acute phase after stroke, and no more than 400 days after the stroke to minimize confounding due to subsequent development of other unrelated causes of cognitive impairment. Each stroke case was matched on age, sex, and cohort to a control subject. Controls were selected from the same study population and had to be dementia free at the index date (date of stroke in the corresponding case) and to survive for and attend the same clinical, imaging and cognitive evaluations as their case. Age matching was performed to within 1 year. One-hundred-seventy-four cases were successfully matched using these criteria. Stroke cases and controls underwent neurological and cognitive evaluation in a visit approximately 6 months after the stroke index date. Four pairs were excluded because dementia was diagnosed before the stroke date among the stroke-cases. In addition, we excluded 31 pairs in which either the case or control did not have cognitive evaluation within the defined time frame, and 7 pairs in which data on educational achievement was incomplete. Thus, 132 pairs of cases and controls were available for analysis (figure 1).
Figure 1.
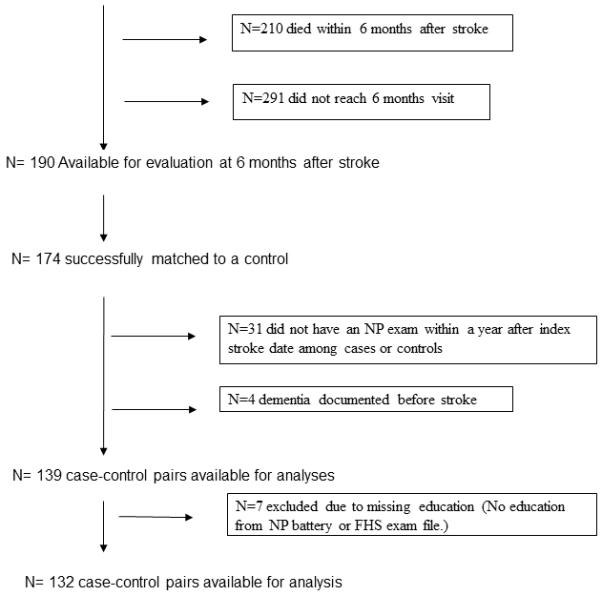
Study group composition.
Dementia surveillance
All FHS participants remain under continuous screening and surveillance for development of dementia.13 In brief, dementia screening was done by administrating the MMSE test during the routine exams. Participants who were suspected to have possible cognitive decline based on the Mini-Mental State Examination score or additional health status information underwent in-depth assessment that included neurological and neuropsychological evaluations. A final decision regarding presence of dementia, its type, severity and date of onset was done by a review committee using information from cognitive evaluations as well as data from primary care physicians, hospitals and nursery home records, structured family interviews, brain imaging and autopsy data when available. Dementia was diagnosed according to the criteria of the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition.14 Where appropriate, persons were also identified as having mild cognitive impairment (MCI) using Petersen’s criteria15 and type and date of onset of MCI were assigned.
Stroke surveillance
Stroke surveillance was done by daily monitoring of admissions to the only general hospital in the town and by reviewing medical records, laboratory tests and imaging results on FHS participants requested from general practitioner offices, emergency departments, and imaging facilities in the area. If a participant saw a physician or was admitted to the hospital, visited an emergency department, or underwent any brain imaging between biennial examinations for symptoms that were suggestive of transient ischemic attack or stroke, a stroke neurologist from the Framingham Study visited the person within 48 hours. In addition, at every FHS visit, and at annual health status updates, all participants are asked whether they have suffered a stroke or transient ischemic attack and are specifically questioned about symptoms suggestive of a stroke, such as sudden speech or vision changes and hemiplegia. A panel of 3 investigators (at least 2 neurologists) adjudicated the diagnosis of stroke in each case, based on review of all relevant medical results and on the assessment of the study neurologist who had examined the participant, and in addition made a judgment about stroke type, severity and anatomic features (laterality, size, deep or. superficial location and presence of associated neurological deficits). An independent tracking system is in place to gather information on participants regardless of whether they still reside in the vicinity of Framingham, Massachusetts. This study was approved by the Institutional Review Board at Boston University and informed consent was obtained from all subjects.
Cognitive tests
Subjects were administered a neuropsychological test battery using standard administration protocols and trained examiners. Details of tests administered and normative values for the FHS Original and Offspring cohorts have been published.16, 17 We selected a subset of tests from the cognitive battery that were representative of several cognitive domains: The immediate and delayed recall components of the Logical Memory test (LMi and LMd) provide measures of short- and long-term verbal memory. The immediate and delayed recall components of the Visual Reproductions test assess short- and long-term visuospatial memory (VRi and VRd). The Paired Associate Learning test (PAS; immediate and delayed recall) measures the ability to learn new information. The Similarities test (SIM) measures abstract reasoning skills. Digit span forward (DSF) is a measure of attention and Digit span backwards (DSB) as well as the Controlled word association test (COWAT) are measures of executive function. The Hooper visual organization test (HVOT) is a measure of visual perception. The Boston Naming Test (BNT) measures language abilities. Block design is a measure of visuospatial and motor skills and the Wechsler Adult Intelligence Scale- revised (WAISR) Information subscale and the Wide Range Achievement test (WRAT) measure premorbid educational achievement.
Definition of covariates
We used previously described and validated components of the Framingham Stroke Risk Profile (FSRP),11 including age, sex, systolic blood pressure (SBP), diabetes, prevalent non-stroke cardiovascular disease (CVD), the presence of atrial fibrillation and smoking status, as baseline covariates. The MMSE score before the stroke date served as a measure of pre-stroke cognitive function. Educational achievement was defined as a four-class variable (<high-school degree, high-school degree only, some college or ≥college degree). All the covariates used in this study were measured at the last examination before the date of stroke among the cases and before the index date among the controls.
Statistical analysis
Comparisons between baseline characteristics of different groups were performed using chi-square tests, Fisher’s exact tests, two-sample t-tests, or wilcoxon rank sum tests, as appropriate. Outcome variables were natural log-transformed, as necessary, to reduce skewness. Differences in cognitive performance in stroke cases and controls were assessed using linear regression models, with cognitive score as the dependent variable and case/control status as the independent variable. The beta coefficient from this model represents the difference in cognitive score between cases and controls (reference group). Model 1 is adjusted for age, sex, education level, cohort (Original or Offspring), and pre-stroke MMSE score. Model 2 is further adjusted for SBP, diabetes, prevalent CVD, prevalent atrial fibrillation and current smoking.
In a secondary analysis we excluded 9 participants (7 cases and 2 controls) who were retrospectively diagnosed by the FHS neurologic committee as having had mild cognitive impairment at the time of the stroke/ index date . All analyses were performed using SAS version 9.2 (Cary, NC). A p-value of <0.05 was considered statistically significant.
Results
The baseline characteristics of participants with stroke who survived to the cognitive assessment (n=481; figure 1) and were and were not included in the study are presented in table 1. These two groups did not differ in age, sex, stroke characteristics and vascular risk factors. Stroke participants who were not included were slightly more educated, and a greater percentage had their stroke occur outside of Massachusetts as compared to participants included in the study. In addition, the time period between the FHS examination at which covariate data was collected and the date of stroke was longer for those not included in the study, probably because they lived further away and attended FHS examinations less frequently.
Table 1.
Baseline characteristics of stroke cases included and not included in the study
| Stroke cases included (N=132) |
Stroke cases not included (N=349) |
P-value | ||
|---|---|---|---|---|
| Age at stroke (years) | 76.8±9.4 | 76.6±10.2 | 0.876 | |
| Time difference between covariate assessment and date of stroke (years) |
2.9±4.9 | 5.3±7.9 | <0.001 | |
| Women | 71 (53.8) | 211 (60.5) | 0.19 | |
| Education | < High-school degree | 8 (6.2) | 11 (3.7) | 0.046 |
| High-school degree | 26 (20.2) | 69 (21.1) | ||
| Some college | 55 (42.6) | 93 (31.1) | ||
| ≥College degree | 40 (31.0) | 126 (42.1) | ||
| Stroke type | Non-lacune Atherothrombotic brain infarction |
68 (51.5) | 164 (47.0) | 0.521 |
| Lacune Atherothrombotic brain infarction |
25 (18.9) | 59 (16.9) | ||
| Cardio embolism | 31 (23.5) | 93 (26.7) | ||
| Intracerebral hemorrhage | 8 (6.1) | 25 (7.2) | ||
| Subarachnoid hemorrhage | 0 (0.0) | 7 (2.0) | ||
| Other | 0 (0.0) | 1 (0.3) | ||
| Stroke side= right (vs. left, both, or none) | 56 (42.4) | 141 (40.4) | 0.687 | |
| Prevalent diabetes | 32 (27.6) | 59 (21.9) | 0.224 | |
| Prevalent hypertension | 103 (81.8) | 246 (75.0) | 0.127 | |
| Prevalent cardiovascular disease | 42 (31.8) | 108 (31.0) | 0.854 | |
| Current smokers | 27 (21.4) | 69 (20.2) | 0.766 | |
| Stroke occurred outside Massachusetts | 10 (8.6) | 115 (35.0) | <0.001 | |
Values are n (%) or mean ± SD.
For the 132 stroke cases included in the study, the mean interval between the date of stroke and the cognitive evaluation was 0.6±0.1 years, with a minimum of 108 and a maximum of 396 days. Table 2 presents the baseline characteristics of stroke cases and controls. Seventy-one percent were part of the Original cohort and the rest were part of the Offspring cohort. The participants’ mean age at the time of the cognitive evaluation was 77.4 years and 54% were women. Stroke cases were more likely to be current smokers and to have diabetes before the stroke, and they had higher pre-stroke mean SBP compared to controls. The pre-stroke median MMSE score was not significantly different comparing cases and controls. At the time of the cognitive evaluation (~6 months after stroke or stroke index date for controls), 20 (15.5%) and 4 (3%) individuals were already diagnosed with mild dementia among the stroke cases and controls, respectively (p<0.001) (table 2).
Table 2.
Baseline characteristics of cases and controls
| Case | Control | P-value | ||
|---|---|---|---|---|
| (N=132) | (N=132) | |||
| Offspring (vs Original) cohort† | 38 (28.8) | 38 (28.8) | 0.999 | |
| Age at cognitive assessment (years)† | 77.4±9.4 | 77.4±9.4 | 0.953 | |
| Women† | 71 (53.8) | 71 (53.8) | 0.999 | |
| Education | <High-school degree | 14 (10.6) | 10 (7.6) | 0.835 |
| High-school degree | 37 (28.0) | 38 (28.8) | ||
| Some college | 46 (34.9) | 50 (37.9) | ||
| ≥College degree | 35 (26.5) | 34 (25.8) | ||
| Current smokers | 27 (20.9) | 13 (9.9) | 0.014 | |
| Systolic blood pressure (mm Hg) | 146.3±21.9 | 139.4±19.4 | 0.007 | |
| Diastolic blood pressure (mm Hg) | 74.7±11.0 | 74.1±10.3 | 0.640 | |
| Hypertension treatment | 75 (57.7) | 74 (57.4) | 0.957 | |
| Prevalent diabetes | 30 (27.3) | 18 (15.4) | 0.028 | |
| Prevalent atrial fibrillation | 13 (9.9) | 8 (6.1) | 0.255 | |
| Prevalent cardiovascular disease | 43 (32.6) | 30 (22.7) | 0.074 | |
| Mild dementia at stroke | 7 (5.3) | 2 (1.5) | 0.172 | |
| Mild dementia at cognitive assessment | 20 (15.5) | 4 (3.0) | <0.001 | |
| MMSE score, median (25th, 75th percentile) | 28.0 (27, 29) | 28.0 (26, 29) | 0.110 | |
| Time difference between covariate assessment and date of stroke (years) |
2.90±4.91 | 3.08±4.92 | 0.758 | |
| ApoEε4 genotype | 21 (19.8) | 31 (26.1) | 0.268 | |
Values are n (%) or mean ± SD.
Abbreviations: MMSE=Mini-Mental State Examination; ApoE ε4=Apolipoprotein E ε4.
†Matching factor
Overall, stroke cases had lower scores on the majority of tests comprising the cognitive assessment (table 3). After adjustments for age, sex, education, cohort (Original or Offspring) and pre-stroke MMSE, statistically significant differences were found for tests of verbal and visual memory (LMi: β=−1.35±0.52; P=0.01; LMd: β=−1.23±0.56; P=0.03; VRi: β=−1.34±0.41; P=0.001; VRd: β=−1.04±0.42; P=0.014), verbal learning-immediate (PASi: β=−1.29±0.49; P=0.010), abstract reasoning (SIM: β=−1.71±0.67; P=0.011), language (BNT: β=−0.29±0.07; P=<0.001), executive function (DSB: β=−0.62±0.19; P=0.001 and COWAT: β=−7.33±1.66; P=<0.001) and visuospatial and motor skills (block design: β=−3.15±0.94; P=0.001). Stroke was not significantly related to tests of delayed verbal learning (PASd), attention (DSF), visual perception (HVOT) or to tests of pre-morbid intelligence (WAIS-R and WRAT).
Table 3.
Association of clinical stroke with cognitive performance
| Outcome | Cases | Controls | Model 1† | Model 2‡ | |||||
|---|---|---|---|---|---|---|---|---|---|
| N | Mean ± SD | N | Mean ± SD | P-value | ß±SE | P-value | ß±SE | P-value | |
| LMi | 129 | 7.2±4.7 | 129 | 9.0±4.0 | 0.001 | −1.35±0.52 | 0.010 | −1.27±0.60 | 0.035 |
| LMd | 125 | 5.9±4.8 | 125 | 7.8±4.3 | 0.002 | −1.23±0.56 | 0.029 | −1.18±0.66 | 0.075 |
| VRi | 119 | 4.3±3.5 | 119 | 6.1±3.4 | <0.001 | −1.34±0.41 | 0.001 | −1.03±0.47 | 0.028 |
| VRd | 115 | 3.3±3.3 | 115 | 4.8±3.5 | 0.001 | −1.04±0.42 | 0.014 | −0.78±0.49 | 0.110 |
| PASi | 114 | 10.9±4.3 | 114 | 12.4±3.4 | 0.005 | −1.29±0.49 | 0.010 | −1.31±0.57 | 0.023 |
| PASd | 27 | 7.2±1.8 | 27 | 7.8±1.6 | 0.210 | −0.62±0.45 | 0.177 | −0.64±0.57 | 0.274 |
| SIM | 117 | 10.8±5.9 | 117 | 13.2±5.2 | <0.001 | −1.71±0.67 | 0.011 | −1.33±0.74 | 0.076 |
| DSF | 128 | 5.7±1.9 | 128 | 6.1±1.2 | 0.063 | −0.23±0.20 | 0.260 | −0.15±0.23 | 0.531 |
| DSB | 122 | 3.8±1.6 | 122 | 4.6±1.2 | <0.001 | −0.62±0.19 | 0.001 | −0.53±0.21 | 0.015 |
| HVOT* | 29 | −2.4±0.7 | 29 | −2.0±0.6 | 0.049 | −0.24±0.13 | 0.072 | −0.23±0.13 | 0.090 |
| BNT* | 119 | −0.7±0.7 | 119 | −0.4±0.5 | <0.001 | −0.29±0.07 | <0.001 | −0.27±0.08 | 0.002 |
| COWAT | 114 | 23.1±13.2 | 114 | 32.0±11.9 | <0.001 | −7.33±1.66 | <0.001 | −7.36±1.87 | <0.001 |
| Block design | 86 | 16.3±7.7 | 86 | 19.9±5.7 | <0.001 | −3.15±0.94 | 0.001 | −3.02±1.06 | 0.005 |
| WAIS-R | 56 | 16.5±6.1 | 56 | 18.3±4.1 | 0.071 | −1.24±0.79 | 0.120 | −0.64±0.93 | 0.496 |
| WRAT* | 27 | −2.5±0.5 | 27 | −2.2±0.4 | 0.070 | −0.23±0.13 | 0.075 | −0.21±0.15 | 0.170 |
Variables are log-transformed.
Model 1: adjusted for age, sex, cohort (Original/Offspring), education and pre-stroke Mini-Mental State Examination (MMSE)
Model 2: Model 1 + systolic blood pressure, diabetes, cardiovascular disease, atrial fibrillationand current smoking.
Abbreviations: SD = standard deviation; SE = standard error; LMi = Logical Memory - immediate recall; LMd = Logical Memory –delayed recall; VRi = Visual Reproductions - immediate recall; VRd = Visual Reproductions –delayed recall; PASi = Paired Associates-intermediate recall; PASd= Paired Associates- delayed recall; SIM = Similarities test; DSF = Digit Span Forward; DFB = Digit Span Backward; HVOT = Hooper Visual Organization Test; BNT = Boston Naming Test; COWAT = Controlled oral association fluency (phonemic fluency F-A-S); WAIS-R = Wechsler Adult Intelligence Scale- Revised; WRAT=Wide Range Achievement test.
After further adjustment for vascular risk factors, most associations were attenuated, except the test of immediate verbal learning (PASi) and a test of verbal fluency (COWAT) which remained strongly associated with stroke. While the associations of stroke with LMd, VRd and SIM were no longer significant after the adjustment, the associations with LMi (β=−1.27±0.60; P=0.035), VRi (β=−1.03±0.47; P=0.028), PAS (β=−1.31±0.57; P=0.023), DSB (β=−0.53±0.21; P=0.015), BNT (β=−0.27±0.08; P=0.002), COWAT (β=−7.36±1.87; P=<0.001) and block design (β=−3.02±1.06; P=0.005) remained statistically significant (table 3). Secondary analysis excluding persons with mild cognitive impairment at the time of stroke/ index date produced similar results, except for LMi which remained significant when adjusting for age, sex, cohort, education and pre-stroke MMSE but not after additional adjustment for vascular risk factors (table S1).
Discussion
Our findings suggest that stroke among dementia-free individuals may be followed by poor cognitive performance in multiple domains, independently of pre-stroke cognitive function or vascular risk factors. Individuals with a verified clinical stroke performed worse in all cognitive domains assessed. After accounting for pre-stroke cognition and vascular risk factors, stroke was inversely related to measures of short-term episodic memory, visuospatial skills, language and executive function.
Stroke increases the risk of Alzheimer’s disease18, dementia4-6 and mild cognitive impairment.19-21 However, the influence of stroke on performance in specific cognitive tests among a representative community sample of dementia-free individuals has not been adequately studied. To our knowledge, two studies have previously related stroke to continuous measures of cognitive function.22, 23 In both these studies, however, history of stroke was determined through self-report with a subsequent confirmation through medical records, and vascular risk factors could be assessed only after stroke occurred, thus exposing the analyses to potential biases. Most importantly, these studies have not accounted for the potential variance in cognitive function prior to stroke. Indeed, previous literature suggests an association between cognitive performance in various domains and risk of future stroke, independently of vascular risk factors. 7, 8, 24
Our findings confirm the intuitive suggestion that stroke itself would directly affect subsequent cognitive function in dementia-free individuals, as opposed to being merely a marker of the effect of underlying vascular risk factors which these two conditions share.2 Adjustment for vascular risk factors only slightly attenuated the associations of stroke with cognitive measures, indicating a minor role of these risk factors in the presence of stroke. This finding is strengthened by a previous Framingham study of dementia after stroke in which vascular risk factors did not alter the magnitude of association between stroke and subsequent risk of clinical dementia. 4 In addition, two systematic reviews of factors associated with post-stroke dementia have shown that the direct effect of stroke on risk of dementia is substantial and immediate, and is over and above the risk from pre-stroke vascular risk factors.5, 6 The importance of stroke itself on dementia risk has also been demonstrated in prior studies by the fact that stroke characteristics such as severity, location, type, multiple strokes and volume of infarct are important determinants of dementia after stroke,6, 25 and by the findings that the multi-domain pattern of deficits after stroke is more pronounced than that seen after TIA and acute coronary events, despite similar overall vascular risk burdens.26, 27 The cognitive function of some of the individuals with poorer cognitive performance ~6 months after stroke may slowly decline, even in the absence of new clinically apparent ischemic events.28 The mechanisms through which stroke can cause subtle cognitive deficits and gradual cognitive decline are not completely clear. An initial stroke increases the likelihood of subsequent cerebrovascular events, and these could contribute to further decline in cognitive performance. It is also possible that stroke causes an abrupt reduction in structural reserve which in turn results in an earlier manifestation of clinical cognitive decline. In addition, cerebral ischemia could potentiate the neurodegenerative process. Nevertheless, we cannot rule-out the possibility that factors prior to stroke such as lower cognitive reserve or a subclinical vascular brain injury resulting in worse cognitive function among the stroke cases placed them at higher risk of both stroke and clinical dementia. This possibility, though, is less plausible, as we did not observe significant differences in pre-stroke MMSE and in prevalence of mild cognitive impairment between cases and controls.
In the present study, individuals who experienced clinical stroke demonstrated poorer cognitive performance across all domains tested. Tasks of memory and executive function as well as measures of language, visuospatial and motor skills were related to the stroke, while tasks associated with retention such as VRd and PASd, with reasoning, visual perception and pre-morbid intelligence were not. The fact that the association of COWAT, a measure of executive function, with stroke was not attenuated after controlling for vascular risk factors emphasizes the large impact of stroke on this cognitive domain in the current sample as compared to the impact of stroke risk factors. DSF, although a measure of attention, did not significantly differ between cases and control, probably because it is not demanding enough and thus does not have the sensitivity to distinguish between these groups. Our findings may suggest a pattern of cognitive deficits which ismore indicative of frontal lobe functional disturbance, also characterizing vascular cognitive impairment in prior studies that did not study matched controls.29, 30 However, unlike Knopman et al. who demonstrated an association of history of stroke only with non-amnestic mild cognitive impairment and with impairment in non-memory cognitive domains,23 stroke in the current study was also associated with a deficit in memory, which is also supported by other studies.31
Strengths of our study are as follows: first, this is the first study of cognitive function after stroke that thoroughly accounts for pre-stroke dementia status and uses a comprehensive cognitive assessment. Second, strokes were carefully verified in a prospective manner. Third, all the vascular risk factors adjusted for were measured before stroke had occurred. Although many individuals were excluded from this study, we were able to show that this was mainly due to their moving to a different geographic location and was unrelated to stroke severity or to other baseline characteristics. Yet, there was not enough statistical power to further examine the effect of different types, severities or location of stroke, as well as concomitant magnetic resonance imaging indices on subsequent cognitive performance. Stroke characteristics such as location and side may explain the effect of stroke on the cognitive domains found in this study through stroke outcomes other than cognitive impairment such as language problems. However, because stroke types and sides do not differ between participants included in the study and other FHS participants with stroke, we suggest that the cognitive domains affected by stroke in the current study are representative of the general population of stroke patients. Moreover, we cannot rule out the possibility of residual confounding by factors available only on a small proportion of participants such as lipid subtype profile and statin use.
In post-stroke settings, a careful screening for executive function deficits is needed in order to further assess the impact of these cognitive deficits on function, instrumental activities of daily living and risk of subsequent stroke. Moreover, rehabilitation protocols should emphasize the need for cognitive screening in stroke patients.
Supplementary Material
Acknowledgments
This work was supported by the dedication of the Framingham Heart Study participants, the National Heart, Lung and Blood Institute’s Framingham Heart Study (Contract No. N01-HC-25195) and by grants from the National Institute of Neurological Disorders and Stroke (NS17950), the National Heart, Lung and Blood Association (HL93029, U01HL 096917) and the National Institute of Aging (AG08122, AG16495, AG033193, AG031287, P30AG013846). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke, the National Heart Lung and Blood Institute, the National Institute of Aging or the National Institutes of Health.
Source of funding: Supported by National Institute of Neurological Disorders and Stroke (NS17950), the National Heart, Lung and Blood Association (HL93029, U01HL 096917) and the National Institute of Aging (AG08122, AG16495, AG033193, AG031287, P30AG013846).
Footnotes
Conflicts of interests: None declared.
References
- 1.Gorelick PB, Scuteri A, Black SE, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42:2672–713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gorelick PB. Risk factors for vascular dementia and Alzheimer disease. Stroke. 2004;35:2620–2. doi: 10.1161/01.STR.0000143318.70292.47. [DOI] [PubMed] [Google Scholar]
- 3.Elias MF, Sullivan LM, D’Agostino RB, et al. Framingham stroke risk profile and lowered cognitive performance. Stroke. 2004;35:404–9. doi: 10.1161/01.STR.0000103141.82869.77. [DOI] [PubMed] [Google Scholar]
- 4.Ivan CS, Seshadri S, Beiser A, et al. Dementia after stroke: the Framingham Study. Stroke. 2004;35:1264–8. doi: 10.1161/01.STR.0000127810.92616.78. [DOI] [PubMed] [Google Scholar]
- 5.Savva GM, Stephan BC. Epidemiological studies of the effect of stroke on incident dementia: a systematic review. Stroke. 2010;41:e41–6. doi: 10.1161/STROKEAHA.109.559880. [DOI] [PubMed] [Google Scholar]
- 6.Pendlebury ST, Rothwell PM. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: a systematic review and meta-analysis. Lancet Neurol. 2009;8:1006–18. doi: 10.1016/S1474-4422(09)70236-4. [DOI] [PubMed] [Google Scholar]
- 7.Elkins JS, Knopman DS, Yaffe K, Johnston SC. Cognitive function predicts first-time stroke and heart disease. Neurology. 2005;64:1750–5. doi: 10.1212/01.WNL.0000161850.01792.77. [DOI] [PubMed] [Google Scholar]
- 8.DeFries T, Avendano M, Glymour MM. Level and change in cognitive test scores predict risk of first stroke. J Am Geriatr Soc. 2009;57:499–505. doi: 10.1111/j.1532-5415.2008.02132.x. [DOI] [PubMed] [Google Scholar]
- 9.Wiberg B, Lind L, Kilander L, Zethelius B, Sundelof JE, Sundstrom J. Cognitive function and risk of stroke in elderly men. Neurology. 2010;74:379–85. doi: 10.1212/WNL.0b013e3181ccc516. [DOI] [PubMed] [Google Scholar]
- 10.Kase CS, Wolf PA, Kelly-Hayes M, Kannel WB, Beiser A, D’Agostino RB. Intellectual decline after stroke: the Framingham Study. Stroke. 1998;29:805–12. doi: 10.1161/01.str.29.4.805. [DOI] [PubMed] [Google Scholar]
- 11.Dawber TR, Meadors GF, Moore FE., Jr Epidemiological approaches to heart disease: the Framingham Study. Am J Public Health Nations Health. 1951;41:279–81. doi: 10.2105/ajph.41.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feinleib M, Kannel WB, Garrison RJ, McNamara PM, Castelli WP. The Framingham Offspring Study. Design and preliminary data. Prev Med. 1975;4:518–25. doi: 10.1016/0091-7435(75)90037-7. [DOI] [PubMed] [Google Scholar]
- 13.Seshadri S, Wolf PA, Beiser A, et al. Lifetime risk of dementia and Alzheimer’s disease. The impact of mortality on risk estimates in the Framingham Study. Neurology. 1997;49:1498–504. doi: 10.1212/wnl.49.6.1498. [DOI] [PubMed] [Google Scholar]
- 14.Diagnostic and statistical manual of mental disorders : DSM-IV. 4th ed American Psychiatric Association; Washington, D.C.: 1994. [Google Scholar]
- 15.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–94. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 16.Au R, Seshadri S, Wolf PA, et al. New norms for a new generation: cognitive performance in the framingham offspring cohort. Exp Aging Res. 2004;30:333–58. doi: 10.1080/03610730490484380. [DOI] [PubMed] [Google Scholar]
- 17.Farmer ME, White LR, Kittner SJ, et al. Neuropsychological test performance in Framingham: a descriptive study. Psychol Rep. 1987;60:1023–40. doi: 10.1177/0033294187060003-201.1. [DOI] [PubMed] [Google Scholar]
- 18.Honig LS, Tang MX, Albert S, et al. Stroke and the risk of Alzheimer disease. Arch Neurol. 2003;60:1707–12. doi: 10.1001/archneur.60.12.1707. [DOI] [PubMed] [Google Scholar]
- 19.Petrovitch H, White L, Masaki KH, et al. Influence of myocardial infarction, coronary artery bypass surgery, and stroke on cognitive impairment in late life. Am J Cardiol. 1998;81:1017–21. doi: 10.1016/s0002-9149(98)00082-4. [DOI] [PubMed] [Google Scholar]
- 20.Mariani E, Monastero R, Ercolani S, et al. Vascular risk factors in mild cognitive impairment subtypes. Findings from the ReGAl project. Dement Geriatr Cogn Disord. 2007;24:448–56. doi: 10.1159/000110653. [DOI] [PubMed] [Google Scholar]
- 21.Srikanth VK, Anderson JF, Donnan GA, et al. Progressive dementia after first-ever stroke: a community-based follow-up study. Neurology. 2004;63:785–92. doi: 10.1212/01.wnl.0000137042.01774.33. [DOI] [PubMed] [Google Scholar]
- 22.Reitz C, Luchsinger JA, Tang MX, Manly J, Mayeux R. Stroke and memory performance in elderly persons without dementia. Arch Neurol. 2006;63:571–6. doi: 10.1001/archneur.63.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Knopman DS, Roberts RO, Geda YE, et al. Association of prior stroke with cognitive function and cognitive impairment: a population-based study. Arch Neurol. 2009;66:614–9. doi: 10.1001/archneurol.2009.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu L, Fratiglioni L, Guo Z, Winblad B, Viitanen M. Incidence of stroke in relation to cognitive function and dementia in the Kungsholmen Project. Neurology. 2000;54:2103–7. doi: 10.1212/wnl.54.11.2103. [DOI] [PubMed] [Google Scholar]
- 25.Sachdev PS, Brodaty H, Valenzuela MJ, et al. Clinical determinants of dementia and mild cognitive impairment following ischaemic stroke: the Sydney Stroke Study. Dement Geriatr Cogn Disord. 2006;21:275–83. doi: 10.1159/000091434. [DOI] [PubMed] [Google Scholar]
- 26.Volonghi I, Pendlebury ST, Welch SJ, Mehta Z, Rothwell PM. Cognitive outcomes after acute coronary syndrome: a population based comparison with transient ischaemic attack and minor stroke. Heart. 2013;99:1509–14. doi: 10.1136/heartjnl-2013-304207. [DOI] [PubMed] [Google Scholar]
- 27.Pendlebury ST, Mariz J, Bull L, Mehta Z, Rothwell PM. MoCA, ACE-R, and MMSE versus the National Institute of Neurological Disorders and Stroke-Canadian Stroke Network Vascular Cognitive Impairment Harmonization Standards Neuropsychological Battery after TIA and stroke. Stroke. 2012;43:464–9. doi: 10.1161/STROKEAHA.111.633586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sachdev PS, Brodaty H, Valenzuela MJ, Lorentz LM, Koschera A. Progression of cognitive impairment in stroke patients. Neurology. 2004;63:1618–23. doi: 10.1212/01.wnl.0000142964.83484.de. [DOI] [PubMed] [Google Scholar]
- 29.Sachdev PS, Brodaty H, Valenzuela MJ, et al. The neuropsychological profile of vascular cognitive impairment in stroke and TIA patients. Neurology. 2004;62:912–9. doi: 10.1212/01.wnl.0000115108.65264.4b. [DOI] [PubMed] [Google Scholar]
- 30.Kandiah N, Narasimhalu K, Lee J, Chen CL. Differences exist in the cognitive profile of mild Alzheimer’s disease and subcortical ischemic vascular dementia. Dement Geriatr Cogn Disord. 2009;27:399–403. doi: 10.1159/000210387. [DOI] [PubMed] [Google Scholar]
- 31.Snaphaan L, de Leeuw FE. Poststroke memory function in nondemented patients: a systematic review on frequency and neuroimaging correlates. Stroke; a journal of cerebral circulation. 2007;38:198–203. doi: 10.1161/01.STR.0000251842.34322.8f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


