Abstract
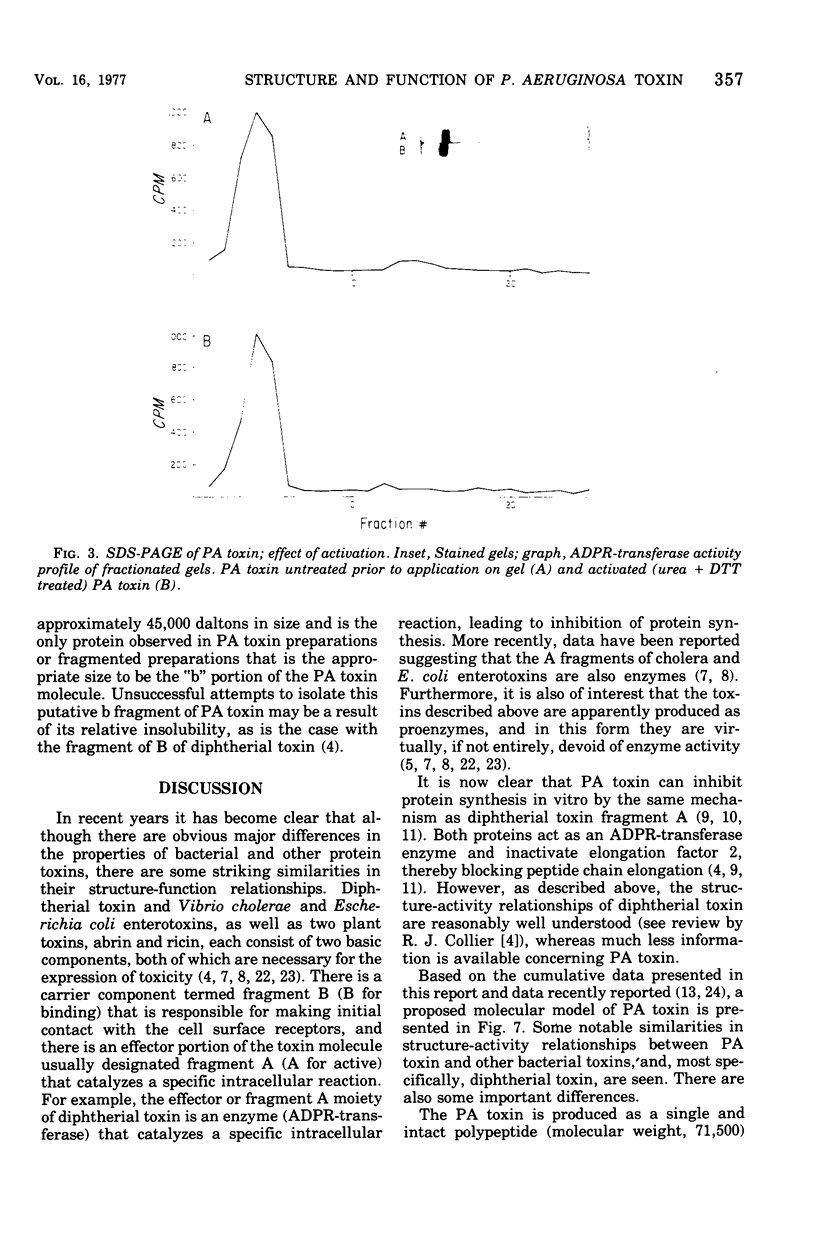
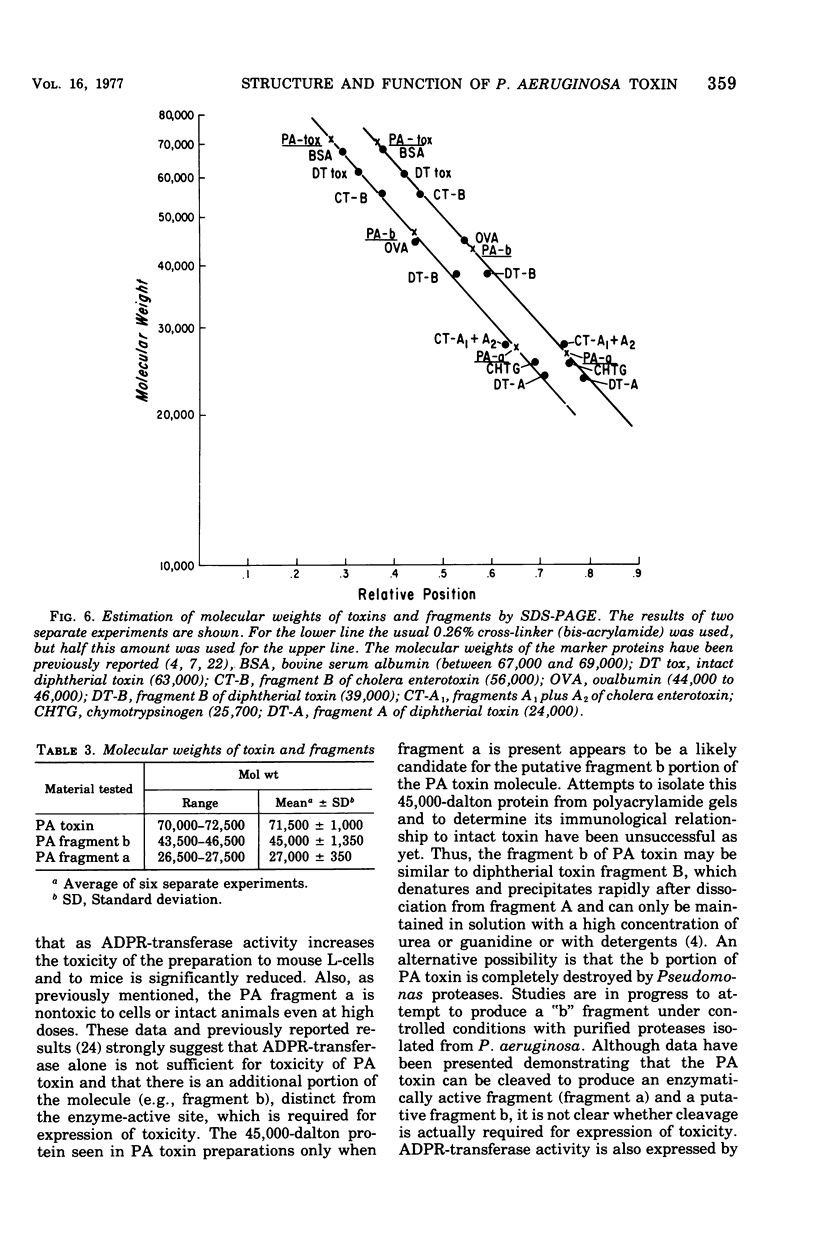
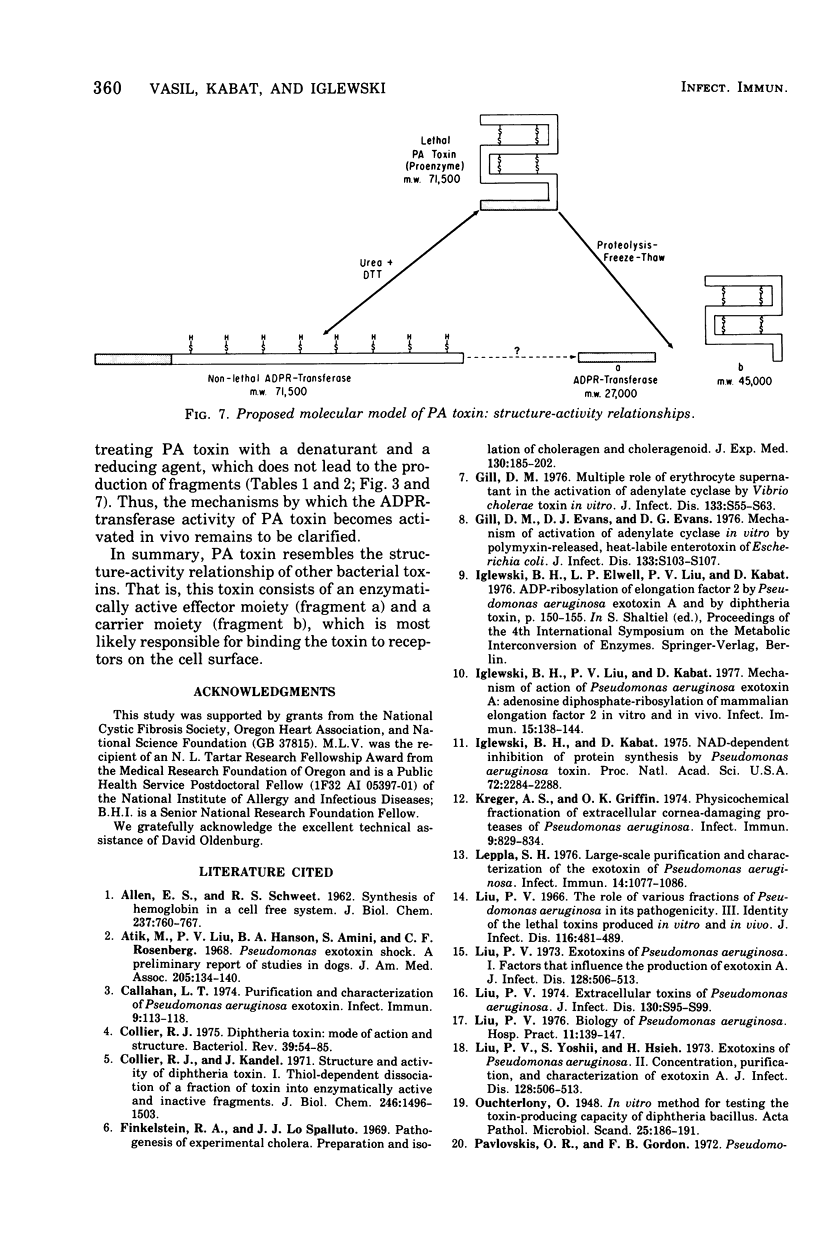
The relation of the structure of Pseudomonas aeruginosa exotoxin A (PA toxin) to its enzymatic activity (adenosine 5'-diphosphate-ribosyl transferase) in vitro and to its toxicity in vivo was examined. PA toxin is produced as a single polypeptide chain with a molecular weight of about 71,500. PA toxin is produced by Pseudomonas as a toxic proenzyme that lacks enzymatic activity. Adenosine 5'-diphosphate-ribosyl transferase activity is expressed when the molecule is denatured and reduced or when its is cleaved by Pseudomonas proteases to yield an enzymatically active 27,000-dalton fragment (fragment a). A 45,000-dalton protein is tentatively identified as the enzymatically inactive fragment b of PA toxin. Enzymatically active forms of the toxin lack toxicity for mouse L-cells or mouse lethality. Thus, it is concluded that the native toxin proenzyme is required for toxicity and that a structural rearrangement must precede its intracellular activity.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLEN E. H., SCHWEET R. S. Synthesis of hemoglobin in a cell-free system. I. Properties of the complete system. J Biol Chem. 1962 Mar;237:760–767. [PubMed] [Google Scholar]
- Atik M., Liu P. V., Hanson B. A., Amini S., Rosenberg C. F. Pseudomonas exotoxin shock. A preliminary report of studies in dogs. JAMA. 1968 Jul 15;205(3):134–140. doi: 10.1001/jama.205.3.134. [DOI] [PubMed] [Google Scholar]
- Callahan L. T., 3rd Purification and characterization of Pseudomonas aeruginosa exotoxin. Infect Immun. 1974 Jan;9(1):113–118. doi: 10.1128/iai.9.1.113-118.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier R. J. Diphtheria toxin: mode of action and structure. Bacteriol Rev. 1975 Mar;39(1):54–85. doi: 10.1128/br.39.1.54-85.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier R. J., Kandel J. Structure and activity of diphtheria toxin. I. Thiol-dependent dissociation of a fraction of toxin into enzymically active and inactive fragments. J Biol Chem. 1971 Mar 10;246(5):1496–1503. [PubMed] [Google Scholar]
- Finkelstein R. A., LoSpalluto J. J. Pathogenesis of experimental cholera. Preparation and isolation of choleragen and choleragenoid. J Exp Med. 1969 Jul 1;130(1):185–202. doi: 10.1084/jem.130.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill D. M. Multiple roles of erythrocyte supernatant in the activation of adenylate cyclase by Vibrio cholerae toxin in vitro. J Infect Dis. 1976 Mar;133 (Suppl):55–63. doi: 10.1093/infdis/133.supplement_1.s55. [DOI] [PubMed] [Google Scholar]
- Iglewski B. H., Kabat D. NAD-dependent inhibition of protein synthesis by Pseudomonas aeruginosa toxin,. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2284–2288. doi: 10.1073/pnas.72.6.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglewski B. H., Liu P. V., Kabat D. Mechanism of action of Pseudomonas aeruginosa exotoxin Aiadenosine diphosphate-ribosylation of mammalian elongation factor 2 in vitro and in vivo. Infect Immun. 1977 Jan;15(1):138–144. doi: 10.1128/iai.15.1.138-144.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreger A. S., Griffin O. K. Physicochemical fractionation of extracellular cornea-damaging proteases of Pseudomonas aeruginosa. Infect Immun. 1974 May;9(5):828–834. doi: 10.1128/iai.9.5.828-834.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppla S. H. Large-scale purification and characterization of the exotoxin of Pseudomonas aeruginosa. Infect Immun. 1976 Oct;14(4):1077–1086. doi: 10.1128/iai.14.4.1077-1086.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P. V. Exotoxins of Pseudomonas aeruginosa. I. Factors that influence the production of exotoxin A. J Infect Dis. 1973 Oct;128(4):506–513. doi: 10.1093/infdis/128.4.506. [DOI] [PubMed] [Google Scholar]
- Liu P. V. Exotoxins of Pseudomonas aeruginosa. I. Factors that influence the production of exotoxin A. J Infect Dis. 1973 Oct;128(4):506–513. doi: 10.1093/infdis/128.4.506. [DOI] [PubMed] [Google Scholar]
- Liu P. V. Extracellular toxins of Pseudomonas aeruginosa. J Infect Dis. 1974 Nov;130 (Suppl)(0):S94–S99. doi: 10.1093/infdis/130.supplement.s94. [DOI] [PubMed] [Google Scholar]
- Liu P. V. The roles of various fractions of Pseudomonas aeruginosa in its pathogenesis. 3. Identity of the lethal toxins produced in vitro and in vivo. J Infect Dis. 1966 Oct;116(4):481–489. doi: 10.1093/infdis/116.4.481. [DOI] [PubMed] [Google Scholar]
- Lui P. V. Biology of Pseudomonas aeruginosa. Hosp Pract. 1976 Jan;11(1):139–147. [PubMed] [Google Scholar]
- Pavlovskis O. R., Gordon F. B. Pseudomonas aeruginosa exotoxin: effect on cell cultures. J Infect Dis. 1972 Jun;125(6):631–636. doi: 10.1093/infdis/125.6.631. [DOI] [PubMed] [Google Scholar]
- Pavlovskis O. R., Voelker F. A., Shackelford A. H. Pseudomonas aeruginosa exotoxin in mice: histopathology and serum enzyme changes. J Infect Dis. 1976 Mar;133(3):253–259. doi: 10.1093/infdis/133.3.253. [DOI] [PubMed] [Google Scholar]
- Refsnes K., Olsnes S., Pihl A. On the toxic proteins abrin and ricin. Studies of their binding to and entry into Ehrlich ascites cells. J Biol Chem. 1974 Jun 10;249(11):3557–3562. [PubMed] [Google Scholar]
- Vasil M. L., Liu P. V., Iglewski B. H. Temperature-dependent inactivating factor of Pseudomonas aeruginosa exotoxin A. Infect Immun. 1976 May;13(5):1467–1472. doi: 10.1128/iai.13.5.1467-1472.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]