Abstract
The insulin-like growth factor type 1 receptor (IGF-1R) plays an essential role in the development of numerous cancers. Figitumumab (CP) is not only a monocloncal antibody, it also has agonist activity on IGF-1R. The antitumor activity of CP in esophageal squamous cell carcinoma (ESCC) is still unclear. In our study, we identified IGF-1R as an independent prognostic factor in ESCC patients, and investigated the antitumor effects of CP in ESCC cell lines. CP suppressed tumor growth and sensitized cells to chemotherapeutic drugs. In addition, CP inhibited cell proliferation, migration, colony forming activity and anti-apoptosis induced by IGF-1. Our results showed that CP not only inhibited IGF-1 induced receptor autophosphorylation and downstream signaling, but also triggered β-arrestin1 and G protein-coupled receptor kinases (GRKs) mediated ERK1/2 activation, indicating CP as a biased agonist for IGF-1R. Inhibition of ERK1/2 enhanced the antitumor activity of CP. Furthermore, CP was a more powerful agonist for IGF-1R down-regulation than IGF-1, and dysregulation of β-arrestin1 and GRKs affected this down-regulation. Thus, we demonstrated antitumor activities of CP on ESCC, and as a biased agonist, CP induced ERK1/2 activation and receptor down-regulation required β-arrestin1 and GRKs, suggesting a promising role for targeting IGF-1R in ESCC.
Esophageal cancer is characterized by its apparent geographical distribution, and mostly occurs in eastern Asia1,2,3. In China, it is the fourth most common cause of mortality, whereby 95% of esophageal cancers are pathologically diagnosed as esophageal squamous cell carcinoma (ESCC)2,4. At the time of diagnosis, 50% of patients have either unresectable tumors or radiographically detectable metastases. Even after complete surgical resection, the 5 year survival is still unsatisfactory, and in the cases for unresectable ESCC tumors, treatments are limited and less effective3,5,6.
Molecular targeted therapy supplies a useful and hopeful approach to combat various tumors7. The insulin-like growth factor (IGF) signaling pathway is a promising candidate8. Elevations of serum IGF-I and/or IGF binding protein 3 (IGFBP3) not only increase the risk of developing several cancers, but also correlate with cancer patients' worse survival9,10,11. The insulin-like growth factor type 1 receptor (IGF-1R) belongs to the receptor tyrosine kinases (RTK) family12. Following ligand binding, the IGF-1R is autophosphorylated and then activates multiple downstream signaling pathways, including PI3-kinase/Akt and mitogen activated protein kinases (MAPKs), which are considered to be essential for cell proliferation, migration, metastasis and anti-apoptosis13,14,15. Furthermore, the IGF-1R knockout mouse embryonic fibroblast cells are resistance to malignant transformation by several common oncogenes, or viruses, indicating that IGF-1R plays a critical role in developing a cancer13. Unlike the EGFR or other RTKs, IGF-1R gene is seldom mutated in human cancers, but ectopic expression of IGF-1R are reported in many human malignancies13,16. In ESCC, both IGF-IR and its ligands are overexpressed in cancer tissues compared with the normal epithelium17,18. Except for the most abundant source of serum IGF-1, IGF-1 is produced by the parotid, palatine, and submandibular salivary glands in a free form, and continuously bathes the lumen of the esophagus19,20. The functions of IGF-I action may be underestimated and could play a more important role in esophageal malignancies19,20. All of above indicates IGF-1R to be a rather interesting and prominent target for anti-cancer therapy in ESCC.
Inhibition of IGF signaling with therapeutic intent can be achieved by several approaches, including blocking the ligand or receptor with neutralizing antibodies, or small molecular receptor kinase inhibitors which lead to growth inhibition, cell cycle arrest and apoptosis of vary tumor cells8,15. Figitumumab (CP-751871, CP) is a selective human IgG2 monoclonal antibody targeting the IGF-1R. The results of phase 2 clinical trials of CP were encouraging in the treatment of prostate, lung, breast, colorectal cancers and Ewing's sarcoma, however results of the phase 3 clinical trial was disappointing due to the adverse effects and discouraging responsiveness in unselected patients8,21. Hence, Pfizer has chosen to terminate clinical trials with CP, which is to say that, should we throw the baby out with the bathwater8?
In order to cover this gap between the promising results in vitro and the disappointing clinical results, it was indicated that the canonical paradigm of IGF-1R as a RTK was not efficient to explain the observations of the clinical results and the outcomes induced by IGF-1R activation. Under IGF-1 binding, IGF-1R can utilize the components of G protein coupled receptor (GPCR) signaling machineries, including heterotrimeric G proteins, β-arrestins, and GPCR kinases (GRKs) to activate various signaling cascades including the desensitization of the receptors and also the MAPK/Akt signaling pathways22,23,24. CP not only blocks the IGF-1R phosphorylation and downstream signaling pathways, it also mediates receptor internalization and degradation25,26. Furthermore, the binding of CP to IGF-1R induced β-arrestin1 dependent ERK1/2 activation, or so-called ‘β-arrestin1 biased signaling' in Ewing's sarcoma cell lines. Like the classification of agonists of GPCRs, CP is now considered as the ‘β-arrestin1 biased agonists' for IGF-1R25. However the antitumor effect and molecular mechanisms of CP in ESCC and whether the GRKs are involved in the desensitization of IGF-1R following CP binding are still unknown.
Results
Immunohistochemical analysis of IGF-1R in ESCC tissues
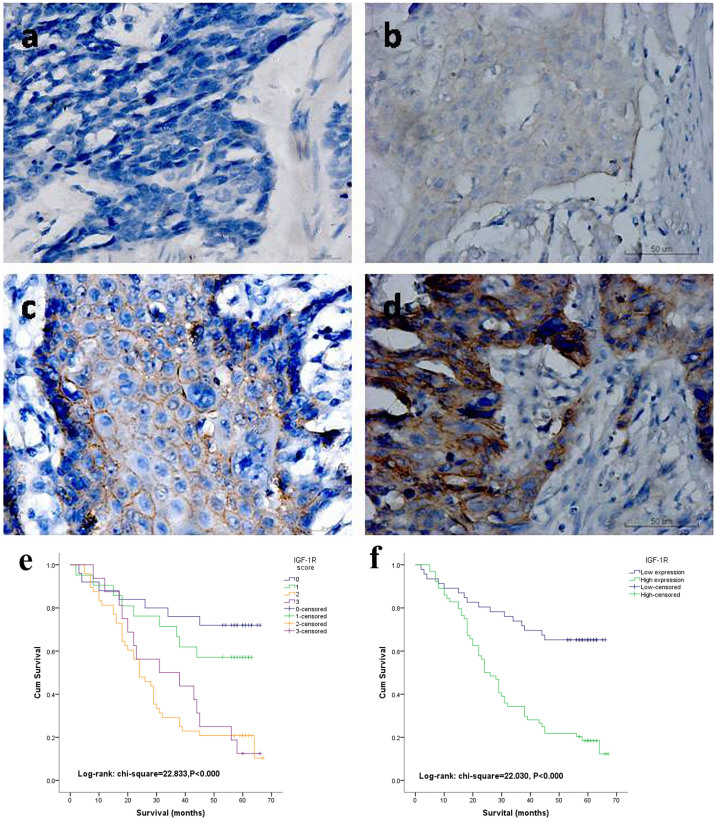
First, the expression of IGF-1R was determined in paraffin embedded ESCC tissues using immunohistochemical staining. 110 completely resected specimens from ESCC patients were obtained, whom of which, did not receive any preoperative chemoradiation therapies. There were 93 male and 17 female patients, whereby the mean age was 58.22 years. 25s of those patients did not exhibit expression of IGF-1R, whereas, 21 showed weak expression and, 48 showed moderate expression of IGF-1R. The remaining 16 patients exhibited strong expression of IGF-1R (Shown in Figures 1a, 1b, 1c and 1d). The patients were then classified into two groups; as low (including negative and weak expression of IGF-1R) and high (moderate and strong expression of IGF-1R included) expression groups for further analysis. The basic demographics of the patients stratified by IGF-1R expression are shown in Table 1. Expression of IGF-1R correlated with gender (P = 0.002), and no other correlations were found. The mean overall survival of low IGF-1R expression group was 46.46 ± 20.046 months compared with 30.89 ± 18.750 months of the high expression group. Clear distinctions were observed from the Kaplan-Meier survival curves in both of the four and two categories of IGF-1R expression (Shown in Figure 1e and 1f), and the P values were less than 0.001 for both. In multivariate Cox analysis for the patients' overall survival, IGF-1R expression together with lymph node metastasis and cell differentiation were independent and significant prognostic factors (Table 2).
Figure 1. Immunohistochemistry in resected human ESCC tumors.
(a) Negative expression of IGF-1R (original magnification x400). (b) Weak cytoplasmic and membranous expression of IGF-1R in ESCC cells (x400). (c) Moderate and mainly membranous expression of IGF-1R in ESCC cells (x400). (d) Strong membranous expression of IGF-1R in ESCC cells (x400). Kaplan–Meier life-table analyses of the overall survival (OS) rate according to four categories (e) and two categories (f) of IGF-1R expression levels (P<0.000 and 0.000 respectively).
Table 1. Patient characteristics.
| IGF-R expression | |||
|---|---|---|---|
| Characteristic | Low | High | P |
| Age | 0.417 | ||
| <65 | 23 | 37 | |
| ≥65 | 23 | 27 | |
| Gender | 0.002 | ||
| Male | 33 | 60 | |
| Female | 13 | 4 | |
| Cell differentiation | 0.569 | ||
| Well | 2 | 3 | |
| Moderate | 42 | 53 | |
| Poor | 2 | 6 | |
| Tumor location | 0.267 | ||
| Upper | 7 | 4 | |
| Middle | 26 | 37 | |
| Lower | 13 | 23 | |
| T stage | 0.446 | ||
| T1 | 6 | 4 | |
| T2 | 14 | 19 | |
| T3 | 26 | 41 | |
| Lymph node metastasis | 0.24 | ||
| No | 33 | 39 | |
| Yes | 13 | 25 | |
| TNM | 0.665 | ||
| I | 6 | 5 | |
| II | 31 | 41 | |
| III | 8 | 16 | |
| IV | 1 | 2 | |
| Tumor diameter(cm)Mean ± SD | 3.6 ± 1.72 | 4.0 ± 1.44 | 0.184 |
| OS(months)Mean ± SD | 46.46 ± 20.046 | 30.89 ± 18.750 | 0.000 |
Table 2. Multivariate proportional hazards (Cox) regression analyses according to overall survival.
| Overall Survival | ||
|---|---|---|
| Variables in the equation | P | Hazard ratio (95.0% CI) |
| IGF-1R(low versus high) | 0.002 | 2.962(1.636–5.363) |
| Lymph node metastasis(No versus yes) | 0.003 | 2.234(1.327–3.763) |
| Cell differentiation | ||
| Well | 0.056 | |
| Moderate | 0.384 | 1.877(0.455–7.736) |
| Poor | 0.058 | 4.578(0.949–22.081) |
Antitumor effects of CP in ESCC cell lines
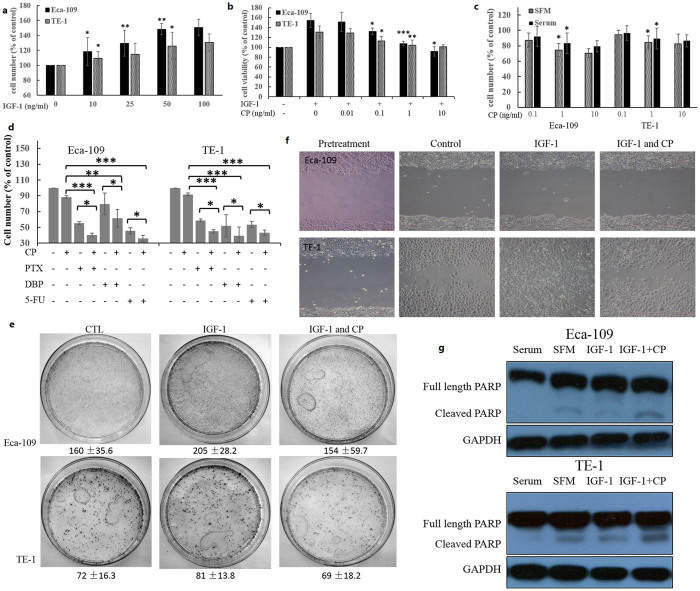
As 77.27% of the patients had expression of IGF-1R whereby the expression of IGF-1R was a strong predictor of patients' overall survival, the IGF-1R could be an interesting target in treating ESCC. Next, we utilized the CP to explore the antitumor effect of IGF-1R inhibition. First, we investigated the proliferation of the two ESCC cell lines, Eca-109 and TE-1, with addition of the IGF-1. Cells were incubated with varying doses of IGF-1 for 48 hours, and the effects on proliferation were measured using the CCK-8 assay. Both of the cell lines were responsive to IGF-1 stimulation, and Eca-109 cells seemed to be more sensitive to IGF-1 stimulation (Figure 2a). Cells were then pretreated with various doses of CP for 1 hour and then stimulated with 50 ng/ml IGF-1. CP was able to completely block the proliferation and growth effect induced by IGF-1 in both of the cell lines (Figure 2b). In addition, CP itself inhibited the cell proliferation of ESCC cells under both serum and serum free conditions (Figure 2c). It is indicated that IGF-1R activation mediates the resistance of chemotherapeutic therapies in various cancers17,27. Our results showed that CP increased the sensitivity of chemotherapeutic drugs such as paclitaxel (PTX), cisplatin (DBP) and 5-fluorouracil (5-FU) to both of the ESCC cell lines (Figure 2d).
Figure 2. Antitumor activity of CP on ESCC cell lines.
(a) Cells were plated, allowed to adhere and serum starvation for 12 hours, and then treated with indicated concentration of IGF-1 for 48 h. Number of viable cells are displayed as percentage of untreated cells. The SEM are displayed from 3 independent replicates. (b) Cells were handled as above, and then treated with 50 ng/ml IGF-1 and different concentrations of CP for 48 h. Number of cells are displayed as percentage of untreated cells. (c) Cells were plated, allowed to adhere overnight and then treated with indicated concentrations of CP in either SFM or with serum for 96 h. Number of cells are displayed as percentage of untreated cells. (d) Cells were plated, allowed to adhere, and serum starvation for 12 h. Cells were first treated with 1 ng/ml CP for 24 h, and then treated with paclitaxel (PTX), cisplatin (DBP) and 5-fluorouracil (5-FU) for another 24 h. Number of cells are displayed as percentage of untreated cells. (e) Cells (103/plate) were seeded onto 100 mm culture plates and incubated with indicated treatment for 14 days, then cell layer was methanol fixed and stained with 0.05% crystal violet solution containing 25% of methanol. (f) Cells were plated in six-well chambers, grown normally for 24 h and starved overnight. Cells were cut with a cell scraper and five images were captured along the cut surface. Additional images were captured 48 h later. (g) Cells were plated, allowed to adhere and serum starvation for 12 h. After 48 h of indicated treatment, cells were collected with LDS sample buffer and protein samples were analyzed by western blot for PARP and GAPDH. The student's t-test or one-way ANOVA were used for statistical analysis properly. *p<0.05; **p<0.01; ***p<0.001.
The wound-healing assay and colony formation assay were used to determine the migration and colony forming activity of the ESCC cells. CP inhibited IGF-1 induced colony forming activity for both of the cell lines and migration ability of TE-1 cells, but Eca-109 cells seemed to display little migration ability even with IGF-1 stimulation (Figure 2e and 2f). Immunobloting of cleaved PARP was also used to determine the apoptosis of the treated cells. The results showed that CP suppressed the IGF-1 induced anti-apoptotic effects of ESCC cell lines (Figure 2g).
CP competes with IGF-1 binding to the receptor and inhibits IGF-IR autophosphorylation
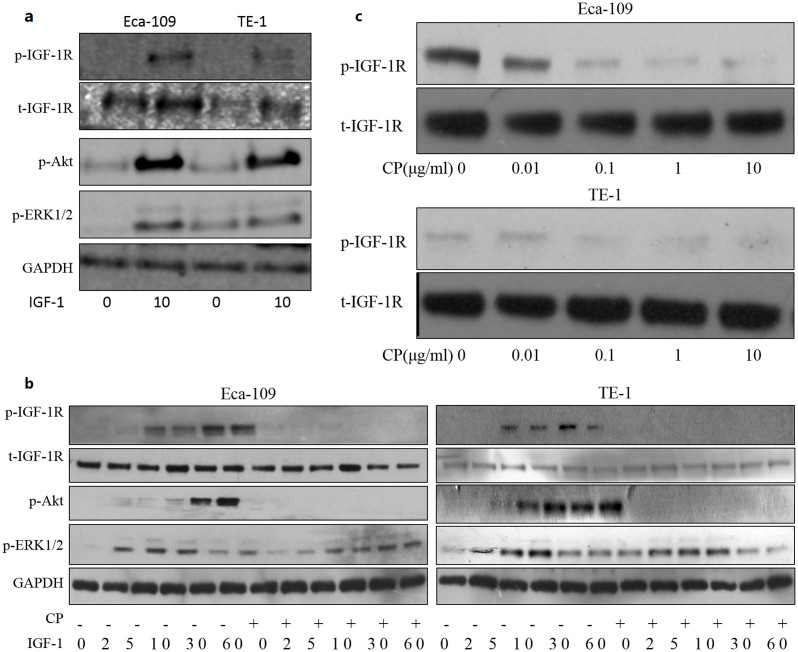
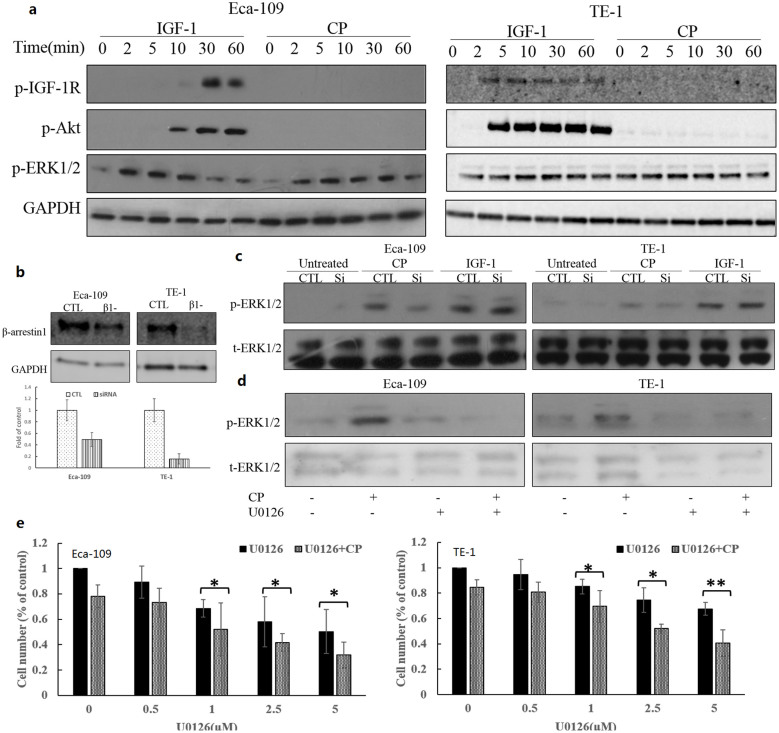
First, IGF-1R expression and responsive to IGF-1 stimulation of Eca-109 and TE-1 were investigated by stimulating the cells for 10 minutes. Eca-109 had a relative higher expression of IGF-1R. Both of the cell lines displayed phosphorylation of IGF-1R and Akt following IGF-1 stimulation. ERK1/2 were activated in both Eca-109 cells with low basal p-ERK1/2 and TE-1 with high level of basal p-ERK1/2 (Figure 3a). Next, effects of CP on tyrosine phosphorylation of IGF-1R β-subunit were determined. Figure 3b showed the effect of CP on IGF-IR autophosphorylation. Following preincubation with CP or PBS for 1 hour, cells were stimulated with IGF-I (50 ng/mL) at different time points. Cells were lysed and immunoblottings were used to detect the effect of the treatment. IGF-1 induced IGF-1R phosphorylation in a time dependent manner. Phosphorylation of IGF-1R continued up to 60 minutes, and the same occurred on phosphorylation of Akt. However, the ERK1/2 phosphorylation reached its peak at 10 minutes and then decreased markedly. CP was able to completely inhibit IGF-1 induced IGF-1R phosphorylation. Also, the downstream Akt phosphorylation of IGF-1R signaling was also completely blocked by CP treatment. However, the ERK1/2 phosphorylation was partially blocked and postponed in both of the cell lines. Furthermore, effects of CP were also studied in cells culturing with growth medium in order to mimic the physiological conditions in the human body. Cells were splitted into 24 well plates and allowed to attach overnight, then treated with vary concentrations of CP for 1 hour, cells were lysed and analyzed with immunoblotting. CP inhibited the phosphorylation of IGF-1R in a dose-dependent manner (Figure 3c). Thus, our results demonstrated that short-term treatment of CP suppressed IGF-1 and serum–induced phosphorylation of IGF-1R.
Figure 3. CP competes with IGF-1 binding to the receptor.
(a) Cells were plated, allowed to adhere and serum starvation for 12 hours. Cells were stimulated with 50 ng/ml IGF-1 for 10 minutes, protein samples were then collected and analyzed by immuboblotting. (b) Cells were plated, allowed to adhere and serum starvation for 12 hours. Cells were pretreated with CP or PBS for 1 h and then treated with IGF-1 for indicated times. Protein samples were collected and analyzed by western blot for p-IGF-1R, p-Akt, p-ERK1/2, t-IGF-1R and GAPDH. (c) Cells were plated, allowed to adhere overnight and then treated with indicated concentration of CP in growth medium. Protein samples were harvested and analyzed by western blot for p-IGF-1R and total-IGF-1R. Cropped blots were used in the figures and the gels were run under the same experimental conditions. Representative full length blots were shown in Supplementary information.
CP-induced IGF-1R down-regulation was partly dependent on β-arrestin1 binding to IGF-1R
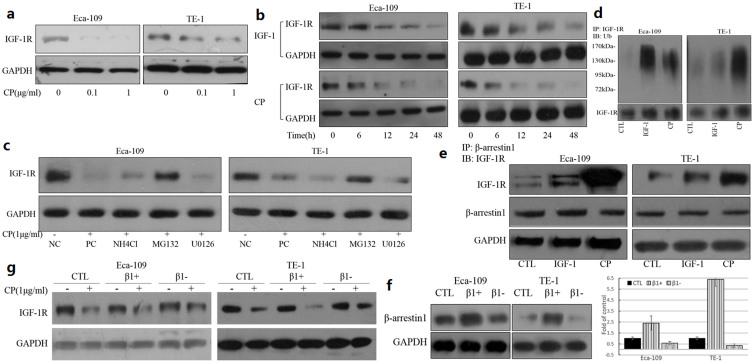
IGF-1 as well as several IGF-1R monoclonal antibodies were demonstrated to have the ability of down-regulating IGF-1R25,28. The next experiments were designed to investigate the effects of CP on IGF-1R down-regulation in ESCC cell lines. Cells were seeded into 24 well plates and serum starved overnight to abolish the effect of the serum, then stimulated with various doses of CP and at different time points. As indicated in figures 4a and 4b, CP induced IGF-1R down-regulation occurred in a dose- and time-dependent manner. CP was a more powerful inducer of IGF-1R down-regulation compared to IGF-1. To investigate whether the degradation was via a proteasome or lysosome pathway and whether the ERK1/2 phosphorylation was required for the down-regulation, MG132, NH4Cl and U0126 were used to inhibit the proteasome pathway, the lysosome pathway and the ERK1/2 activation respectively. Treatment with MG132 could reverse the CP induced IGF-1R down-regulation greatly, and the NH4Cl could also reverse the down-regulation effect partially (Figure 4c), suggesting that the degradation of IGF-1R induced by CP was mainly through the proteasome pathway.
Figure 4. CP-induced IGF-1R down-regulation was partly dependent on β-arrestin1.
IGF-1R was down-regulated by CP in a dose- and time- dependent manner. Cells were plated, allowed to adhere and serum starvation for 12 h. Cells were treated with indicated amounts of CP for 24 h (a), or with 50 ng/ml IGF-1 or 1 ug/ml CP for indicated time points (b), and the cells were lysed with LDS sample buffer. Protein samples were analyzed by western blot for IGF-1R and GAPDH. (c) Cells were handled as above, and treated the cells with lysosomal inhibitor (NH4Cl), proteasome inhibitor (MG132), MEK inhibitor (U0126) or PBS for 1 h. After 24 h of CP treatment, cells were lysed, and protein samples were analyzed by western blot for IGF-1R and GAPDH. NC, negative control; PC, positive control; (d) CP induced IGF-1R ubiquitination. Cells were plated, allowed to attach, serum starved for 12 h and stimulated with 50 ng/ml IGF-1 or 1 ug/ml CP for 10 minutes and the cells were lysed. IGF-1R was immunoprecipitated and the immunoprecipitated proteins analyzed by WB for ubiquitin and IGF-1R. (e) CP induced β-arrestin1 recruitment to the IGF-1R. Cells were handled as above. β-arrestin1 was immunoprecipitated and the immunoprecipitated proteins were analyzed by WB for IGF-1R. The whole lysates were analyzed by WB for β-arrestin1and GAPDH as a loading control. (f) and (g) β-arrestin1 involvement of CP induced degradation. Cells were plated, allowed to attach, and transiently transfected with plasmids expressing β-arrestin1 or β-arrestin1 siRNA for 48 h. Cells were serum starved for 12 h and then treated with CP for 24 h. Cells were lysed and protein samples were analyzed by WB for β-arrestin1, IGF-1R and GAPDH. Cropped blots were used in the figures and the gels were run under the same experimental conditions. Representative full length blots were shown in Supplementary information.
Receptor ubiquitination has been implicated in modulating IGF-1R activity by IGF-1 or other ligands widely23,25,29. We aimed to investigate whether the IGF-1R was ubiquitinated with CP. Cells were serum starved overnight, and treated with PBS, 50 ng/ml IGF-1 or 1 ug/ml CP for 10 minutes, cell lysates were harvested and IGF-1R was immunoprecipitated, whereby the ubiquitinations were analysed by immunoblotting. Ligand-dependent ubiquitination of the IGF-1R was clearly detected for both the IGF-1 and CP stimulation in the two cell lines (Figure 4d). Considering that β-arrestin1 is the adaptor protein for E3 ligase involved in IGF-1 induced IGF-1R ubiquitination, our further results indicated that CP was much more potent than IGF-1 in recruiting β- arrestin1 to the receptor in ESCC cell lines (Figure 4e). However, traditional co-IP protocol was used here, whereby antibody fragments and antigens were co-eluted, resulting in a strong band at 45–60 kDa for the heavy chain of antibody analyzed by WB. The molecular weight of β-arrestin1 is 49 kDa, indicating an overlap with the heavy chain of the antibody in WB. In addition, we hypothesized that the IP efficiencies were assumed to be constant. So the whole cell lysates were analyzed by WB for β- arrestin1 and GAPDH as a loading control in order to use the similar amount of total proteins for immunoprecipitation. Overexpression of β-arrestin1 by plasmid transfection enhanced the CP induced IGF-1R degradation and down-regulation of β-arrestin1 by siRNA transfection partially reversed the IGF-1R degradation (Figure 4f and 4g). Taken together, these experiments displayed that CP stimulated β-arrestin1 interaction with IGF-1R, and induced subsequent receptor ubiquitination and degradation via the proteasome pathway.
β-arrestin1 dependent ERK1/2 signaling activation indicates CP as a biased agonist for IGF-1R
It is reported that CP directly activates ERK1/2 signaling in a β-arrestin1 biased mechanism in Ewing's sarcoma cell lines25. We therefore investigated the direct effects of CP on IGF-1R signaling in ESCC cells by detection of the kinetics of IGF-1- or CP-mediated activation of the ERK1/2 pathway and Akt pathway downstream of IGF-1R signaling. Upon IGF-1 stimulation, the IGF-1R was gradually phosphorylated for up to 60 minutes, demonstrating an increase in its kinase activity. Kinetic of Akt phosphorylation was in accordance with phosphorylation of IGF-1R, whereby ERK1/2 phosphorylation decreased markedly after 10 minutes (Figures 3b and 5a). In the case of CP direct stimulation, IGF-1R and Akt phosphorylation were undetectable; however, clear ERK1/2 phosphorylation induced by CP were indicated in both of the ESCC cell lines (Figure 5a). ERK1/2 activation levels were lower and postponed compared with that of IGF-1 stimulated activation, suggesting that the majority of ERK1/2 phosphorylation was dependent on the IGF-1R kinase activity, and part of ERK1/2 activation was independent of IGF-1R kinase activation, possibly through a β-arrestin1 mediated mechanism. Eca-109 and TE-1 cells were then transfected with β-arrestin1 siRNA to down-regulate β-arrestin1 expression (Figure 5b). As demonstrated in figure 5c, CP induced ERK1/2 phosphorylation was greatly decreased in the absence of β-arrestin1, whereas IGF-1 induced ERK1/2 phosphorylation was slightly affected. These results indicated a CP-induced and β-arrestin1–mediated ERK1/2 signaling activation downstream of IGF-1R signaling transduction in ESCC cells.
Figure 5. β-arrestin1 dependent ERK1/2 signaling activation indicates CP as a biased agonist for IGF-1R.
(a) CP induced ERK1/2 activation.Cells were plated, allowed to adhere and serum starved for 12 hours. Cells were treated with IGF-1 or CP for indicated times and were lysed. Protein samples were collected and analyzed by western blot for p-IGF-1R, p-Akt, p-ERK1/2, t-IGF-1R and GAPDH. (b) and (c) β-arrestin1 involvement of CP induced ERK1/2 activation. Cells were plated and transfected with β-arrestin1 siRNA for 48 h. Transfection efficiency was detected by WB for β-arrestin1 and GAPDH. Cells were starved and treated with IGF-1 or CP for 10 min, and were then lysed. Protein samples were analyzed by WB for p-ERK1/2 and total-ERK1/2. CTL, control; si, β-arrestin1 siRNA transfected; (d) Cells were plated, allowed to attach, and serum starved for 12 h. Then cells were pretreated with MEK inhibitor (U0126) or PBS for 1 h, and treated with CP or not for 10 min. Cells were lysed and protein samples were analyzed by WB for p-ERK1/2 and t-ERK1/2. (e) Combinational effects between CP and U0126 treatment. Cells were plated, allowed to adhere, and serum starved for 12 h. Cells were treated with 1 ng/ml CP with indicated concentrations of U0126 for 48 h. Number of cells are displayed as percentage of untreated cells. One-way ANOVA were used for statistical analysis properly. *p<0.05; **p<0.01. Cropped blots were used in the figures and the gels were run under the same experimental conditions. Representative full length blots were shown in Supplementary information.
Considering the ability of ERK1/2 activation of CP, this may be responsible for the clinical resistance to CP when treating cancer patients; therefore we investigated the combination effect of inhibiting ERK1/2 by U0126 and CP treatment. With 1 uM U0126 pretreatment of the cells, the ERK1/2 phosphorylation induced by IGF-1 or CP was completely inhibited (Figure 5d). Inhibition of ERK1/2 made the cells more sensitive to CP treatment, especially for the TE-1 cells, which exhibited high basal ERK1/2 activation and were initially not so sensitive to CP treatment (Figure 5e). These data indicated promising combination of ERK1/2 inhibitors and CP in future clinical trials.
Involvement of GRK 2 and GRK 6 in CP induced signaling and receptor down-regulation further validated the existence of biased manner of IGF-1R signaling
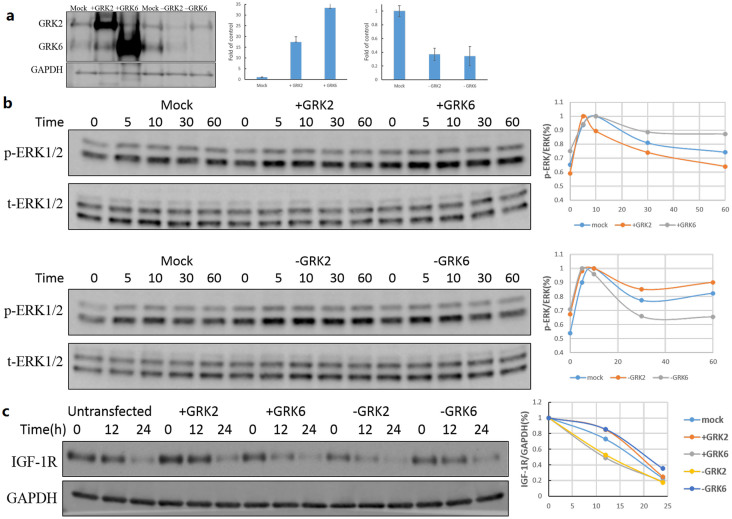
IGF-1 induced IGF-1R activation can not only utilize β-arrestin1 but also GRKs of GPCRs signaling machinery as regulators22,23,30. So, the next step was to investigate whether GRKs were involved in the effects of CP. It was indicated that GRK2 decreased the ERK1/2 activation, and prevented receptor degradation, whereas GRK6 acted on the contrary. For these series of experiments, we used HEK-293T cells, which expressed relatively very higher level of GRK2 compared to GRK6. HEK 293T cells, transfected either with GRK2/GRK6 plasmids for overexpression or respective siRNA for down-regulation, were used in subsequent experiments (Figure 6a). Figure 6b showed that with overexpression of GRK6 and lower expression of GRK2, there was an increase in ERK1/2 activation when the cells were treated with 1 ug/ml CP, whereas lower expression of GRK6 and overexpression of GRK2 decreased the ERK1/2 activation slightly. As for degradation of IGF-1R, GRK2 could prevent the receptor to be down-regulated, and GRK6 accelerated the down-regulation process greatly. The above results demonstrated the regulatory roles of GRKs in CP induced IGF-1R signaling and down-regulation.
Figure 6. Involvement of GRK 2 and GRK 6 in CP induced signaling and receptor down-regulation.
(a) Cells were plated, and transiently transfected with plasmids expressing GRK2 or GRK6 or with siRNAs for GRK2 or GRK6 for 48 hours. Cells were lysed and protein samples were analyzed by WB for GRK2, GRK6 and GAPDH. (b) Involvement of GRKs in CP induced signaling. Transfected cells were splitted into 24 well-plates, allowed to attach and serum starved for 12 h. Cells were then stimulated with 1 ug/ml CP for indicated times, and were lysed then. Protein samples were analyzed by WB for p-ERK1/2 and t-ERK1/2. (c) Involvement of GRKs in CP induced receptor down-regulation. Transfected cells were splitted into 24 well-plates, allowed to attach and serum starved for 12 h. Cells were then stimulated with 1 ug/ml CP for indicated times, and were lysed then. Protein samples were analyzed by WB for IGF-1R and GAPDH. Cropped blots were used in the figures and the gels were run under the same experimental conditions. Representative full length blots were shown in Supplementary information.
Discussion
Although overexpression of IGF-IR and the association between its expression and patients' survival have been reported in various human solid tumors8,13,14, to our knowledge, controversies still exist with expression of IGF-1R as a prognostic factor in ESCC18,31,32. Using banked esophageal tissues from a well-characterized series of patients who underwent complete surgical resection and subsequent standard comprehensive therapy for ESCC, we evaluated expression levels of IGF-IR with immunohistochemical staining. We found that 77.27% of the 110 ESCC patients expressed IGF-1R. Differences between IGF-IR expression and age, tumor differentiation, tumor location, T stage and lymph node metastasis were not found, however, male patients seemed to express higher levels of IGF-1R than female patients. It was reported that IGF-1R was up-regulated by either androgens or estrogens, indicating that the expression levels of IGF-1R may exist gender differences33,34. In addition, males had significantly higher serum IGF-1 levels than females in the age groups 50–69 (P < 0.05)35, in which group the majority of ESCC patients were. These two reasons may explain partly why there are gender differences in IGF-1R expression. Multivariate cox analysis indicated that level of IGF-1R expression was a significant and independent prognostic factor after adjusting for age, gender, tumor differentiation, T stage and lymph node metastasis. This is the first reason why IGF-1R should be considered as an interesting target. The second reason is owing to the peculiar context of IGF-1 function in ESCC. There are four sources of IGF-1 that can be used by the ESCC cells. The first source is where there an abundance IGF-1, and it comes from the serum, whereby most of the IGF-1 binds IGFBPs9. The second source is the IGF-1 secreted by the salivary gland, where they exist in free form and can directly affect the ESCC cells19,20. The third source is the paracrine IGF-1 secreted by the macrophage in the tumor stromal31. Lastly, autocrine IGF is secreted by ESCC cells themselves17,18,31. All the above indicate that the IGF-1R is important in the development and progression of ESCC. These reasons make the IGF-1R a more attractive and promising target in treating ESCC than in other cancers.
Inhibition of IGF-1R signaling pathway by shRNA, among other ways has been implicated to have antitumor activities in ESCC18,36,37. IGF-1R monoclonal antibodies displayed in vitro and in vivo antitumor effects in various cancers. One important humanized IgG2 antibody Figitumumab (CP751871, CP), not only has good antitumor activity, also has a good pharmacokinetic distribution38. In this paper, we attempted to investigate the antitumor effect of CP in ESCC. Our results showed that CP not only completely inhibited the cell proliferation induced by IGF-1, but also suppressed cell growth by itself under both serum free and containing conditions. In growth medium, CP treatment of the cells blocked the binding of IGF-1/IGF-2 in the serum to IGF-1R, decreased IGF-1R phosphorylation (Figure 3b), and then inhibited the cell proliferation. However, it also decreased cell proliferation under serum free conditions. There were IGF-1 and also IGF-2 secretion by the ESCC cells themselves17,18,31, so this autocrine IGF loop provided an explanation to the decreased cell growth under serum free conditions. IGF-I is responsible for a variety of cell-specific functions, including cell migration, colony forming activity and anti-apoptotic effect13,14,15. All of these effects could potentially be affected by IGF-IR antagonism in the clinic. In our study we detected effects of CP that were considered to be critical as a potential therapeutic agent. CP reversed the anti-apoptotic effects induced by IGF-1 of the tumor cells. IGF-1also mediates chemoresistence to several chemotherapeutic drugs in ESCC and other cancers. Our results showed that CP treatment enhanced the activities of various chemotherapeutic drugs. At last, we verified that CP suppressed the colony forming activity and cell migration induced by IGF-1 in ESCC, which we believe is of paramount importance for the potential use of CP in clinic.
As an IGF-1R monoclonal antibody, CP not only blocks the ligand-receptor interaction but also down-regulates receptor, whereby the down-regulated receptor then may be more effective than blocking ligand-receptor interaction as treatment25,26. Here, we showed that CP blocked the IGF-1R phosphorylation induced by both IGF-1 and serum. Akt activation was completely abolished by the CP treatment, but ERK1/2 activation was only partially inhibited, which may be the reason the cells were not so highly responsive to CP treatment. CP was a more powerful agent in degrading IGF-1R than IGF-1. It was suggested that ubiquitination of IGF-1R upon ligand or monoclonal antibody treatment were required for receptor internalization, sequestration and degradation39,40. Three E3 ligases Mdm2, NEDD4, and c-Cbl were implicated in this ubiquitination process41. β- arrestin1 is required and acts as an adaptor protein for Mdm2 binding to the IGF-1R29. We confirmed that treatment with either IGF-1 or CP induced clear ubiquitination of IGF-1R, and that β- arrestin1 was recruited to the IGF-1R following treatment. Compared to IGF-1, CP induced an increased recruitment of β- arrestin1 to the IGF-1R, this maybe one explanation of the more powerful degradation ability of CP than IGF-1. Overexpression of β- arrestin1 enhanced the receptor degradation, and vice versa. Whether the ubiquitinated IGF-1R is then internalized and degraded from proteasome or lysosome pathway is still controversial28,40. Our data indicated that the proteasome pathway was involved in degradation of IGF-1R β subunit, however, we did not know whether IGF-1R α subunit was also degraded via the proteasome pathway, given that it was reported that theαand β subunit utilized different pathways40. Degradation of IGF-1R by CP through proteasome pathway was in accordance with the results in which the IGF-1R used the Mdm2 as an E3 ligase upon IGF-1 stimulation29.
From our findings we therefore concluded that the signaling activation and receptor down-regulation were separated events, although they may affected each other to some extent, which could not be explained by the canonical IGF-1R activation model. Leonard Girnita's group recommended a novel IGF-1R activation paradigm where an unbalanced manner or biased manner existed in modulating IGF-1R activity41,42. In this model, IGF-1R induces canonical kinase signaling, and also β-arrestin dependent signaling, heterotrimeric G protein signaling as well as β-arrestin mediated receptor desensitization. This model is borrowed from the GPCRs, and IGF-1R now can be considered as an untypical GPCR. Like the classification of agonist for GPCRs, agonists for IGF-1R are divided into balanced agonists, which activate kinase signaling, G protein as well as β-arrestin signaling, and biased agonists which activate either kinase/G protein signaling or β-arrestin signaling, according to this new model. Furthermore CP is classified as β-arrestin biased agonist or ligand for IGF-1R. Our results showed that CP itself could induce ERK1/2 activation in a time dependent manner without any detectable phosphorylation of IGF-1R and Akt. ERK1/2 activation was mediated by β-arrestin1, indicating that ERK1/2 signaling was β-arrestin1 biased signaling. We verified that CP was a biased agonist for the IGF-1R. Considering the ERK1/2 activation induced by CP, we attempted to inhibit ERK1/2 with a MEK inhibitor U0126 in order to get enhanced antitumor effects with CP treatment. Addition of U0126 made the cells more sensitive to CP treatment especially in TE-1 cells, which were initially more resistant to CP treatment. That is to say that CP induced ERK1/2 activation has a role in the resistance to CP treatment. Combination of ERK1/2 inhibitors to CP would make the inhibition of IGF-1R more suitable for clinical usage.
In the course of GPCRs, GRKs phosphorylate serine residues on the GPCRs, and then provide the binding site for β-arrestins to desensitize the G protein signaling and induce β-arrestin biased signaling43. It was reported that IGF-1R also utilized GRK2 and GRK6 to phosphorylate serine residues on C-terminal of IGF-1R, recruited β-arrestin1 to mediate receptor desensitization and induced β-arrestin1 dependent signaling under IGF-1 stimulation23,30. As we have shown there is β-arrestin1 dependent degradation and signaling with CP treatment, but it is unclear whether GRKs are included in this process. We found that degradation of IGF-1R was affected by the GRKs, which were involved in receptor desensitization. Furthermore, our results indicated that overexpression of GRK6 or lower expression of GRK2 enhanced β-arrestin1 biased signaling, and vice versa. These findings provides us with more evidence to verify that IGF-1R can act as an untypical GPCR, whereby CP is a β-arrestin1 biased agonist for IGF-1R, and binding of CP to IGF-1R requires GRK2 and GRK6 phosphorylating the serine residues of the IGF-1R, β-arrestin1 is then recruited to the receptor, leading to receptor ubiquitination and down-regulation, simultaneously inducing the β-arrestin1 biased ERK1/2 activation. This biased mechanism of IGF-1R may supply a new therapeutic view to overcome the gaps between preclinical and clinical use of IGF-1R inhibition in cancer treatment.
Methods
All the patients involved gave a signed consent for the study, and the study was approved by the Hospital Ethical Committee. All methods used in this study were carried out in accordance with the approved guidelines.
Patients
One hundred and ten consecutive patients who were operated for ESCC between 2008 and 2009 were included in our study. All patients had curative enbloc esophageal resection and radical lymph node dissection without preoperative radiochemotherapy. Patients who died within 1 month after surgery were considered perioperative failures and were excluded from our study.
Cell lines and Reagents
Eca-109 and HEK-293T cells were cultured in DMEM and TE-1 cells were cultured in RPMI-1640 medium, supplemented with 10% fetal bovine serum (FBS; Sigma-Aldrich, St. Louis) at 37°C and 5% CO2. Anti-IGF-1R(H60), anti-GRK2(C15), anti-GRK6(C-20), anti-ubiquitin(P4D1), β-arrestin-1(K16) and anti-GAPDH(FL-335)antibodies were purchased from Santa Cruz Biotechnology Inc.(Santa Cruz, CA, USA). Antibodies for IGF-1R, phosphor-IGF-1R (Tyr 1135), phosphor-ERK1/2(Thr202/Tyr204) and phosphor-Akt (S473) were from Cell Signaling Technology (Danvers, USA). Antibodies against β-arrestin-1 was purchased from abcam(Cambridge, MA). IGF-1was from Life Technologies. The other reagents were from Beyotime (Jiangsu, China).
Immunohistochemical staining, evaluation and statistical analysis
Immunohistochemical staining was performed as previously described44 and PBS was used as the negative control instead of the IGF-1R antibody. The results were scored by 2 pathologists (SHC and DW), following an established 4-step scale (0, 1, 2 and 3). Tumors were subsequently categorized as negative, weakly positive, moderately positive or strongly positive (>50% of cells have 2 or 3 score staining)18. Expression of IGF-IR was assessed for associations with clinicopathological characteristics using the following statistical tests: Student's t-test, the Mann–Whitney test, the chi-square two-tailed test and Fisher's exact test. Cumulative survival rates were calculated by the Kaplan–Meier method. The difference between the survival curves was analyzed by the log-rank test. Factors related to survival were analyzed by Cox's proportional hazards regression model. The statistical significance of differences was determined by one-way analysis of variance or two-factor factorial analysis of variance. P values of 0.05 were considered to indicate statistical significance.
Cell Viability Assay
Cells were incubated in 96-well tissue culture plates, and cell viability was measured by CCK-8 assay (Beyotime, China) according to the manufacturer's instructions. Absorbance was measured at 490 nm. Cell number was calculated from a standard curve of measurements from known numbers of cells. Triplicate cultures were performed on all experiments.
Colony forming activity
Cells (103/plate) were seeded onto 100 mm culture plates and incubated with different treatment for 14 days, the cell layer was then methanol fixed and stained with 0.05% crystal violet solution containing 25% of methanol. Foci were defined to be groups of cells containing 50 cells or more.
Wound healing assay
Wound healing assays were performed using a modification of the procedure described by Imsumran et al.18. Briefly, six-well chambers were prepared by scratching registration marks onto the slide surface. TE1 cells were plated, grown normally for 48 h and starved overnight. Cells were cut with a cell scraper and five images were captured along the cut surface. Additional images were captured 24 h later.
Transient transfection
The plasmid expressing β-arrestin1-flag, GRK2 and GRK6 were kind gifts from Leonard Girnita (Karolinska Institutet, Stockholm, Sweden). Cells were cultured at 90% confluency in six-well plates and transfected with plasmids using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. SiRNA for β-arrestin-1 was purchased from Cell Signaling Technology and siRNAs for GRK2 and GRK6 were from Life Technologies. Cells were plated at 40–60% confluences when transfected with vary siRNAs with Lipofectamine 2000 for 48 hours.
Immunoprecipitation and Western blotting
Cells were lysed with 500 μL IP lysis buffer [20mM Tris(pH7.5), 150mM NaCl, 1% Triton X-100, with protease inhibitor tablet (Roche)]. 500 μg of protein was incubated with Dynabeads protein G (10 μL) (Invitrogen) and 1 μg antibody overnight at 4°C on a rotator platform. The immunoprecipitates were collected on a magnetic holder, the supernatant was discarded, and the beads were washed three times with lysis buffer and then dissolved in the sample buffer for western blotting.
Protein samples were dissolved in lithium dodecyl sulfate (LDS) sample buffer (Invitrogen) and analyzed by SDS/PAGE with 4–12% Bis-Tris gel (Invitrogen). After separation, the proteins were transferred to nitrocellulose membranes. Membranes were then blocked for 1 h at room temperature in 5% (wt/vol) skimmed milk powder and 0.1% (vol/vol) Tween 20 in Tris-buffered saline (TBS), pH 7.5 (TBS-T). Appropriate primary antibody was incubated overnight at 4°C. Following washing three times in TBS-T, the membrane was incubated with a HRP-labeled secondary antibody (Santa Cruz) for 1 h. The detection was performed with enhanced chemiluminescent substrate (Pierce) and exposed to X-ray film.
Author Contributions
T.H.Z., Q.L. and J.J.D. conceived and designed the experiments. T.H.Z., H.C.S. and W.D. performed most of the biological experiments. T.H.Z. and X.Q. analyzed the data. T.H.Z., Q.L. and J.J.D. wrote the main manuscript text. All the authors reviewed the manuscript.
Supplementary Material
Supplement 1
Acknowledgments
The work was supported by Provincial science and technology development plan of Shandong (2012GG0021836), Provincial Natural Science Foundation of Shandong (ZR2013HZ001) and the National Natural Science Foundation of China (project 81301728). We would like to thank Marina Ilic (Karolinska Institutet, Sweden) and Godwin Tong (University of Glasgow, UK) for critical reading of the manuscript. We would also like to acknowledge all patients involved in this study.
References
- Jemal A., Center M. M., DeSantis C. & Ward E. M. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev 19, 1893–1907 (2010). [DOI] [PubMed] [Google Scholar]
- Lin Y. et al. Epidemiology of esophageal cancer in Japan and China. J. Epidemiol. 23, 233 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napier K. J., Scheerer M. & Misra S. Esophageal cancer: A Review of epidemiology, pathogenesis, staging workup and treatment modalities. World. J. Gastrointest. Oncol. 6, 112 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo P. & Li K. Trends in esophageal cancer mortality in China during 1987–2009: age, period and birth cohort analyzes. Cancer Epidemiol. 36, 99–105 (2012). [DOI] [PubMed] [Google Scholar]
- Law S. & Wong J. Current management of esophageal cancer. J. Gastrointest. Surg. 9, 291–310 (2005). [DOI] [PubMed] [Google Scholar]
- Berry M. F. Esophageal cancer: staging system and guidelines for staging and treatment. J. Thorac. Dis. 6, S289 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyers C. Targeted cancer therapy. Nature 432, 294–297 (2004). [DOI] [PubMed] [Google Scholar]
- Yee D. Insulin-like growth factor receptor inhibitors: baby or the bathwater? J. Natl. Cancer Inst. 104, 975–981 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz O., Eroglu A., Dag E., Karaoglanoglu N. & Yilmaz A. Serum levels of IGF-I and IGFBP-III and their relation with carcinoembryonic antigen and carbohydrate antigen 19-9 in cases of esophageal cancer. Int. J. Clin. Pract. 60, 1604–1608 (2006). [DOI] [PubMed] [Google Scholar]
- Rinaldi S. et al. Serum levels of IGF-I, IGFBP-3 and colorectal cancer risk: results from the EPIC cohort, plus a meta-analysis of prospective studies. Int. J. Cancer 126, 1702–1715 (2010). [DOI] [PubMed] [Google Scholar]
- Key T. J., Appleby P. N., Reeves G. K. & Roddam A. W. Insulin-like growth factor 1 (IGF1), IGF binding protein 3 (IGFBP3), and breast cancer risk: pooled individual data analysis of 17 prospective studies. Lancet Oncol 11, 530–542 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favelyukis S., Till J. H., Hubbard S. R. & Miller W. T. Structure and autoregulation of the insulin-like growth factor 1 receptor kinase. Nat. Struct. Mol. Biol. 8, 1058–1063 (2001). [DOI] [PubMed] [Google Scholar]
- Baserga R., Peruzzi F. & Reiss K. The IGF-1 receptor in cancer biology. Int. J. Cancer 107, 873–877 (2003). [DOI] [PubMed] [Google Scholar]
- Pollak M. The insulin and insulin-like growth factor receptor family in neoplasia: an update. Nature Rev. Cancer 12, 159–169 (2012). [DOI] [PubMed] [Google Scholar]
- Gualberto A. & Pollak M. Emerging role of insulin-like growth factor receptor inhibitors in oncology: early clinical trial results and future directions. Oncogene 28, 3009–3021 (2009). [DOI] [PubMed] [Google Scholar]
- Ouban A., Muraca P., Yeatman T. & Coppola D. Expression and distribution of insulin-like growth factor-1 receptor in human carcinomas. Hum. Pathol. 34, 803–808 (2003). [DOI] [PubMed] [Google Scholar]
- Liu Y.-C. et al. Autocrine stimulation by insulin-like growth factor I is involved in the growth, tumorigenicity and chemoresistance of human esophageal carcinoma cells. J. Biomed. Sci. 9, 665–674 (2002). [DOI] [PubMed] [Google Scholar]
- Imsumran A. et al. Insulin-like growth factor-I receptor as a marker for prognosis and a therapeutic target in human esophageal squamous cell carcinoma. Carcinogenesis 28, 947–956 (2007). [DOI] [PubMed] [Google Scholar]
- Costigan D. C., Guyda H. J. & Posner B. I. Free Insulin-Like Growth Factor I (IGF-I) and IGF-II in Human Saliva*. J Clin Endoc Metabol 66, 1014–1018 (1988). [DOI] [PubMed] [Google Scholar]
- Tchorzewski M. et al. Role of insulin-like growth factor-I in esophageal mucosal healing processes. J. Lab. Clin. Med. 132, 134–141 (1998). [DOI] [PubMed] [Google Scholar]
- Gualberto A. Figitumumab (CP-751,871) for cancer therapy. Expert Opin. Biol. Ther. 10, 575–585 (2010). [DOI] [PubMed] [Google Scholar]
- Girnita L. et al. Beta-arrestin and Mdm2 mediate IGF-1 receptor-stimulated ERK activation and cell cycle progression. J Biol Chem 282, 11329–11338, 10.1074/jbc.M611526200 (2007). [DOI] [PubMed] [Google Scholar]
- Zheng H. et al. Selective recruitment of G protein-coupled receptor kinases (GRKs) controls signaling of the insulin-like growth factor 1 receptor. Proc Natl Acad Sci USA 109, 7055–7060 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalle S., Ricketts W., Imamura T., Vollenweider P. & Olefsky J. M. Insulin and insulin-like growth factor I receptors utilize different G protein signaling components. J. Biol. Chem. 276, 15688–15695 (2001). [DOI] [PubMed] [Google Scholar]
- Zheng H. et al. β-Arrestin–biased agonism as the central mechanism of action for insulin-like growth factor 1 receptor–targeting antibodies in Ewing's sarcoma. Proc Natl Acad Sci USA 109, 20620–20625 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garofalo C. et al. Efficacy of and resistance to anti-IGF-1R therapies in Ewing's sarcoma is dependent on insulin receptor signaling. Oncogene 30, 2730–2740 (2011). [DOI] [PubMed] [Google Scholar]
- Juan H. C. et al. Insulin-like growth factor 1 mediates 5-fluorouracil chemoresistance in esophageal carcinoma cells through increasing survivin stability. Apoptosis 16, 174–183, 10.1007/s10495-010-0555-z (2011). [DOI] [PubMed] [Google Scholar]
- Ohtani M., Numazaki M., Yajima Y. & Fujita-Yamaguchi Y. Mechanisms of antibody-mediated insulin-like growth factor I receptor (IGF-IR) down-regulation in MCF-7 breast cancer cells. Biosci. Trends 3, 131–138 (2009). [PubMed] [Google Scholar]
- Girnita L., Girnita A. & Larsson O. Mdm2-dependent ubiquitination and degradation of the insulin-like growth factor 1 receptor. Proc Natl Acad Sci USA 100, 8247–8252, 10.1073/pnas.1431613100 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Z. et al. GRK2 negatively regulates IGF-1R signaling pathway and cyclins' expression in HepG2 cells. J Cell Physiol 228, 1897–1901, 10.1002/jcp.24353 (2013). [DOI] [PubMed] [Google Scholar]
- Doyle S. L. et al. IGF-1 and its receptor in esophageal cancer: association with adenocarcinoma and visceral obesity. Am. J. Gastroenterol. 107, 196–204, 10.1038/ajg.2011.417 (2012). [DOI] [PubMed] [Google Scholar]
- Kalinina T. et al. Insulin-like growth factor-1 receptor as a novel prognostic marker and its implication as a cotarget in the treatment of human adenocarcinoma of the esophagus. Int J Cancer 127, 1931–1940, 10.1002/ijc.25196 (2010). [DOI] [PubMed] [Google Scholar]
- Pandini G. et al. Androgens up-regulate the insulin-like growth factor-I receptor in prostate cancer cells. Cancer Res. 65, 1849–1857 (2005). [DOI] [PubMed] [Google Scholar]
- Pandini G. et al. 17β-Estradiol up-regulates the insulin-like growth factor receptor through a nongenotropic pathway in prostate cancer cells. Cancer Res. 67, 8932–8941 (2007). [DOI] [PubMed] [Google Scholar]
- Bayram F. et al. Epidemiologic survey: reference ranges of serum insulin-like growth factor 1 levels in Caucasian adult population with immunoradiometric assay. Endocrine 40, 304–309 (2011). [DOI] [PubMed] [Google Scholar]
- Piao W. et al. Insulin-like growth factor-I receptor blockade by a specific tyrosine kinase inhibitor for human gastrointestinal carcinomas. Mol. Cancer Ther. 7, 1483–1493, 10.1158/1535-7163.MCT-07-2395 (2008). [DOI] [PubMed] [Google Scholar]
- Wang Y. et al. Targeting for insulin-like growth factor-I receptor with short hairpin RNA for human digestive/gastrointestinal cancers. J. Gastroenterol. 45, 159–170, 10.1007/s00535-009-0151-6 (2010). [DOI] [PubMed] [Google Scholar]
- Cohen B. D. et al. Combination therapy enhances the inhibition of tumor growth with the fully human anti–type 1 insulin-like growth factor receptor monoclonal antibody CP-751,871. Clin. Cancer Res. 11, 2063–2073 (2005). [DOI] [PubMed] [Google Scholar]
- Mao Y. et al. Polyubiquitination of insulin-like growth factor I receptor (IGF-IR) activation loop promotes antibody-induced receptor internalization and down-regulation. J Biol Chem 286, 41852–41861, 10.1074/jbc.M111.288514 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broussas M. et al. Molecular mechanisms involved in activity of h7C10, a humanized monoclonal antibody, to IGF-1 receptor. Int J Cancer 124, 2281–2293, 10.1002/ijc.24186 (2009). [DOI] [PubMed] [Google Scholar]
- Worralti C. et al. Novel mechanisms of regulation of IGF-1R action: functional and therapeutic implications. Ped Endocrinol. Rev. 10, 473–484 (2013). [PubMed] [Google Scholar]
- Girnita L., Worrall C., Takahashi S. I., Seregard S. & Girnita A. Something old, something new and something borrowed: emerging paradigm of insulin-like growth factor type 1 receptor (IGF-1R) signaling regulation. Cell. Mol. Life Sci., 10.1007/s00018-013-1514-y (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefkowitz R. J. Historical review: a brief history and personal retrospective of seven-transmembrane receptors. Trends Pharmacol. Sci. 25, 413–422, 10.1016/j.tips.2004.06.006 (2004). [DOI] [PubMed] [Google Scholar]
- Zhang T. et al. High expression of PRDM14 correlates with cell differentiation and is a novel prognostic marker in resected non-small cell lung cancer. Med. Oncol. 30, 1–7 (2013). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement 1