Abstract
Perinatal hypoxia results in neuronal and endothelial cell damage. The main purpose of this study was to investigate the correlation of soluble intercellular adhesion molecule 1 (sICAM-1) expression and peripheral blood changes in perinatal asphyxia with neuronal injury markers in low birth weight (LBW) neonates. We compared the concentrations of serum sICAM-1, neuron-specific enolase (NSE) and antibodies specific for NR2 glutamate receptors in 29 asphyxiated and 20 control infants using standard enzyme immunoassay procedures. The mean total concentrations of sICAM-1 and neuron-specific proteins (NSE and NR2-specific antibodies) were higher in the asphyxiated infants than in the control infants. The serum sICAM-1 concentrations significantly correlated with Apgar scoring and with the pH and lactate data from capillary or arterial cord blood. No significant correlation between serum concentrations of neuron specific proteins and blood changes of asphyxia was found. Therefore, endothelial sICAM-1 expression levels might be accepted as an indicator of the severity of perinatal asphyxia in LBW infants.
Even with widespread advances in perinatal technology and knowledge, perinatal asphyxia remains a severe condition that causes considerable mortality and long-term morbidity1,2. Oxygen deprivation during labor is the most common cause of neuronal injury in premature infants3. Hypoxia-induced changes occur in various organs in newborns and include neuronal injury and generalized endothelial dysfunction4. Hyperstimulation of glutamate receptors is one of the central features of the pathogenesis of neuronal injury5. Excessive stimulation of glutamate receptor/ion channel complexes causes a cascade of intracellular events that results in apoptosis and/or necrosis6. Previous investigations have demonstrated that high concentration of antibodies specific for N-methyl-D-aspartate glutamate receptors are reliable indicators of hypoxic ischemic encephalopathy (HIE) after birth asphyxia7. Following acute neuronal injury, the levels of neuron-specific proteins increase in neuronal cell bodies. Neuron-specific enolase (NSE), a dimeric isozyme of the glycolytic enzyme enolase, is found in the cytoplasm of neurons and can be used as an identifying marker for all neuron types in vivo and in vitro8. An increase in serum concentrations of NSE reflects neuronal cell injury, and this increase is positively correlated with the degree of injury9.
After ischemia and reperfusion of hypoxic tissue, leukocytes are recruited to the injured area through various adhesion proteins. One of these proteins, soluble intercellular adhesion molecule 1 (sICAM-1), is an important in the trans-endothelial migration of leukocytes during inflammation10,11. Previous studies have demonstrated increases in serum sICAM-1 levels during the first month of life in healthy neonates, suggesting a progressive increase in the activation of the neonatal immune system12. Most previous studies also confirmed that in infants, the activation of adhesion molecules occurs during inflammation in response to infection13,14. The roles of sICAM-1 in cell–cell adhesion, extravasation, and infection are more fully understood than its role in hypoxia in infants, as there are few reports on adhesion molecules in hypoxic conditions. The relationship between neuronal tissue injury and adhesion molecule activation after birth asphyxia has not yet been investigated.
The objective of this study was to assess the concentrations of sICAM-1 in asphyxiated and non-asphyxiated low birth weight infants (LBW) infants and to examine the association of asphyxia parameters and cell adhesion molecule activation with neuronal injury marker concentrations. We estimated the presence of neuronal injury on the basis of increased concentrations of neuron-specific proteins, such as NSE and NR2-specific antibodies.
Results
The characteristics of the subjects are presented in Table 1. No significant differences in intrauterine growth, birth type or maternal parameters were found between groups. By contrast, the groups were significantly different in terms of the 1 min and 5 min Apgar scores, as well as the capillary or arterial cord blood pH and blood lactate concentrations. These findings confirm that infants in the asphyxiated group experienced hypoxia, as determined by assessing both clinical and laboratory parameters. All of the hypoxic infants were resuscitated in the delivery room. HIE was detected in 72.4% of the asphyxiated infants, and 44.8% of asphyxiated newborns also had a different grade of IVH.
Table 1. Important characteristics and clinical parameters for the infants.
| Characteristics | Asphyxiated infants | Non-asphyxiated infants |
|---|---|---|
| Number of subjects (N) | 29 | 20 |
| Birth weight, g | 1990.9 | 2100.6 |
| (1510–2300) | (1590–2550) | |
| Gestational age, week | 33.6 | 34.2 |
| (32–38) | (33–39) | |
| Intrauterine growth restriction | 8 (27.6) | 4 (20) |
| Gender M/F | 13/16 | 10/10 |
| Maternal age, year | 26.5 | 24.2 |
| (19–32) | (19–30) | |
| Maternal preeclampsia | 4 (13.8) | 3 (15) |
| Cesarean section | 7 (24.1) | 4 (20) |
| 1-min Apgar score | 1.62 (0–3)* | 7.9 (7–8) |
| 5-min Apgar score | 2.7 (1–3)* | 8.3 (7–9) |
| Capillary or arterial cord blood pH | 6.71* | 7.31 |
| (6.58–6.89) | (7.23–7.37) | |
| Capillary or arterial cord blood lactate, mmol/L | 14.1* | 4.8 |
| (7.3–20.6) | (2.2–6.9) | |
| Free flow oxygen∧ | 2 (6.9) | 4 (20) |
| Bag and mask ventilation∧ | 20 (69) | - |
| Intubation∧ | 7 (24.1) | - |
| HIE, stage I | 14 (48.3) | 3 (15) |
| HIE, stage II–III | 7 (24.1) | - |
| IVH, grade I | 8 (27.6) | - |
| IVH, grade II–III | 5 (17.2) | - |
Data are shown as the mean (range) or n (%).
∧Results indicate that resuscitation was required in the delivery room.
*There is a significant difference between the study groups (p < 0.001).
As shown in Figure 1, the mean total concentrations of sICAM-1 and neuron-specific proteins were higher in the asphyxiated infants than in the control infants, and significant differences were found in the sICAM-1 levels (on day 3), the NSE levels (on days 1 and 3), and the NR2-specific antibody levels (on day 1) between the groups.
Figure 1. Mean total concentrations of sICAM-1, NSE and NR2-specific antibodies in the study groups.
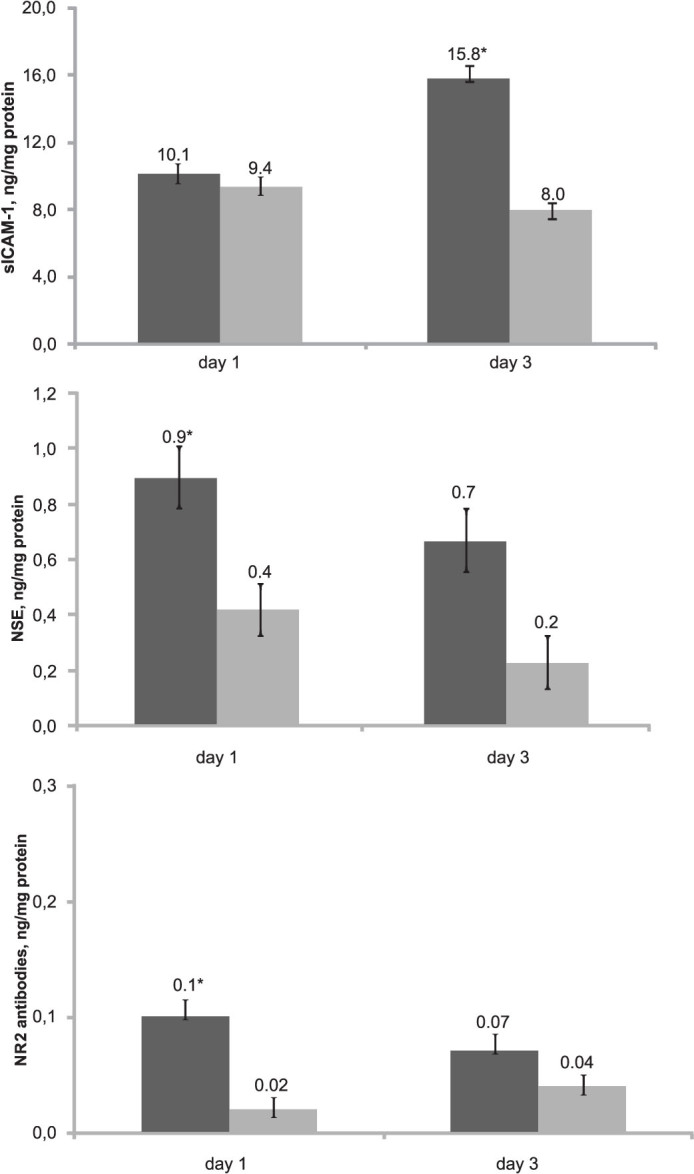
The error bars indicate the standard error of the mean. The black bars represent the asphyxiated infants, and the gray bars represent the control infants. *p < 0.05 vs the control infants.
We analyzed the correlation between the parameters of asphyxia and the levels of sICAM-1, NSE and NR2 antibodies (Table 2). It has been shown that sICAM-1 concentrations were significantly negatively correlated with the Apgar score and with the capillary or arterial cord blood pH, and were significantly positively correlated with the lactate concentrations from capillary or arterial cord blood. The peripheral blood NSE levels were significantly correlated with the Apgar score, but no correlation between NSE and peripheral blood changes was observed. As shown in Table 2, there was no significant correlation between the NR2-specific antibody levels and the asphyxia parameters.
Table 2. Spearman's rank-order correlation coefficients between the biochemical markers and parameters of asphyxia.
| Parameter | sICAM, ng/mg protein | NSE, ng/mg protein | NR2-specific antibodies, ng/mg protein |
|---|---|---|---|
| Apgar score | −0.52* | −0.58* | 0.09 |
| Capillary or arterial cord blood pH | −0.89* | −0.32 | 0.33 |
| Capillary or arterial cord blood lactate, mmol/L | 0.81* | 0.36 | 0.24 |
*p < 0.05 significance for Spearman's rank-order correlation coefficient.
Discussion
During hypoxia, the endothelium becomes inflamed, and this process is associated with leukocyte accumulation21. In the early days of extrauterine life, the vascular endothelium is exposed to high concentrations of inflammatory stimuli, and it can become further dysfunctional if exposed to a hypoxic environment, which is the main cause of endothelial dysfunction22. ICAM-1 is an important messenger molecule in leukocyte–endothelial cell stimulation23. Therefore, we hypothesized that endothelial sICAM-1 expression correlates with hypoxia-induced changes in the peripheral blood in asphyxiated newborns. The results of previous studies on the activation of intercellular adhesion molecules during hypoxia or during inflammation in response to infection in infants are controversial. Intercellular adhesion plays a significant role in the activation of the neonatal immune system, and measuring the concentrations of sICAM-1 can be used as a highly sensitive diagnostic tool in newborns suspected of infection13,14,24,25. Xanthou et al. detected increases in the serum levels of sICAM-1 and other inflammatory markers in neonates who had experienced perinatal asphyxia26. These authors confirmed that, based on the correlation between the increased concentrations of sICAM-1 and the severity of the perinatal insult, neonates with a high risk of brain damage could be identified. The results of a similar study concluded that cord serum sICAM-1 measurements have no diagnostic relevance for neonatal infection27. It should be noted that the increase in sICAM-1 expression might not only arise from injured neuronal areas. In hypoxic conditions, it is possible that adhesion molecules are produced by the activated endothelium in all injured vasculature. Therefore, it is difficult to accept the idea that an increase in adhesion molecule concentrations would be a good predictor of neuronal injury. In line with our previous investigations28, we observed increases in serum sICAM-1 and neuron-specific marker concentrations in newborns under hypoxic conditions. However, sICAM-1 concentrations were significantly correlated with the peripheral blood parameters of asphyxia, whereas it was not found correlation between NSE and NR2-specific antibody concentrations and blood changes of perinatal asphyxia. This result provides a strong case for accepting sICAM-1 concentration as a marker of endothelial inflammation under hypoxic conditions.
Perinatal asphyxia results in neuronal injury and leads to endothelial damage and acidosis, which causes multiple organ insufficiency and results in complications of extrauterine adaptation. The significance of neuron specific proteins and other types of biochemical markers for the early prognosis and evaluation of the severity of hypoxia has been investigated29. According to our results, the levels of NSE and antibodies specific for glutamate receptors are not speciflic enough markers to serve as indicators of acute hypoxia. As demonstrated in previous studies, elevated levels of serum brain-specific proteins are of limited value for predicting long-term neurodevelopmental outcomes after birth asphyxia30. We show that the severity of perinatal asphyxia in newborns can be assessed by measuring the concentration of sICAM-1 in the peripheral blood, and this is of great clinical significance. First, we conclude that serum sICAM-1 levels can be considered an important indicator for predicting the severity of birth asphyxia. Second, the results of our study show that the relationship between the severity of perinatal asphyxia and the elevated levels of adhesion molecules in LBW infants might not be causal and that the early assessment of endothelial sICAM-1 expression might be useful for early therapeutic decisions.
Based on the results of our study on LBW infants, we conclude that asphyxia causes neuronal and endothelial cell injury in LBW infants and that one of the indicators of perinatal asphyxia is the endothelial expression of adhesion molecules. Our findings show that endothelial sICAM-1 expression levels might be an indicator of the severity of perinatal asphyxia. A significant correlation between intercellular adhesion molecule levels and the severity of asphyxia-induced changes in the peripheral blood would be useful for predicting the long-term outcomes in infants who experienced perinatal asphyxia and would provide a basis for further investigations of the effect of vascular endothelial adhesion molecule expression on the development of future neurosomatic problems.
Methods
Patient population and study design
This study was conducted in accordance with the approved guidelines of the Azerbaijan National Committee on Bioethics and Ethics of Science and Technology. The parents of all of the infants provided written consent for participation in this study after receiving complete information on the study's scope and purpose.
Forty-nine LBW infants born between November 2011 and January 2012 at a gestational age of 32–39 weeks and a birth weight of 1510–2550 g were recruited for this study. The study was not blinded, and the patients were classified as asphyxiated newborns (n = 29) or control infants (n = 20). The diagnosis of asphyxia was determined by a persistent Apgar score of 0 to 3 for more than 5 min, an initial capillary or arterial cord blood pH value of less than 7.00, an initial capillary or arterial cord blood lactate value of greater than 7.00 mmol/L, neonatal neurologic sequelae (e.g., seizures, coma, and hypotonia) and multiple organ involvement (e.g., kidney, lungs, liver, heart, and intestines), in accordance with the American Academy of Pediatrics and the American College of Obstetrics and Gynecology guidelines15,16. The control group was composed of neonates who fulfilled all of the following criteria: an uncomplicated maternal history, a 5 min Apgar score of 7 or more, capillary or arterial cord blood pH of 7.00 or higher, a normal delivery after an uncomplicated pregnancy in the same hospital during the same time period, no neurologic manifestations, normal cranial ultrasound, and no medication during the neonatal period. The study exclusion criteria for both groups included birth weights lower than 1000 g or higher than 2600 g, the death of a newborn within the first 3 days of life, transfer to another unit, and clinical or laboratory evidence of congenital infection, neonatal sepsis, maternal drug addiction, or congenital malformation.
Data on maternal preeclampsia, infant gender, type of delivery, resuscitation measures in the delivery room, and anthropometric measurements (e.g., weight, body length, head and chest circumferences) were included in the analysis. Obstetric data were collected from the hospital records, and intrapartum and neonatal data were collected prospectively.
Blood gases were measured within 30 min of delivery. The gestational age was based on the date of the mother's most recent menstrual period and an ultrasonogram and was confirmed using the scale by Ballard et al.17. The condition of intrauterine growth restriction was defined as a newborn having estimated fetal anthropometric parameters below the 10th percentile for his/her gestational age and gender, which was confirmed at birth18. The severity of hypoxic-ischemic encephalopathy (HIE) was estimated based on the clinical behavior of the neonate 24 hours postpartum by using the Sarnat score19. A cranial ultrasound was performed on day 3 of life using 5 and 7.5 MHz sector transducers. Intraventricular hemorrhage (IVH) was classified into the following four grades according to the Papile description: grade I IVH was confined to the germinal matrix, grade II IVH had mild or no dilatation of the lateral ventricle, grade III IVH had patent ventricular dilatation, and grade IV IVH extended into the adjacent brain parenchyma20.
Blood collection
Venous blood was collected on days 1 and 3 of life. No venous punctures were performed for the sole purpose of study-related analysis. The blood samples were collected in EDTA-containing tubes and centrifuged for 15–20 min. The serum samples were frozen at −70°C. Grossly hemolyzed samples were not included in the analysis.
Measurement of the concentrations of adhesion molecules and neuronal injury markers in the peripheral blood by ELISA
The sICAM-1 and NSE levels were determined using USCN kits (Life Science Inc., Wuhan, China), and antibodies specific for the NR2 subunit of the NMDA receptor were detected by using Gold Dot NR2 Antibody ELISA kits (CIS Biotech, Inc., Decatur, GA, USA). All tests were performed according to standard enzyme immunoassay procedures. The levels of sICAM-1 and both neuron-specific proteins are expressed in ng/mg of total protein in the sample.
Statistical analysis
The data in the study groups were nonparametric. Significant differences between the asphyxiated and control groups were determined using the Mann-Whitney U-test to assess differences in the levels of sICAM-1, NSE and NR2-specific antibodies. The same test was used to assess differences in the quantitative variables between groups. The qualitative variables, such as gender, maternal preeclampsia, Cesarean section, resuscitation measures used in the delivery room, and the severity of HIE and IVH, were compared using Fisher's Exact test. In all instances, significance was set at p < 0.05.
Author Contributions
S.H. initiated the project. S.H. and N.P. carried out the theoretical design and analysis, designed the devices and the studies, and wrote the manuscript. O.P. collected the patient data and contributed the computing resources. S.H. reviewed the manuscript. M.G. and A.O. contributed to the biomarker analyses and participated in the analysis of the results.
Acknowledgments
The authors sincerely thank the Science Development Foundation under the President of the Azerbaijan Republic for providing the reagent kits. We also thank the staff of the Clinical Biochemistry Laboratory of Azerbaijan Medical University for assistance with the biomarker analyses.
References
- Bryce J., Boschi-Pinto C., Shibuya K. & Black R. E. WHO Child Health Epidemiology Reference Group. WHO estimates of the causes of death in children. Lancet 365, 1147–52 (2005). [DOI] [PubMed] [Google Scholar]
- Al-Macki N., Miller S. P., Hall N. & Shevell M. The spectrum of abnormal neurologic outcomes subsequent to term intrapartum asphyxia. Pediatr. Neurol. 41, 399–405 (2009). [DOI] [PubMed] [Google Scholar]
- Graham E. M., Ruis K. A., Hartman A. L., Northington F. J. & Fox H. E. Asystematic review of the role of intrapartum hypoxia-ischemia in the causation of neonatal encephalopathy. Am. J. Obstet. Gynecol. 6, 587–595 (2008). [DOI] [PubMed] [Google Scholar]
- Volpe J. J. Hypoxic-ischemic encephalopathy: Clinical aspects. In:: Neurology of the Newborn (eds Volpe J. J.) 331–396 (WB Saunders Co, Philadelphia, 2000). [Google Scholar]
- Choi D. W. Glutamate neurotoxicity and diseases of the nervous system. Neuron 1, 623–634 (1998). [DOI] [PubMed] [Google Scholar]
- White B. C. et al. Brain ischemia and reperfusion: molecular mechanisms of neuronal injury. J. Neurol. Sci. 179, 1–33 (2000). [DOI] [PubMed] [Google Scholar]
- Dambinova S. A. et al. Blood test detecting auto antibodies to N-methyl-D-aspartate neuroreseptors for evaluation of patients with transient ischemic attack and stroke. Clin. Chem. 49, 1752–1762 (2003). [DOI] [PubMed] [Google Scholar]
- Schmechel D., Marangos P. J., Zis A. P., Brightman M. & Goodwin F. K. Brain enolases as specific markers of neuronal and glial cells. Science 199, 313–315 (1978). [DOI] [PubMed] [Google Scholar]
- Selakovic V., Raicevic R. & Radenovic L. The increase of neuron-specific enolase in cerebrospinal fluid and plasma as a marker of neuronal damage in patients with acute brain infarction. J. Clin. Neurosci. 12, 542–547 (2005). [DOI] [PubMed] [Google Scholar]
- Scholz D. et al. Expression of adhesion molecules is specific and time-dependent in cytokine-stimulated endothelial cells in culture. Cell Tissue Res. 284, 415–423 (1996). [DOI] [PubMed] [Google Scholar]
- Butcher E. C. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell 67, 1033–1036 (1991). [DOI] [PubMed] [Google Scholar]
- Phocas I. et al. Soluble intercellular adhesion molecule-1 in newborn infants. Eur. J. Pediatr. 157, 153–156 (1998). [DOI] [PubMed] [Google Scholar]
- Austgulen R., Arntzen K. J., Haereid P. E., Aag S. & Døllner H. Infections in neonates delivered at term are associated with increased serum levels of ICAM-1 and E-selectin. Acta Paediatr. Suppl. 91, 92–97 (2002). [DOI] [PubMed] [Google Scholar]
- Distefano G. et al. Concentration of blood ICAM-1s in the newborn at risk of infection. A prospective study in the first 2 weeks of life. Pediatr. Med. Chir. 19, 27–30 (1997). [PubMed] [Google Scholar]
- Chen Z. L. et al. Clinical study on improving the diagnostic criteria for neonatal asphyxia. Zhonghua Er Ke Za Zhi 44, 167–172 (2006). [PubMed] [Google Scholar]
- Yildizdas D., Yapicoglu H., Yilmaz H. L., & Sertdemir Y. Correlation of simultaneously obtained capillary, venous and arterial blood gases of patients in a paediatric intensive care unit. Arch. Dis. Child 89, 176–180 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard J. L., Khoury J. C. & Wedig K. New Ballard Score, expanded to include extremely premature infants. J. Pediatr. 119, 417–23 (1991). [DOI] [PubMed] [Google Scholar]
- Mandruzzato G., Meir Y. J., Natale R. & Maso G. Antepartal assessment of IUGR fetuses. Perinat. Med. 3, 222–229 (2001). [DOI] [PubMed] [Google Scholar]
- Sarnat H. B. & Sarnat M. S. Neonatal encephalopathy following fetal distress: a clinical and electroencephalographic study. Arc. Neurol. 33, 695–706 (1976). [DOI] [PubMed] [Google Scholar]
- Papile L. S., Burstein J. & Burstein R. Incidence and evolution of the subependymal intraventricular hemorrhage: a study of infants with weights less than 1500 grams. J. Pediatr. 92, 529–534 (1978). [DOI] [PubMed] [Google Scholar]
- Granger D. N. & Senchenkova E. Inflammation and the Microcirculation (Morgan & Claypool Life Sciences, San Rafael, 2010). [PubMed] [Google Scholar]
- Zyuzkov G. N., Dygai A. M. & Goldberg E. D. Changes in granulocytic hemopoietic stem and their mechanisms during hypoxia of different genesis. Bull. Exp. Biol. Med. 139, 279–282 (2005). [DOI] [PubMed] [Google Scholar]
- Weston R. M., Jones N. M., Jarrott B. & Callaway J. K. Inflammatory cell infiltration after endothelin-1-induced cerebral ischemia: histochemical and myeloperoxidase correlation with temporal changes in brain injury. J. Cereb. Blood Flow Metab. 27, 100–114 (2007). [DOI] [PubMed] [Google Scholar]
- Døllner H., Vatten L. & Austgulen R. Early diagnostic markers for neonatal sepsis Comparing C-reactive protein, interleukin-6, soluble tumour necrosis factor receptors and soluble adhesion molecules. J. Clin. Epidemiol. 54, 1251–1257 (2001). [DOI] [PubMed] [Google Scholar]
- Jaber S. M., Hamed E. A. & Hamed S. A. Adhesion molecule levels in serum and cerebrospinal fluid in children with bacterial meningitis and sepsis. J. Pediatr. Neurosci. 4, 76–85 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xanthou M. et al. Inflammatory mediators in perinatal asphyxia and infection. Acta. Paediatr. Suppl. 91, 92–97 (2002). [DOI] [PubMed] [Google Scholar]
- Daraï E. et al. Soluble intercellular adhesion molecule 1 in umbilical cord serum: potential for the diagnosis of neonatal infections. Fetal Diagn. Ther. 17, 167–172 (2002). [DOI] [PubMed] [Google Scholar]
- Huseynova S. et al. Endothelial Dysfunction and Cellular Adhesion Molecule Activation in Preterm Infants with Hypoxic Ischemic Encephalopathy. AIJCR 2, 47–52 (2012). [Google Scholar]
- Naithani M. & Simalti A. K. Biochemical Markers in Perinatal Asphyxia. J. Nepal Paediatr. Soc. 2, 151–156 (2011). [Google Scholar]
- Nagdyman N., Grimmer I., Scholz T. & Müller C. h. Predictive value of brain-specific proteins in serum for neurodevelopmental outcome after birth asphyxia. Ped. Res. 2, 270–275 (2003). [DOI] [PubMed] [Google Scholar]


