Abstract
Angiogenesis is one of the important hallmarks of psoriasis. The extension of the superficial microvascular structure and activated pro-angiogenic mediators in psoriasis seem to be important factors involved in the pathology. According to the changes of superficial microvasculature in psoriatic lesions, anti-angiogenic treatment could be a promising therapeutic strategy for psoriasis. The aim of this study was to construct an in vitro vascularized psoriatic skin substitute for fundamental research. Psoriatic fibroblasts and keratinocytes were isolated from psoriatic plaque biopsies, while healthy fibroblasts and keratinocytes, as well as microvascular endothelial cells, were isolated from healthy skin biopsies of cosmetic breast surgery. Psoriatic and healthy skin substitutes with and without endothelial cells were produced using the self-assembly approach. Afterward the substitutes were examined by histology, immunofluorescence studies, and three-dimensional (3D) confocal microscopy. Histological analysis and immunofluorescence staining of specific markers for endothelial cells (von Willebrand, PECAM-1 [CD31], and VE-cadherin [CD144]) and basement membrane component (collagen IV) demonstrated that endothelial cells have the ability to form capillary-like tubes. Moreover, the 3D branched structure of the capillary-like structures and an eagle eye view of them were observed by confocal microscopy. Also the semiquantification of capillary-like tubes (CLTs) was carried out with a 3D eagle eye view of substitutes, and more CLTs were observed in psoriatic substitutes. These results suggest that it is possible to observe 3D capillary-like structures in the self-assembled psoriatic skin substitutes, which could become a good in vitro testing model for anti-angiogenic drug research, and facilitate the study of this complex pathology, which links angiogenesis to its development.
Key words: : angiogenesis, endothelial cells, psoriasis, skin, tissue engineering
Introduction
Psoriasis is a recurrent and chronic inflammatory skin disorder, with the principal manifestations including an activated immune system, epidermal hyperproliferation, and abnormal vascular patterns. With chronic inflammation, the endothelial cells (ECs) lining the venules proliferate and migrate into the tissue, resulting in an increase in the microvascular density, which is mediated by angiogenesis.1–5
The mechanism of vascular pattern alterations in psoriatic skin is explained by the venulization phenomenon. This refers to the elongation of the capillary loops in psoriatic skin due to the division of ECs of the extrapapillary venous parts causing the venous limb to enlarge and the arterial limb to become shorter.1–4 As a consequence, the capillaries are wider, dilated, and tortuous, which results in the expansion of existing blood vessels in psoriatic skin.6–10 These alterations in microvascular structure are necessary for the survival of inflammatory cells and resident cells within the tissue because of their increased metabolic needs and hypoxia. The altered vascular structure is also required for the modifications to the matrix that support leukocyte extravasation.11,12
In addition to these microvascular structural abnormalities in psoriatic skin, there is elevated blood flow in lesional skin compared to nonlesional skin.13 There is also a greater number of circulating ECs due to increased EC division and migration.14 The role of microvascular changes in the pathology of psoriasis remains a mystery. Some researchers suggest that these alterations appear during the initial stages of psoriatic plaque development even before epidermal hyperplasia is histologically observable.15–17 This assumes that angiogenesis is one of the first signs of psoriasis and plays an active role in the complex pathology of the disease.
There are two principal challenges with psoriasis: the first is the poorly understood pathology, and the second is the absence of a permanent cure. These problems make psoriasis an attractive area of inquiry for researchers. In addition, the easy availability of psoriatic cells from a skin biopsy makes psoriasis a model chronic inflammatory disease with which to study the complex pathology of chronic inflammation and to develop new therapeutic strategies for psoriasis and similar disorders.
An in vitro, three-dimensional (3D) engineered skin psoriatic tissue model was prepared in the laboratory using the self-assembly (SA) method without any induction agents or scaffold-like biomaterials.18 The goals of this study were to improve the existing model with the addition of ECs to reconstruct capillary-like structures (CLSs), and to determine the feasibility of the construction method and the physical characterization methods. It was hoped that the resulting model would be easily accessible for the purpose of studying the linked biology of angiogenesis and inflammation, thus widening its scope beyond psoriasis research.
Materials and Methods
Human dermal microvascular EC isolation, purification and expansion
Fresh pieces of skin were harvested and washed in phosphate-buffered saline (PBS) containing penicillin (100 IU/mL)–gentamicin (25 μg/mL) and fungizone (0.5 μg/mL). The skin was then cut into small strips in a laminar flow cabinet and incubated overnight at 4°C in a thermolysin solution with HEPES buffer (500 μg/mL). The epidermis was peeled away from the dermis, and the dermal pieces were then compressed and pressure applied with forceps to extrude the ECs. The extracted ECs were cultured on gelatin-coated six-well plates in Endothelial Cell Growth Media-2 microvascular (EGM-2 MV) + SingleQuots Kit media with supplements and growth factors (Lonza) for 24 h. The cells were then left until they reached 85%–90% confluence. The purification of the ECs was performed using anti-human CD31 dynabeads (Invitrogen). Briefly, the cells were trypsinized, centrifuged (300 g), and counted. Afterwards, they were incubated with beads for 30 min at 4°C and agitated. At the end of the incubation period, ECs attached to the beads were selected with a magnet, and the supernatant was removed. These cells were cultured in gel-coated six-well plates, and their media changed three times a week. EC cultures from passage 2 and three different cell populations of human dermal microvascular EC (HDMECs; 37XX, 48XX, and 51XX) were used for all experiments.
Preparation of endothelialized skin substitutes
The skin substitutes were prepared using the SA method.18–20 Psoriatic and normal dermal fibroblasts (passage 5, 8000 cells/cm2) were seeded onto the plates and cultivated for 3 weeks with Dulbecco-Vogt modification of Eagle's medium (DME). The HDMECs (12,000 cells/cm2) were seeded onto the fibroblast sheets and cultivated for an additional week with 1:1 DME:EGM-2 MV medium with supplements and growth factors. The endothelialized fibroblast sheets were then transposed and cultivated for 7 days to form the dermal component. Afterwards, keratinocytes (passage 2, 300,000 cells/cm2) were seeded onto the dermal component to form the epidermal layer and cultivated for 1 week under the same culture conditions using Dulbecco-Vogt modification of Eagle's medium with Ham's F12 in a 3:1 ratio (DH) and EGM-2 MV medium mixture (1:1) with supplements and growth factors. Finally, the substitutes were raised to the air–liquid interface and cultivated in a 1:1 mixture of DH without the epidermal growth factor (EGF) and EGM-2 MV medium with supplements and growth factors for 3 weeks. Throughout the production process, the culture medium was changed three times a week and the corresponding medium freshly supplemented with ascorbic acid (50 μg/mL). The experiments were repeated at least three times with several replications of different psoriatic (49 XY, 64XX, and 65XX) and healthy (18XX, 38XX, and 18XX) cell populations (Fig. 1).
FIG. 1.
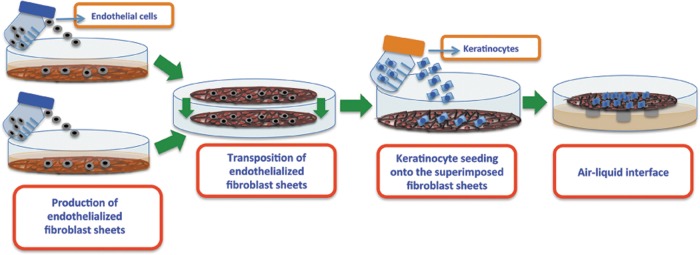
Schematic representation of the in vitro production of endothelialized substitutes using the self-assembly approach.
Histological analysis of skin substitutes
Biopsies were done on three different areas of each subject. The harvested tissue was fixed in HistoChoice (Amreco) solution overnight at room temperature. The following day, the samples were embedded in paraffin and refrigerated. Cross-sections (5 μm) were cut and stained with Masson's trichrome.
Immunofluorescence staining
Indirect immunolabelings were performed on the optimal cutting temperature (OCT) compound cross-sections (5 μm and 25 μm). The primary antibodies were used as follows: vascular endothelial cadherin (VE-cadherin, CD144, R&D systems; 1:100), von Willebrand factor (vWF, DAKO; 1:200) antibody, platelet-endothelial cellular adhesion molecule-1 (PECAM-1, CD31, Invitrogen; 1:100) antibody, type IV collagen (Coll IV, Abcam; 1:400) antibody, filaggrin (Abcam; 1:500), loricrin (Cedarlane; 1:1000), keratin 10 (Monosan; 1:200), and KI67 (BD pharmingen; 1:300). Alexa 488 and Alexa 594 were used as secondary antibodies, but the secondary antibody was mixed with Hoechst 33258 (Sigma-Aldrich; 1/100) to stain the cell nuclei. The Coll IV synthesis of the ECs was analyzed under the same conditions as the SA process. HDMECs were seeded into nongelatinized flasks and cultivated in the same medium mixtures as the substitutes for the same time intervals. The monolayer ECs were fixed and stained with CD31 and Coll IV.
Whole-mount immunofluorescence staining
At the end of the air–liquid period, the substitutes were plunged into ice-cold acetone (4°C) for 24 h in glass petri dishes. Afterwards, the substitutes were washed several times with PBS and floated in the primary antibody solution for 24 h at 4°C under very gentle agitation. The substitutes were then washed again with PBS and floated in the secondary antibody solution containing Hoechst 33258 (1:100) at 4°C for 24 h. After the last washing period, the substitutes were slided and covered with coverslips and mounting medium. The following day, nail polish was applied around the slides and the samples were observed with a Zeiss LSM 700 laser-scanning confocal microscopy system. The semiquantification of CLSs in the substitutes was performed using the 3D whole-mount-image photographs. The photographs were obtained by several z-stack series scans of Zen software (Zeiss). The image analysis was carried out using Imaris 7.0.0 software between certain size scales of the 3D surfaces. The calculation was based on the individual number and volume ratio (%) of the CD31-marked structures that were greater than 1000 μm.
Statistics
Error bars represent the standard error. Statistical significance was determined using a nonparametric Mann-Whitney U test (p<α=0.07). All statistical analysis was performed using SPSS 21.
Results
Macroscopy and histology of skin substitutes
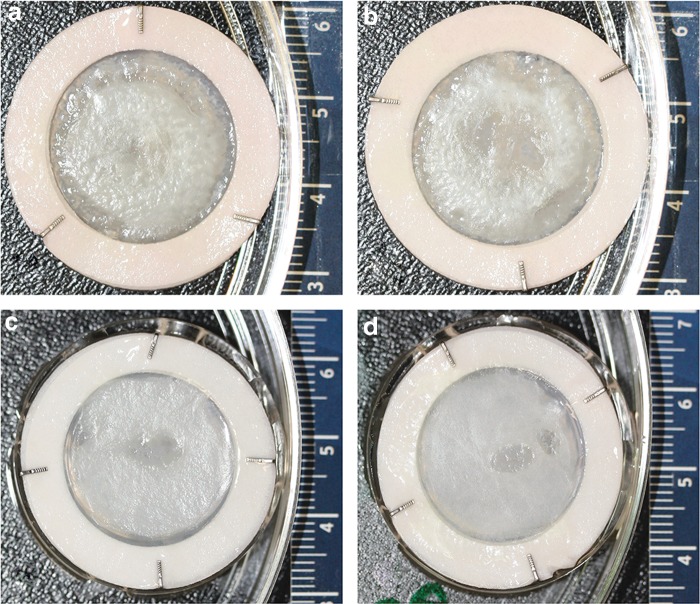
Psoriatic skin substitutes (Ps) and healthy skin substitutes (Hs) were prepared with or without ECs. The Ps showed a rough epidermal structure compared to Hs. However, there was no major difference between the substitutes with or without ECs (Fig. 2). Masson's trichrome staining of Ps showed increased viable epidermal thickness (excluding the stratum corneum) and total epidermal thickness compared to the Hs (Fig. 3). In addition, greater retention of nuclei within the stratum corneum cells in Ps was observed. Openings lined with ECs were observed in the dermal part of the endothelialized substitutes (Fig. 3b, 3e). The structure of the openings consisted of multiple connected ECs, in which the bulged nuclei of individual ECs (indicated by arrows in Fig. 3c, 3f) were clearly seen. On the other hand, as expected, there were no openings in the dermal part of the nonendothelialized substitutes (Fig. 3a, 3d).
FIG. 2.
Macroscopic analysis. The structural and physical observations of whole substitutes were analyzed by a digital camera system (Canon EOS Rebel XSI-450D) for the collection of magnified pictures of the whole substitutes before the preparation of biopsies. Psoriatic skin substitute without (a) or with (b) endothelial cells (ECs), healthy skin substitute without (c) or with (d) ECs. The experiments were performed with three different psoriatic (49XY, 64XX, and 65XX) and healthy (18XX, 38XX, and 18XX) cell populations.
FIG. 3.
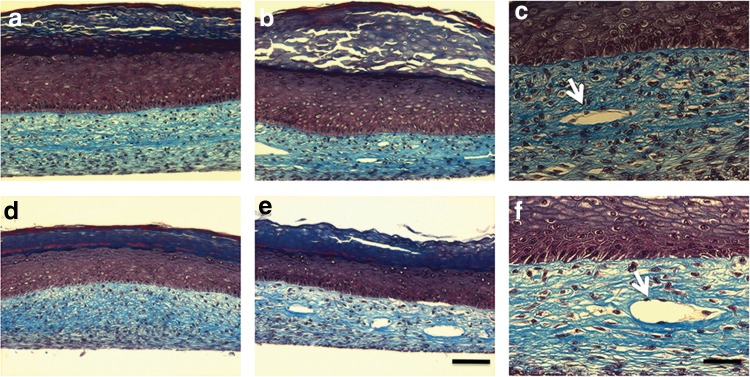
Histological analysis with Masson's trichrome staining. Psoriatic skin substitutes without (a) or with (b, c) ECs. Healthy skin substitutes without (d) or with (e, f) ECs. 10× magnification for a, b, d, and e; scale bar=100 μm. 20× magnification for c and f; scale bar=50 μm. Arrows indicate the capillary tubes. These results showed that the dermal portions of the substitutes were well-fused, and also after the seeding of ECs onto the fibroblast sheets, ECs could penetrate, migrate into the matrix; then divide, elongate, and break up this matrix by enzyme secretion; and spontaneously organize to restore the capillary-like structures (CLSs).
Immunofluorescent labeling of CLSs and basement membrane deposition around CLSs
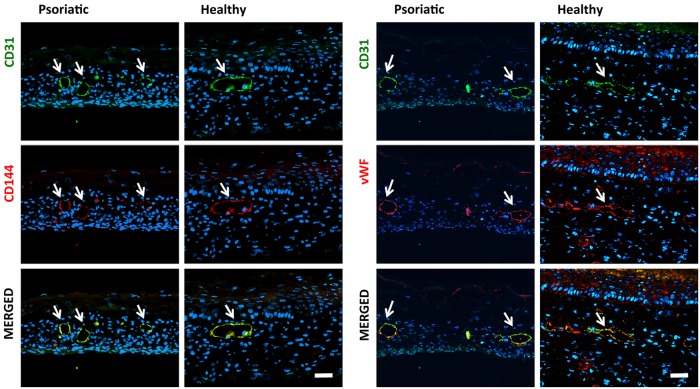
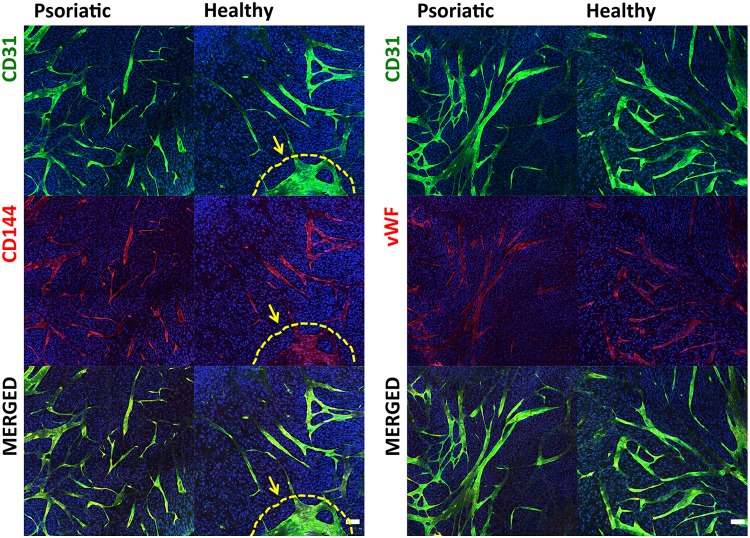
CD31 and CD144 are cell-surface adhesion molecules produced by ECs. CD144 plays an important role in the formation of single-EC layers in vascular maturation. The first two columns of Figure 4 show that the ECs organized and rebuilt the tube-like structures in which lumen exists surrounded by ECs (Fig. 4). A second immunolabeling study was undertaken to determine whether ECs retained their function and character during in vitro substitute preparation procedures. The most important functions of ECs are the synthesis and secretion of the blood-clotting protein vWF (factor VIII). vWF, which is also secreted by megakaryocytes and exists in circulating blood, is deposited into plasma, into the Weibel Palade bodies of ECs, and onto the subendothelial matrix. vWF and CD31 were used together to observe if the CD31-labeled ECs secreted vWF. The double-stained samples (Fig. 4, second two columns) demonstrated that the HDMECs kept their characteristic features during skin reconstruction. In addition, the vWF-stained samples showed that ECs could secrete a basal membrane (BM) around the CLSs.
FIG. 4.
The immunohistological phenotypes of the CLSs were determined with specific antibodies for the ECs. Immunohistological localization of CD31/CD144 and CD31/vWF of endothelialized psoriatic or healthy substitutes. CD31-positive CLSs are green, CD144- and vWF-positive CLSs are red, and the nuclei of cells are blue (white arrows indicate CLSs; scale bar=50 μm). vWF, von Willebrand factor.
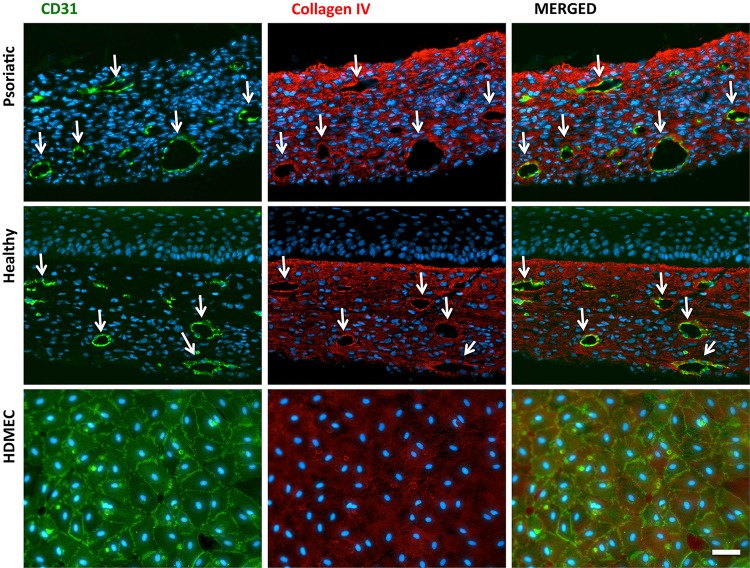
This last immunostaining study was done to determine the organizational and functional stability of CLSs in the substitutes. This was accomplished using anti-Coll IV, an important component of the BM of capillaries and the extracellular matrix that provides mechanical support against blood pressure. ECs secrete a subendothelial BM around the EC layer, which has an important role in the dilatation of capillaries and immune cell extravasation (indicated by arrows in Fig. 5). Additionally, it was proven that ECs were able to secrete their own matrix in vitro by monolayer studies (Fig. 5, horizontal bottom row).
FIG. 5.
Immunohistological localization of collagen IV (red) surrounded capillary-like tubes in the endothelialized psoriatic and healthy substitutes; ECs are labeled with CD31 (green). The last horizontal row indicates the collagen IV secretion of monolayer ECs under the same conditions as the substitutes (arrows indicate CLSs; scale bar=50 μm).
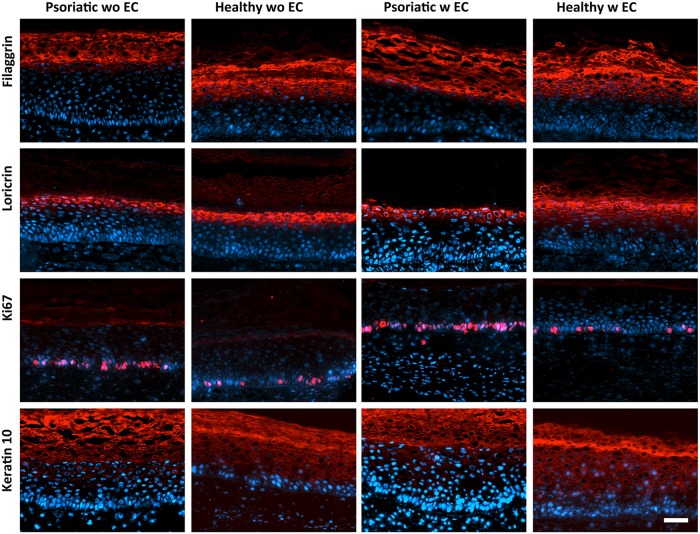
Jean et al.18 used DME as a medium for fibroblast sheets and DH as a medium after keratinocyte seeding. However, in this study, EGM-2 MV media was mixed half and half with those media because substitutes were seeded with ECs. To specify the effects of the medium mixture on the psoriatic character, keratinocyte differentiation markers such as filaggrin, loricrin, keratin 10, and proliferation marker Ki67 were tested.
It was observed that the psoriatic character of the pathological substitutes was retained even if mixed culture mediums were used in the presence of the ECs (Fig. 6). The Ps showed a decreased expression of differentiation markers. Owing to the high epidermal turnover of psoriatic keratinocytes, it is known that keratinocytes could not differentiate well, and epidermal barrier as well as differentiation markers are delayed (keratin 10) and reduced (filaggrin and loricrin). However, an increased number of proliferating keratinocytes were observed in the Ps compared to the Hs with Ki67 staining.
FIG. 6.
Markers of keratinocyte differentiation and proliferation. Expression of filaggrin, loricrin, Ki67, and keratin 10 in psoriatic and healthy skin substitutes with (w) or without (wo) ECs.
Complex capillary network formation and semiquantification
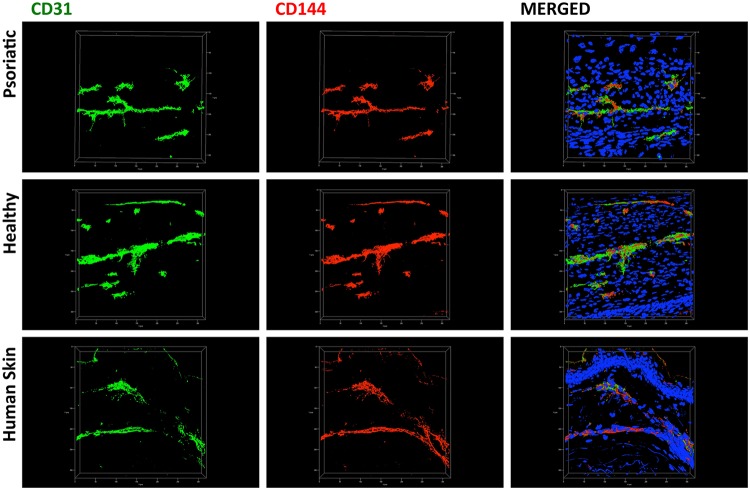
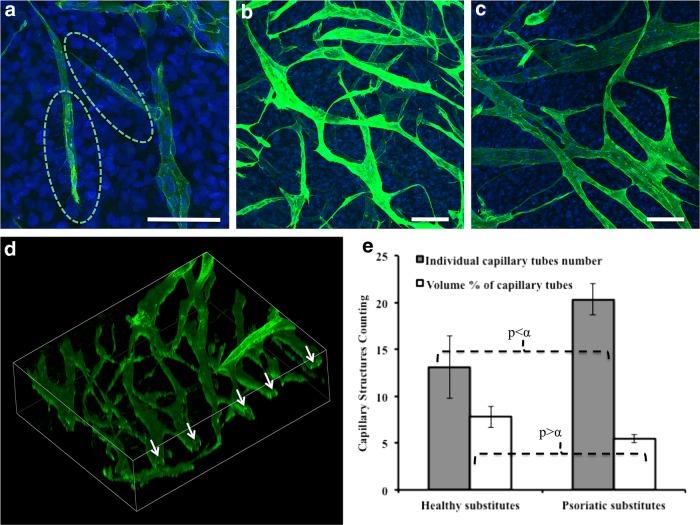
Confocal microscopy images from the 25-μm OCT sections showed the 3D morphological structure and arrangement similarity of CLSs between the substitutes and ex vivo human skin. CLSs had a linear tube-like formation with branches similar to the ex vivo human skin control sample (Fig. 7). The broad perspective observation using a whole-mount staining technique allowed an eagle-eye observation of CLSs in an extensive area without mechanical sectioning (Fig. 8). An interesting observation in the substitutes was the presence of tip cells, which are specialized ECs that promote and guide the capillaries sprouting via their filopodias. The filopodial structure can be clearly seen in Figure 9a within the green broken lines. Moreover, the tip cells were observed after 60 days of culture, which means the network formation was still remodeling actively. Differences in luminosity between sublayers and upper layers showed that there were different networks at different layers, suggesting that ECs migrated to deeper layers and the CLSs anastomosed with different CLSs (Fig. 9b, 9c) in the dermal component. Branches of tube and lumen formation are clearly seen in Figure 9d (the lumens are indicated by arrows).
FIG. 7.
Individual topographical visualization of CLSs in 25-μm cross-sections by double staining with CD31 (green) and CD144 (red) in the endothelialized psoriatic and healthy substitutes. (The cell nuclei were stained with Hoechst [blue]; scale bar=50 μm.) The images were taken by a Zeiss LSM 700 confocal laser scanning microscopy.
FIG. 8.
The two-dimensional views of the whole-mount staining of psoriatic or healthy substitutes were presented with different combinations of double immunofluorescence staining: CD31-CD144 (first set of figures) and CD31-vWf (second set of figures) in endothelialized psoriatic or healthy substitutes (scale bar=100 μm). The ECs proliferated, migrated, and spread all around the substitutes and rebuilt the capillary structures homogeneously while keeping their functionality. Clusters were observed between capillary tubes and ECs in healthy substitutes, which is indicated by the yellow arrow and broken line.
FIG. 9.
Three-dimensional whole-mount immunofluorescence staining of CLSs with CD31 (green). CD31-positive ECs were labeled green, and nonendothelialized substitutes confirmed that there was no nonspecific staining (data not shown). CD31-marked tip cells and their filopodias are indicated by green broken lines (a) (×40 magnification). Psoriatic substitute (b) and healthy substitute (c) (×20 magnification). Lumen formation in the psoriatic endothelialized substitute (d) (arrows indicate the lumens in capillary tubes; scale bar=100 μm). Whole-mount staining enabled semiquantitative analysis on the whole substitutes. Volume percentages (white bars) and individual capillary tube numbers (gray bars) (e) of CLSs (n=3) in the substitutes.
CD31-labeled 3D photographs were used to make a comparative semiquantitative analysis of the CLSs in the substitutes by confocal microscopy software (Imaris 7.0.0). The quantification was done on the basis of the volume percentages of the CLSs and individual CLS number counting per certain volumes. The results showed that there was no significant difference between the volume percentages of the CLSs (p=0.094>α=0.07). However, there was a significant difference in the numbers of CLSs between Hs and Ps (p=0.063<α=0.07). This meant that the volume of the substitutes covered by CLSs was the same, but there were more CLSs in the Ps (Fig. 9e). The quantity variance of CLSs between Ps and Hs showed that, even though the same quantity of ECs were seeded onto the substitutes, there were more CLSs in Ps than in Hs. This observation can be associated with the angiogenic provocation feature of psoriatic cells. In Hs, some EC accumulations or colonies were observed between CLSs (indicated by the yellow broken line and the arrow in Fig. 8) in contrast to what was observed in Ps. This could certainly contribute to the high volume of CLS marked by CD31 per total volume.
Discussion
The pathology of psoriasis has many as yet unexplained mechanisms. There are no specific markers for the prognosis, diagnosis, or progression of psoriasis or for the therapeutic response of treatments.21 The absence of a specific profile that defines the psoriatic phenotype necessitates physiologically relevant and reliable in vitro models to investigate this disorder and develop more effective treatments.
The literature indicates that past psoriatic models were developed using de-epidermized dermis, fibrin gel, and a hybrid of skin tissue engineering and animal modelling.22–24 These models all involved the use of stimulant additives or immune cell injections. However, Jean et al.,18 using the SA method, described an in vitro, 3D tissue-engineered psoriatic-skin model that did not use any scaffold-like supportive material or induction supplements. In our study, that model was taken a step further by introducing HDMECs, and a psoriatic skin model with capillary networks was built and physically characterized.
As with earlier models, the psoriasis form pattern that was developed has the hallmarks of the psoriatic phenotype–altered keratinocyte differentiation and proliferation. Filaggrin is localized in the granular layer of the epidermis where it is deposited in keratohyalin granules. Loricrin, the main component of the cornified-cell envelope, is also stored in the granular layer. The results of our study showed that these two epidermal proteins were reduced in the psoriatic substitutes compared to the healthy counterparts. In addition, the granular layers of the healthy substitutes were more apparent than in the psoriatic substitutes. These differences could be explained by tumor necrosis factor-α (TNF-α), the principal cytokine involved in psoriasis, and its modulated expression of these proteins.25 The suprabasal keratinocyte differentiation marker, keratin 10, is significantly decreased in psoriasis because its synthesis is delayed.26 In our study, keratin 10 was detected in the upper layers of the epidermis in the psoriatic substitutes, whereas it was detected in the deeper layers in the healthy counterparts. That means the healthy keratinocytes start to differentiate earlier than psoriatic ones. KI67 staining confirmed the hyperproliferative character of psoriatic skin models. There were also a higher number of KI67-positive cells in the psoriatic substitutes.
An angiogenic cascade has four steps: tip cell formation, sprout formation, lumen formation, and maturation. These steps were all observed in the in vitro models. The capillary networks extended over many layers of the dermal component and lumen formation confirmed that these structures were not cord-like but well-differentiated capillary-like networks. The last step of the cascade is the maturation of capillaries with basement membranes and coverage with pericytes. Since there were no pericytes in our cultured conditions, this step was inferred by basement membrane formation around the CLS. Berthod et al.27 showed that the pericytes could derive from fibroblast in vitro, which encouraged us to investigate the same effect with our model. The CLSs in psoriatic skin models are more numerous, complex, branched, dense, and chaotic than in healthy substitutes. Because TNF-α can induce tip cell formation,28 more tip cell formation is the reason for the more complex CLS formation in the psoriatic substitutes. This observation can also be tied to the angiogenic provocation feature of psoriatic cells.
Some researchers have suggested that the anti-angiogenic effect may be beneficial in the treatment of psoriasis.3,29,30 VEGF-based31 and Tie2-based32 psoriatic models, and the healing of psoriatic lesions related to vascular improvements with conventional9,17,33 or new treatments,30,34–37 assuming that microvascular abnormalities in psoriasis play a role in the pathogenesis. However, while it is still unknown whether angiogenesis is a cause (primary role) or a consequence (secondary role) in psoriasis pathology, it is clear that inflammation and angiogenesis are related processes. The hypothesis about the healing process suggests that when lesions are resolved with treatment, the microcirculation is slower to return to its normal state.9,33,37 So without a return of the microcirculation to normal state, psoriasis cannot be said to be in remission. This observation indicates that the altered cutaneous microcirculation remains even after the lesions have healed.9,33,37 In conclusion, few trials have been conducted on the effect of existing and new cures on microvascular pattern alterations. Therefore, this vascularized psoriatic-skin model will be a useful tool to evaluate and predict the treatment responses to phenotypic features and microvascular aspects of this serious disease.
Abbreviations Used
- 3D
three-dimensional
- BM
basal membrane
- CLS
capillary-like structure
- DH
Dulbecco-Vogt modification of Eagle's medium with Ham's F12 in a 3:1 ratio
- DME
Dulbecco-Vogt modification of Eagle's medium
- EC
endothelial cell
- EGF
epidermal growth factor
- EGM-2 MV
Endothelial Cell Growth Media-2 microvascular
- HDMEC
human dermal microvascular endothelial cell
- Hs
healthy skin substitutes
- PBS
phosphate-buffered saline
- PECAM-1
platelet-endothelial cellular adhesion molecule-1
- Ps
psoriatic skin substitutes
- OCT
optimal cutting temperature
- TNF-α
tumor necrosis factor-α
- VE-cadherin
vascular endothelial cadherin
- vWF
von Willebrand factor
Acknowledgments
The authors acknowledge financial support from the Natural Sciences and Engineering Research Council of Canada (NSERC) and the Canadian Institutes of Health Research (CIHR) through their joint Collaborative Health Research Projects (CHRP) program. We acknowledge the support of the Réseau ThéCell du FRQS. R. Pouliot is a FRQS career award scholar. The authors gratefully acknowledge Dr. Stéphane Chabaud, Dr. Jacques Soucy, Dr. Dominique Mayrand, Dr. Jessica Jean, and Sébastien Larochelle for their scientific and technical support.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Chua RA, Arbiser JL. The role of angiogenesis in the pathogenesis of psoriasis. Autoimmunity. 2009;42:574–579 [DOI] [PubMed] [Google Scholar]
- 2.Creamer D, Sullivan D, Bicknell R, et al. Angiogenesis in psoriasis. Angiogenesis. 2002;5:231–236 [DOI] [PubMed] [Google Scholar]
- 3.Heidenreich R, Rocken M, Ghoreschi K. Angiogenesis drives psoriasis pathogenesis. Int J Exp Pathol. 2009;90:232–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Micali G, Lacarrubba F, Musumeci ML, et al. Cutaneous vascular patterns in psoriasis. Int J Dermatol. 2010;49:249–256 [DOI] [PubMed] [Google Scholar]
- 5.Raychaudhuri SK, Maverakis E, Raychaudhuri SP. Diagnosis and classification of psoriasis. Autoimmun Rev. 2014;13:490–495 [DOI] [PubMed] [Google Scholar]
- 6.Archid R, Patzelt A, Lange-Asschenfeldt B, et al. Confocal laser-scanning microscopy of capillaries in normal and psoriatic skin. J Biomed Opt. 2012;17:101511. [DOI] [PubMed] [Google Scholar]
- 7.Hern S, Stanton AW, Mellor RH, et al. In vivo quantification of the structural abnormalities in psoriatic microvessels before and after pulsed dye laser treatment. Br J Dermatol. 2005;152:505–511 [DOI] [PubMed] [Google Scholar]
- 8.Qin J, Jiang J, An L, Gareau D, et al. In vivo volumetric imaging of microcirculation within human skin under psoriatic conditions using optical microangiography. Lasers Surg Med. 2011;43:122–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stinco G, Lautieri S, Valent F, et al. Cutaneous vascular alterations in psoriatic patients treated with cyclosporine. Acta Derm Venereol. 2007;87:152–154 [DOI] [PubMed] [Google Scholar]
- 10.Wolberink EA, van Erp PE, de Boer-van Huizen RT, et al. Reflectance confocal microscopy: an effective tool for monitoring ultraviolet B phototherapy in psoriasis. Br J Dermatol. 2012;167:396–403 [DOI] [PubMed] [Google Scholar]
- 11.Costa C, Incio J, Soares R. Angiogenesis and chronic inflammation: cause or consequence? Angiogenesis. 2007;10:149–166 [DOI] [PubMed] [Google Scholar]
- 12.Pober JS, Sessa WC. Evolving functions of endothelial cells in inflammation. Nat Rev Immunol. 2007;7:803–815 [DOI] [PubMed] [Google Scholar]
- 13.Hendriks AG, Steenbergen W, Hondebrink E, et al. Whole field laser Doppler imaging of the microcirculation in psoriasis and clinically unaffected skin. J Dermatolog Treat. 2014;25:18–21 [DOI] [PubMed] [Google Scholar]
- 14.Sochorova R, Svecova D, Sinka L, et al. Increased endothelemia as an indirect marker of changes in the blood vessel endothelium in psoriasis. J Eur Acad Dermatol Venereol. 2004;18:556–559 [DOI] [PubMed] [Google Scholar]
- 15.Barton SP, Abdullah MS, Marks R. Quantification of microvascular changes in the skin in patients with psoriasis. Br J Dermatol. 1992;126:569–574 [DOI] [PubMed] [Google Scholar]
- 16.Goodfield M, Hull SM, Holland D, et al. Investigations of the ‘active’ edge of plaque psoriasis: vascular proliferation precedes changes in epidermal keratin. Br J Dermatol. 1994;131:808–813 [DOI] [PubMed] [Google Scholar]
- 17.Shaker OG, Khairallah M, Rasheed HM, et al. Antiangiogenic effect of methotrexate and PUVA on psoriasis. Cell Biochem Biophys. 2013;67:735–742 [DOI] [PubMed] [Google Scholar]
- 18.Jean J, Lapointe M, Soucy J, et al. Development of an in vitro psoriatic skin model by tissue engineering. J Dermatol Sci 2009;53:19–25 [DOI] [PubMed] [Google Scholar]
- 19.Gibot L, Galbraith T, Huot J, et al. A preexisting microvascular network benefits in vivo revascularization of a microvascularized tissue-engineered skin substitute. Tissue Eng Part A. 2010;16:3199–3206 [DOI] [PubMed] [Google Scholar]
- 20.Rochon MH, Fradette J, Fortin V, et al. Normal human epithelial cells regulate the size and morphology of tissue-engineered capillaries. Tissue Eng Part A. 2010;16:1457–1468 [DOI] [PubMed] [Google Scholar]
- 21.Villanova F, Di Meglio P, Nestle FO. Biomarkers in psoriasis and psoriatic arthritis. Ann Rheum Dis. 2013;72(Suppl 2):ii104–110 [DOI] [PubMed] [Google Scholar]
- 22.Barker CL, McHale MT, Gillies AK, et al. The development and characterization of an in vitro model of psoriasis. J Invest Dermatol. 2004;123:892–901 [DOI] [PubMed] [Google Scholar]
- 23.Guerrero-Aspizua S, García M, Murillas R, et al. Development of a bioengineered skin-humanized mouse model for psoriasis: dissecting epidermal-lymphocyte interacting pathways. Am J Pathol. 2010;177:3112–3124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tjabringa G, Bergers M, van Rens D, et al. Development and validation of human psoriatic skin equivalents. Am J Pathol. 2008;173:815–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim BE, Howell MD, Guttman-Yassky E, et al. TNF-alpha downregulates filaggrin and loricrin through c-Jun N-terminal kinase: role for TNF-alpha antagonists to improve skin barrier. J Invest Dermatol. 2011;131:1272–1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bernerd F, Magnaldo T, Darmon M. Delayed onset of epidermal differentiation in psoriasis. J Invest Dermatol. 1992;98:902–910 [DOI] [PubMed] [Google Scholar]
- 27.Berthod F, Symes J, Tremblay N, et al. Spontaneous fibroblast-derived pericyte recruitment in a human tissue-engineered angiogenesis model in vitro. J Cell Physiol. 2012;227:2130–2137 [DOI] [PubMed] [Google Scholar]
- 28.Sainson RC, Johnston DA, Chu HC, et al. TNF primes endothelial cells for angiogenic sprouting by inducing a tip cell phenotype. Blood. 2008;111:4997–5007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heidenreich R, Rocken M, Ghoreschi K. Angiogenesis: the new potential target for the therapy of psoriasis? Drug News Perspect. 2008;21:97–105 [DOI] [PubMed] [Google Scholar]
- 30.Markham T, Mullan R, Golden-Mason L, et al. Resolution of endothelial activation and down-regulation of Tie2 receptor in psoriatic skin after infliximab therapy. J Am Acad Dermatol 2006;54:1003–1012 [DOI] [PubMed] [Google Scholar]
- 31.Elias PM, Arbiser J, Brown BE, et al. Epidermal vascular endothelial growth factor production is required for permeability barrier homeostasis, dermal angiogenesis, and the development of epidermal hyperplasia: implications for the pathogenesis of psoriasis. Am J Pathol. 2008;173:689–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wolfram JA, Diaconu D, Hatala DA, et al. Keratinocyte but not endothelial cell-specific overexpression of Tie2 leads to the development of psoriasis. Am J Pathol. 2009;174:1443–1458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stinco G, Buligan C, Errichetti E, et al. Clinical and capillaroscopic modifications of the psoriatic plaque during therapy: observations with oral acitretin. Dermatol Res Pract. 2013;2013:781942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Avramidis G, Krüger-Krasagakis S, Krasagakis K, et al. The role of endothelial cell apoptosis in the effect of etanercept in psoriasis. Br J Dermatol. 2010;163:928–934 [DOI] [PubMed] [Google Scholar]
- 35.Campanati A, Goteri G, Simonetti O, et al. Angiogenesis in psoriatic skin and its modifications after administration of etanercept: videocapillaroscopic, histological and immunohistochemical evaluation. Int J Immunopathol Pharmacol. 2009;22:371–377 [DOI] [PubMed] [Google Scholar]
- 36.Goedkoop AY, Kraan MC, Picavet DI, et al. Deactivation of endothelium and reduction in angiogenesis in psoriatic skin and synovium by low dose infliximab therapy in combination with stable methotrexate therapy: a prospective single-centre study. Arthritis Res Ther. 2004;6:R326–R334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stinco G, Buligan C, Maione V, et al. Videocapillaroscopic findings in the microcirculation of the psoriatic plaque during etanercept therapy. Clin Exp Dermatol. 2013;38:633–637 [DOI] [PubMed] [Google Scholar]