Abstract
The research group has detected nitrosative stress and a singular version of nitrosylated serum α-synuclein in serum of Parkinson's disease (PD) patients. Dysfunction of the thyroid gland has been proposed to be linked to this disease. The aim of the study was to know if the thyroid gland is involved in idiopathic PD and nitrosative stress. We studied 50 patients (early and advanced disease patients), 35 controls, and 6 subjects with thyroidectomy. Clinical characteristics, serum thyroperoxidase levels, and 3-nitrotyrosine proteins were analyzed. Enzyme-linked immunosorbent assay and immunoblotting methods were employed. The findings indicated that the prevalence of two thyroid dysfunctions (hyper- or hypothyroidism) was not found to be different in patients relative to controls. However, the levels of the enzyme thyroperoxidase were found to be elevated in early disease patients (p<0.006), not in advanced disease subjects, and these levels were negatively correlated with serum 3-nitrotyrosine proteins (p<0.05), the indicators of nitrosative stress. The thyroidectomized subjects showed very low levels of serum 3-nitrotyrosine proteins (78% reduction vs. controls) and, among these proteins, the nitrosylated serum α-synuclein was nearly absent. These observations lead to the hypothesis that the thyroid gland and thyroperoxidase participate in nitrosylation of serum proteins and they could influence Parkinsonian nitrosative stress as well as nitrosylation of serum α-synuclein, a potentially pathogenic factor. Antioxid. Redox Signal. 21, 2143–2148.
Introduction
The research group has reported the presence of nitrosylation stress in serum and, to a lesser extent, cerebrospinal fluid of early parkinsonian patients, characterized by an excess of 3-nitrotyrosine proteins without changes in nitroalbumin (4, 6). Nitrosative stress also leads to a post-transcriptional modification of serum 3-nitrotyrosine α-synuclein (3NT-αSyn), characterized by an altered profile of tyrosine (Tyr) nitrosylation. Thus, while the nitrosylation of tyrosines in control subjects is balanced between the tyrosine 125/136 residues at the carboxyl terminus and the Tyr39 at the amine terminus of 3NT-αSyn, in early disease patients, there is dominant nitrosylation of the Tyr125/136 residues. In other words, the ratio between both parameters is reliably higher in early parkinsonian patients relative to controls (4), and we propose that it may represent a biomarker for early Parkinson's disease (PD).
It has also been proposed that phagocytic overactivity (through myeloperoxidase excess) could underlie the observed changes, but in contrast to this hypothesis, myeloperoxidase is not found to be augmented in serum (3). Of note is that the thyroid gland is an organ where physiological oxidative stress takes place, and there is a very active thyroid peroxidase or thyroperoxidase (TPO) that is critical for iodination of tyrosine residues on thyroglobulin. The thyroid gland has been linked to PD because some researchers have found more prevalence of some thyroid dysfunctions among PD patients or they have detected sympathetic denervation of the thyroid gland in PD patients, a hallmark of the disease, because it also occurs in other organs such as the heart and rectum (5, 7, 8, 9). The aim was to study the role of the thyroid gland in idiopathic PD.
Innovation.
We have detected that thyroperoxidase is elevated in serum of patients with early Parkinson's disease. This elevation is negatively correlated with serum levels of 3-nitrotyrosine proteins. These proteins are augmented in early Parkinsonism, indicative of nitrosative stress. Furthermore, the thyroid gland mediates nitrosylation of serum proteins because this process is strongly reduced in thyroidectomized subjects where, for instance, the 3-nitrosylated α-synuclein is nearly absent. These observations indicate that the thyroid gland and its enzyme thyroperoxidase participates in nitrosylation of serum proteins, and hence, they may influence Parkinsonian nitrosative stress and nitrosylation of serum α-synuclein, a potentially pathogenic factor.
Results and Discussion
First, we quantified thyroid dysfunction through the frequency of hypo- and hyperthyroidism in our groups of patients and controls. In PD patients, the prevalence of hypothyroidism was 10% and that of hyperthyroidism was 2%. In controls, the prevalence of hypothyroidism was 5.7% and that of hyperthyroidism was 2.8%. Although the hypothyroidism prevalence was nearly twice higher in PD patients than in controls, the differences were not found to be significant. It can be concluded that the most frequent thyroid dysfunctions, hyper- and hypothyroidism, are not more prevalent in PD.
Considering our previous work with blood myeloperoxidase that could participate in nitrosative stress (4), it was decided to evaluate serum levels of thyroid peroxidase, a peroxidase linked to thyroid function, which could also participate in nitrosative stress. Interestingly, TPO was found to be elevated in early disease patients (<0.01), not in advanced PD subjects, as shown in Table 1. Myeloperoxidase was also quantified, for comparison, and was devoid of serum changes, confirming previous results (4). These findings point to a role for TPO on sporadic PD.
Table 1.
Clinical Features of Patients and Serum Levels of Thyroperoxidase and Myeloperoxidase
| PD (n=50) | Control (n=35) | p | |
|---|---|---|---|
| Clinical parameters | |||
| Age (years) | 63.4±2 | 67.9±10 | NS |
| Sex, male, n (%) | 24 (48) | 22 (62) | NS |
| Body–mass index | 24.2±3 | 24.3±3.5 | NS |
| Hypertension, n (%) | 20 (40) | 6 (17) | <0.001 |
| Dyslipidemia, n (%) | 11 (22) | 7 (20) | NS |
| Vitamin A/E supplement | 11 (22) | 7 (20) | NS |
| Statin use, n (%) | 6 (12) | 3 (8.5) | NS |
| Aspirin, n (%) | 8 (16) | 4 (11.4) | NS |
| Hypothyroidism (%) | 5 (10) | 2 (5.7) | NS |
| Hyperthyroidism (%) | 1 (2) | 1 (2.8) | NS |
| Peroxidases | |||
| Myeloperoxidase (ng/ml) | 41.6±6.9 | 29.2±9.5 | NS |
| Thyroperoxidase (ng/ml) | 1.1±0.5 | 0.36±0.1 | NS |
| TPO early PD (ng/ml) | 2.1±0.6a | ||
| TPO advanced PD (ng/ml) | 0.4±0.4 | ||
Mean±SEM. Statistical comparisons were carried out with the χ2 test (qualitative variables) or Student's t-test (quantitative variables).
p<0.01 versus TPO levels of advanced PD patients and controls.
PD, Parkinson's disease; TPO, thyroperoxidase.
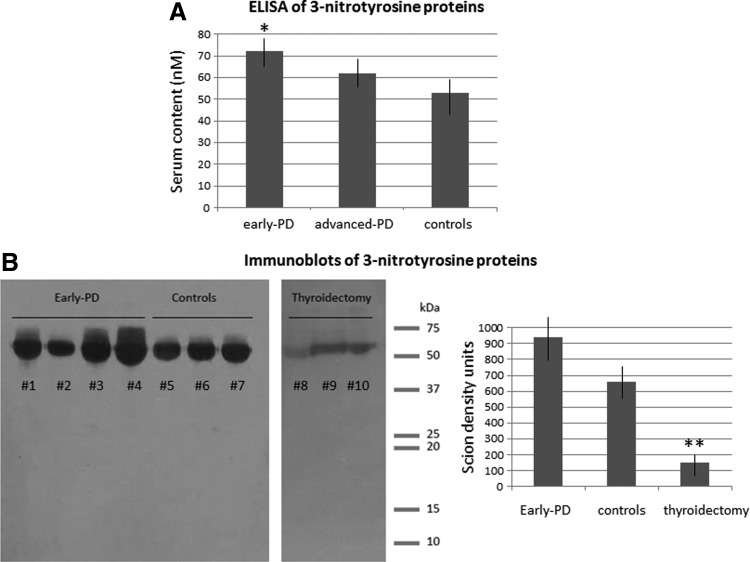
Considering that TPO was found to be elevated in early disease patients where nitrosative stress was detected previously (4), we tried to discern if this change was correlated with nitrosative stress. Nitrosative stress was evaluated through the enzyme-linked immunosorbent assay (ELISA) and immunoblot of serum 3-nitrotyrosine proteins. The one-way analysis of variance (ANOVA) indicated a significant group effect (F2, 45=3.6, p<0.05, n=16 each group), and the post hoc analysis revealed that serum 3-nitrotyrosine protein levels were significantly enhanced in early PD patients (p<0.05 vs. controls), not in advanced PD patients, as shown in Figure 1. Blots revealed a quite similar distribution of 3-nitrotyrosine protein bands in PD patients and controls, but the mean Scion density of 3-nitrotyrosine protein bands was significantly higher in early PD patients (879±61) than in controls (682.3±55, p<0.05), as shown in Figure 1, further indicating that the amount of 3-nitrotyrosine proteins was higher in early PD patients relative to controls. The presence of nitrosative stress in early disease patients, as measured through the elevation of serum 3-nitrotyrosine proteins, was hence confirmed (4).
FIG. 1.
Analysis of 3-nitrotyrosine proteins in patients, controls, and thyroidectomized subjects. (A) Serum levels of 3-nitrotyrosine proteins of early disease patients, advanced disease patients, and control subjects, as measured with the ELISA technique. Mean±SEM, *p<0.05 versus control (Tukey's test). (B) Representative immunoblots of 3-nitrotyrosine proteins of early PD patients, controls, and subjects with thyroidectomy. Bands of proteins between 50 and 75 kDa can be observed, mostly including nitroalbumin (68 kDa). Bands are faint in subjects with thyroidectomy. Right, Scion density values of immunoblot bands of early PD, controls, and subjects with thyroidectomy. Mean±SEM, **p<0.01 versus the remainder groups (Tukey's test). ELISA, enzyme-linked immunosorbent assay; PD, Parkinson's disease.
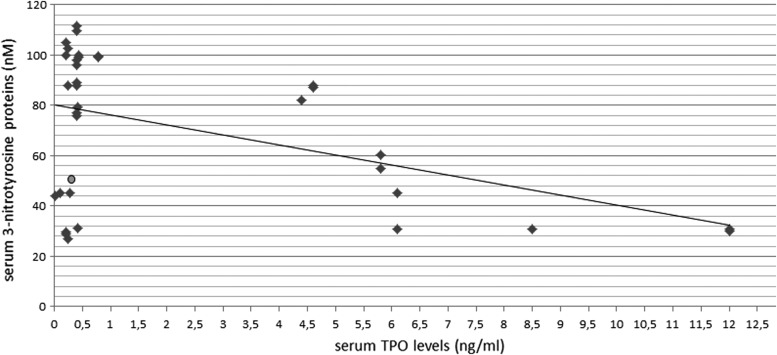
Then, thyroperoxidase and 3-nitrotyrosine protein levels were analyzed in search for statistical correlations. A significant correlation was found between TPO and 3-nitrotyrosine levels in serum of early disease patients (R = −0.46, p<0.006), as illustrated in Figure 2. There is a negative correlation, and the higher the TPO levels the lower the serum 3-nitrotyrosine proteins levels. Thus, in contrast to expectation, the serum thyroperoxidase level is a negative, not positive, indicator of nitrosative stress, as measured through 3-nitrotyrosine proteins γ levels.
FIG. 2.
Correlation between serum levels of thyroperoxidase and 3-nitrotyrosine proteins in early disease patients (Hoehn–Yahr stages 1 and 2, n=34). Correlation was found to be significant (R = −0.46, p<0.006; Pearson's test). The mean value in control subjects is represented as a gray dot.
For further confirming that there is a relationship between the thyroid gland and nitrosylation of serum proteins, 3-nitrotyrosine protein levels in six subjects with subtotal thyroidectomy and without neurological disorder were evaluated. Immunoblots revealed that these subjects presented a faint protein band around 65–68 kDa, with a low mean Scion density (149.9±45, p<0.01 vs. controls, Student's t-test). This value represents a reduction of around 78% relative to controls. These subjects suffered from incomplete ablation of the thyroid gland due to nodular or multinodular goiter. Immunoblot data of representative subjects are shown in Figure 1B.
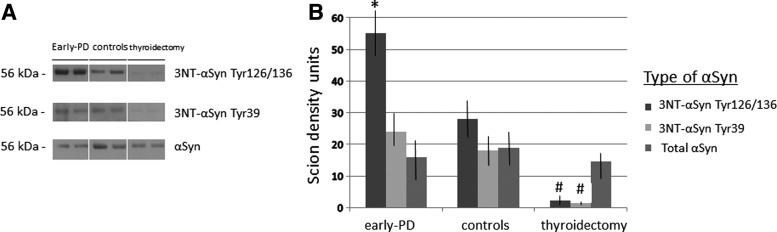
Finally, we studied the levels of 3NT-αSyn because it has been proposed, among 3-nitrotyrosine proteins, as a biomarker candidate for early PD (4). By means of Western blotting and one-step filtered serum (Amicon 50K filters), a 3-nitrotyrosine protein band of ∼56 kDa was detected in serum from patients and controls corresponding to 3NT-αSyn because it was detected with anti-3NT-αSyn antibodies aimed to residues Tyr125/136 or Tyr39. An ∼56 kDa band is characteristic of tetrameric α-synuclein (1). Confirming previous results, the 3NT-αSynTyr125/136 signal was stronger in early PD patients relative to controls (4), as measured with the Scion density program (Fig. 3). However, the 3NT-α-SynTyr39 signal was similar in both groups. Of note is that those subjects with thyroidectomy showed nearly absent 3NT-αSynTyr125/136 and 3NT-αSynTyr39 bands, with low density values (p<0.01 vs. controls and early PD patients; Newman–Keuls), as shown in Figure 3. The density of the band corresponding to wild α-synuclein was similar in every group, indicating that is the nitrosylation profile of the molecule rather than the wild protein what is altered in PD.
FIG. 3.
Analysis of 3NT-αSyn in patients, controls and subjects with thyroidectomy. (A) Representative immunoblots of 3-nitrotyrosine-α-synuclein (after two monoclonal antibodies or mAb against 3NT- αSyn with nitrosylation of tyrosine residues at the carboxyl terminus or 3NT-αSyn Tyr125/136 mAb and the amine terminus or 3NT-αSyn Tyr39 mAb) and wild α-synuclein in early disease patients, controls, and subjects with thyroidectomy. (B) Scion density units of immunoblot bands of 3-nitrotyrosine-α-synuclein (after two monoclonal antibodies or mAb against nitrosylated αSyn with nitrosylation of tyrosine residues at the carboxyl terminus or 3NT-αSyn Tyr125/136 mAb and the amine terminus or 3NT-αSyn Tyr39 mAb) and total α-synuclein in early disease patients, controls, and subjects with thyroidectomy. Mean±SEM, *p<0.05 versus the other bands of the same group; #p<0.01 versus the same bands of controls and early disease patients (Newman–Keuls test). 3NT-αSyn Tyr125/136, 3-nitrotyrosine-α-synuclein with nitrosylated Tyr125/136 residues; 3NT-αSyn Tyr39, 3-nitrotyrosine-α-synuclein with nitrosylated Tyr39 residue; αSyn; α-synuclein; mAb, monoclonal antibody.
In summary, the present study indicates that thyroid dysfunction, as evaluated through the frequency of hyper- and hypothyroidism, is not more prevalent in PD patients than in controls. However, the enzyme thyroperoxidase is found to be significantly elevated in early disease patients, a fact observed for the first time to our knowledge. The elevation of TPO would represent a novel indicator of dysfunction of the thyroid gland in early PD. This enzyme is a peroxidase, critical for iodination of tyrosine residues on thyroglobulin for the production of thyroid hormones. Of note is that peroxidase excess is usually linked to oxidative/nitrosative stress, but TPO levels were negatively correlated with serum 3-nitrotyrosine protein levels. However, this finding indicates that there is a link between thyroperoxidase and nitrosylation of proteins in PD. Another finding confirmed that the thyroid gland is involved in nitrosylation of proteins because, as observed for the first time, the levels of 3-nitrotyrosine proteins are strongly reduced in subjects with subtotal thyroidectomy.
Whether the thyroid gland mediates protein nitrosylation, an excess of protein nitrosylation in early Parkinsonism would be mediated by biochemical modifications within the thyroid gland. In this context, the presence of nitric oxide (NO) in the thyroid gland has been reported as a cofactor of TPO that participates in the process of iodination. We hypothesize that thyroid NO participates in nitrosylation of blood proteins, and an excess of thyroid gland NO could come into place in sporadic PD, a fact that requires further investigation. Bazzara et al. (2) described an inhibitory role of the NO/cGMP pathway on parameters of thyroid hormone biosynthesis and reported that NO inhibits TPO and thyroglobulin mRNA expression. This negative relationship between TPO and nitrosylation was also found in the present study, but in the opposite way. These authors postulate that the long-term inhibition of TPO and thyroid hormonogenesis by NO could be of interest in thyroid pathophysiology (2), and the present data support a critical role in sporadic PD of the triad TPO, thyroid gland function, and nitrosylation of serum proteins. Taken together, it seems that NO overactivity may take place in PD leading to the augmentation of 3-nitrotyrosine proteins, and the elevation of thyroperoxidase may be a homeostatic reaction tending to counteract the excess of nitrosylation of serum proteins.
Finally, since the thyroid gland seems to be critical for nitrosylation of blood proteins, it would be important for the formation of nitrosylated serum α-synuclein, which is nearly absent in thyroidectomized subjects. This protein is altered in early PD where it presents dominant nitrosylation of the tyrosine residues of the carboxyl terminus and it could participate in the pathogenesis of PD (4), pointing to another relationship between the thyroid gland and sporadic PD.
Notes
Study participants
Patients suffering from PD and clinically and SPECT-based diagnosed were included in the study. Patients were classified according to the Hoehn–Yahr staging. All participants were nonsmokers or nonalcohol drinkers. Control subjects were recruited from either the patients' relatives or volunteers without any neurological disorder and subjected to intradural anesthesia for traumatologic surgery in the Macarena Hospital. Thyroidectomized subjects without any neurological disorder were recruited from volunteers. Individuals presenting with any renal, liver, and cardiac dysfunction, malabsorption, autoimmune diseases, AIDS, diabetes mellitus, rheumatoid arthritis, and infectious conditions (oxidative stress markers in peripheral blood may be altered in such conditions) were excluded from both the PD and control groups. Clinical information, such as age, sex, body weight, hypertension, dyslipidemia, fasting blood sugar, coffee drinking, smoking, taking of vitamin A/vitamin E supplement, statins and aspirins, daily levodopa dose, type and dose of dopamine agonists, and rasagiline, was gathered from each patient.
Serum collection and ELISA measures
Five milliliters of blood was collected, after cephalic vein puncture, in gel-coated tubes to induce blood coagulation and to obtain serum (BD Vacuotainer). Serum was centrifuged at 2500 rpm for 10 min, and then, it was aliquoted, coded, and frozen at −80°C. For measuring 3-nitrotyrosine proteins, a commercial kit was used (Oxiselect Nitrotyrosine kit; Cell Biolabs, Inc.), following the manufacturer's instructions. For measuring myeloperoxidase and thyroperoxidase, commercial kits were also used (myeloperoxidase, Human MPO Instant ELISA kit; eBioscience, thyroperoxidase, Thyroid peroxidase Human ELISA kit, ELH-TPX-2; Raybiotech). Serum was not diluted for ELISA.
Western blotting and albumin removal
For removing albumin from serum and better understanding the signal of studying the presence of serum α-synuclein, a low denaturalizing method was used based on filtration with Amicon Ultra 50K (Millipore), based on our previous study (4). We have observed that one-step filtration (not three-times filtration as usual) allows obtaining a filtrate with molecules lower than 60 kDa, and hence, albumin is depleted without the need of magnetic beads or immunoprecipitation. The serum samples were lysated in 10% glycerol, 137 mM NaCl, and 20 mM Tris HCl, pH 7.5, containing peptidase inhibitors (1 μg/ml aproteinin and leupeptin, 1 mM PMSF). Protein levels were quantified by using the Bradford method. Samples were boiled, and aliquots containing 15 μg of protein each were subjected to SDS/polyacrylamide gel electrophoresis. Proteins were transferred electrophoretically to PVDF membranes. Immunolabeling was conducted with primary antibodies for nitrosylated α-synuclein at tyrosine 125/136 residues (anti-nitro-α/β-synuclein antibody, γ Tyr125/136, nSYn12; Millipore), nitrosylated α-synuclein at tyrosine 39 residue (anti-nitro-α/β-synuclein antibody Tyr39, nSYn14; Millipore), and α-synuclein (monoclonal anti-α-synuclein antibody Syn211; Sigma-Aldrich). Primary antibodies were detected with peroxidase-linked secondary antibodies (Santa Cruz Biotechnology), with enhanced chemiluminescence (Amersham, GE HealthCare), and autoradiography. Band densities of resulting autoradiograms were quantified by using the Scion Image program for PC (NIH). Values are given as intensity of bands in arbitrary Scion Units.
Statistical analysis and ethics
We enrolled 35 control subjects, six thyroidectomized subjects, and 50 patients, comprising early disease patients (n=34) and advanced PD patients (n=16). The early period corresponds to Hoehn–Yahr 1 and 2 stages. The advanced period corresponds to Hoehn–Yahr 3 and 4 stages. Differences in nitrosylation markers and clinical characteristics between the PD and control groups were analyzed by the χ2 test or Student's t-test (independent samples). Immunoblot band signals of different groups were analyzed by one-way ANOVA, followed by Tukey's test. When two factors were analyzed, two-way ANOVA was employed (group and type of α-synuclein as variables), followed by the Newman–Keuls test. Two groups were compared with Student's t-test. If needed, normalization was verified with the Shapiro–Wilk test. Correlations were studied with Pearson's test. Informed consent forms, under a protocol approved by the University of Seville and the Macarena Hospital internal ethics and scientific boards, were obtained from all the subjects, and the subjects' consent was obtained according to the Declaration of Helsinki (BMJ 1991; 302: 1194).
Abbreviations Used
- αSyn
α-synuclein
- ANOVA
analysis of variance
- cGMP
cyclic guanosine monophosphate
- ELISA
enzyme-linked immunosorbent assay
- mAb
monoclonal antibody
- MPO
myeloperoxidase
- NaCl
sodium chloride
- NO
nitric oxide
- 3NT-αSyn
3-nitrotyrosine α-synuclein
- 3NT-αSyn-Tyr125/136
α-synuclein with nitrosylated tyrosines 125 and 136
- 3NT-αSyn-Tyr39
α-synuclein with nitrosylated tyrosine 39
- PD
Parkinson's disease
- PMSF
phenylmethylsulfonyl fluoride
- PVDF
polyvinylidene fluoride
- SPECT
single photon emission computerized tomography
- TPO
thyroperoxidase
- Tyr
tyrosine
Acknowledgments
The authors thank Mara Guerra and Silvia Castellano (University of Seville) for their excellent technical assistance, Macarena Rus (Hospital Macarena) for her help with blood collection, Dr. Guillermo Izquierdo for allowing the use of the facilities of the Service of Neurology (Hospital Macarena), Dr. Maria-Isabel Garcia-Sánchez and the Biobanco Hospitalario Macarena (National Biobank Network, Carlos III Health Institute RD09/0076/00080) for the support in the samples collection procedure and storage, Dr. Cinta Calvo and the Service of Nuclear Medicine (Hospital Macarena, Seville) for SPECT analyses and Drs. Rodriguez Quecedo and Lola Rivero for their help. The authors are most grateful to all patients and their partners as well as control subjects who participated in this study. This study was supported by grants to E.F. by Junta de Andalucia (BIO127) and Spanish Ministerio de Sanidad (RETICS, RD06/010/1007; Instituto Carlos III, co-financing with FEDER, European Fund for Regional Development).
References
- 1.Bartels T, Choi JG, and Selkoe DJ. α-Synuclein occurs physiologically as a helically folded tetramer that resists aggregation. Nature 477: 107–110, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bazzara LG, Vélez ML, Costamagna ME, Cabanillas AM, Fozzatti L, Lucero AM, Pellizas CG, and Masini-Repiso AM. Nitric oxide/cGMP signaling inhibits TSH-stimulated iodide uptake and expression of thyroid peroxidase and thyroglobulin mRNA in FRTL-5 thyroid cells. Thyroid 17: 717–727, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Fernández E. Serum protein halogenation and nitrosylation: trait of maintained overstimulation of blood phagocytes in sporadic Parkinson's disease. OA Medical Hypothesis 1: 2–4, 2013 [Google Scholar]
- 4.Fernández E, García-Moreno JM, Martín de Pablos A, and Chacón J. May the evaluation of nitrosative stress through selective increase of 3-nitrotyrosine proteins other than nitroalbumin and dominant tyrosine-125/136 nitrosylation of serum α-synuclein serve for diagnosis of sporadic Parkinson's disease? Antioxid Redox Signal 19: 912–918, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.García-Moreno JM. and Chacón J. Hipotiroidismo enmascarado por enfermedad de Parkinson. Rev Neurol 35: 741–742, 2002 [PubMed] [Google Scholar]
- 6.García-Moreno JM, Martín de Pablos A, García-Sánchez MI, Méndez-Lucena C, Damas-Hermoso F, Rus M, Chacón J, and Fernández E. May serum levels of advanced oxidized protein products serve as a prognostic marker of disease duration in patients with idiopathic Parkinson's disease. Antioxid Redox Signal 18: 1296–1302, 2013 [DOI] [PubMed] [Google Scholar]
- 7.Matsui H, Udaka F, Oda M, Tamura A, Kubori T, Nishinaka K, and Kameyama M. Metaiodobenzylguanidine (MIBG) uptake in Parkinson's disease also decreases at thyroid. Ann Nucl Med 19: 225–229, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Tandeter H, Levy A, Gutman G, and Shvartzman P. Subclinical thyroid disease in patients with Parkinson's disease. Arch Gerontol Geriatr 33: 295–300, 2001 [DOI] [PubMed] [Google Scholar]
- 9.Tipre DN. and Goldstein DS. Cardiac and extracardiac sympathetic denervation in Parkinson's disease with orthostatic hypotension and in pure autonomic failure. J Nucl Med 46: 1775–1781, 2005 [PubMed] [Google Scholar]