Abstract
This research investigated the modification of homopolymer polyacrylonitrile (PAN) fibers for use as an adsorbent for removing arsenic from drinking water. Fibers were chemically modified and cross-linked using combinations of hydrazine hydrate and sodium hydroxide (NaOH) before being loaded with ferric hydroxide using two different iron loading procedures. Effects of reagent concentrations and reaction times on degree of chemical modification and fiber properties were investigated using Fourier transform infrared spectroscopy and ion-exchange measurements. Arsenate adsorption was a function of both the iron loading and the properties of the underlying fiber. For fibers treated with only a single reagent, both Fe3+ and arsenate adsorption could be understood in terms of ion-exchange properties of the fiber surfaces. However, for fibers treated with both hydrazine and NaOH, the ion-exchange properties of the surface could not explain the Fe3+ and arsenate adsorption behavior. The best arsenate removal performance was obtained using the simplest pretreatment procedure of soaking in 10% NaOH at 95°C for 90 min, followed by precipitation coating of ferric hydroxide. This simple preparation procedure involves only two commonly available and inexpensive reagents and can be carried out without any specialized equipment. This suggests that adsorbents based on inexpensive homopolymer PAN fabric may be produced in developing areas of the world where commercial products may not be available.
Key words: : adsorbent, arsenic, ferric hydroxide, polyacrylonitrile
Introduction
Arsenic contamination of underground drinking water supplies is a global problem affecting more than 100 million people (Mandal and Suzuki, 2002). The occurrence of arsenic in groundwater is due to both natural and anthropogenic activities, such as geothermal processes, pesticide use, mining operations, and industrial activities (Stone, 2008; Cheng et al., 2009; Camacho et al., 2011; Feng et al., 2012). The arsenic contamination problem is especially acute in developing parts of the world where the water treatment infrastructure may not be capable of providing suitable treatment. Thus, there is a great need for point-of-use systems for removing arsenic from drinking water.
Most point-of-use treatment methods for arsenic removal utilize adsorbents or ion-exchange media in small flow-through canisters, and a wide variety of treatment media have been developed (Ghimire et al., 2003; Guo and Chen, 2005; Sylvester et al., 2007; Mohan and Pittman, 2007; Deng et al., 2008; Chen et al., 2009; An et al., 2010; Chang et al., 2010; Hoshina et al., 2012). Many adsorbents for arsenic removal incorporate a metal oxide that chemically adsorbs arsenate and arsenite. Since the retention mechanism involves chemical adsorption, metal oxide-based adsorbents have a much higher specificity for arsenic anions compared with other anions that are usually present in potable water, such as chloride and sulfate (Pierce and Moore, 1982; Ghosh and Yuan, 1987; Gao et al., 1995; Suzuki et al., 2000; Dutta et al., 2004; Jang, 2004; Jeong et al., 2007).
Ferric hydroxide is the most commonly employed metal oxide in arsenic adsorbents (Mohan and Pittman, 2007). Ferric hydroxides have been used to coat sand (Panthi and Wareham, 2011), activated carbon (Deliyanni et al., 2013), fiberglass (Wang et al., 2010), cellulose (Guo and Chen, 2005), and polymeric fibers (Lin and SenGupta, 2009). Ferric hydroxide nanoparticles have also been incorporated into conventional granular anion- and cation-exchange media (DeMarco et al., 2003; Cumbal and SenGupta, 2005).
In recent years, there has been an increasing interest in polymeric fibers for arsenic removal (Dominguez et al., 2003; Zhang et al., 2008; Wang et al., 2010; Awual et al., 2012). These fibrous adsorbents have faster adsorption kinetics than granular material due to the shorter diffusional distances required for adsorption (Economy and Dominguez, 2002). The two most commonly used fibers for adsorbent preparation are polyacrylonitrile (PAN) and polypropylene (PP). Vatutsina et al. (2007) tested a variety of PAN- and PP-based cation- and anion-exchange fibers loaded with ferric hydroxide for arsenic removal. They reported that fibers functionalized with both weak base anion and weak acid cation groups showed the highest levels of arsenic removal. Unfortunately, this work did not describe the fiber preparation or iron loading procedures. Lin and SenGupta (2009) investigated a PP-based strong base anion fiber loaded with ferric hydroxide for arsenic and perchlorate removal. Although they reported the iron loading procedure, the fiber was obtained from a commercial source and no details were given on the fiber preparation method.
A previous work modifying PAN fibers for use as anion and cation exchangers first employed a cross-linking step followed by a base catalyzed hydrolysis step (Awual et al., 2008; Soldatov, 2008; Jia and Yang, 2012; Sha et al., 2013). Hydrazine hydrate (HH) and other polyamines, such as diethylenetriamine (DETA) and ethylenediamine, have been used for cross-linking (Zhang et al., 2009a; Yan et al., 2012). The high pH of the hydrazine or polyamine solution may also convert nitrile groups to carboxamide and carboxylate groups via base catalyzed hydrolysis reactions. Further treatment in sodium hydroxide (NaOH) solution also introduces carboxamide and carboxylate functional groups on the fiber. Anion-exchange sites and chelating sites for heavy metal adsorption are sometimes added by a second amination step using reagents such as DETA, hydrazine, and ethylenediamine.
Previous research into the use of modified PAN fibers as arsenic adsorbents employed PAN copolymers and did not investigate how the preparation procedures, such as the degree of hydrazine cross-linking, the extent of base catalyzed hydrolysis, and the method of iron loading, affect arsenic removal (Vatutsina et al., 2007). This research builds on previous efforts modifying PAN fibers by investigating the effect of the preparation procedures on the arsenic adsorption capacity for homopolymer PAN fibers prepared using hydrazine, NaOH, and ferric chloride. Toward that end, homopolymer PAN fibers were chemically modified using hydrazine alone, NaOH alone, and via both hydrazine and NaOH using different treatment times and reagent concentrations. The effect of the method and amount of ferric hydroxide loading on arsenic removal was also investigated. The properties of the fibers were characterized using Fourier transform infrared spectroscopy (FTIR), scanning electron microscopy (SEM), and titrimetric techniques in order to understand how fiber properties affected arsenic removal performance.
Experimental Protocol
Reagents
Homopolymer PAN needle-punched felt fabric with a weight of 500 g/m2 and a fiber diameter of 15 μm was purchased from Heading Filter Material Co., Ltd. Sodium arsenate heptahydrate (99.7% trace metal basis) was used for arsenate, and reagent-grade sodium chloride was used as an inert background electrolyte. Reagent-grade ferric chloride was obtained from Sigma-Aldrich. Adjustments to pH were made using reagent-grade NaOH and HCl. All solutions were prepared using ultrapure water (UPW) with a resistivity of 18.2 MΩ cm.
Fiber preparation
Needle-punched PAN fabric was cut into ∼1 g samples that were then soaked overnight in UPW and dried at 50°C before use. Before iron loading, the fibers were chemically modified using HH and/or NaOH solutions. One preparation procedure employed HH first, followed by NaOH treatment, and a second procedure employed these reagents in reverse order. Fibers were also modified using HH only or NaOH only.
HH treatment involved placing 12.5 g of fabric in 0.5 L of 10%, 20%, 30%, and 35% HH solutions at 100°C under vigorous stirring for 0.5–5 h. The reactions were carried out in a 1.0 L round bottom flask equipped with a heating mantle and a reflux condenser mounted on a magnetic stir plate. The initial pH values of the HH solutions were 11.5 (10%), 13.0 (20%), 13.7 (30%), and 13.9 (35%). The final solution pH values depended on the treatment time and ranged from 10.9 to 13.0. After reacting with HH, the fiber samples were cooled to room temperature followed by repeated rinsing with solutions containing 50% ethanol in UPW. The fibers were then dried at 50°C until constant weight was attained, and the weight gain or loss was recorded.
NaOH treatment involved placing 12.5 g of the fabric into 0.5 L of 10% and 20% NaOH solutions at 95°C for 0.5–3 h in the stirred flask reactor. After NaOH treatment, the fibers were cooled, rinsed multiple times in the ethanol and water solutions, dried, and weighed.
Ferric hydroxide was loaded onto the chemically modified fibers from ferric chloride solutions using two different techniques. The first technique has been previously used by Lin and SenGupta (2009). In this method, 1 g samples of the modified PAN fabric were immersed in 100 mL of 6% FeCl3 in a 50 wt% methanol, 50 wt% UPW solution for 1 h. After removal from the ferric chloride solutions, the fiber samples were then dried at 25°C. After drying, the 1 g fabric samples were immersed in 100 mL of 1% NaOH solution for 0.5 h and then dried at 25°C. The samples were then washed with UPW until a clear run off was obtained. This loading procedure was repeated approximately five times in order to increase the amount of ferric hydroxide loading. The amount of iron loaded onto the fiber samples was determined by a series of acid extractions using 1.0 M HCl solutions (Ulery and Drees, 2008).
The second iron loading procedure was analogous to the first technique except that a precipitation process was carried out in the 6% FeCl3 solution. The pH of the solution was raised to 8.0 by slowly adding 1.0 M NaOH. After 24 h of stirring, the fiber was repeatedly rinsed with UPW to remove excess precipitates and dried at room temperature.
Fiber characterization
Chemical modification of the fibers was assessed using FTIR. The FTIR spectra were recorded using a Thermo Nicolet IR 200 instrument (Thermo Electron) at wavenumbers ranging from 400 to 4,000 cm−1. The surface morphology of the fibers was observed by SEM using a Hitachi S-4800 Type II instrument. The cation-exchange capacity (CEC) and anion-exchange capacity (AEC) of the fibers were determined after conditioning in 1 M HCl or NaOH, respectively, to convert the exchange sites into H+ or free base forms. The fiber samples were then rinsed thoroughly in UPW to remove excess acid or base. To obtain the CEC, about 0.1 g of fiber (H+ form) was immersed into 25 mL of 12.5 mM CaCl2 solution at pH 8.0. After 12 h, the uptake of Ca2+ ions from the solutions was measured using a PerkinElmer Optima 2100 DV inductively coupled plasma optical emission spectrometer (ICP-OES). For determination of the AEC, 0.1 g of fiber (free base form) was shaken in 25 mL of 0.1 M HCl solution at 25°C for 12 h (Ezzeldin et al., 2010). The solution was then analyzed for Cl− via titration with a standardized 0.1 M AgNO3 solution using chromate as an indicator (Zhang et al., 2011).
Arsenic adsorption experiments
Adsorption isotherms for arsenate at 25°C were determined at a pH value of 8 in 15 mM NaCl background electrolyte solutions. NaCl was selected as the background electrolyte as the Cl− ion does not chemically adsorb to ferric hydroxide, but Cl− would compete with HAsO42− for ion-exchange sites. These experiments were carried out in nominally 500 mL flasks, each of which contained 100 μg/L initial As(V) concentration in 500 mL of solution. Approximately 0.1 g of the fabric was added to each flask and vigorously stirred for 24 h. Sampling between 1 and 24 h elapsed found that 24 h was sufficient to reach apparent equilibrium. After 24 h elapsed, a 10 mL sample was taken and the solution was respiked with 1.0 mL of a 50 mg/L As(V) solution and stirred for 24 h before sampling. This process was repeated approximately 12 times. Thus, each adsorption isotherm was obtained from a single fabric sample. Before analysis, the aqueous samples were filtered with 0.45 μm syringe filters to remove any colloidal or suspended material. Arsenic concentrations were determined using ICP-OES.
Results and Discussion
Hydrazine and NaOH treatment
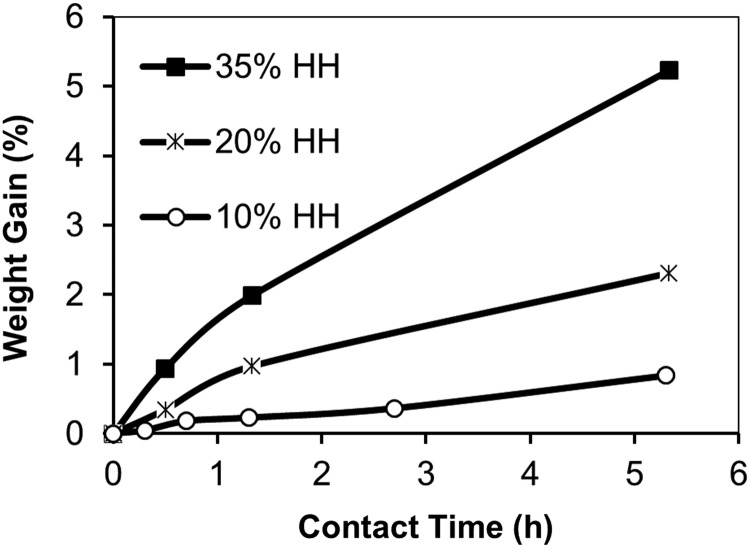
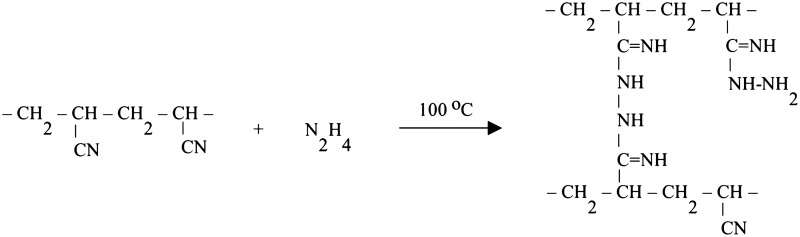
Figure 1 shows the increase in the mass of the fabric versus elapsed time for treatment with HH concentrations of 10%, 20%, and 35%. A PAN reaction with hydrazine occurs through a nucleophilic attack on the nitrile groups by the lone pair of electrons on the nitrogen atoms in hydrazine (Karaivanova and Badev, 1986). Bonding of hydrazine to adjacent polymer chains results in cross-linking, as illustrated by (Zhang et al., 2009b):
FIG. 1.
Relative increase in mass of polyacrylonitrile (PAN) fabric after a reaction with 10% hydrazine hydrate (HH), 20% HH, and 35% HH at 100°C.
In addition to cross-linking and amination of the fibers, treatment with hydrazine may also introduce carboxamide and carboxylate groups as a result of base catalyzed hydrolysis reactions (Nesteronok et al., 2012). Therefore, both cation- and anion-exchange groups are expected on HH-treated PAN fibers. The AEC and CEC of the HH-treated fibers are shown in Table 1. Increasing HH concentrations increased both the AEC and CEC of the fibers. For each HH concentration, the AEC ranged from 82% to 285% greater than the CEC.
Table 1.
Cation- and Anion-Exchange Capacities of Modified Polyacrylonitrile Fibers
| Sample | CEC (meq/g) | AEC (meq/g) |
|---|---|---|
| 10% HH | 0.23 | 0.42 |
| 20% HH | 0.32 | 0.79 |
| 35% HH | 0.35 | 1.00 |
| 10% NaOH | 2.67 | 0.20 |
| 10% NaOH/20% HH | 1.68 | 0.51 |
| 20% HH/10% NaOH | 0.85 | 0.50 |
| Purolite A-100 | — | 1.89 (1.25a) |
| Purolite C-104 | 2.65 (3.64a) | — |
Also shown for comparative purposes are data measured for Purolite C-104 weak acid cation-exchange beads and Purolite A-100 weak base anion-exchange beads.
Manufacturer's specifications.
AEC, anion-exchange capacity; CEC, cation-exchange capacity; HH, hydrazine hydrate.
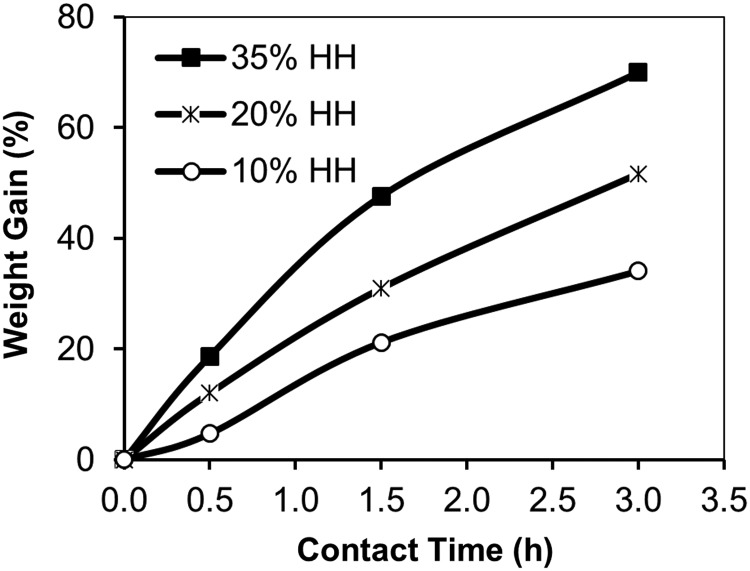
Figure 2 shows the weight gain for the HH-treated fibers as a function of treatment time during a subsequent treatment step in 10% NaOH. The greater the HH concentration in the first step, the greater the weight gain during the NaOH step. Treatment with NaOH results in conversion of nitrile groups to carboxamides, according to
FIG. 2.
Increase in weight of 10% HH, 20% HH, and 35% HH-reacted PAN fabric during alkali hydrolysis in 10% NaOH at 95°C.
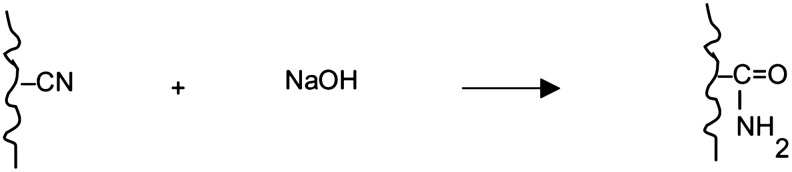
The carboxamides may then undergo oxidation to carboxylate groups, with the release of ammonia, according to
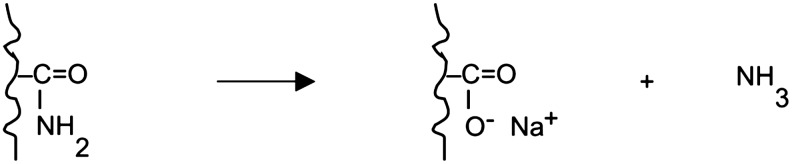
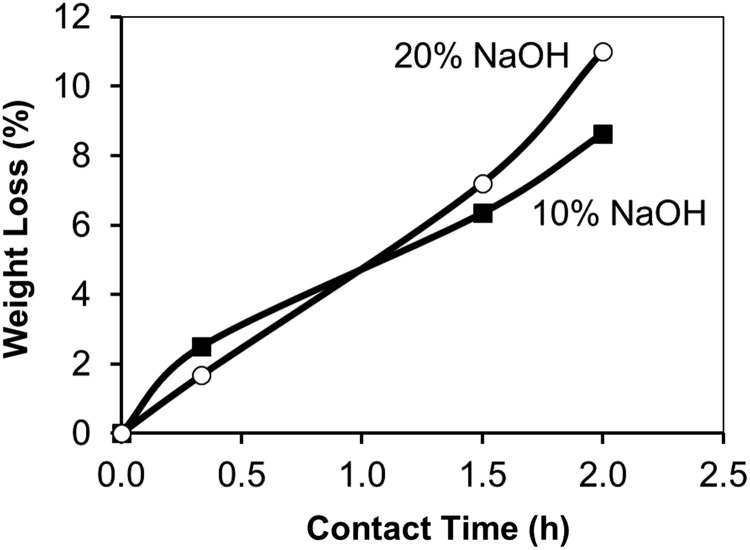
In the absence of cross-linking with HH, the PAN fabric slowly dissolves in NaOH solutions. Figure 3 compares the dissolution behavior of PAN fabric in NaOH that was not subjected to HH cross-linking. The weight loss shown in the absence of cross-linking contrasts with the weight gain found in the fibers first treated with HH. The slow dissolution of unmodified fabric during alkali treatment can be attributed to transition of nitrile into hydrophilic carboxamide and carboxylate groups, which then promote dissolution of the fiber (Lohokare et al., 2006; Yan et al., 2012). Although similar dissolution behavior was found in 10% and 20% NaOH solutions over the first 2 h elapsed, the destruction and complete dissolution of the fabric in 20% NaOH was obtained after ∼2.5 h. In addition, without prior cross-linking, the color of the fiber treated with 10% NaOH gradually changed from light orange to light pink after 2.5 h.
FIG. 3.
Weight loss of PAN fabric samples during 10% NaOH and 20% NaOH treatment at 95°C.
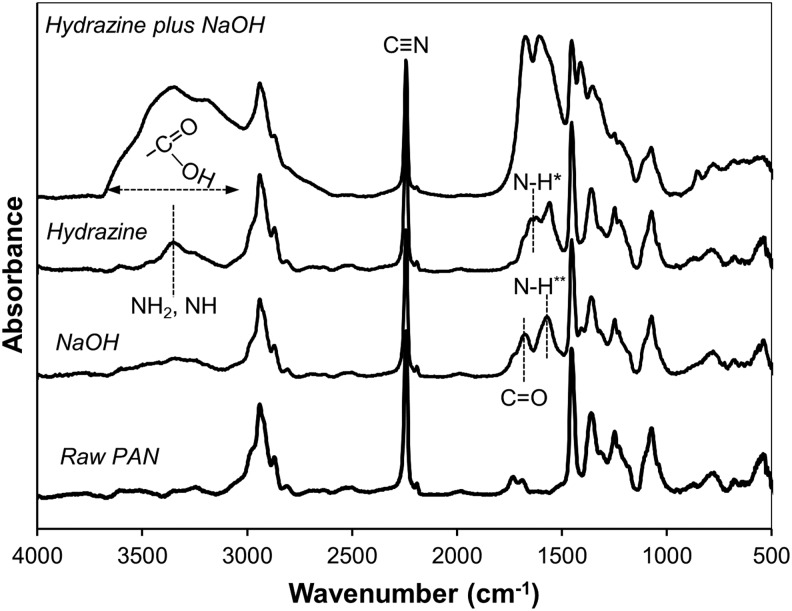
FTIR spectroscopy was used to assess chemical changes in the fabric after HH and NaOH treatment. The FTIR spectra of modified PAN fibers shows the presence of different functional groups than those of the original PAN fiber, as shown in Fig. 4. The characteristics peaks for untreated PAN fibers are assigned as follows: 2,239 cm−1 (C N stretching), 2,942 cm−1 (C-H stretching), and 1,454 cm−1 (C-H stretching on backbone of PAN fiber) (Anderson et al., 2004). After a reaction with NaOH, two new peaks at 1,561 and 1,654 cm−1 correspond to the NH group in carboxamide and the carbonyl group (C
N stretching), 2,942 cm−1 (C-H stretching), and 1,454 cm−1 (C-H stretching on backbone of PAN fiber) (Anderson et al., 2004). After a reaction with NaOH, two new peaks at 1,561 and 1,654 cm−1 correspond to the NH group in carboxamide and the carbonyl group (C O) in carboxamide and carboxylate. The NaOH-treated fiber also shows a small band ranging from 3,100 to 3,600 cm−1, corresponding to the hydroxyl group (OH) in carboxylate, which verifies the formation of carboxylate cation-exchange groups. In the hydrazine modified fiber, the band centered at 3,414 cm−1 corresponds to the stretching vibration of amine and amino (NH, NH2) groups, confirming that anion-exchange groups have been introduced. A bending vibration of NH in amines appears at 1,652 cm−1 and at 1,561 cm−1, a bending vibration for NH in amide also appears in the HH-treated fibers. In the HH/NaOH-treated fiber, the strong band ranging from 3,000 to 3,630 cm−1 corresponds to the stretching vibration bands of amine groups, and also the stretching vibration of hydroxyl groups (OH) in carboxylates. A comparison of spectra for 10% and 35% HH-treated fibers (data not shown) shows that the peak heights associated with these groups are a factor of ∼2 higher in the 35% solution versus the 10% solution, which is consistent with the anion-exchange data shown in Table 1. In addition, the peak of
O) in carboxamide and carboxylate. The NaOH-treated fiber also shows a small band ranging from 3,100 to 3,600 cm−1, corresponding to the hydroxyl group (OH) in carboxylate, which verifies the formation of carboxylate cation-exchange groups. In the hydrazine modified fiber, the band centered at 3,414 cm−1 corresponds to the stretching vibration of amine and amino (NH, NH2) groups, confirming that anion-exchange groups have been introduced. A bending vibration of NH in amines appears at 1,652 cm−1 and at 1,561 cm−1, a bending vibration for NH in amide also appears in the HH-treated fibers. In the HH/NaOH-treated fiber, the strong band ranging from 3,000 to 3,630 cm−1 corresponds to the stretching vibration bands of amine groups, and also the stretching vibration of hydroxyl groups (OH) in carboxylates. A comparison of spectra for 10% and 35% HH-treated fibers (data not shown) shows that the peak heights associated with these groups are a factor of ∼2 higher in the 35% solution versus the 10% solution, which is consistent with the anion-exchange data shown in Table 1. In addition, the peak of 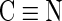 stretching is reduced in the HH-treated fibers, indicating that a reaction of
stretching is reduced in the HH-treated fibers, indicating that a reaction of 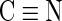 has occurred.
has occurred.
FIG. 4.
Fourier transform infrared spectroscopy spectra of untreated and treated fiber in 10% NaOH (2 h), 35% HH (3 h), and 35% HH (3 h) plus 10% NaOH (2 h). *amine groups; **carboxamide groups.
The weight gains associated with HH treatment suggest that hydrazine not only reacted with nitrile groups on the surface of the fiber, but also reacted with nitrile groups inside the fiber or was absorbed into the fiber. The weight gain corresponds to 0.3 mmol/g for 10% HH and 1.7 mmol/g for 35% HH. The unit molecular weight of PAN indicates that there are 18.9 mmol of nitrile groups per gram of untreated fiber. Thus, in the 35% HH solution, the molar hydrazine uptake is equivalent to 9% of the moles of CN. Based on the fiber diameter of 15 μm, only 0.01% of the nitrile groups reside in the surface (∼5Å) layer of the fiber. Hydrazine incorporation inside the fiber is consistent with the SEM images in Fig. 5 that show a 7% increase in the diameter of the fibers after 2 h of the 35% HH treatment. Incorporation of hydrazine into the fiber may result from the fact that the treatments were conducted at 100°C, which is above the ∼95°C glass temperature of PAN (Beevers, 1964).
FIG. 5.
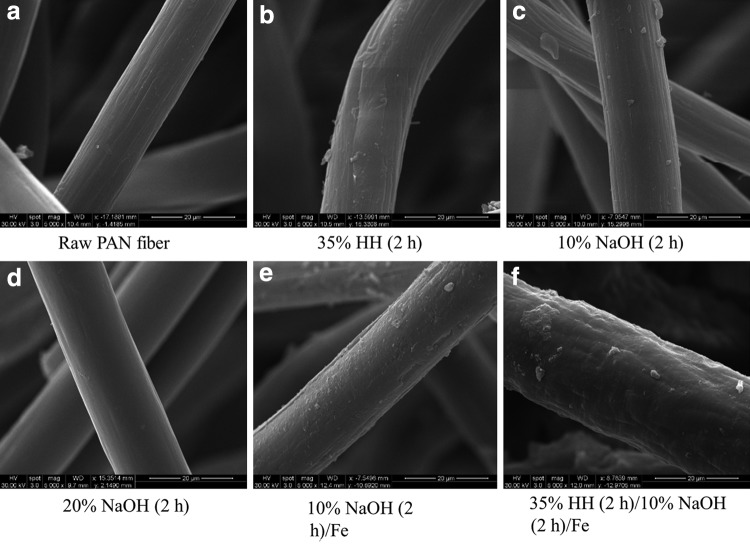
Scanning electron microscopy images of PAN fiber samples.
SEM images show that the fibers become thicker after modification, without any strong impact on their overall morphology (Fig. 5a–c). The surfaces of the untreated fiber look slightly wavy in Fig. 5a. Figure 5d has comparatively smoother surfaces as a result of fiber swelling in 20% NaOH. The combination of HH and NaOH treatment resulted in a ∼12% increase in fiber diameter from 15.71 to 17.64 μm. Nonuniform iron loading can be seen in HH/NaOH-treated fiber as compared with fibers with only NaOH treatment, as shown in Fig. 5e–f.
Iron loading
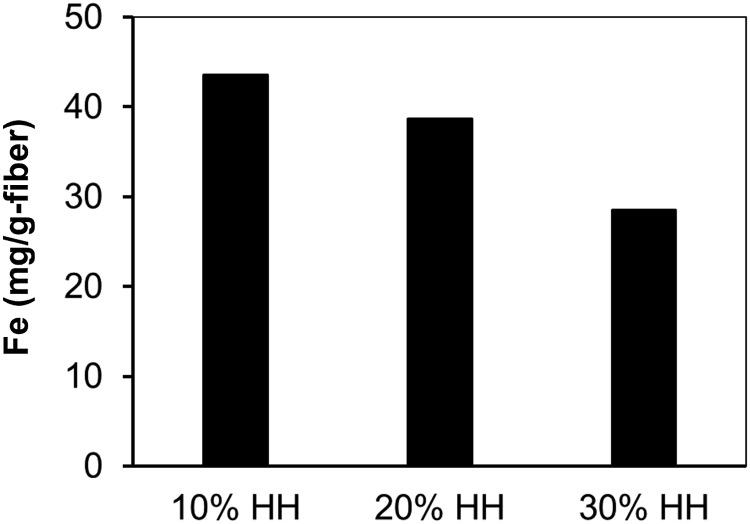
The effect of the chemical modification steps on the amount of iron that could be loaded onto the fibers was investigated using the dipping and precipitation iron loading procedures. Figure 6 compares the iron loading using the dipping procedure for fabric samples that were treated with HH for 3 h. The iron loading decreases by increasing hydrazine concentration. This can likely be attributed to the increasing number of cationic groups with increasing HH concentration, as shown by the AEC data in Table 1, and that cationic amine groups show a low affinity for adsorbing ferric chloride. X-ray diffraction of the iron-loaded fibers did not show any crystalline phases, indicating that the final product on the fiber surfaces was amorphous ferric hydroxide. SEM images indicated that the HH-treated fibers were uniformly coated with the ferric hydroxide.
FIG. 6.
Iron loading for fabric samples treated with 10% HH, 20% HH, and 30% HH for 3 h at 100°C. Fabric loaded via a single dip in 6% FeCl3 dissolved in 50% methanol/50% ultrapure water (UPW).
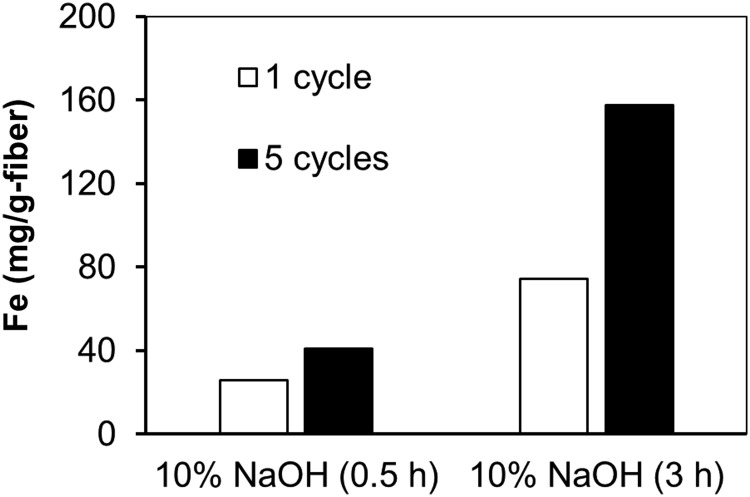
Figure 7 compares the iron loading on fabric samples treated with 10% NaOH for 0.5 and 3.0 h using 1 and 5 dipping cycles. The longer NaOH treatment that resulted in greater conversion of nitrile into carboxylate groups resulted in the fibers having a greater affinity for adsorbing ferric chloride. A comparison of iron loadings between 1 and 5 dipping cycles shows that there was a 59% increase for the 0.5-h-treated sample and a 112% increase for the 3-h-treated sample.
FIG. 7.
Iron loading for fabric samples treated with 10% NaOH for 0.5 and 3.0 h at 95°C. Fabric loaded via 1 or 5 dipping cycles in 6% FeCl3 dissolved in 50% methanol/50% UPW.
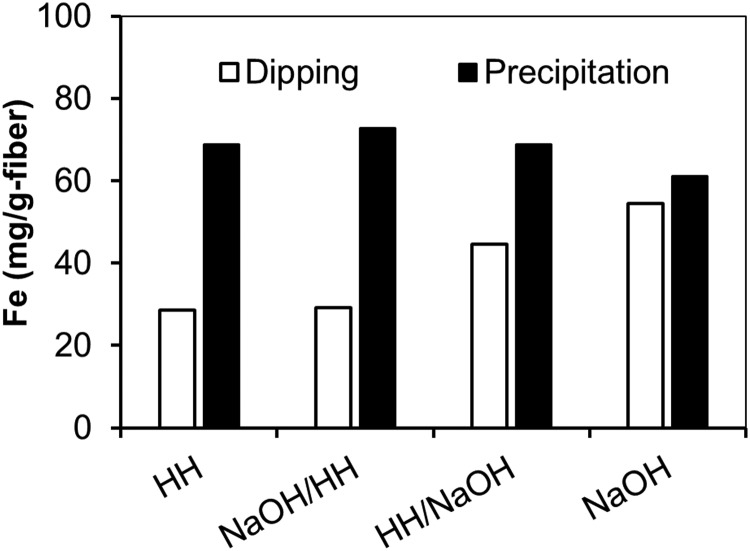
Figure 8 compares the iron loading on fibers treated only with 30% HH or 10% NaOH, with fibers treated first with 30% HH followed by 10% NaOH (HH/NaOH), and with fibers treated first with 10% NaOH followed by 30% HH (NaOH/HH). Using the dipping method, the highest amount of iron loading was found on the NaOH-treated fiber and the lowest loading was observed for the HH-treated fiber. Thus, the NaOH treatment that introduces more cation-exchange groups provides a more attractive surface for ferric chloride adsorption than the hydrazine treatment which introduces more anion-exchange groups. Figure 8 also shows the iron loadings using the single-step precipitation method. In all cases, higher iron loadings were obtained using the precipitation method. However, there was no trend of higher iron loading on fibers with more CEC. This suggests that the surface properties had a smaller effect on the iron loading using the precipitation method. The iron loadings in Figs. 6–8 are similar to those reported in previous investigations of ferric hydroxide-loaded fibers, which ranged from 17 to 120 mg/g as Fe (Vatutsina et al., 2007; Lin and SenGupta, 2009; Wang et al., 2010).
FIG. 8.
Iron loading for fabric samples treated with 30% HH (2 h), 10% NaOH (2 h), 30% HH (2 h)/10% NaOH (2 h), and 10% NaOH (2 h)/30% HH (2 h) at 95°C. Fabric loaded via 1 dipping cycle or 1 precipitation step in 6% FeCl3 dissolved in 50% methanol/50% UPW.
Stability of the ferric hydroxide loaded on the fabric samples was investigated by vigorously stirring the fabric samples for 48 h in 500 mL of 15 mM NaCl solution. In all cases, less than 1% of the loaded iron was found in the solution, and there were no observable particles in solution.
Arsenic adsorption
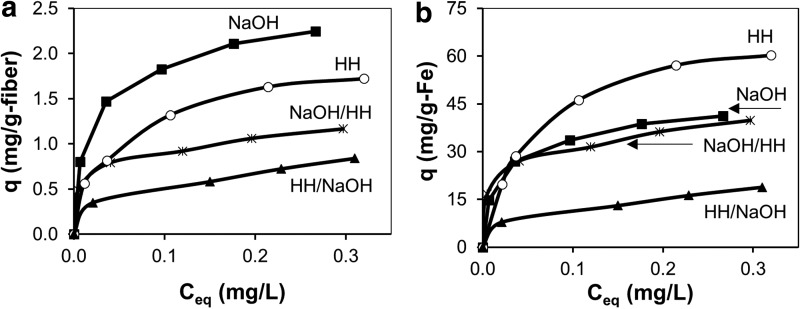
Only some of the arsenic adsorption behavior on the fibers can be rationalized in terms of previously reported mechanisms. Figure 9a shows arsenic adsorption normalized per gram of dry fabric for four different fiber samples loaded with iron using the dipping procedure. Arsenic adsorption on all four samples showed good conformance to the Freundlich isotherm model, as indicated by the high coefficients of determination (R2) shown in Table 2. The greatest amount of adsorption was observed in the fibers treated with only NaOH, which had the highest CEC (2.67 meq/g) and the lowest AEC (0.20 meq/g). In previous studies investigating arsenic adsorption on ferric hydroxide-loaded ion-exchange beads and ion-exchange fibers, arsenate uptake was positively correlated with AEC and negatively correlated with CEC (Cumbal and SenGupta, 2005; Vatutsina et al., 2007). Thus, the high arsenic adsorption on the NaOH-treated fibers cannot be explained by previously reported effects of the ion-exchange sites. A likely explanation for the high arsenic adsorption on the NaOH-treated fibers is the 23–90% higher iron loading, as compared with the other fibers.
FIG. 9.
(a) Arsenate adsorption isotherms normalized per gram of dry fiber by iron-loaded PAN fabric having different chemical modifications. Iron was loaded via a single dipping cycle. (b) Arsenate adsorption isotherms normalized per gram of iron for the data in (a).
Table 2.
Freundlich Isotherm Parameters for Adsorption of Arsenate at a pH Value of 8
| Method | ||||||
|---|---|---|---|---|---|---|
| Dipping | Precipitation | |||||
| Sample | KF | n | R2 | KF | n | R2 |
| 10% NaOH (2 h) | 3.45 | 0.28 | 0.9794 | 10.20 | 0.45 | 0.9765 |
| 10% NaOH (2 h)/30% HH (2 h) | 1.49 | 0.21 | 0.9933 | 10.82 | 0.55 | 0.9083 |
| 30% HH (2 h) | 2.73 | 0.35 | 0.9892 | 6.04 | 0.26 | 0.9442 |
| 30% HH (2 h)/10% NaOH (2 h) | 1.14 | 0.31 | 0.9760 | 2.73 | 0.24 | 0.9681 |
q is adsorbed phase concentration, Ceq is aqueous phase equilibrium concentration, and KF and n are the fitted parameters of the equation: 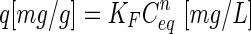 .
.
In addition to the iron loading affecting arsenic adsorption, there is also an effect of the surface chemistry of the fibers. Figure 9b shows the same arsenic adsorption data normalized per gram of iron on the fibers. The fibers treated only with HH show the highest arsenic adsorption per gram of iron. These fibers had the highest AEC and the lowest CEC. The high arsenic adsorption per gram of iron on these fibers is consistent with previous observations that positively charged anion-exchange sites increase arsenic adsorption to ferric hydroxide by increasing anion concentrations near the surface of the fibers due to electrostatic effects. Very little of the arsenate uptake by the hydrazine-treated fibers can be attributed to ion exchange on positively charged functional groups. In separate experiments with samples treated only with HH, there was negligible arsenate adsorption in the absence of the ferric hydroxide coating. At an aqueous arsenic concentration of 0.40 mg/L (in 15 mM NaCl), there was only 0.012 mg/g of arsenic adsorption. This represents less than 1% of the uptake by the ferric hydroxide-loaded fiber at the same aqueous concentration.
Arsenic uptake by fibers treated with both HH and NaOH was difficult to understand from the perspective of iron loading or the ion-exchange properties of the underlying support. The fibers with the HH/NaOH treatment showed the lowest amount of arsenic adsorption per gram of fiber or per gram of iron in Fig. 9. The AEC of these fibers (0.50 meq/g) was similar to the NaOH/HH-treated fibers (0.51 meq/g), but the CEC of the HH/NaOH (0.85 meq/g) was significantly less than that for the NaOH/HH (1.68 meq/g) treatment. Thus, rather than diminishing arsenic uptake, the extra CEC of the NaOH/HH fibers increased arsenic adsorption (Fig. 9a), despite having 52% less iron than the HH/NaOH fibers. The net result of this effect is a more than a factor of two increase in arsenic uptake per gram of iron for the NaOH/HH-treated fibers as compared with the HH/NaOH treatment. These results indicate that there are complex interactions between the fiber, the ferric hydroxide, and the arsenic which require further investigation.
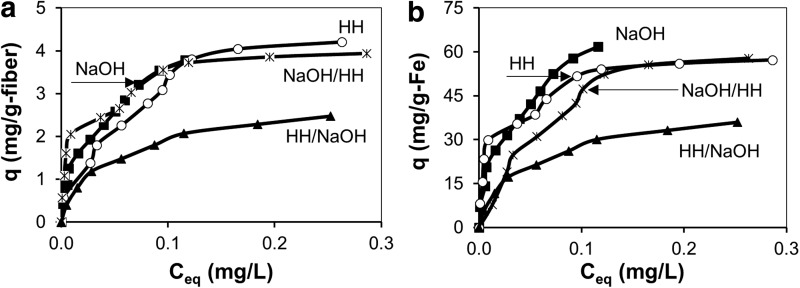
Figure 10a shows arsenic adsorption normalized per gram of dry fiber for the four different treatment procedures for the precipitation-loaded fibers. Freundlich isotherm parameters for this data are listed in Table 2. The maximum arsenic uptake on the HH, NaOH/HH, and NaOH-treated fibers was similar, with the HH/NaOH fiber showing significantly less arsenic removal. Figure 10b shows the same arsenic adsorption data normalized per gram of iron on the fibers. Here, the NaOH-treated fibers show a similar maximum uptake per gram of iron. This contrasts with the behavior shown in Fig. 9b, and indicates that the fiber surface properties of the precipitation-loaded samples have a smaller effect on arsenic adsorption than for the dip-loaded samples. This conclusion is supported by data from the other fibers. A comparison of Figs. 9b and 10b shows that the arsenic uptake per gram of iron is greater for NaOH, NaOH/HH, and HH/NaOH fibers when the iron loading is high (Fig. 10b) than when the iron loading is low (Fig. 9b). Thus, the fiber surface properties on these samples inhibit arsenate adsorption, with thicker iron coatings leading to less inhibition. In contrast, the maximum adsorption per gram of iron is higher for the HH-treated fibers when the iron loading is low (Fig. 9b) than when it is high (Fig. 10b). Thus, the fiber surface properties on the HH-treated samples enhance arsenic adsorption, with thinner iron coatings leading to greater enhancement. Although thicker iron coatings diminish the effects of fiber surface properties, the substantially lower arsenic uptake by the HH/NaOH-treated fibers in Fig. 10b indicates that the fiber surface properties still affect arsenic adsorption, even at high iron loadings.
FIG. 10.
(a) Adsorption isotherm of arsenate by iron-loaded PAN fiber having different chemical modifications. Iron was loaded via the precipitation method. (b) Arsenate adsorption isotherms normalized per gram of iron for the data in (a).
Conclusions
This research showed how the preparation procedures affected the properties and arsenate adsorption performance of ferric hydroxide-loaded modified homopolymer PAN fibers. In contrast to previous studies, cross-linking using HH or other polyamine was not necessary in order to prevent PAN fiber disintegration during NaOH treatment. This may result from the fact that this study employed homopolymer PAN fibers which are more resistant to disintegration in hot alkaline solutions. The amount of arsenate adsorption was affected by both the amount of iron loaded on the fibers and the underlying fiber surface chemistry. Treatment with only HH not only yielded the most favorable surface chemistry for arsenic adsorption but also yielded the lowest amount of iron uptake using the dipping loading method. Increasing the iron loading reduced, but did not completely eliminate, the effects of fiber surface chemistry on arsenic adsorption. Several factors make the NaOH-only treatment the preferred method of fiber preparation. These factors are high arsenic adsorption capacity, one-step procedure, lower costs, wider availability, and toxicity avoidance of using NaOH versus HH.
Acknowledgments
Grant number P42 ES004940 from the National Institutes of Environmental Health Sciences (NIEHS), National Institutes of Health (NIH), supported this work. The views of the authors do not necessarily represent those of the NIEHS, NIH.
Author Disclosure Statement
No competing financial interests exist.
References
- An B., Fu Z., Xiong Z., Zhao D., and SenGupta A.K. (2010). Synthesis and characterization of a new class of polymeric ligand exchangers for selective removal of arsenate from drinking water. React. Funct. Polym. 70, 497 [Google Scholar]
- Anderson R.J., Bendell D.J., and Groundwater P.W. (2004). Organic Spectroscopic Analysis. Cambridge: Royal Society of Chemistry [Google Scholar]
- Awual M.R., Shenashen M.A., Yaita T., Shiwaku H., and Jyo A. (2012). Efficient arsenic(V) removal from water by ligand exchange fibrous adsorbent. Water Res. 46, 5541. [DOI] [PubMed] [Google Scholar]
- Awual M.R., Urata S., Jyo A., Tamada M., and Katakai A. (2008). Arsenate removal from water by a weak-base anion exchange fibrous adsorbent. Water Res. 42, 689. [DOI] [PubMed] [Google Scholar]
- Beevers R.B. (1964). Dependence of the glass transition temperature of polyacrylonitrile on molecular weight. J. Polym. Sci. Part A. 2, 5257 [Google Scholar]
- Camacho L.M., Gutierrez M., Herrera M.T.A., and Villalba M.D.L. (2011). Occurrence and treatment of arsenic in groundwater and soil in northern Mexico and Southwestern USA. Chemosphere 83, 211. [DOI] [PubMed] [Google Scholar]
- Chang Q., Lin W., and Ying W.C. (2010). Preparation of iron-impregnated granular activated carbon for arsenic removal from drinking water. J. Hazard. Mater. 184, 515. [DOI] [PubMed] [Google Scholar]
- Chen A.S.C., Lipps J.P., McCall S., and Wang L. (2009). Arsenic Removal from Drinking Water by Adsorptive Media. Environmental Protection Agency, EPA/600/R-09/067 [Google Scholar]
- Cheng H., Hu Y., Luo J., Xu B., and Zhao J. (2009). Geochemical processes controlling fate and transport of arsenic in acid mine drainage (AMD) and natural systems. J. Hazard. Mater. 165, 13. [DOI] [PubMed] [Google Scholar]
- Cumbal L., and SenGupta A.K. (2005). Arsenic removal using polymer-supported hydrated iron(III) oxide nanoparticles: role of donnan membrane effect. Environ. Sci. Technol. 39, 6508. [DOI] [PubMed] [Google Scholar]
- Deliyanni E., Bandosz T.J., and Matis K.A. (2013). Impregnation of activated carbon by iron oxyhydroxide and its effect on arsenate removal. J. Chem. Technol. Biotechnol. 88, 1058 [Google Scholar]
- DeMarco M.J., SenGupta A.K., and Greenleaf J.E. (2003). Arsenic removal using a polymeric/inorganic hybrid sorbent. Water Res. 37, 164. [DOI] [PubMed] [Google Scholar]
- Deng S.B., Yu G., and Xie S.H. (2008). Enhanced adsorption of arsenate on the aminated fibers: sorption behavior and uptake mechanism. Langmuir 24, 10961. [DOI] [PubMed] [Google Scholar]
- Dominguez L., Economy J., Benak K., and Mangun C.L. (2003). Anion exchange fibers for arsenate removal derived from a vinylbenzyl chloride precursor. Polym. Adv. Technol. 14, 632 [Google Scholar]
- Dutta P.K., Ray A.K., Sharma V.K., and Millero F.J. (2004). Adsorption of arsenate and arsenite on titanium dioxide suspensions. J. Colloid Interface Sci. 278, 270. [DOI] [PubMed] [Google Scholar]
- Economy J., and Dominguez L. (2002). Polymeric ion-exchange fibers. Ind. Eng. Chem. Res. 41, 6436 [Google Scholar]
- Ezzeldin H.A., Apblett A., and Foutch G.L. (2010). Synthesis and properties of anion exchangers derived from chloromethyl styrene codivinylbenzene and their use in water treatment. Int. J. Polym. Sci. DOI: 10.1155/2010/684051 [DOI] [Google Scholar]
- Feng Q., Zhang Z., Ma Y., He X., Zhao Y., and Chai Z. (2012). Adsorption and desorption characteristics of arsenic onto ceria nanoparticles. Nanoscale Res. Lett. 7, 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y., SenGupta A.K., and Simpson D. (1995). A new hybrid inorganic sorbent for heavy metals removal. Water Res. 29, 2195 [Google Scholar]
- Ghimire K.N., Inoue K., Yamaguchi H., Makino K., and Miyajima T. (2003). Adsorptive separation of arsenate and arsenite anions from aqueous medium using orange waste. Water Res. 37, 4945. [DOI] [PubMed] [Google Scholar]
- Ghosh M.M., and Yuan J.R. (1987). Adsorption of inorganic arsenic and organicoarsenicals on hydrous oxides. Environ. Prog. 3, 150 [Google Scholar]
- Guo X., and Chen F. (2005). Removal of arsenic by bead cellulose loaded with iron oxyhydroxide from groundwater. Environ. Sci. Technol. 39, 6808. [DOI] [PubMed] [Google Scholar]
- Hoshina H., Takahashi M., Kasai N., and Seko N. (2012). Adsorbent for arsenic(V) removal synthesized by radiation-induced graft polymerization onto nonwoven cotton fabric. Int. J. Organic Chem. 2, 173 [Google Scholar]
- Jang J.H. (2004). Surface chemistry of hydrous ferric oxideandhematite as based on their reactions with Fe(II) and U(VI) [Ph.D. Dissertation]. Department of Civil and Environmental Engineering, The Pennsylvania State University [Google Scholar]
- Jeong Y., Maohong F., Leeuwen J.V., and Belczyk J.F. (2007). Effect of competing solutes on arsenic(V) adsorption using iron and aluminum oxides. J. Environ. Sci. 19, 910. [DOI] [PubMed] [Google Scholar]
- Jia Z., and Yang Y. (2012). Study on Structure and Properties of Polyacrylonitrile Fiber Modified by Hydrazine Hydrate. Switzerland: Trans Tech Publications [Google Scholar]
- Karaivanova S., and Badev A. (1986). Modification of polyacrylonitrile fibers with hydrazine and hydroxylamine in aqueous medium. Die Angew. Makromol. Chem. 140, 1 [Google Scholar]
- Lin J.C., and SenGupta A.K. (2009). Hybrid anion exchange fibers with dual binding sites: Simultaneous and reversible sorption of perchlorate and arsenate. Environ. Eng. Sci. 26, 1673 [Google Scholar]
- Lohokare H.R., Kumbharkar S.C., Bhole Y.S., and Kharul U.K. (2006). Surface modification of polyacrylonitrile based ultrafiltration membrane. J. Appl. Polym. Sci. 101, 4378 [Google Scholar]
- Mandal B.K., and Suzuki K.T. (2002). Arsenic round the world: a review. Talanta 58, 201. [PubMed] [Google Scholar]
- Mohan D., and Pittman C.U. (2007). Arsenic removal from water/wastewater using adsorbents—a critical review. J. Hazard. Mater. 142, 1. [DOI] [PubMed] [Google Scholar]
- Nesteronok P.V., and Soldatov V.S. (2012). Acid-base properties of ion exchangers. IV. Synthesis and potentiometric analysis of polyampholytes on the base of polyacrylonitrile fibers. Solvent Extr. Ion Exch. 30, 414 [Google Scholar]
- Panthi S.R., and Wareham D.G. (2011). Removal of arsenic from water using the adsorbent: New Zealand iron-sand. J. Environ. Sci. Health Part A 46, 1533. [DOI] [PubMed] [Google Scholar]
- Pierce M.L., and Moore C.B. (1982). Adsorption of As(III) and As(V) on amorphous iron hydroxide. Water Res. 6, 1247 [Google Scholar]
- Sha B., Wang J., Zhou L., Zhang X., Han L., and Zhao L. (2013). Adsorption of organic amines from wastewater by carboxyl group-modified polyacrylonitrile fibers. J. Appl. Polym. Sci. DOI: 10.1002/APP.38643 [DOI] [Google Scholar]
- Soldatov V.S. (2008). Synthesis and the main properties of fiban fibrous ion exchangers. Solvent Extr. Ion Exch. 26, 457 [Google Scholar]
- Stone R. (2008). Arsenic and paddy rice: a neglected cancer risk? Science 321, 184. [DOI] [PubMed] [Google Scholar]
- Suzuki T.M., Bomani J.O., Matsunaga H., and Yokoyama Y. (2000). Preparation of porous resin loaded with crystalline hydrous zirconium oxide and its application to the removal of arsenic. React. Funct. Polym. 43, 165 [Google Scholar]
- Sylvester P., Westerhoff P., Moller T., Badruzzaman M., and Boyd O. (2007). A hybrid sorbent utilizing nanoparticles of hydrous iron oxide for arsenic removal from drinking water. Environ. Eng. Sci. 24, 104 [Google Scholar]
- Ulery A.L., and Drees L.R. (2008). Methods of soil analysis. Part 5—Mineralogical. Methods. Madison, WI: Soil Science Society of America, Inc [Google Scholar]
- Vatutsina O.M., Soldatov V.S., Sokolova V.I., Johann J., Bissen M., and Weissenbacher A. (2007). A new hybrid (polymer/inorganic) fibrous sorbent for arsenic removal from drinking water. React. Funct. Polym. 67, 184 [Google Scholar]
- Wang J., Li X., Ince J.S., Yue Z., and Economy J. (2010). Iron oxide-coated on glass fibers for arsenic removal. Sep. Sci. Technol. 45, 1058 [Google Scholar]
- Yan S., Zhao M., Lei G., and Wei Y. (2012). Novel tetrazole-functionalized absorbent from polyacrylonitrile fiber for heavy-metal ion adsorption. J. Appl. Polym. Sci. 125, 382 [Google Scholar]
- Zhang G., Meng H., and Ji S. (2009a). Hydrolysis differences of polyacrylonitrile support membrane and its influence on polyacrylonitrile-based membrane performances. Desalination 242, 313 [Google Scholar]
- Zhang J., Li R., Zhang K., and Yang M. (2011). Potentiometric detection for chloride in copper sulfate pentahydrate recycled from printed circuit board. Presented at the 5th International Conference on Bioinformatics and Biomedical Engineering, Wuhan, China, May10 [Google Scholar]
- Zhang L., Zhang X., Li P., and Zhang W. (2009b). Effective Cd2+ chelating fiber based on polyacrylonitrile. React. Funct. Polym. 69, 48 [Google Scholar]
- Zhang X., Jiang K., Tian Z., Huang W., and Zhao L. (2008). Removal of arsenic in water by an ion-exchange fiber with amino groups. J. Appl. Polym. Sci. 110, 3934 [Google Scholar]