Abstract
Serotonin, 5-hydroxytryptamine, is a systemic bioactive amine that acts in the gut and brain. As a substrate of myeloperoxidase in vitro, serotonin is oxidized to tryptamine-4,5-dione (TD), which is highly reactive with thiols. In this work, we successively prepared a monoclonal antibody to quinone-modified proteins and found that the antibody preferentially recognizes the TD–thiol adduct. Using the antibody, we observed that the chloride ion, the predominant physiological substrate for myeloperoxidase in vivo, is not competitive toward the enzyme catalyzed serotonin oxidation process, suggesting that serotonin is a plausible physiological substrate for the enzyme in vivo. Immunocytochemical analyses revealed that TD staining was observed in the cytosol of SH-SY5Y neuroblastoma cells while blot analyses showed that some cellular proteins were preferentially modified. Pull-down analyses confirmed that the cytoskeletal proteins tubulins, vimentin, and neurofilament-L were modified. When pure tubulins were exposed to micromolar levels of synthetic TD, self-polymerization was initially enhanced and then suppressed. These results suggest that serotonin oxidation by myeloperoxidase or the action of other oxidants could cause functional alteration of cellular proteins, which may be related to neurodegeneration processes or irritable bowel syndrome.
Keywords: 5-Hydroxytryptamine; Tryptamine 4,5-dione; Quinone; Adduct; Antibody; Neuronal cells
Abbreviations: TD, tryptamine-4,5-dione; PMNs, polymorphonuclear leukocytes; AD, Alzheimer’s disease; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; NAC, N-acetyl-l-cysteine; DTT, dithiothreitol; XOD, xanthine oxidase; TCEP, Tris[2-carboxyethyl] phosphine hydrochloride; KLH, keyhole limpet hemocyanin; PBS, phosphate-buffered saline; BSA, bovine serum albumin; TPBS, PBS containing 0.05% Tween 20; HRP, horseradish peroxidase; TMB, 3,3′,5,5′-tetramethylbenzidine reagent; ELISA, enzyme-linked immunosorbent assay; AP, alkaline phosphatase; NBT, nitroblue tetrazolium; NEM, N-ethylmaleimide; DMEM, Dulbecco’s modified Eagle’s medium; SDS, sodium dodecyl sulfate; TTBS, Tris-buffered saline containing 0.05% Tween-20; O.D., optical density; 5HIAA, 5-hydroxyindoleacetic acid; 5OH-Trp, 5-hydroxytryptophan; HOCl, hypochlorous acid
Highlights
-
•
Antibody to quinone-modified protein was established and characterized.
-
•
Modification of protein by quinone was dependent on myeloperoxidase but independent of chloride ion concentration.
-
•
We immunochemically found that cellular proteins were preferentially modified.
-
•
Quinone modulated polymerization of tubulins in vitro.
Introduction
Serotonin, which is a well-known monoamine neurotransmitter, has multifunctional bioactivity including modulation of intestinal movements and blood clotting. Serotonin in brain induces biosignals via serotonin receptors on the cellular membrane. These bioactivities have been intensively investigated since the discovery of serotonin in the 1930s. However, there are few reports on serotonin oxidation. Serotonin is oxidized by superoxide [1] or myeloperoxidase [2], forming a reactive quinone, tryptamine-4,5-dione (TD), and a dimer of serotonin. The dimer of serotonin is formed by copper oxidation [3], a respiratory burst of activated microglia [4] or activated neutrophils [2]. TD covalently reacts with the thiol [5,6] and inactivation of enzymes via the formation of quinone adducts has been reported [7,8]. Neutrophils, which contain myeloperoxidase in their azurophilic granules, or purified myeloperoxidase causes aggregation of the protein [9]. Stimulation of polymorphonuclear leukocytes (PMNs) with serotonin increased serotonin binding to PMN proteins [10]. These reports suggest that covalent modification of serotonin-derived species on protein molecules might be triggered by myeloperoxidase activity in vivo. A computer-aided docking study showed that serotonin is a plausible substrate of myeloperoxidase [11]. Myeloperoxidase may contribute to the development of Alzheimer's disease (AD) as suggested by its expression in the brain of AD patients where it is also co-localized with Aβ protein [12], which is one possible initiator for AD. Taken together, this information supports the idea that myeloperoxidase could oxidize serotonin in the brain.
In a previous study, we also detected the in vitro formation of a covalent adduct of a serotonin moiety with a model thiol protein, glyceraldehyde 3-phosphate dehydrogenase (GAPDH), using serotonin or biotinylated serotonin as the substrate for myeloperoxidase [13]. The adduction of the serotonin oxidation products with the thiol moiety was determined to occur via a quinone or serotonin radical by use of N-acetyl-l-cysteine (NAC) as a model of a thiol residue. When protein was used instead of NAC, quinone adducts but not serotonin–thiol adducts were identified on the protein molecule (Fig. 1). In addition, the adduction of TD has been shown to generate a tryptamine-4,5-diol–protein adduct, which rapidly converts to the corresponding quinone adduct [5]. However, the molecular mechanism and biological significance of protein modification by serotonin oxidation products has not been fully investigated because of the lack of an analytical tool for detection of modification in a cell or tissue.
Fig. 1.
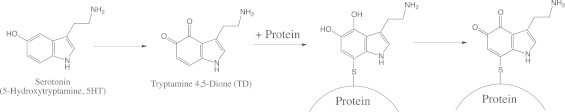
Scheme for adduct formation of serotonin oxidation products onto a protein.
Herein, we report the study of cytoskeletal proteins as targets of TD by pull-down methods using a novel antibody to TD-modified proteins. The modification on the proteins was also induced using biotinylated TD probe and the biotin-incorporated proteins were then captured with avidin linked agarose.
Materials and methods
Materials
Serotonin and dithiothreitol (DTT) were purchased from Wako Pure Chemicals. Human myeloperoxidase was obtained from Planta Natural Products. Xanthine oxidase from bovine milk (XOD; type X4500) and GAPDH (from rabbit) were purchased from Sigma. Acetaldehyde was purchased from Merck. CanGetSignal-1 and -2 were purchased from TOYOBO. Tris[2-carboxyethyl] phosphine hydrochloride (TCEP) was purchased from Nacalai Tesque Inc. Biotinylated serotonin (serotonin–biotin) was prepared by reacting sulfo-NHS-LC-biotin (Thermo Scientific) with serotonin [13].
Synthesis of tryptamine-4,5-dione
TD was freshly synthesized with minor modifications [7]. Briefly, 1 mg of serotonin was dissolved in 500 µl of water and then added to potassium nitrosodisulfonate (Aldrich), which is known as Fremy's reagent. After 1 min, the mixture was applied to a Supelco Discovery DSC-18 (C18) solid phase extraction column (500 mg, SPE). The SPE column was washed with 2 ml of water, and the purple colored compound was eluted with 1% formic acid in water/CH3CN (25/75) and then collected. The TD concentration was measured by absorbance at 350 nm using its extinction coefficient [14]. TD–biotin was synthesized from a serotonin–biotin conjugate according to the method referenced above.
Preparation of immunogen
Keyhole limpet hemocyanin (Imject KLH, Pierce) was used as a carrier protein. Before exposure to TD, the KLH was treated with DTT to regenerate the thiol moiety as described previously [15]. Briefly, 20 mg of KLH was dissolved in 4 M guanidine-HCl (pH 8.5) and 20 mg of DTT was then added. After saturation with N2, the solution was sealed and then stirred at room temperature overnight in the dark. The solution was dialyzed against phosphate-buffered saline (PBS) at 4 °C for 1 day with 3 exchanges of PBS. The dialyzed KLH (2 mg/ml) was incubated with synthetic TD (1 mM) in PBS and after 1 h, the solution was dialyzed at 4 °C against PBS overnight with two exchanges of PBS. As the positive control antigen, commercial bovine serum albumin (BSA; lipid-free) was exposed to TD and then further processed as described above. The samples were washed with 4 volumes of ice-cold acetone and centrifuged at 4 °C for 10 min. The precipitates were dissolved in PBS and stored at −80 °C.
Preparation of monoclonal antibody
A total of 150 µl of immunogen (1 mg/ml) was mixed with 350 µl of PBS and 500 µl of Freund's complete adjuvant. A total of 250 µl of the mixture (final 150 µg/ml of KLH) was injected into a Balb/c mouse. After 1 month, the mouse was boosted with 250 µl of the immunogen, which was mixed with 150 µl of the KLH (1 mg/ml) and 350 µl of PBS with 500 µl of Freund's incomplete adjuvant. Two weeks after the second immunization, the mouse was immunized into the tail vein and the abdominal cavity with 250 µl of the KLH in PBS. Three days after the final immunization, the spleen from the mouse was fused with myeloma cells by the polyethylene glycol method as described previously [16]. Hybridomas were selected by using TD-modified BSA as an antigen by noncompetitive enzyme-linked immunosorbent assay (ELISA) as described below. Finally 5 clones (1B7, 1A11, 2E12, 1E3, and 1D11) were obtained. The antibodies were collected from serum free culture medium (Hybridoma-SFM, GIBCO) by cultivation of the clones and then purified by a Protein G column (Hi-Trap IgG, GE Healthcare). All antibodies were determined as IgG1 subclass, which was estimated by immunochromatography (Iso-gold Rapid Mouse-Monoclonal Isotyping Kit, Bioassay Works). Antibody 1B7 was mainly used for the following analyses.
Characterization of antibody
Competitive ELISA
TD–BSA (50 µl, 0.01 mg/ml in PBS) was coated onto a microtiter plate (Nunc, Maxisorp) and kept at 4 °C overnight. The plate was washed with 200 µl of PBS containing 0.05% Tween 20 (TPBS) three times. Competitor (50 µl) and antibody (50 µl) were dissolved in 1% BSA–PBS, and then were added into the wells of the plate. The plate was incubated for 2 h at 37 °C and then treated with anti-mouse IgG-horseradish peroxidase (HRP) for 1 h at room temperature. The color development was achieved using 3,3′,5,5′-tetramethylbenzidine reagent (TMB; Kirkegaard & Perry Laboratories Inc.) and terminated by the addition of 1 M phosphoric acid.
Noncompetitive ELISA
Samples (50 µl, 0.01 mg/ml in PBS) were coated at 4 °C onto the microtiter plate overnight. The plate was washed with 200 µl of TPBS 3 times, and 100 µl of antibody in 1% BSA–PBS was added and incubated at 37 °C for 2 h. The binding of the antibody was evaluated by using the anti-mouse IgG antibody labeled with HRP. The color development was done by TMB as described previously. In some cases, alkaline phosphatase (AP)-labeled anti-mouse IgG antibody was also used instead of the anti-mouse IgG–HRP conjugate in order to avoid artifact peroxidase activity derived from the antigen, myeloperoxidase. In this case, after the reaction of antibody with antigen, anti-mouse IgG–AP conjugate in the CanGetSignal-2 reagent was added and incubated for 1 h. The plate was then treated with p-nitrophenyl phosphate in 10% diethanolamine–HCl buffer (pH 9.8) containing 0.5 mM MgCl2.
Modifications of protein by oxidized serotonin
Serotonin and its related compounds including biotinylated serotonin were used as substrates of the myeloperoxidase system and the modified proteins were analyzed by slot-blot/quinone staining and ELISA. The system contained myeloperoxidase (50 nM), XOD (1/1000), substrate (0.1 mM) and GAPDH (0.7 mg/ml) in 50 mM phosphate buffer containing 1 mM diethylenediaminetetraacetic acid (pH 7.4) and reactions were started by adding acetaldehyde (10 mM). After 10 min, the reaction was terminated by the addition of allopurinol (0.1 mM) and the modified proteins were separated from the reaction mixture by a microspin-column (Bio-Rad).
Detection of quinones on slot-blotted membrane by redox-cycling staining
Proteins were blotted with a slot-blot apparatus (Bio-Dot SF, Bio-Rad). Briefly, 200 µl of GAPDH (0.01 mg/ml in PBS) was blotted onto a polyvinylidene difluoride membrane and washed with 200 µl of PBS. The membrane was removed from the blot apparatus and washed with water for 5 min and then soaked with nitroblue tetrazolium (NBT)/alkaline glycinate buffer (NBT 4 mg/20 ml alkaline–glycinate buffer) for 2 h in the dark [17]. The membrane image was analyzed by ImageJ software (version 1.41).
Estimation of biotin incorporation into protein
Samples (50 µl, 0.01 mg/ml in PBS) were coated at 4 °C onto the microtiter plate for overnight. The plate was washed with 200 µl of TPBS three times and 100 µl of streptavidin–HRP polymer (Ultrasensitive, Sigma) in 1% BSA–PBS was added and incubated at 37 °C for 1 h. The color development was done using TMB as described above.
Identification of antigen from the reaction mixture of tryptamine dione and N-acetyl cysteine
Synthetic TD (5 mM) was mixed with NAC (5 mM) in phosphate buffer, pH 7.4. After 15 min, the reaction mixture was injected into an HPLC equipped with a Develosil Combi-RP column (20×100 mm, Nomura Chemical Co., Ltd.) using 0.1% acetic acid/CH3CN (9/1) as an eluent at a flow rate of 5 ml/min. The eluate was monitored using a photodiode array detector and then fractionated every minute (5 ml each). The fractions were concentrated with a centrifugal evaporator and used for competitive ELISA as described above. Some fractions were also analyzed by liquid chromatography mass spectrometry using API3000 (AB Sciex) and Agilent 1100 with a Develosil ODS-SR-5 column (2×150 mm, Nomura Chemical Co., Ltd.) with the same solvent at a flow rate of 0.2 ml/min. A positive Q1 scan from 50 to 600 and Q1-multiple ion scan at 352.1 were performed.
Preparation of thiol-proteins
DTT–treated thiol–refreshed BSA (DTT–BSA)
BSA (25 mg) was dissolved in 2.25 ml of water. To the solution, 250 µl of DTT (3 mg/ml) was added and reacted for 2 h. The DTT–treated BSA was concentrated using the centrifugal filtration apparatus Vivaspin 6 (7500g, 4 °C, 30 min). The remaining protein was further centrifuged with an additional 5 ml of 0.1 M phosphate buffer (pH 7.4) to wash off the residual DTT. The protein was then collected and immediately used.
N–Ethylmaleimide–treated free thiol–blocked BSA (NEM-BSA)
10 mg of NEM (Sigma) was added to 1 ml of BSA (10 mg in 0.1 M phosphate buffer, pH 7.2) and reacted for 2 h at room temperature. The reaction mixture was applied to a PD-10 gel filtration column (GE Healthcare) and fractionated. The fractions containing protein was further applied to the Vivaspin 6 (7500g, 4 °C, 30 min) and concentrated protein was collected and stored at −20 °C until use.
Free thiol estimation
To estimate free thiols in the protein, 100 µl of 5, 5′-dithiobis(2-nitrobenzoic acid) dissolved at 3 mg/ml of water, 400 µl of phosphate buffer, and 500 µl of protein (2 mg/ml in phosphate buffer) were mixed and incubated at room temperature for 15 min in the dark. The absorbance at 412 nm was measured and the thiol concentration was estimated using the extinction coefficient of 13,600 M-1 cm−1.
Cultured cells
The neuroblastoma cell line SH-SY5Y was obtained from DS Pharma-Biomedical Company (ECACC). The cells were maintained in Dulbecco's modified Eagle's medium/Ham's F12 (Ham's F-12/DMEM) containing 15% fetal bovine serum.
Immunocytochemistry
SH-SY5Y cells were cultivated on an eight-well chamber slide (Lab-Tek II, Nunc). The slide was washed and replaced with serum-free DMEM medium in order to exclude hypoxanthine, which is included in the Ham's F-12/DMEM medium and is known to be a substrate of XOD. Myeloperoxidase (50 nM), XOD (1/1000), acetaldehyde (10 mM), and serotonin (0.1 mM) were added to the well and further incubated for 30 min in a CO2 incubator. As a positive control, 50 µM of synthetic TD was also added to the well. The wells were washed with PBS and fixed in methanol at −20 °C for 10 min. After treatment with 0.5% Triton X100 in PBS for 10 min at room temperature, the slide was washed with PBS and reacted with the 1B7 antibody (2 µg/ml) in PBS containing 1% BSA for 1 h at room temperature. The slide was washed and incubated with FITC-conjugated anti-mouse immunoglobulin and peritoneum iodide (for nuclei) in PBS at room temperature for 1 h in the dark. The slide was then washed, and an antifade reagent (ProLong Gold, Invitrogen) was added before analysis by confocal microscopy.
Immunoprecipitation by antibody beads
SH-SY5Y cells were exposed to various concentrations of synthetic TD for 15 min in serum-free DMEM and then washed with ice-cold PBS. The cells were lysed by Mammalian Protein Extraction Reagent (M-PER) with HALT protease inhibitor cocktail (Pierce) and then immunoprecipitated by 1B7 mAb which had been conjugated to NHS-activated Sepharose 4 Fast Flow beads (GE Healthcare). The precipitates were eluted with 0.1 M glycine–HCl (pH 3.0) and then neutralized by 1 M aqueous Tris. The sample was applied to gels for further analyses as described below.
Pull-down assay with avidin–agarose
SH-SY5Y cells were exposed to synthetic TD–biotin and lysed as described above. The biotin-tagged protein was precipitated by avidin–agarose (A9207, Sigma). The precipitates were applied to gels and further analyzed as described below.
Gel and Western blot analysis
Protein samples were mixed with sodium dodecyl sulfate (SDS) loading buffer without 2-mercaptoethanol. The proteins were applied to two gels for SDS polyacrylamide gel electrophoresis. In one gel, the protein was stained with SYPRO Orange or SYPRO Ruby (Invitrogen) according to the manufacturer's recommendations. The protein in the other gel was electrotransferred onto an Immobilon-P transfer membrane (Millipore). The membrane was then blocked with 4% Block Ace (Dainihon Sumitomo Seiyaku) aqueous solution for 1 h at room temperature. After washing the membrane three times for 10 min each with TTBS (Tris–buffered saline containing 0.05% Tween-20), the membrane was incubated with the primary antibody (0.5 µg/ml dilution in TTBS or CanGetSignal-1) for 1 h at room temperature. After washing again, the membrane was incubated with anti-mouse IgG–HRP conjugate at a 1:10,000 dilution in TTBS or CanGetSignal-2 for 1 h at room temperature. After washing, the membrane was visualized using ECL-Plus or ECL-prime detection reagent (GE Healthcare). The gel images were captured by Lumino Image Analyzer LAS-1000plus (FUJIFILM) and analyzed with Image Gauge software (ver. 3.4).
Polymerization assay of tubulin
The polymerization assay was performed using a commercial Tubulin Polymerization Assay kit (Cytoskeleton Inc.). Porcine tubulin was incubated with or without synthetic TD at 37 °C and the changes in optical density (O.D.) at 340 nm were recorded for 30 min with a microplate reader according to the manufacturer's recommendations. As a control, a tubulin solution was kept at 4 °C for 30 min. After the microplate reader measurement, the intact sample and TCEP-reduced sample were collected and applied to gels. Blot analyses were performed as shown in the above section. TCEP was added to the sample at a concentration of 25 mM for 30 min at room temperature.
Results
Preparation and characterization of an antibody to TD-modified protein
To confirm the covalent modification of cellular proteins by TD, a monoclonal antibody to a TD-modified protein was prepared as described in Materials and methods. Among the obtained antibodies, 1B7 mAb was further characterized. As TD is known to preferentially react with thiols [5], we prepared three types of BSA, DTT–treated BSA, NEM–treated BSA, and native BSA and the effect of free thiol on the generation of immunoreactivity by exposure of TD was examined. The relative amounts of free thiols on BSA varied depending on its type, at 9.7, 0.2, and 1 for DTT–treated BSA, NEM–treated BSA, and native BSA, respectively. The reactivity of antibody toward the BSAs after exposure to TD increased with an increase in the free thiol content of the BSAs (Fig. S1A), suggesting that the antibody recognized the TD-modified thiol moiety. The adduction of TD on the protein molecules, which was evaluated by using TD–biotin, was observed even with the thiol-depleted BSA, NEM–BSA (Fig. S1B). This suggests that the major epitope of the TD-modified protein recognized by the antibody is a TD-modified thiol moiety but TD adduction itself is not restricted to cysteine residues, i.e., amino residues could possibly be targeted by TD.
Epitope analysis of the established antibody
To further confirm the epitope of 1B7 mAb, TD and NAC were reacted and the products were then fractionated by HPLC (Fig. S2A and B). The antibody reacted with the fraction containing two products (retention times at 14 and 16 min). From the UV–vis scan and the mass spectrometry analysis, the product at 16 min was identified as the adduct of NAC with TD, 7-S-(N-acetylcysteinyl)tryptamine-4,5-dione [13]. The former product was unstable and had the same 352 [M+H]+ ion as the latter product, 7-S-(N-acetylcysteinyl)tryptamine-4,5-dione. This suggests that the former product is a transient diol-type adduct [5,6].
Comparison of immunoreactivity among modified proteins exposed to myeloperoxidase-treated indoles
It has been reported that melatonin is also modified by myeloperoxidase [18]. A metabolite of serotonin, 5-hydroxyindoleacetic acid (5HIAA), might be oxidized by myeloperoxidase as well. On the basis of structural similarity, 5-hydroxytryptophan (5OH-Trp), the precursor of serotonin, is another plausible target of myeloperoxidase. Melatonin, 5HIAA, 5OH-Trp and serotonin were treated with the myeloperoxidase system in the presence of GAPDH and the modified proteins were then blotted onto a membrane. After exposure to the myeloperoxidase system, 5HIAA, 5OH-Trp, and serotonin were converted to their respective quinones as estimated by the results from the NBT-reduction reagent, indicating that the quinone moieties were formed from the three indoles, in particular 5HIAA, but not melatonin (Fig. 2A). When the immunoreactivities against quinone-modified GAPDH were examined, the antibody only reacted with GAPDH exposed to myeloperoxidase-treated serotonin (Fig. 2B). This suggests that the established antibody specifically recognizes oxidized serotonin-derived protein modifications.
Fig. 2.
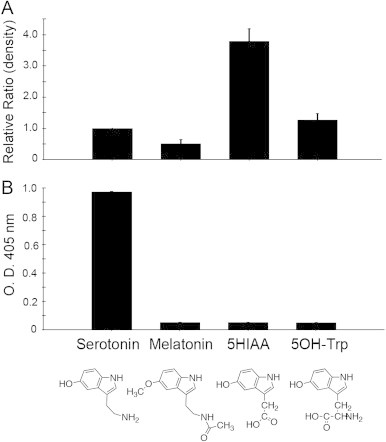
Comparison of the formation of antigenicity with chemical adduction of indoles treated with myeloperoxidase. (A) The chemical adduction of indoles on the protein molecule was evaluated by quinone redox staining following the reaction and slot blotting. (B) The immunoreactivity was estimated by ELISA followed by the reaction of indoles with the myeloperoxidase/xanthine oxidase/acetaldehyde enzyme system. The bar shows the relative intensity of the staining to serotonin (substrate). The structures of four substrates for myeloperoxidase are shown.
Effect of chloride ion on enzymatic serotonin-derived protein modification
Myeloperoxidase is known to generate reactive hypochlorous acid (HOCl) from the substrates hydrogen peroxide and chloride ion under physiological conditions. When the buffer concentration of chloride ion was varied in the enzymatic serotonin oxidation system, the immunoreactivity against modified proteins was the same even at 150 mM NaCl, which is the physiological concentration of chloride ion (Fig. 3). This suggests that serotonin oxidation and successive modification of protein are not competitive with the enzymatic generation of HOCl.
Fig. 3.
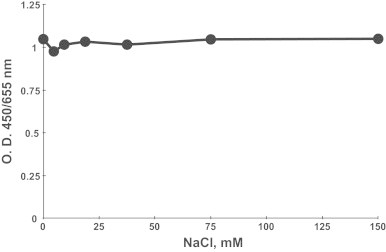
Effect of chlorine ion on myeloperoxidase-dependent protein modification by serotonin. GAPDH was exposed to the myeloperoxidase/xanthine oxidase/acetaldehyde system in phosphate buffer (pH 7.4) with various concentrations of NaCl. The modification of GAPDH with TD generated by the enzyme was estimated by ELISA.
Generation of cellular antigen in neuroblastoma cells treated with serotonin
TD may be a neurotoxin because it has a highly reactive quinone moiety. Proteins in SH-SY5Y neuroblastoma cells were modified by the serotonin oxidation system, which included the complete enzyme system and substrates (Fig. 4). This suggested that the serotonin oxidation product, TD, modified cellular proteins. Positive immunostains were also observed in cell lysates and some bands seemed to be selectively modified by TD in a dose-dependent manner (Fig. 5). The intensities of positive bands by immunostaining were not matched with those by protein staining, indicating that the adduction onto proteins was not random.
Fig. 4.
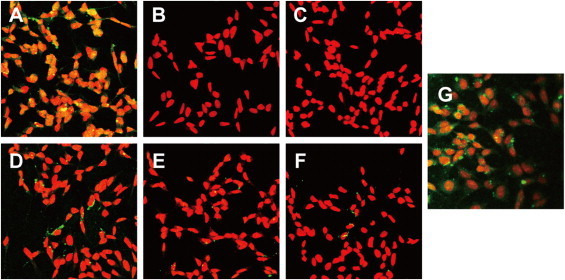
Immunocytostaining of neuroblastoma SH-SY5Y cells incubated with myeloperoxidase/acetaldehyde/xanthine oxidase/serotonin or synthetic TD. The cells were treated in serum-free medium for 30 min with the enzyme system (complete, A), the omission of the component (C–F), or 50 µM synthetic TD (G). The untreated cells are also shown in B. After the treatment, cells were fixed with methanol and permeabilized with 0.5% Triton X-100. The cells were then incubated with the antibody (1B7), followed by secondary antibody (FITC labeled, green). The nuclei were stained with peritoneum iodide (red). A, complete; B, control (untreated cells); C, without serotonin; D, without acetaldehyde; E, without myeloperoxidase; F, without xanthine oxidase; and G, TD (50 µM). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 5.
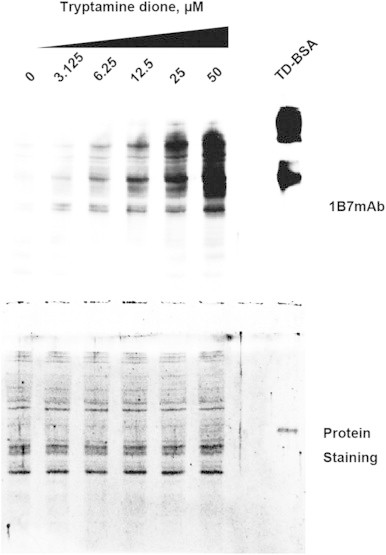
Dose-dependent formation of TD-modified cellular proteins. SH-SY5Y cells were exposed to various concentrations of synthetic TD and the cells were then lysed. Separated proteins were stained with SYPRO Orange. The proteins were blotted onto a membrane and quinone-modified proteins were stained by the specific antibody 1B7.
Identification of target proteins
Immunoprecipitation using the 1B7 specific antibody was then performed. Since the amounts of modified proteins were not enough to analyze by mass spectrometry, we used antibodies to some cytoskeletal proteins, which have been reported as predominant targets for some nucleophilic reagents [19–22]. As shown in Fig. 6A, the cytoskeletal proteins ⍺,β-tubulins (55 kDa) and vimentin (58 kDa) were detected on the blot membrane. Neurofilament-L (70 kDa) was weakly detected. GAPDH (35 kDa) and β-actin (50 kDa), which are often modified by a nucleophilic reagent, were not identified in the precipitate.
Fig. 6.
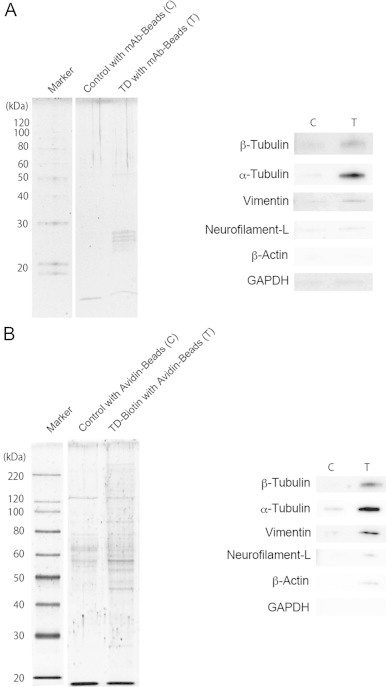
Identification of TD-modified proteins with specific antibodies. (A) Immunoprecipitation of TD-modified proteins by 1B7 mAb-beads. (B) Precipitation of biotinylated proteins by avidin-beads. The cells were exposed to 100 µM of synthetic TD or TD–biotin and then lysed. The pull-down assay was performed as described in Materials and methods. The precipitated proteins were stained with SYPRO Ruby.
The cells were exposed to TD–biotin and the quinone-modified proteins tagged by the biotin moiety were then precipitated by using avidin beads. Some proteins, especially those 40–70 kDa, were separated in the gel (Fig. 6B). ⍺,β-Tubulins, vimentin, neurofilament-L, and β-actin were also identified on the blotted membrane.
Polymerization assay of tubulins exposed to TD
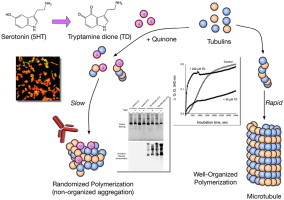
The protein modifications by serotonin oxidation products may induce changes in the function of the proteins. As shown in Fig. 6A and B tubulins were clearly modified by TD. Therefore, in the current study, we focused on tubulins, which were allowed to self-polymerize in the presence of guanosine triphosphate, and then the effect of TD on polymerization of tubulin was examined. During incubation, tubulins were polymerized/self-assembled in a time-dependent fashion and the addition of 200 µM TD to tubulins significantly enhanced the polymerization, particularly during the initial phase (Fig. 7). Fifty micromolar TD also promoted the polymerization at first, but within 10 min, the control tubulin polymerization proceeded faster. As shown in Fig. 8, highly aggregated tubulin was observed at the top of the gel (stacking gel) and quinone-modified proteins were detected in TD-treated tubulins even after reduction with TCEP. These results suggest that the larger than expected increase in O.D. at 340 nm by TD was due to the nonspecific aggregation of tubulins rather than the polymerization/self-assembly of tubulins, which is a well-organized event (Fig. 9).
Fig. 7.
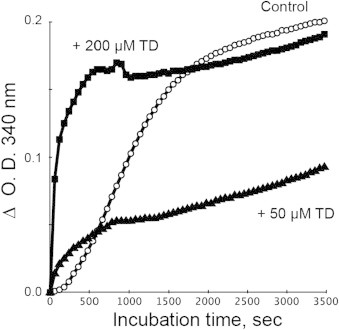
Effect of TD on polymerization of tubulins. Tubulins were incubated with guanosine triphosphate in the presence or absence of TD, and the aggregation and/or polymerization of tubulins was confirmed by monitoring the O.D. at 340 nm.
Fig. 8.
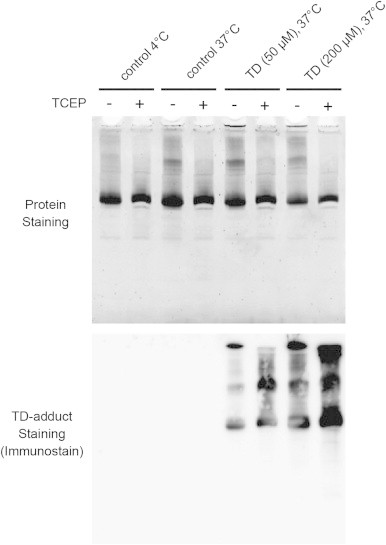
Immunoblot analyses of tubulin exposed to TD. The reaction was done as described in the legend to Fig. 7. The samples were then applied with or without reduction by TCEP. The protein was stained with SYPRO Orange and quinone-modified tubulins were then detected by blot analyses.
Fig. 9.
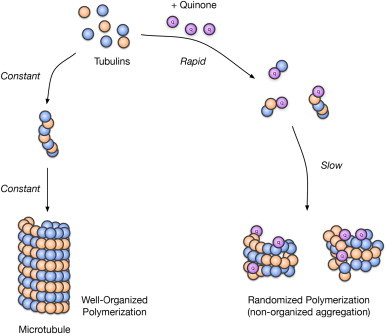
Plausible scheme for modulation of tubulin aggregation by TD exposure.
Discussion
Serotonin has several beneficial biological activities in vivo but, it may also contribute to the development of some diseases such as irritable bowel syndrome or depression. Perfusion of serotonin causes protein carbonylation in the heart, possibly through ROS production and inhibition of monoamine oxidase B [23]. Serotonin in the plasma of patients with vascular dementia is normally higher than that in the control [24]. These findings suggest that increased plasma levels of serotonin with carotid atherosclerotic plaques may be involved in the pathogenesis and progression of vascular dementia. When serotonin is oxidized by various oxidants and peroxidases, the highly reactive quinone, TD, is then formed [1,2]. However, there are only a few reports on TD generation in vivo. For example, in zebra fish treated with methylmercury, extracellular serotonin was decreased while TD increased [25]. Thus, it is likely that the reactive quinone may contribute to certain biological events.
Serotonin is one possible substrate for peroxidases in vivo. As shown in Fig. 3, the generation of a quinone adduct was consistently observed in the presence of various concentrations of chloride ion, which should be the main substrate of myeloperoxidase in vivo. This suggests that chloride ion does not compete with serotonin as a substrate of myeloperoxidase.
In a previous study, we found by mass spectrometry that myeloperoxidase uses serotonin as the substrate and the formed product, TD, covalently reacts with the peptide containing Cys152 and Cys156 in human GAPDH [13]. The adduction of quinone was also confirmed by biotin-labeled serotonin as a substrate of myeloperoxidase or TD–biotin followed by detection with streptavidin-peroxidase or streptavidin–AP. In the current study, we have successfully developed an immunochemical method to detect quinone–protein adducts by preparing a novel antibody. The TD–thiol adduct is considered to be the predominant epitope for the antibody (Fig. S2) but the antibody may also cross-react with a TD-amine adduct (Fig. S1A).
TD is a putative neurotoxin. However, it is difficult to detect TD in biological samples because of its high reactivity. Once conjugated with biomolecules, the adduct becomes relatively stable [13]. The established antibody may prove useful for identifying the contribution of TD to the development/progression of some diseases including serotonin-related diseases. In this study, the target proteins were isolated using both antibody beads and avidin beads with biotinylated TD as a model compound. Whereas the signals for TD–biotin were stronger than those from antibody beads, similar results were obtained. TD–biotin is a good probe for screening target proteins even though it is a synthetic compound. Our novel antibody should be useful to identify serotonin-derived oxidative modifications.
Previous papers have reported that these cytoskeletal proteins are modified by nucleophiles in cells [19–22]. We immunochemically identified cytoskeletal proteins as targets for the quinone in neuronal cells but could not verify the identities of these proteins by mass spectrometry because there was insufficient precipitated protein. Though our previous study showed that GAPDH, as a thiol model protein, was modified by TD in vitro [13], we could not detect TD-modification of GAPDH in the cells. The proteins on the blotted membrane, in particular, in the higher molecular weight region, remain to be identified.
We found that polymerization of tubulins is affected by TD treatment even at micromolar levels, suggesting that TD modification of a protein changes the nature and/or function of the protein. Modification of functional proteins by reactive TD may play an important part in some serotonin-related diseases.
Acknowledgments
We thank Asuna Maeda for technical assistance in preparing serotonin–biotin. We are also thankful to Dr. Akari Ishisaka for her critical reading of this manuscript.
Contributor Information
Yoji Kato, Email: yojikato@shse.u-hyogo.ac.jp.
Shigeki Ono, Email: shigekix0505@gmail.com.
Noritoshi Kitamoto, Email: kitamoto@shse.u-hyogo.ac.jp.
Anthony J. Kettle, Email: tony.kettle@otago.ac.nz.
Appendix A. Supplementary material
Supplementary data associated with this article can be found in the online version at doi:dx.doi.org/10.1016/j.redox.2014.08.004.
Appendix A. Supplementary material
Supplementary data
References
- 1.Wrona M.Z., Dryhurst G. Oxidation of serotonin by superoxide radical: implications to neurodegenerative brain disorders. Chemical Research in Toxicology. 1998;11:639–650. doi: 10.1021/tx970185w. 9625732 [DOI] [PubMed] [Google Scholar]
- 2.Ximenes V.F., Maghzal G.J., Turner R., Kato Y., Winterbourn C.C., Kettle A.J. Serotonin as a physiological substrate for myeloperoxidase and its superoxide-dependent oxidation to cytotoxic tryptamine-4,5-dione. Biochemical Journal. 2010;425:285–293. doi: 10.1042/BJ20090776. 19828014 [DOI] [PubMed] [Google Scholar]
- 3.Jones C.E., Underwood C.K., Coulson E.J., Taylor P.J. Copper induced oxidation of serotonin: analysis of products and toxicity. Journal of Neurochemistry. 2007;102:1035–1043. doi: 10.1111/j.1471-4159.2007.04602.x. 17663749 [DOI] [PubMed] [Google Scholar]
- 4.Huether G., Fettkötter I., Keilhoff G., Wolf G. Serotonin acts as a radical scavenger and is oxidized to a dimer during the respiratory burst of activated microglia. Journal of Neurochemistry. 1997;69:2096–2101. doi: 10.1046/j.1471-4159.1997.69052096.x. 9349555 [DOI] [PubMed] [Google Scholar]
- 5.Jiang X.R., Wrona M.Z., Alguindigue S.S., Dryhurst G. Reactions of the putative neurotoxin tryptamine-4,5-dione with l-cysteine and other thiols. Chemical Research in Toxicology. 2004;17:357–369. doi: 10.1021/tx020084k. 15025506 [DOI] [PubMed] [Google Scholar]
- 6.Wrona M.Z., Singh S., Dryhurst G. Influence of l-cysteine on the oxidation chemistry of serotonin. Bioorganic Chemistry. 1994;22:421–445. [Google Scholar]
- 7.Jiang X.R., Dryhurst G. Inhibition of the alpha-ketoglutarate dehydrogenase and pyruvate dehydrogenase complexes by a putative aberrant metabolite of serotonin, tryptamine-4,5-dione. Chemical Research in Toxicology. 2002;15:1242–1247. doi: 10.1021/tx020029b. 12387620 [DOI] [PubMed] [Google Scholar]
- 8.Wrona M.Z., Dryhurst G. A putative metabolite of serotonin, tryptamine-4,5-dione, is an irreversible inhibitor of tryptophan hydroxylase: possible relevance to the serotonergic neurotoxicity of methamphetamine. Chemical Research in Toxicology. 2001;14:1184–1192. doi: 10.1021/tx010037c. 11559032 [DOI] [PubMed] [Google Scholar]
- 9.Jasin H.E. Generation of IgG aggregates by the myeloperoxidase-hydrogen peroxide system. Journal of Immunology (Baltimore, Md.: 1950) 1983;130:1918–1923. 6300234 [PubMed] [Google Scholar]
- 10.Salman-Tabcheh S., Guérin M.C., Torreilles J. Potential role of the peroxidase-dependent metabolism of serotonin in lowering the polymorphonuclear leukocyte bactericidal function. Free Radical Research. 1996;24:61–68. doi: 10.3109/10715769609088000. 8747893 [DOI] [PubMed] [Google Scholar]
- 11.Hallingbäck H.R., Gabdoulline R.R., Wade R.C. Comparison of the binding and reactivity of plant and mammalian peroxidases to indole derivatives by computational docking. Biochemistry. 2006;45:2940–2950. doi: 10.1021/bi051510e. 16503648 [DOI] [PubMed] [Google Scholar]
- 12.Reynolds W.F., Rhees J., Maciejewski D., Paladino T., Sieburg H., Maki R.A., Masliah E. Myeloperoxidase polymorphism is associated with gender specific risk for Alzheimer's disease. Experimental Neurology. 1999;155:31–41. doi: 10.1006/exnr.1998.6977. 9918702 [DOI] [PubMed] [Google Scholar]
- 13.Kato Y., Peskin A.V., Dickerhof N., Harwood D.T., Kettle A.J. Myeloperoxidase catalyzes the conjugation of serotonin to thiols via free radicals and tryptamine-4,5-dione. Chemical Research in Toxicology. 2012;25:2322–2332. doi: 10.1021/tx300218f. 23009681 [DOI] [PubMed] [Google Scholar]
- 14.Singh S., Wrona M.Z., Dryhurst G. Synthesis and reactivity of the putative neurotoxin tryptamine-4,5-dione. Bioorganic Chemistry. 1992;20:189–203. [Google Scholar]
- 15.Zheng J., Hammock B.D. Development of polyclonal antibodies for detection of protein modification by 1,2-naphthoquinone. Chemical Research in Toxicology. 1996;9:904–909. doi: 10.1021/tx960014b. 8828928 [DOI] [PubMed] [Google Scholar]
- 16.Kato Y., Kawai Y., Morinaga H., Kondo H., Dozaki N., Kitamoto N., Osawa T. Immunogenicity of a brominated protein and successive establishment of a monoclonal antibody to dihalogenated tyrosine. Free Radical Biology and Medicine. 2005;38:24–31. doi: 10.1016/j.freeradbiomed.2004.09.013. 15589368 [DOI] [PubMed] [Google Scholar]
- 17.Paz M.A., Flückiger R., Boak A., Kagan H.M., Gallop P.M. Specific detection of quinoproteins by redox-cycling staining. Journal of Biological Chemistry. 1991;266:689–692. 1702437 [PubMed] [Google Scholar]
- 18.Ximenes V.F., Silva S.O., Rodrigues M.R., Catalani L.H., Maghzal G.J., Kettle A.J., Campa A. Superoxide-dependent oxidation of melatonin by myeloperoxidase. Journal of Biological Chemistry. 2005;280:38160–38169. doi: 10.1074/jbc.M506384200. 16148002 [DOI] [PubMed] [Google Scholar]
- 19.Pamplona R., Dalfó E., Ayala V., Bellmunt M.J., Prat J., Ferrer I., Portero-Otín M. Proteins in human brain cortex are modified by oxidation, glycoxidation, and lipoxidation. Effects of Alzheimer disease and identification of lipoxidation targets. Journal of Biological Chemistry. 2005;280:21522–21530. doi: 10.1074/jbc.M502255200. 15799962 [DOI] [PubMed] [Google Scholar]
- 20.Jeong M.S., Kang J.H. Acrolein, the toxic endogenous aldehyde, induces neurofilament-L aggregation. BMB Reports. 2008;41:635–639. doi: 10.5483/bmbrep.2008.41.9.635. 18823586 [DOI] [PubMed] [Google Scholar]
- 21.Van Laar V.S., Mishizen A.J., Cascio M., Hastings T.G. Proteomic identification of dopamine-conjugated proteins from isolated rat brain mitochondria and SH-SY5Y cells. Neurobiology of Disease. 2009;34:487–500. doi: 10.1016/j.nbd.2009.03.004. 19332121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mi L., Hood B.L., Stewart N.A., Xiao Z., Govind S., Wang X., Conrads T.P., Veenstra T.D., Chung F.L. Identification of potential protein targets of isothiocyanates by proteomics. Chemical Research in Toxicology. 2011;24:1735–1743. doi: 10.1021/tx2002806. 21838287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu L., Marcocci L., Wong C.M., Park A.M., Suzuki Y.J. Serotonin-mediated protein carbonylation in the right heart. Free Radical Biology and Medicine. 2008;45:847–854. doi: 10.1016/j.freeradbiomed.2008.06.008. 18616998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ban Y., Watanabe T., Miyazaki A., Nakano Y., Tobe T., Idei T., Iguchi T., Ban Y., Katagiri T. Impact of increased plasma serotonin levels and carotid atherosclerosis on vascular dementia. Atherosclerosis. 2007;195:153–159. doi: 10.1016/j.atherosclerosis.2006.09.005. 17049533 [DOI] [PubMed] [Google Scholar]
- 25.Maximino C., Araujo J., Leão L.K., Grisolia A.B., Oliveira K.R., Lima M.G., Batista Ede J., Crespo-López M.E., Gouveia A., Jr., Herculano A.M. Possible role of serotoninergic system in the neurobehavioral impairment induced by acute methylmercury exposure in zebrafish (Danio rerio) Neurotoxicology and Teratology. 2011;33:727–734. doi: 10.1016/j.ntt.2011.08.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data


