Abstract
Obesity and excessive inflammation/oxidative stress are pathophysiological forces associated with kidney dysfunction. Although we recently showed that heme-oxygenase (HO) improves renal functions, the mechanisms are largely unclear. Moreover, the effects of the HO-system on podocyte cytoskeletal proteins like podocin, podocalyxin, CD2-associated-protein (CD2AP) and proteins of regeneration/repair like beta-catenin, Oct3/4, WT1 and Pax2 in renal tissue from normoglycemic obese Zucker-fatty rats (ZFs) have not been reported.
Treatment with hemin reduced renal histo-pathological lesions including glomerular-hypertrophy, tubular-cast, tubular-atrophy and mononuclear cell-infiltration in ZFs. These were associated with enhanced expression of beta-catenin, Oct3/4, WT1, Pax2 and nephrin, an essential transmembrane protein required for the formation of the scaffoldings of the podocyte slit-diaphragm, permitting the filtration of small ions, but not massive excretion of proteins, hence proteinuria. Besides nephrin, hemin also enhanced other important podocyte-regulators including, podocalyxin, podocin and CD2AP. Correspondingly, important markers of renal dysfunction such as albuminuria and proteinuria were reduced, while creatinine clearance increased, suggesting improved renal function in hemin-treated ZFs. The renoprotection by hemin was accompanied by the reduction of inflammatory/oxidative mediators including, macrophage-inflammatory-protein-1α, macrophage-chemoattractant-protein-1 and 8-isoprostane, whereas HO-1, HO-activity and the total-anti-oxidant-capacity increased. Contrarily, the HO-inhibitor, stannous-mesoporphyrin nullified the reno-protection by hemin.
Collectively, these data suggest that hemin ameliorates nephropathy by potentiating the expression of proteins of repair/regeneration, abating oxidative/inflammatory mediators, reducing renal histo-pathological lesions, while enhancing nephrin, podocin, podocalyxin, CD2AP and creatinine clearance, with corresponding reduction of albuminuria/proteinuria suggesting improved renal function in hemin-treated ZFs. Importantly, the concomitant potentiation regeneration proteins and podocyte cytoskeletal proteins are novel mechanisms by which hemin rescue nephropathy in obesity.
Keywords: Heme oxygenase, beta-catenin, Podocalyxin, Oct-3/4, Podocin, CD2-associated protein
Highlights
-
•
Renal dysfunction is common in obesity.
-
•
Novel mechanisms by which heme-oxygenase (HO) rescue kidney failure are unveiled.
-
•
HO enhance podocyte cytoskeletal proteins like podocin, podocalyxin and CD2AP.
-
•
HO enhance proteins of regeneration/repair like beta-catenin, Oct3/4, WT1 and Pax2.
Introduction
The escalation of obesity and kidney disease is all segments of the population, including children is a challenging health concern [1–4]. In obesity, excessive inflammatory/oxidative insults may result to morphological defects in important components of the filtration apparatus of the kidney such as the glomerulus, leading to proteinuria and renal insufficiency [5–8]. Thus, a healthy glomerulus is essential for effective filtration. Generally, the aperture of the renal filtration barrier is regulated by the podocyte slit-diaphragm of the glomerulus, allowing small molecules like ions to selectively pass through, but not larger protein molecules [9–13]. The major constituents of the podocyte slit-diaphragm include nephrin, podocin, podocalyxin and CD2-associated protein (CD2AP) [12]. Defects in these fundamental podocyte proteins cause proteinuria [9–13]. Therefore, novel strategies capable of potentiating the expression of nephrin, podocin, podocalyxin, CD2AP and abating inflammatory/oxidative insults would be beneficial in renal insufficiency.
Heme oxygenase (HO) is an important cytoprotective enzyme with two main isoforms HO-1 (inducible) and HO-2 (constitutive) that catalyzes the breakdown of pro-oxidant heme to produce biliverdin/bilirubin and carbon monoxide with anti-oxidant and anti-inflammatory effects, while the iron formed enhances the synthesis of ferritin with anti-oxidant properties [14]. Although we recently reported the renoprotective effects of the HO system [15–18], the mechanisms are not completely elucidated. Whether the HO system enhances proteins of repair/regeneration like beta-catenin, Oct3/4, WT1 and Pax2 [19–23] in obese normoglycemic Zucker rats (ZF) to improve renal function remains unclear. Similarly, the effects of the HO system on important podocyte regulators such as podocin, podocalyxin and CD2AP in ZFs have not been reported. Therefore, this study was designed to investigate the effects of the HO-inducer, hemin on beta-catenin, Oct3/4, WT1, Pax2, podocin, podocalyxin, CD2AP, nephrin, inflammation, oxidative stress and correlate changes in these factors to renal function in ZFs. Moreover, the effects of the HO system on the expression of beta-catenin, Oct3/4, WT1, Pax2, podocin, podocalyxin, CD2AP in the kidney of normoglycemic obese ZF rats have not been reported. Therefore, our findings will offer novel insights on the role of hemin therapy in kidney dysfunction in obesity.
Materials and methods
Animals, treatment groups and biochemical assays
Our experimental protocol was approved by the Animal Ethics Committee of University of Saskatchewan and is in conformity with the Guide for Care and Use of Laboratory Animals stipulated by the Canadian Council of Animal Care and the National Institutes of Health (NIH Publication no. 85-23, revised 1996). Twelve-week old male Zucker fatty (ZF) rats and sex/age-matched littermates Zucker lean (ZL) rats were purchased from Charles River Laboratories (Willington, MA, USA). The animals were housed at 21 °C with 12-h light/dark cycles, were fed with standard laboratory chow and had access to drinking water ad libitum.
The HO-inducer, hemin (30 mg/kg i.p., Sigma, St. Louis, MO) and the HO-inhibitor, stannous mesoporphyrin [(SnMP) 2 mg/100 g body weight i.p., Porphyrin Products (Logan, UT, USA)] were prepared as we previously reported and administered biweekly for 8 weeks [10,35,36]. At 16 weeks of age, the animals were randomly divided into the following experimental groups (n=6 per group): (A) controls (ZF and ZL), (B) hemin-treated ZF and ZL, (C) ZF+hemin+nMP and (D) ZF+vehicle dissolving hemin and SnMP.
During the treatment period we measured body-weight and fasting glucose on a weekly basis. Body-weight was determined by means of a digital balance (Model Mettler PE1600, Mettler Instruments Corporation, Greifensee, Zurich, Switzerland), while fasting glucose was measured by means of a diagnostic auto-analyzer (BD, Franklin Lakes, NJ) after 6 h of fasting as we previously reported [35,37]. After the 8-week treatment period, the animals were placed in metabolic cages for 24 h urine collection. Proteinuria, albuminuria and creatinine were measured as previously reported [15,16,18]. On the day of killing, the animals were weighed, anesthetized with pentobarbital sodium (50 mg/kg i.p.), and the kidneys removed and weighed with an analytical balance (Precisa Instruments Ltd., Dietikon, Switzerland) as we previously reported [24,25].
Renal HO activity was determined spectrophotometrically as we previously reported [10,38,39], while ELISA kits were used for the determination of HO-1 (Stressgen-Assay Design, Ann Arbor, MI, USA), macrophage inflammatory protein-1α and macrophage-chemoattractant protein-1 (OmniKine™, Assay Biotechnology Company Inc., Sunnyvale, CA) and EIA kits for 8-isoproatane and total-anti-oxidant capacity (Cayman Chemical, Ann Arbor, MI, USA) as we previously reported [15,16,26].
Histology, morphological and immunohistochemical analyzes of kidney tissue
Histology and morphometric analyzes of the kidney were done as we previously reported [16,27]. In brief, whole kidney sections of 5 µm thickness were cut from paraffin embedded tissue, treated with hematoxylin and eosin staining and examined using a virtual microscope (Aperio Scan Scope Model CS, Aperio Technology Inc., CA). The images were magnified at 100× and 200×. Morphological evaluation was done by a blinded researcher who randomly took 20 snaps shots per slide per group (n=6, 120 images per experimental group). The images were analyzed using Aperio Image Scope V11.2.0.780 software (Aperio, e-Pathology Solution, CA) and scored semi-quantitatively [16,17,27,28].
Immunohistochemistry was done as we previously described [15,18,26,29]. Sections of 5 µm of whole kidney were treated with bovine serum albumin in phosphate buffered saline to avoid non-specific staining and incubated overnight with ED1 antibody (1:5 dilution, Santa Cruz Biotechnology, CA), and subsequently with goat anti-mouse IgG for 30 min (1:200 dilution; Jackson ImmunoResearch Laboratories, Inc., ME, USA). Immunohistochemical staining was done using the standard avidin–biotin complex method. The chromogen, 3,3′-diaminobenzidine (DAB) was used at the final detection step. Thereafter, the kidney sections were scanned using a virtual microscope (Aperio Scan Scope, Model CS, Aperio Technology Inc., CA) and macrophages (brown from immune-stained sections) quantified by counting the positively-stained ED1 cells by a blinded researcher under 200× magnification in 15 randomized non-overlapping fields. Only distinct ED1-stained cells from the different experimental groups were taken into consideration.
Western immunoblotting
The kidney was homogenized (1:10, w:v) in 10 mM Tris-buffered saline (20 mM Tris–HCl, pH 7.4, 0.25 M sucrose, and 1 mM EDTA) in the presence of a cocktail of protease inhibitors as we previously reported [30]. Thereafter, proteins were extracted, quantified and aliquots of 100 µg loaded on a 10% SDS-polyacrylamide gel for October 3/4, pax-2, beta-catenin, WT1, nephrin, podocalyxin, podocin and CD2AP. The fractionated proteins were electrophoretically transferred into nitrocellulose paper and non-specific bindings blocked with 3% non-fat milk.
The blots were incubated overnight with primary antibodies [Santa Cruz Biotechnology, Santa Cruz, CA, USA, c-kit (sc-365504), October 3/4 (sc-5279), pax-2 (sc-130387), WT1 (sc-192), nephrin (sc-377246), podocalyxin (sc-10506), podocin (sc-21009) and CD2AP (sc-9137)] and with beta-catenin ([E247] ab32572, Abcam Inc., Cambridge, MA, USA). After washing in milk, the blots were incubated with anti-rabbit IgG conjugated to horseradish peroxide (Bio-Rad, CA, USA), and the immuno-reactivity visualized using enhanced horseradish peroxide/luminol chemiluminescence reagent (PerkinElmer Life Sciences, Boston, MA, USA) and densitometric analysis was done using UN-SCAN-IT software (Silk Scientific, Orem, Utah, USA). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibody (g8795) (Sigma, St. Louis, MO, USA) was used as a control.
Statistical analysis
All the data expressed in the present study are mean±SEM from at least four independent experiments unless otherwise stated. Statistical analyzes were done using two-way ANOVA, by means of Statistical Analysis System (SAS), software, version 9.3 (SAS Institute Inc., Cary, NC, USA) and Student's t-test. In addition to ANOVA, multiple pairwise comparisons between groups were undertaken with Bonferroni corrected p-values to ascertain which groups differed from each other. Group differences at the level of p<0.05 were considered statistically significant.
Results
Upregulating the HO system with hemin improves kidney function in obese normoglycemic ZF rats
The administration of hemin to ZFs significantly enhanced HO-1 and HO-activity (Fig. 1A and B). Interestingly, the potentiation of the HO system by hemin was associated with marked reduction of albuminuria and proteinuria, whereas creatinine clearance was greatly increased, suggesting improved renal function in hemin-treated ZFs (Table 1). In contrast, the co-administration of the HO-inducer, hemin together with the HO-inhibitor SnMP nullified the effects of hemin on HO-1 and HO-activity (Fig. 1A and B), and correspondingly annulled the renoprotective effects of hemin, which was evidenced by the restoration of elevated albuminuria and proteinuria, with reduced creatinine clearance (Table 1). Hemin also increased HO-1 and HO-activity in ZL-control rats, although the effects of hemin on were less-intense in ZF.
Fig. 1.
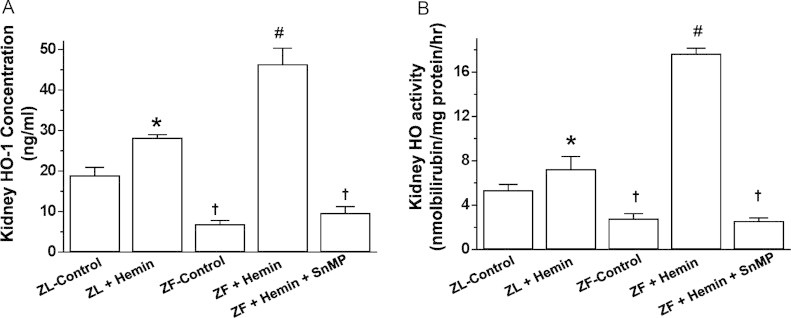
Effects of the heme oxygenase (HO)-inducer hemin and the HO-inhibitor sodium mesoporphyrin (SnMP) on HO-1 and HO-activity in the kidneys of ZFs and ZL. Treatment with hemin robustly increased (A) HO-1 concentration and (B) HO-activity, whereas SnMP abolished the effects. Bars represent mean±SEM; n=6 rats per group. * p<0.01 vs ZL-Control; † p<0.01 vs ZL-Control; #p<0.01 vs ZF+Hemin+SnMP or ZF-control.
Table 1.
Effect of the heme oxygenase (HO) inducer, hemin and the HO-blocker stannous mesoporphysin (SnMP) on physiological variables in Zucker fatty (ZF) and Zucker lean (ZL) rats.
| Parameters |
Animal groups |
|||||
|---|---|---|---|---|---|---|
| ZL Control | ZL+Hemin | ZF Control | ZF+Hemin | ZF+Hemin+SnMP | ZF+Vehicle | |
| Body weight (g) | 475.8±7.3 | 451.5±6.5 | 785.9±11.6† | 732.4±8.1€ | 717.6±10.5€ | 792.8±9.3 |
| Fasting glucose (mmol/L) | 7.3±0.5 | 6.2±0.1§ | 8.5±0.2† | 6.9±0.4€ | 9.2±0.3€ | 8.7±0.2 |
| Albuminuria (mg/24 h) | 1.8±0.2 | 1.3±0.1 | 19.6±2.8† | 8.9±1.6⁎ | 21.7±3.1⁎ | 18.3±2.5 |
| Proteinuria (mg/24 h) | 4.1±0.3 | 3.3±0.2 | 72.9±8.6† | 24.5±3.2⁎⁎ | 80.3±6.4⁎⁎ | 75.4±5.3 |
| Creatinine Clearance (ml min/g kidney) | 4.3±0.4 | 4.6±0.3 | 2.4±0.2†† | 3.9±0.3⁎ | 2.2±0.1⁎⁎ | 2.3±0.2 |
p<0.05 vs ZL.
p<0.05 vs ZF.
p<0.05.
p<0.01 vs ZL control.
p<0.05.
p<0.01 vs ZF-control or ZL-Control, n=6 per group.
We also measured fasting glucose levels in all experimental groups (Table 1). ZFs and ZL-controls were normoglycemic. Treatment with hemin slightly reduced fasting glucose levels in ZFs, while co-treatment of hemin with SnMP reversed the effect (Table 1). Hemin also slightly reduced fasting blood glucose levels in ZL-controls.
The administration of hemin and SnMP caused loss of body-weight in ZFs and ZL-controls (Table 1). In hemin-treated ZLs, hemin-treated ZFs and the hemin+SnMP treated ZFs the loss of body weight were 5.1%, 6.8%, and 8.7% respectively (Table 1). The loss of weight may not be due to toxicity because our previous study indicated that indices of toxicity such as plasma gamma-glutamyltransferase, aspartate aminotransferase and alanine aminotransferase were within normal range [31,32].
The vehicle dissolving hemin and SnMP had no effect on the measured parameters.
Hemin abated the elevated levels of 8-isoprostane, macrophage chemoattractant protein-1 (MCP-1) and macrophage inflammatory protein-1 alpha (MIP-1α) in the kidneys of ZFs
Given that elevated levels of MCP-1, MIP-1α and excessive oxidative stress are implicated in nephropathy [6–8], we measured the levels of MCP-1, MIP-1α and 8-isoprostane, an index of oxidative stress [33].
Our results indicate that in ZFs, the basal levels of MCP-1 and MIP-1α were significantly elevated (Fig. 2A and B). Interestingly, treatment with hemin greatly attenuated the levels of MCP-1 and MIP-1α in ZFs. The effect of hemin was more effective against MCP-1 than MIP-1α as hemin restored the levels of MCP-1 to comparable levels as in ZL-controls while MIP-1α was attenuated by 48.5% (Fig. 2A and B). On the other hand, co-treatment of hemin and the HO-blocker, SnMP nullified the effects of hemin on MCP-1 and MIP-1α.
Fig. 2.
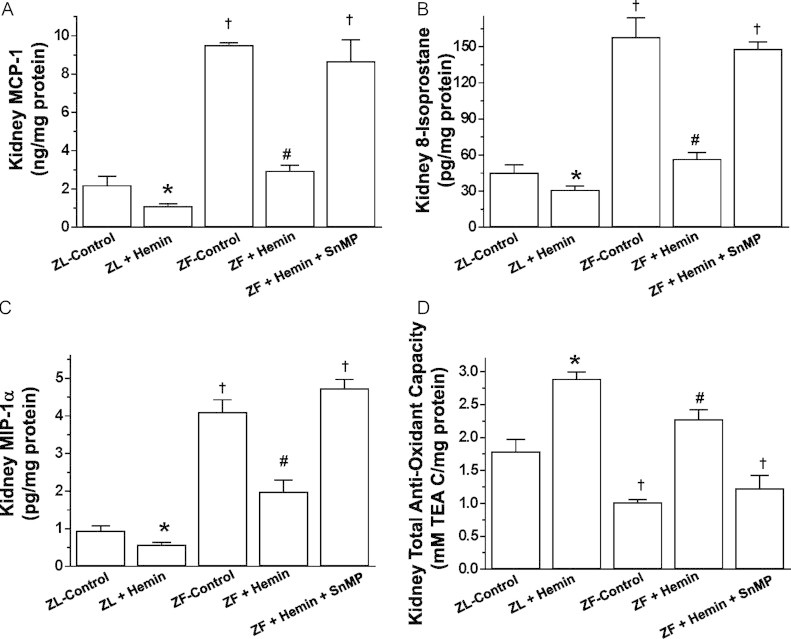
Effects of the heme oxygenase (HO)-inducer hemin and the HO-inhibitor sodium mesoporphyrin (SnMP) on macrophage chemo-attractant protein-1 (MCP-1), macrophage inflammatory protein-1α (MIP-1α), 8-isoprostane and the total-anti-oxidant capacity in the kidneys of ZFs and ZL. Treatment with hemin (A) reduced MCP-1, (B) suppressed MIP-1α, (C) reduced 8-isoprostane, but (D) increased the total-anti-oxidant capacity, whereas SnMP nullified the effects of hemin. Bars represent mean±SEM; n=6 rats per group. * p<0.01 vs ZL-Control; † p<0.01 vs ZL-Control; #p<0.01 vs ZF+Hemin+SnMP or ZF-control.
Hemin therapy was also effective against the oxidative stress marker, 8-isoprostane (Fig. 2C). The basal levels of 8-isoprostane in ZFs were markedly elevated, but were reduced by hemin, while the co-administration of hemin and SnMP abolished the effect of hemin. To further explore the effects of an upregulated HO system by hemin on oxidative stress we measured the total-anti-oxidant capacity. In ZFs, the basal level of the total anti-oxidant capacity was markedly reduced as compared to the ZL-control, but was robustly enhanced by hemin (Fig. 2D). On the other hand, the co-administration of hemin and in SnMP annulled the effect of hemin on the total anti-oxidant capacity.
Hemin also reduced MCP-1, MIP-1α, 8-isoprostane and enhanced the total-anti-oxidant capacity in ZL-controls.
Hemin suppressed macrophage infiltration in the kidney
To further explore the effects of hemin on inflammation, we use the ED-1 antibody to assess macrophage infiltration in the kidney by immunohistochemistry (Fig. 3A). We observed that sections of renal tissue from ZL-controls were almost devoid of the dark brown ED1 positive staining that characterizes macrophage infiltration, and thus inflammatory activity. In contrast, kidney sections from untreated ZF-controls were characterized by marked increase in the number of ED-1 positive staining for macrophage (Fig. 3A). Interestingly, treatment with hemin markedly reduced the number of ED-1 stained macrophage, suggesting reduction of macrophage infiltration and reduced inflammation in hemin-treated ZFs. Further assessment of macrophage infiltration by quantitative analyzes revealed that hemin therapy significantly reduced the ED1 score of renal tissue sections (Fig. 3B).
Fig. 3.
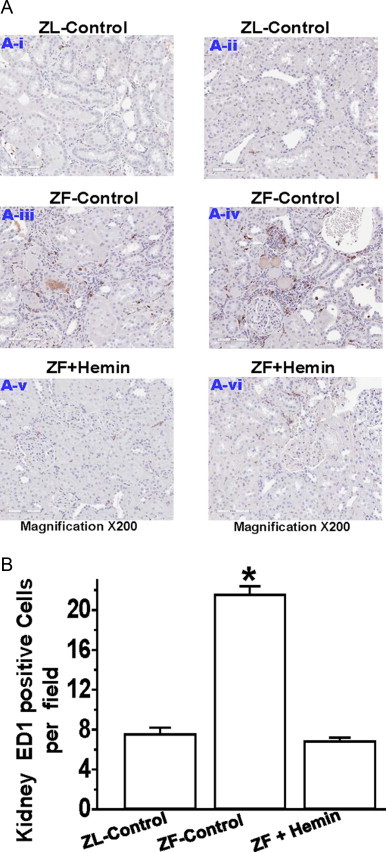
Effect of hemin on macrophage infiltration in the kidney. (A) Representative images of kidney section from different rats indicating the macrophage infiltration. ED1-positive cells stained dark brown in renal sections were increased in untreated ZF-controls (panels A-iii and A-iv) than in ZL-controls (panels A-i and A-ii), but were reduced by hemin therapy (panels A-v and A-vi) (magnification ×200). (B) Quantitative analyzes per field revealed that macrophage infiltration in untreated ZF-controls were markedly elevated than in ZL-control, but was significantly reduced by hemin. Bars represent mean±SEM; n=6 rats per group (* p<0.01 vs all groups).
Hemin therapy improve kidney morphology
Since elevated inflammation and oxidative stress are known to compromise kidney morphology [5–8], causing renal insufficiency, we investigated whether the hemin-dependent suppression of oxidative stress/inflammation (Figs. 2 and 3) would be accompanied by renal morphological amelioration in ZFs. Our results indicate that kidney sections from control-ZL were almost devoid of pathological signs in the cortex and the medulla besides a few areas of mild congestion (Fig. 4A). However, kidney sections from untreated ZFs were characterized by severe morphological lesions such as glomerular hypertrophy, glomerular atrophy, tubular-cast, tubular-atrophy and mononuclear cell-infiltration. Interestingly, the administration of hemin therapy to ZFs resulted in marked reduction of these lesions (Fig. 4A). Further evaluation of renal lesions by semi-quantitative analysis revealed that hemin therapy significantly abated renal lesions in ZFs (Fig. 4B).
Fig. 4.
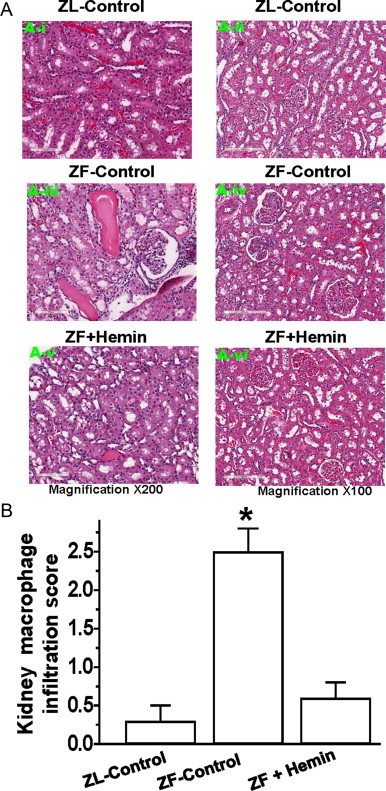
Effect of hemin on renal histo-pathological lesions. (A) Representative images of renal histo-pathological lesions. ZL-controls (panels A-i and A-ii) were almost devoid of lesions in the cortex and the medulla, but untreated ZF-controls (panels A-iii and A-iv) had severe histo-pathological kidney lesions including glomerular hypertrophy, glomerular atrophy, tubular-cast, tubular-atrophy, tubular fibrosis and mononuclear cell-infiltration. Interestingly, in hemin-treated ZFs (panels A-v and A-vi), these histo-pathological lesions were greatly reduced. (B) Semi-quantitative morphological analyzes reveal that hemin significantly attenuated the lesions. Bars represent mean±SEM; n=6 rats per group (* p<0.01 vs all groups).
Hemin therapy enhance proteins of regeneration in the kidney
To investigate the mechanisms by which hemin therapy improves kidney morphology, we measured the expression of proteins of regeneration such as beta-catenin, Oct3/4 and Pax2 [19–23] in renal tissue. Our results indicate that the basal protein expressions of beta-catenin, Oct3/4 and pax2 in ZFs were significantly reduced as compared to the ZL-control (Fig. 5A–C). However, treatment with hemin significantly enhanced the aberrant expressions of beta-catenin, Oct3/4 and pax2.
Fig. 5.
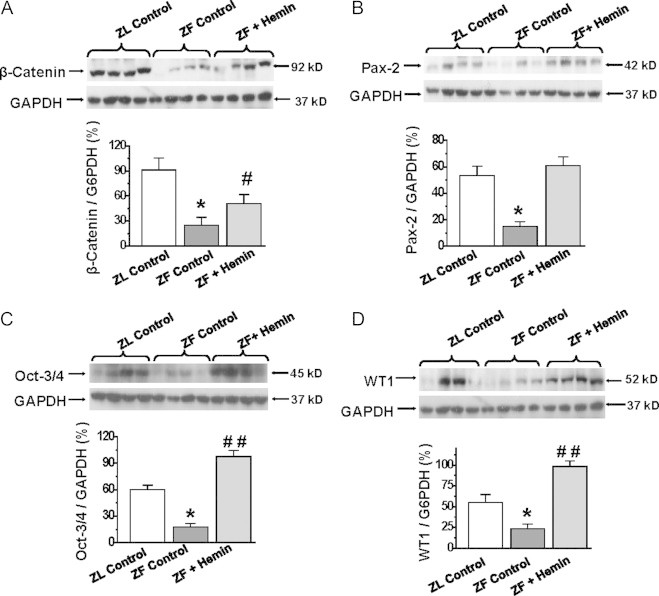
Effects of hemin on the expression of proteins of regeneration in the kidneys. Western immunoblotting and relative densitometry of the expressed proteins normalized by GAPDH that reveals that treatment with hemin (A) increased the expression of β-catenin, (B) potentiated the expression of oct-3/4, (C) enhanced the expression of Pax-2 and (D) increased the expression of WT1 in ZFs. Bars represent mean±SEM; n=4 rats per group.
Since developing nephrons are positive for WT1 expression [22,23], we measured this protein (Fig. 5D). In ZFs, the expression of WT1 was significantly reduced as compared to ZL-controls. Interestingly, hemin therapy robustly enhanced the expression of WT1 in ZFs (Fig. 5D).
Hemin therapy potentiates proteins associated with podocyte and glomerular function in the kidneys of ZFs
Since podocalyxin, CD2AP, podocin and nephrin [9–13] are important proteins necessary for the formation of the podocyte slit-diaphragm of the glomerular barrier that regulates filtration, allowing small molecules to pass through but not larger protein molecules [9–13], we investigated the effects of hemin on these proteins. Moreover, defects in these fundamental podocyte proteins cause proteinuria [9–13].
Our results indicate that in untreated ZF-controls, the basal expressions of nephrin, podocalyxin, podocin and CD2AP were significantly reduced as compared to the ZL-control (Fig. 6A–D). However, treatment with hemin robustly enhanced the expressions of nephrin, podocalyxin, podocin and CD2AP in ZFs.
Fig. 6.
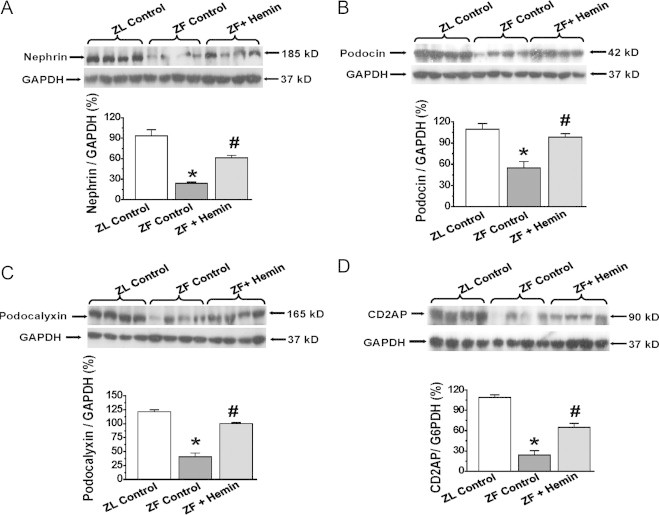
Effects of hemin on the expression of nephrin, podocalyxin, podocin, and CD2-associated protein (CD2AP) in the kidneys. Western immunoblotting and relative densitometry of the expressed proteins normalized by GAPDH indicates hemin (A) increased the expression of nephrin, (B) potentiated the expression of podocalyxin, (C) enhanced the expression of podocin and (D) increased the expression of CD2AP in ZFs. Bars represent mean±SEM; n=4 rats per group.
Discussion
The present study suggests that an unregulated HO system with hemin is a potent renoprotective strategy against obesity-induced renal abnormalities. In obesity excessive oxidative stress and elevated inflammation are among the pathophysiological forces that compromise renal morphology and function [5–8]. Importantly, our study unveils for the first time that hemin therapy potentiates proteins of regeneration/repair such as WT1, beta-catenin, Oct3/4 and Pax2 [19–23] to ameliorate kidney histo-pathological lesions such as glomerular hypertrophy, glomerular atrophy, tubular-cast, tubular-atrophy and mononuclear cell-infiltration. Interestingly, the restoration of kidney morphology by hemin was associated with the concomitant suppression of oxidative/inflammatory insults, alongside the potentiation of several transmembrane proteins including podocin, podocalyxin, CD2AP and nephrin which are important for the formation of the podocyte slit-diaphragm that regulates the aperture size of the glomerular filtration barrier, selectively allowing small molecules like ions to filter through, but not larger molecules like proteins [9–13]. Defects in podocin, podocalyxin, CD2AP and nephrin are known to cause proteinuria, and thus nephropathy [9–13]. Consistently, our study unveiled aberrant expression of podocin, podocalyxin, CD2AP and nephrin which was associated with elevated albuminuria/proteinuria and reduced creatinine clearance, and thus renal insufficiency in ZFs. However, treatment with hemin potentiated the expression of podocin, podocalyxin, CD2AP and nephrin in tandem, with corresponding reduction of albuminuria/proteinuria which interestingly was associated with increased creatinine clearance, and thus improved kidney function. On the other hand, blockade of the HO system with the HO-inhibitor, SnMP abolished the effects of hemin and re-instating elevated levels of albuminuria/proteinuria and reduced levels of creatinine clearance as observed in ZF-controls, suggesting that an upregulated HO system is renoprotective. Therefore it is possible that the multifaceted mechanisms by which an upregulated HO system by hemin rescues nephropathy in ZFs includes: (i) the amelioration of kidney histo-pathological lesions; (ii) the potentiation of proteins of repair/regeneration such as WT1, beta-catenin, Oct3/4 and Pax2; and (iii) the potentiation of podocalyxin, CD2AP, podocin and nephrin.
Hemin administration caused a slight loss of body weight and also improved glucose metabolism. We recently showed that upregulating the HO system with hemin potentiates insulin signaling and glucose metabolism in different diabetic including non-obese Goto-Kakizaki rats [34,35] and Zucker diabetic fatty rat [31], a genetically obese leptin receptor-deficient (fa/fa) model [36,37] and streptozotocin induced diabetes [38]. Similarly, we also observed improved insulin-signaling/glucose metabolism in uninephrectomized deoxycorticosterone-acetate (DOCA)-hypertension [39] and in spontaneously hypertensive rats [40,41]. Interestingly, the anti-diabetic effect of hemin was accompanied by enhanced insulin-sensitivity [31,34,35,38], alongside the potentiation of agents that enhance glucose metabolism, including adiponectin, adenosine monophosphate-activated-protein-kinase (AMPK), aldolase-B and GLUT4. Correspondingly, hemin improved intraperitoneal glucose-tolerance (IPGTT), reduced insulin-tolerance (IPITT), lowered insulin resistance (HOMA-IR index), and the inability of insulin to enhance GLUT4 was overturned [31,34,35,38].
Although hemin therapy caused a slight loss of body weight, toxicity is quite unlikely because important toxicity indices such as plasma gamma-glutamyltransferase, aspartate aminotransferase and alanine aminotransferase were within normal range [31,32]. Rather, the improved metabolism in hemin-treated animals may lead to improved catabolism, better caloric dispensation and loss of weight.
Collectively, our study suggests that upregulating the HO system with hemin improves kidney function by ameliorating histo-pathological lesions, alleviating oxidative/inflammatory insults and reducing albuminuria/proteinuria, while concomitantly enhancing nephrin, podocin, podocalyxin, CD2AP and increasing creatinine clearance. Thus HO-inducers may be explored as in the design of novel drugs against nephropathy, especially when it is co-morbid with obesity.
Conflict of interest
The authors have no conflict of interest that could be perceived as prejudicing the impartiality of the research reported. The authors have nothing to disclose.
Acknowledgements
This work was supported by a grant from the Heart & Stroke Foundation of Saskatchewan, Canada to Dr. Joseph Fomusi Ndisang.
References
- 1.Krmar R.T., Bárány P. Chronic kidney disease: obesity in children with end-stage renal disease. Nature Reviews. Nephrology. 2013;9:707–708. doi: 10.1038/nrneph.2013.223. 24145321 [DOI] [PubMed] [Google Scholar]
- 2.Satirapoj B., Supasyndh O., Mayteedol N., Punpanich D., Chaiprasert A., Nata N., Ruangkanchanasetr P., Kanjanakul I., Choovichian P. Obesity and its relation to chronic kidney disease: a population-based, cross-sectional study of a Thai army population and relatives. Nephrology. 2013;18:229–234. doi: 10.1111/nep.12023. 23279639 [DOI] [PubMed] [Google Scholar]
- 3.Nugent R.A., Fathima S.F., Feigl A.B., Chyung D. The burden of chronic kidney disease on developing nations: a 21st century challenge in global health. Nephron. Clinical Practice. 2011;118:c269–c277. doi: 10.1159/000321382. 21212690 [DOI] [PubMed] [Google Scholar]
- 4.Murray C.J., Richards M.A., Newton J.N., Fenton K.A., Anderson H.R., Atkinson C., Bennett D., Bernabé E., Blencowe H., Bourne R., Braithwaite T., Brayne C., Bruce N.G., Brugha T.S., Burney P., Dherani M., Dolk H., Edmond K., Ezzati M., Flaxman A.D., Fleming T.D., Freedman G., Gunnell D., Hay R.J., Hutchings S.J., Ohno S.L., Lozano R., Lyons R.A., Marcenes W., Naghavi M., Newton C.R., Pearce N., Pope D., Rushton L., Salomon J.A., Shibuya K., Vos T., Wang H., Williams H.C., Woolf A.D., Lopez A.D., Davis A. UK health performance: findings of the Global Burden of Disease Study 2010. Lancet. 2013;381:997–1020. doi: 10.1016/S0140-6736(13)60355-4. 23668584 [DOI] [PubMed] [Google Scholar]
- 5.Briffa J.F., McAinch A.J., Poronnik P., Hryciw D.H. Adipokines as a link between obesity and chronic kidney disease. American Journal of Physiology. Renal Physiology. 2013;305:F1629–F1636. doi: 10.1152/ajprenal.00263.2013. 24107418 [DOI] [PubMed] [Google Scholar]
- 6.Navarro-González J.F., Mora-Fernández C., Muros de Fuentes M., García-Pérez J. Inflammatory molecules and pathways in the pathogenesis of diabetic nephropathy. Nature Reviews. Nephrology. 2011;7:327–340. doi: 10.1038/nrneph.2011.51. 21537349 [DOI] [PubMed] [Google Scholar]
- 7.Lo D.J., Weaver T.A., Kleiner D.E., Mannon R.B., Jacobson L.M., Becker B.N., Swanson S.J., Hale D.A., Kirk A.D. Chemokines and their receptors in human renal allotransplantation. Transplantation. 2011;91:70–77. doi: 10.1097/TP.0b013e3181fe12fc. 21441854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh D.K., Winocour P., Farrington K. Oxidative stress in early diabetic nephropathy: fueling the fire. Nature Reviews. Endocrinology. 2011;7:176–184. doi: 10.1038/nrendo.2010.212. 21151200 [DOI] [PubMed] [Google Scholar]
- 9.Marshall S.M. The podocyte: a potential therapeutic target in diabetic nephropathy? Current Pharmaceutical Design. 2007;13:2713–2720. doi: 10.2174/138161207781662957. 17897015 [DOI] [PubMed] [Google Scholar]
- 10.Menne J., Meier M., Park J.K., Boehne M., Kirsch T., Lindschau C., Ociepka R., Leitges M., Rinta-Valkama J., Holthofer H., Haller H. Nephrin loss in experimental diabetic nephropathy is prevented by deletion of protein kinase C alpha signaling in-vivo. Kidney International. 2006;70:1456–1462. doi: 10.1038/sj.ki.5001830. 16955103 [DOI] [PubMed] [Google Scholar]
- 11.Satchell S.C., Tooke J.E. What is the mechanism of microalbuminuria in diabetes: a role for the glomerular endothelium? Diabetologia. 2008;51:714–725. doi: 10.1007/s00125-008-0961-8. 18347777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakatsue T., Koike H., Han G.D., Suzuki K., Miyauchi N., Yuan H., Salant D.J., Gejyo F., Shimizu F., Kawachi H. Nephrin and podocin dissociate at the onset of proteinuria in experimental membranous nephropathy. Kidney International. 2005;67:2239–2253. doi: 10.1111/j.1523-1755.2005.00328.x. 15882266 [DOI] [PubMed] [Google Scholar]
- 13.Wang Y., Wang D.H. Protective effect of TRPV1 against renal fibrosis via inhibition of TGF-β/Smad signaling in DOCA-salt hypertension. Molecular Medicine. 2011;17:1204–1212. doi: 10.2119/molmed.2011.00063. 21792478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ndisang J.F. Role of heme oxygenase in inflammation, insulin-signalling, diabetes and obesity. Mediators of Inflammation. 2010;2010:359732. doi: 10.1155/2010/359732. 20508722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ndisang J.F., Jadhav A., Mishra M. The heme oxygenase system suppresses perirenal visceral adiposity, abates renal inflammation and ameliorates diabetic nephropathy in Zucker diabetic fatty rats. PloS One. 2014;9:e87936. doi: 10.1371/journal.pone.0087936. 24498225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ndisang J.F., Jadhav A. Hemin therapy improves kidney function in male streptozotocin-induced diabetic rats: role of the heme oxygenase/atrial natriuretic peptide/adiponectin axis. Endocrinology. 2014;155:215–229. doi: 10.1210/en.2013-1050. 24140713 [DOI] [PubMed] [Google Scholar]
- 17.Ndisang J.F., Jadhav A. Heme arginate therapy enhanced adiponectin and atrial natriuretic peptide, but abated endothelin-1 with attenuation of kidney histopathological lesions in mineralocorticoid-induced hypertension. Journal of Pharmacology and Experimental Therapeutics. 2010;334:87–98. doi: 10.1124/jpet.109.164871. 20392817 [DOI] [PubMed] [Google Scholar]
- 18.Jadhav A., Torlakovic E., Ndisang J.F. Hemin therapy attenuates kidney injury in deoxycorticosterone acetate-salt hypertensive rats. American Journal of Physiology. Renal Physiology. 2009;296:F521–F534. doi: 10.1152/ajprenal.00510.2007. 19116243 [DOI] [PubMed] [Google Scholar]
- 19.Heneidi S., Simerman A.A., Keller E., Singh P., Li X., Dumesic D.A., Chazenbalk G. Awakened by cellular stress: isolation and characterization of a novel population of pluripotent stem cells derived from human adipose tissue. PloS One. 2013;8:e64752. doi: 10.1371/journal.pone.0064752. 23755141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Villanueva S., Carreño J.E., Salazar L., Vergara C., Strodthoff R., Fajre F., Céspedes C., Sáez P.J., Irarrázabal C., Bartolucci J., Figueroa F., Vio C.P. Human mesenchymal stem cells derived from adipose tissue reduce functional and tissue damage in a rat model of chronic renal failure. Clinical Science. 2013;125:199–210. doi: 10.1042/CS20120644. [DOI] [PubMed] [Google Scholar]
- 21.Sodhi K., Puri N., Kim D.H., Hinds T.D., Stechschulte L.A., Favero G., Rodella L., Shapiro J.I., Jude D., Abraham N.G. PPARdelta binding to heme oxygenase 1 promoter prevents angiotensin II-induced adipocyte dysfunction in Goldblatt hypertensive rats. International Journal of Obesity. 2014;38:456–465. doi: 10.1038/ijo.2013.116. 23779049 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22.Zhang J., Pippin J.W., Krofft R.D., Naito S., Liu Z.H., Shankland S.J. Podocyte repopulation by renal progenitor cells following glucocorticoids treatment in experimental FSGS. American Journal of Physiology. Renal Physiology. 2013;304:F1375–F1389. doi: 10.1152/ajprenal.00020.2013. 23486009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Watanabe N., Kato M., Suzuki N., Inoue C., Fedorova S., Hashimoto H., Maruyama S., Matsuo S., Wakamatsu Y. Kidney regeneration through nephron neogenesis in medaka. Development, Growth & Differentiation. 2009;51:135–143. doi: 10.1111/j.1440-169X.2009.01090.x. 19207184 [DOI] [PubMed] [Google Scholar]
- 24.Jadhav A., Ndisang J.F. Heme arginate suppresses cardiac lesions and hypertrophy in deoxycorticosterone acetate-salt hypertension. Experimental Biology and Medicine. 2009;234:764–778. doi: 10.3181/0810-RM-302. 19429856 [DOI] [PubMed] [Google Scholar]
- 25.Ndisang J.F., Jadhav A. Upregulating the heme oxygenase system suppresses left ventricular hypertrophy in adult spontaneously hypertensive rats for 3 months. Journal of Cardiac Failure. 2009;15:616–628. doi: 10.1016/j.cardfail.2009.02.003. 19700139 [DOI] [PubMed] [Google Scholar]
- 26.Salley T.N., Mishra M., Tiwari S., Jadhav A., Ndisang J.F. The heme oxygenase system rescues hepatic deterioration in the condition of obesity co-morbid with type-2 diabetes. PloS One. 2013;8:e79270. doi: 10.1371/journal.pone.0079270. 24260182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jadhav A., Ndisang J.F. Treatment with heme arginate alleviates adipose tissue inflammation and improves insulin sensitivity and glucose metabolism in a rat model of human primary aldosteronism. Free Radical Biology & Medicine. 2012;53:2277–2286. doi: 10.1016/j.freeradbiomed.2012.10.529. 23089228 [DOI] [PubMed] [Google Scholar]
- 28.Jadhav A., Tiwari S., Lee P., Ndisang J.F. The heme oxygenase system selectively enhances the anti-inflammatory macrophage-m2 phenotype, reduces pericardial adiposity, and ameliorated cardiac injury in diabetic cardiomyopathy in Zucker diabetic fatty rats. Journal of Pharmacology and Experimental Therapeutics. 2013;345:239–249. doi: 10.1124/jpet.112.200808. 23442249 [DOI] [PubMed] [Google Scholar]
- 29.Tiwari S., Mishra M., Jadhav A., Gerger C., Lee P., Weber L., Ndisang J.F. The risk of heart failure and cardiometabolic complications in obesity may be masked by an apparent healthy status of normal blood glucose. Oxidative Medicine and Cellular Longevity. 2013;2013:253657. doi: 10.1155/2013/253657. 24454978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ndisang J.F., Wang R. Age-related alterations in soluble guanylyl cyclase and cGMP pathway in spontaneously hypertensive rats. Journal of Hypertension. 2003;21:1117–1124. doi: 10.1097/00004872-200306000-00011. 12777948 [DOI] [PubMed] [Google Scholar]
- 31.Ndisang J.F., Lane N., Jadhav A. The heme oxygenase system abates hyperglycemia in Zucker diabetic fatty rats by potentiating insulin-sensitizing pathways. Endocrinology. 2009;150:2098–2108. doi: 10.1210/en.2008-0239. 19106228 [DOI] [PubMed] [Google Scholar]
- 32.Ndisang J.F., Lane N., Jadhav A. Crosstalk between the heme oxygenase system, aldosterone, and phospholipase C in hypertension. Journal of Hypertension. 2008;26:1188–1199. doi: 10.1097/HJH.0b013e3282fad93d. 18475157 [DOI] [PubMed] [Google Scholar]
- 33.Delanty N., Reilly M.P., Pratico D., Lawson J.A., McCarthy J.F., Wood A.E., Ohnishi S.T., Fitzgerald D.J., FitzGerald G.A. 8-epi PGF2 alpha generation during coronary reperfusion. A potential quantitative marker of oxidant stress in vivo. Circulation. 1997;95:2492–2499. doi: 10.1161/01.cir.95.11.2492. 9184579 [DOI] [PubMed] [Google Scholar]
- 34.Ndisang J.F., Jadhav A. Up-regulating the hemeoxygenase system enhances insulin sensitivity and improves glucose metabolism in insulin-resistant diabetes in Goto-Kakizaki rats. Endocrinology. 2009;150:2627–2636. doi: 10.1210/en.2008-1370. 19228889 [DOI] [PubMed] [Google Scholar]
- 35.Ndisang J.F., Lane N., Jadhav A. Upregulation of the heme oxygenase system ameliorates postprandial and fasting hyperglycemia in type 2 diabetes. American Journal of Physiology. Endocrinology and Metabolism. 2009;296:E1029–E1041. doi: 10.1152/ajpendo.90241.2008. 19208858 [DOI] [PubMed] [Google Scholar]
- 36.Finegood D.T., McArthur M.D., Kojwang D., Thomas M.J., Topp B.G., Leonard T., Buckingham R.E. Beta-cell mass dynamics in Zucker diabetic fatty rats. Rosiglitazone prevents the rise in net cell death. Diabetes. 2001;50:1021–1029. doi: 10.2337/diabetes.50.5.1021. 11334404 [DOI] [PubMed] [Google Scholar]
- 37.Kuhlmann J., Neumann-Haefelin C., Belz U., Kalisch J., Juretschke H.P., Stein M., Kleinschmidt E., Kramer W., Herling A.W. Intramyocellular lipid and insulin resistance: a longitudinal in vivo 1H-spectroscopic study in Zucker diabetic fatty rats. Diabetes. 2003;52:138–144. doi: 10.2337/diabetes.52.1.138. 12502504 [DOI] [PubMed] [Google Scholar]
- 38.Ndisang J.F., Jadhav A. Heme oxygenase system enhances insulin sensitivity and glucose metabolism in streptozotocin-induced diabetes. American Journal of Physiology. Endocrinology and Metabolism. 2009;296:E829–E841. doi: 10.1152/ajpendo.90783.2008. 19190261 [DOI] [PubMed] [Google Scholar]
- 39.Ndisang J.F., Jadhav A. The heme oxygenase system attenuates pancreatic lesions and improves insulin sensitivity and glucose metabolism in deoxycorticosterone acetate hypertension. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2010;298:R211–R223. doi: 10.1152/ajpregu.91000.2008. 19864334 [DOI] [PubMed] [Google Scholar]
- 40.Ndisang J.F., Lane N., Syed N., Jadhav A. Up-regulating the heme oxygenase system with hemin improves insulin sensitivity and glucose metabolism in adult spontaneously hypertensive rats. Endocrinology. 2010;151:549–560. doi: 10.1210/en.2009-0471. 20016031 [DOI] [PubMed] [Google Scholar]
- 41.Ndisang J.F. The heme oxygenase system selectively modulates proteins implicated in metabolism, oxidative stress and inflammation in spontaneously hypertensive rats. Current Pharmaceutical Design. 2014;20:1318–1327. doi: 10.2174/13816128113199990551. 23978103 [DOI] [PubMed] [Google Scholar]


