Abstract
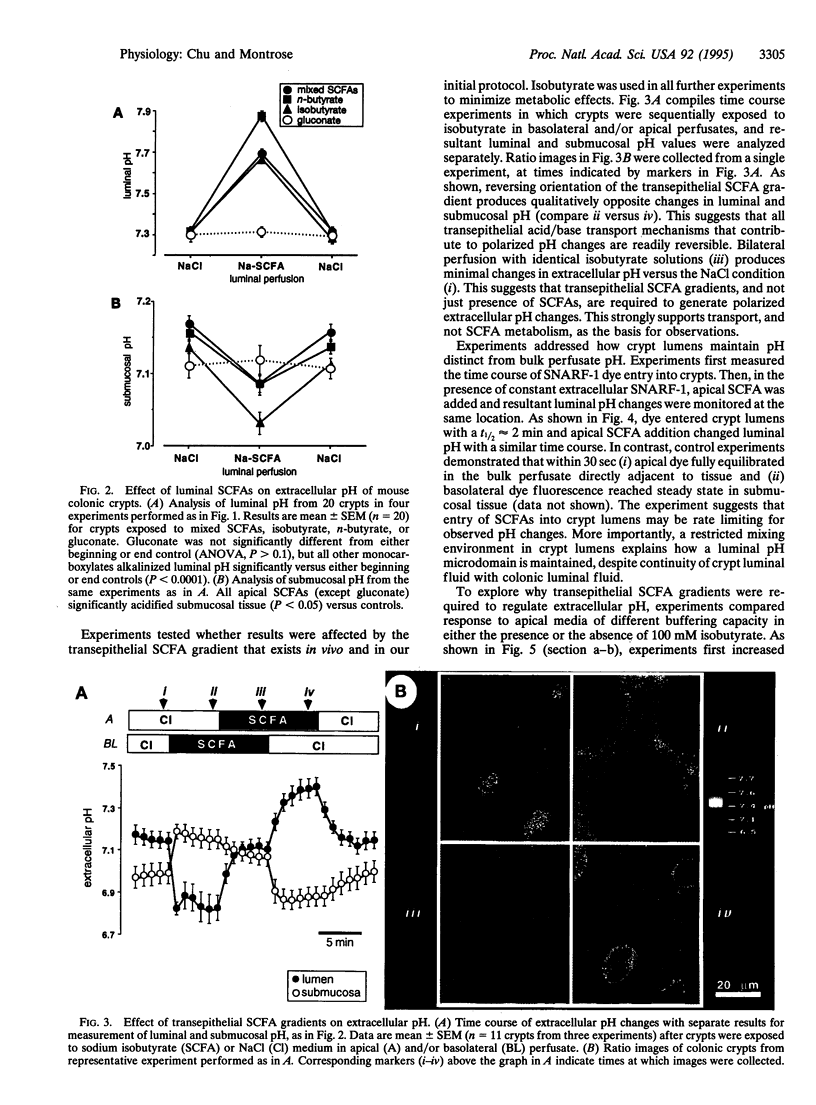
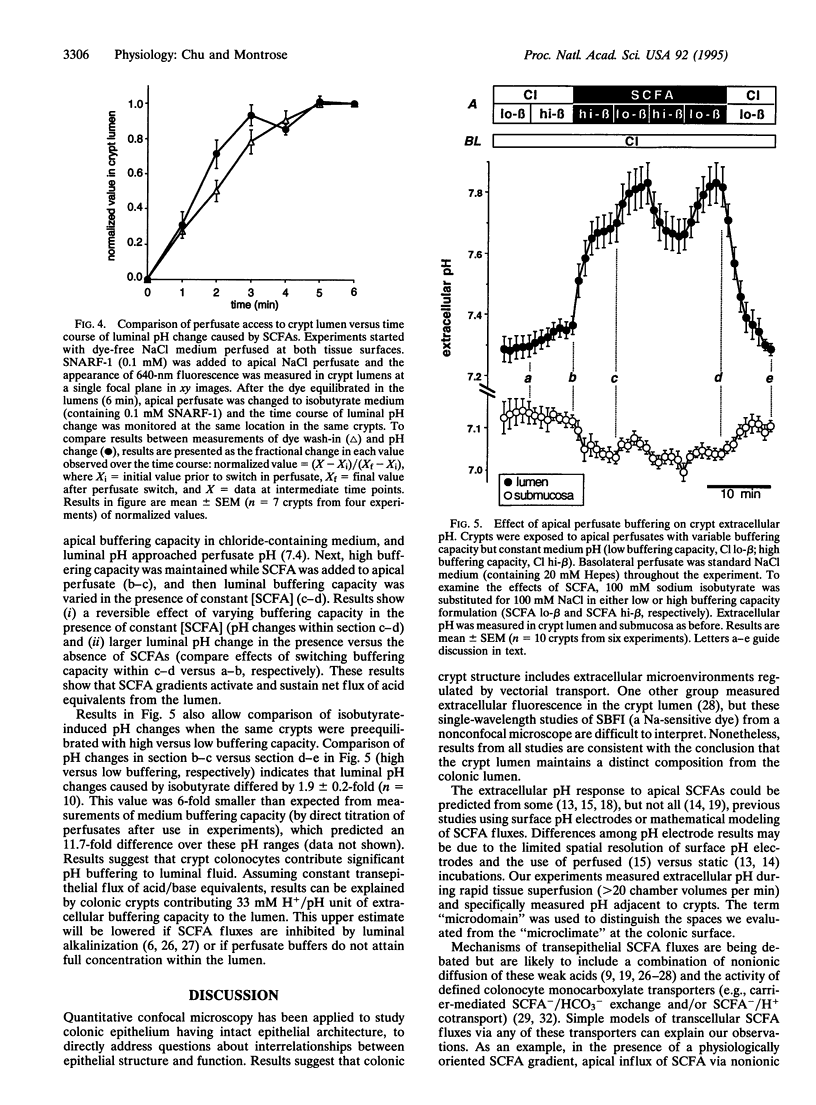
It has been suggested that transepithelial gradients of short-chain fatty acids (SCFAs; the major anions in the colonic lumen) generate pH gradients across the colonic epithelium. Quantitative confocal microscopy was used to study extracellular pH in mouse distal colon with intact epithelial architecture, by superfusing tissue with carboxy SNARF-1 (a pH-sensitive fluorescent dye). Results demonstrate extracellular pH regulation in two separate microdomains surrounding colonic crypts: the crypt lumen and the subepithelial tissue adjacent to crypt colonocytes. Apical superfusion with (i) a poorly metabolized SCFA (isobutyrate), (ii) an avidly metabolized SCFA (n-butyrate), or (iii) a physiologic mixture of acetate/propionate/n-butyrate produced similar results: alkalinization of the crypt lumen and acidification of subepithelial tissue. Effects were (i) dependent on the presence and orientation of a transepithelial SCFA gradient, (ii) not observed with gluconate substitution, and (iii) required activation of sustained vectorial acid/base transport by SCFAs. Results suggest that the crypt lumen functions as a pH microdomain due to slow mixing with bulk superfusates and that crypts contribute significant buffering capacity to the lumen. In conclusion, physiologic SCFA gradients cause polarized extracellular pH regulation because epithelial architecture and vectorial transport synergize to establish regulated microenvironments.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANNISON E. F., PENNINGTON R. J. The metabolism of short-chain fatty acids in the sheep. III Formic, eta-valeric and some branched-chain acids. Biochem J. 1954 Aug;57(4):685–692. doi: 10.1042/bj0570685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassnett S., Reinisch L., Beebe D. C. Intracellular pH measurement using single excitation-dual emission fluorescence ratios. Am J Physiol. 1990 Jan;258(1 Pt 1):C171–C178. doi: 10.1152/ajpcell.1990.258.1.C171. [DOI] [PubMed] [Google Scholar]
- Binder H. J., Mehta P. Short-chain fatty acids stimulate active sodium and chloride absorption in vitro in the rat distal colon. Gastroenterology. 1989 Apr;96(4):989–996. doi: 10.1016/0016-5085(89)91614-4. [DOI] [PubMed] [Google Scholar]
- Bookstein C., DePaoli A. M., Xie Y., Niu P., Musch M. W., Rao M. C., Chang E. B. Na+/H+ exchangers, NHE-1 and NHE-3, of rat intestine. Expression and localization. J Clin Invest. 1994 Jan;93(1):106–113. doi: 10.1172/JCI116933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bödeker D., Shen Y., Kemkowski J., Höller H. Influence of short-chain fatty acids on ammonia absorption across the rumen wall in sheep. Exp Physiol. 1992 Mar;77(2):369–376. doi: 10.1113/expphysiol.1992.sp003597. [DOI] [PubMed] [Google Scholar]
- Cummings J. H., Pomare E. W., Branch W. J., Naylor C. P., Macfarlane G. T. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. 1987 Oct;28(10):1221–1227. doi: 10.1136/gut.28.10.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSoignie R., Sellin J. H. Propionate-initiated changes in intracellular pH in rabbit colonocytes. Gastroenterology. 1994 Aug;107(2):347–356. doi: 10.1016/0016-5085(94)90158-9. [DOI] [PubMed] [Google Scholar]
- Diener M., Helmle-Kolb C., Murer H., Scharrer E. Effect of short-chain fatty acids on cell volume and intracellular pH in rat distal colon. Pflugers Arch. 1993 Aug;424(3-4):216–223. doi: 10.1007/BF00384345. [DOI] [PubMed] [Google Scholar]
- Diener M., Rummel W., Mestres P., Lindemann B. Single chloride channels in colon mucosa and isolated colonic enterocytes of the rat. J Membr Biol. 1989 Apr;108(1):21–30. doi: 10.1007/BF01870422. [DOI] [PubMed] [Google Scholar]
- Garcia C. K., Goldstein J. L., Pathak R. K., Anderson R. G., Brown M. S. Molecular characterization of a membrane transporter for lactate, pyruvate, and other monocarboxylates: implications for the Cori cycle. Cell. 1994 Mar 11;76(5):865–873. doi: 10.1016/0092-8674(94)90361-1. [DOI] [PubMed] [Google Scholar]
- Gutknecht J., Tosteson D. C. Diffusion of weak acids across lipid bilayer membranes: effects of chemical reactions in the unstirred layers. Science. 1973 Dec 21;182(4118):1258–1261. doi: 10.1126/science.182.4118.1258. [DOI] [PubMed] [Google Scholar]
- Gäbel G., Vogler S., Martens H. Short-chain fatty acids and CO2 as regulators of Na+ and Cl- absorption in isolated sheep rumen mucosa. J Comp Physiol B. 1991;161(4):419–426. doi: 10.1007/BF00260803. [DOI] [PubMed] [Google Scholar]
- Harig J. M., Soergel K. H., Barry J. A., Ramaswamy K. Transport of propionate by human ileal brush-border membrane vesicles. Am J Physiol. 1991 May;260(5 Pt 1):G776–G782. doi: 10.1152/ajpgi.1991.260.5.G776. [DOI] [PubMed] [Google Scholar]
- Harris P. J., Chatton J. Y., Tran P. H., Bungay P. M., Spring K. R. pH, morphology, and diffusion in lateral intercellular spaces of epithelial cell monolayers. Am J Physiol. 1994 Jan;266(1 Pt 1):C73–C80. doi: 10.1152/ajpcell.1994.266.1.C73. [DOI] [PubMed] [Google Scholar]
- Holtug K., McEwan G. T., Skadhauge E. Effects of propionate on the acid microclimate of hen (Gallus domesticus) colonic mucosa. Comp Biochem Physiol Comp Physiol. 1992 Dec;103(4):649–652. doi: 10.1016/0300-9629(92)90160-r. [DOI] [PubMed] [Google Scholar]
- Holtug K. Mechanisms of absorption of short chain fatty acids--coupling to intracellular pH regulation. Acta Vet Scand Suppl. 1989;86:126–133. [PubMed] [Google Scholar]
- Høverstad T., Midtvedt T., Bøhmer T. Short-chain fatty acids in intestinal content of germfree mice monocontaminated with Escherichia coli or Clostridium difficile. Scand J Gastroenterol. 1985 Apr;20(3):373–380. doi: 10.3109/00365528509091667. [DOI] [PubMed] [Google Scholar]
- Jackson M. J., Williamson A. M., Dombrowski W. A., Garner D. E. Intestinal transport of weak electrolytes: Determinants of influx at the luminal surface. J Gen Physiol. 1978 Mar;71(3):301–327. doi: 10.1085/jgp.71.3.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köckerling A., Fromm M. Origin of cAMP-dependent Cl- secretion from both crypts and surface epithelia of rat intestine. Am J Physiol. 1993 May;264(5 Pt 1):C1294–C1301. doi: 10.1152/ajpcell.1993.264.5.C1294. [DOI] [PubMed] [Google Scholar]
- Laverty G., Holtug K., Elbrønd V. S., Ridderstråle Y., Skadhauge E. Mucosal acidification and an acid microclimate in the hen colon in vitro. J Comp Physiol B. 1994;163(8):633–641. doi: 10.1007/BF00369513. [DOI] [PubMed] [Google Scholar]
- Lucas M. L. A contribution to analysis of three-compartment models for intestinal weak electrolyte absorption. Am J Physiol. 1984 Nov;247(5 Pt 1):G463–G467. doi: 10.1152/ajpgi.1984.247.5.G463. [DOI] [PubMed] [Google Scholar]
- Mascolo N., Rajendran V. M., Binder H. J. Mechanism of short-chain fatty acid uptake by apical membrane vesicles of rat distal colon. Gastroenterology. 1991 Aug;101(2):331–338. doi: 10.1016/0016-5085(91)90008-9. [DOI] [PubMed] [Google Scholar]
- Montrose M. H., Friedrich T., Murer H. Measurements of intracellular pH in single LLC-PK1 cells: recovery from an acid load via basolateral Na+/H+ exchange. J Membr Biol. 1987;97(1):63–78. doi: 10.1007/BF01869615. [DOI] [PubMed] [Google Scholar]
- Pedley K. C., Naftalin R. J. Evidence from fluorescence microscopy and comparative studies that rat, ovine and bovine colonic crypts are absorptive. J Physiol. 1993 Jan;460:525–547. doi: 10.1113/jphysiol.1993.sp019485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen K. U., Wood J. R., Schulze G., Heintze K. Stimulation of gallbladder fluid and electrolyte absorption by butyrate. J Membr Biol. 1981;62(3):183–193. doi: 10.1007/BF01998164. [DOI] [PubMed] [Google Scholar]
- Rechkemmer G., Wahl M., Kuschinsky W., von Engelhardt W. pH-microclimate at the luminal surface of the intestinal mucosa of guinea pig and rat. Pflugers Arch. 1986 Jul;407(1):33–40. doi: 10.1007/BF00580717. [DOI] [PubMed] [Google Scholar]
- Reynolds D. A., Rajendran V. M., Binder H. J. Bicarbonate-stimulated [14C]butyrate uptake in basolateral membrane vesicles of rat distal colon. Gastroenterology. 1993 Sep;105(3):725–732. doi: 10.1016/0016-5085(93)90889-k. [DOI] [PubMed] [Google Scholar]
- Roediger W. E. Utilization of nutrients by isolated epithelial cells of the rat colon. Gastroenterology. 1982 Aug;83(2):424–429. [PubMed] [Google Scholar]
- Rowe W. A., Blackmon D. L., Montrose M. H. Propionate activates multiple ion transport mechanisms in the HT29-18-C1 human colon cell line. Am J Physiol. 1993 Sep;265(3 Pt 1):G564–G571. doi: 10.1152/ajpgi.1993.265.3.G564. [DOI] [PubMed] [Google Scholar]
- Rowe W. A., Lesho M. J., Montrose M. H. Polarized Na+/H+ exchange function is pliable in response to transepithelial gradients of propionate. Proc Natl Acad Sci U S A. 1994 Jun 21;91(13):6166–6170. doi: 10.1073/pnas.91.13.6166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rönnau K., Guth D., von Engelhardt W. Absorption of dissociated and undissociated short-chain fatty acids across the colonic epithelium of guinea-pig. Q J Exp Physiol. 1989 Jul;74(4):511–519. doi: 10.1113/expphysiol.1989.sp003298. [DOI] [PubMed] [Google Scholar]
- Rübsamen K., von Engelhardt W. Absorption of Na, H ions and short chain fatty acids from the sheep colon. Pflugers Arch. 1981 Aug;391(2):141–146. doi: 10.1007/BF00657005. [DOI] [PubMed] [Google Scholar]
- Sellin J. H., DeSoignie R., Burlingame S. Segmental differences in short-chain fatty acid transport in rabbit colon: effect of pH and Na. J Membr Biol. 1993 Nov;136(2):147–158. doi: 10.1007/BF02505759. [DOI] [PubMed] [Google Scholar]
- Umesaki Y., Yajima T., Yokokura T., Mutai M. Effect of organic acid absorption on bicarbonate transport in rat colon. Pflugers Arch. 1979 Feb 14;379(1):43–47. doi: 10.1007/BF00622903. [DOI] [PubMed] [Google Scholar]
- Weigand E., Young J. W., McGilliard A. D. Volatile fatty acid metabolism by rumen mucosa from cattle fed hay or grain. J Dairy Sci. 1975 Sep;58(9):1294–1300. doi: 10.3168/jds.S0022-0302(75)84709-6. [DOI] [PubMed] [Google Scholar]





