Abstract
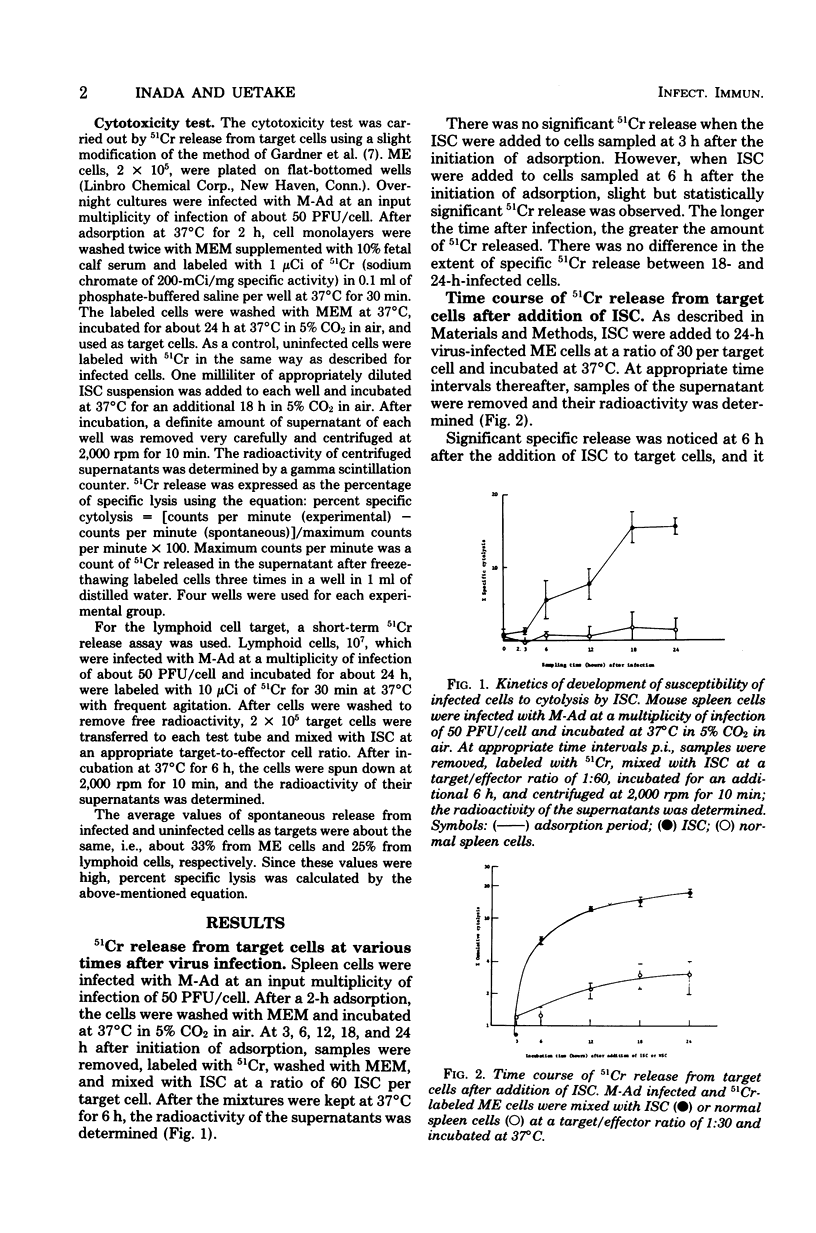
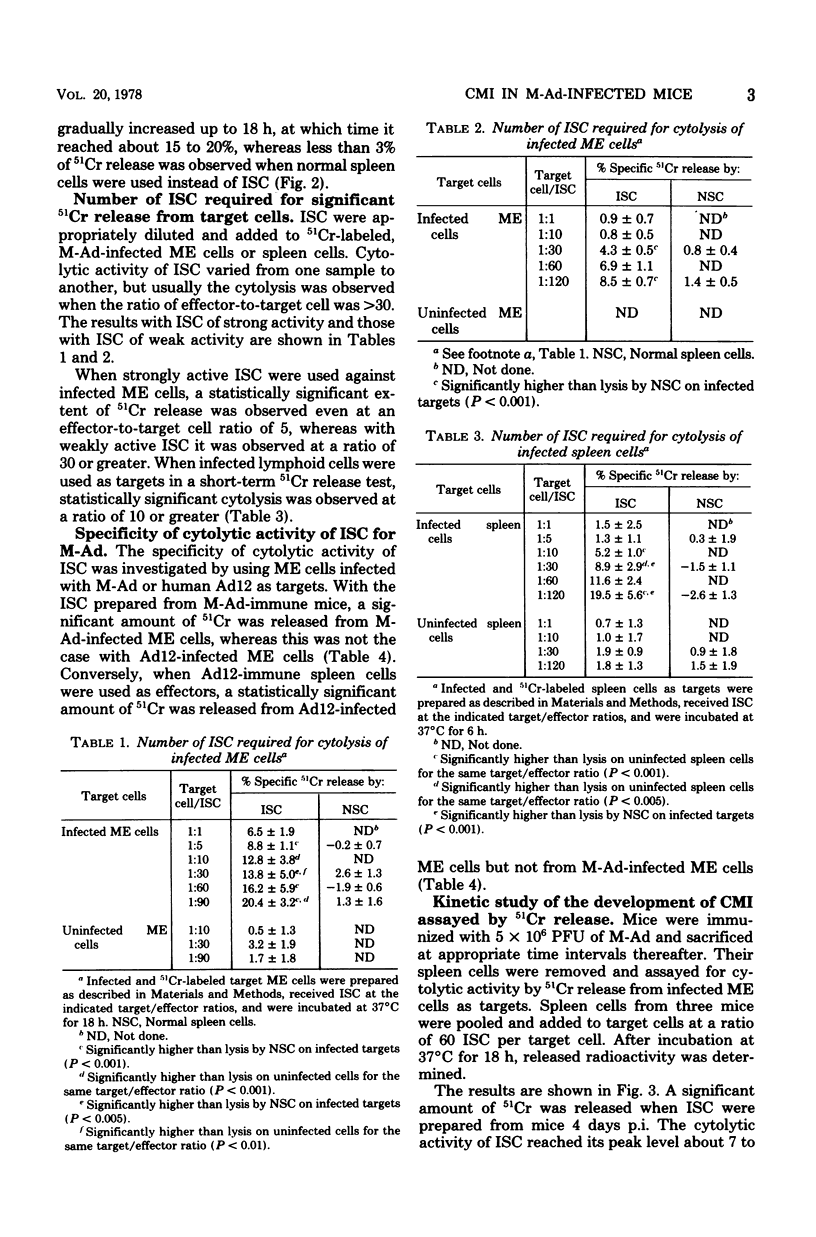
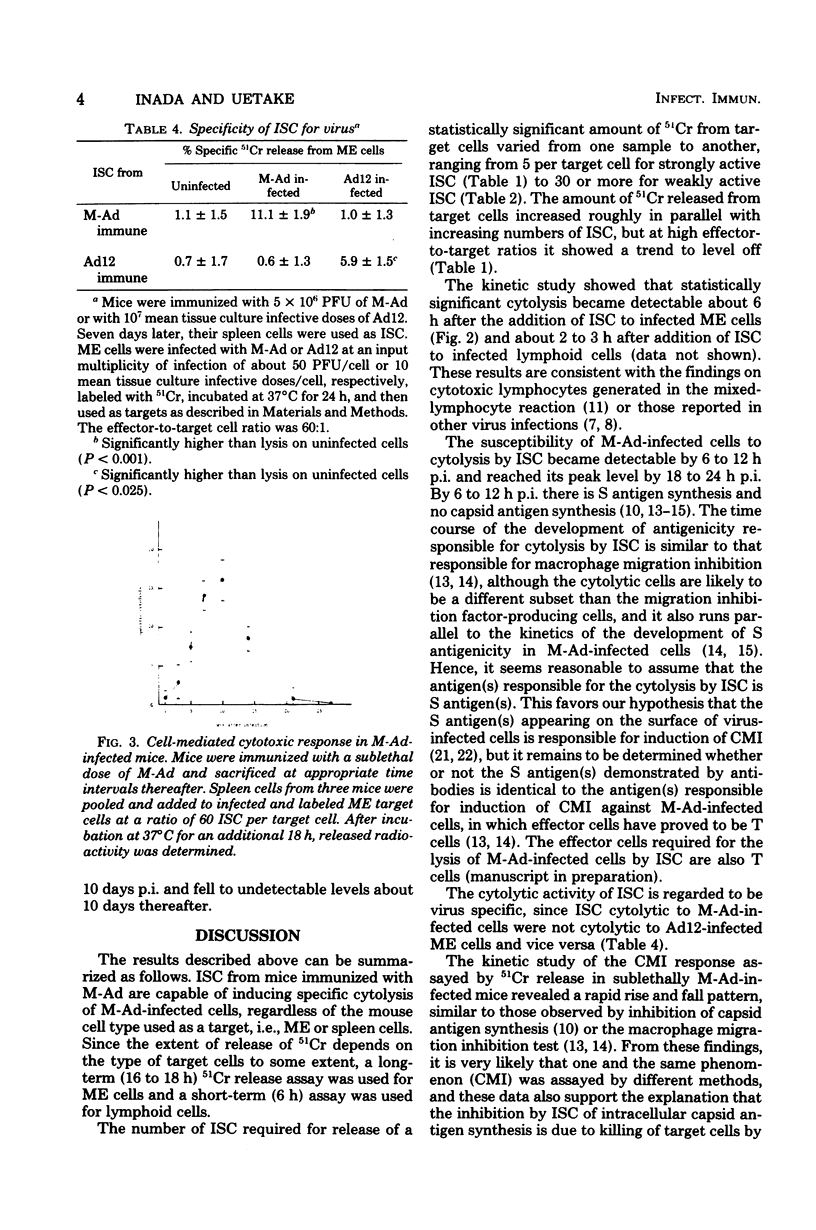
Immune spleen cells (ISC) from mice immunized with a sublethal dose of mouse adenovirus (M-Ad) were shown by the 51Cr release test to be cytotoxic to target mouse embryonic cells or lymphoid cells infected with M-Ad. The number of ISC required for release of statistically significant amounts of 51Cr from target cells varied from one sample to another, ranging from 5 to greater than or equal to 30 ISC per target cell. When 24-h-infected mouse embryonic cells were used as targets, the release of 51Cr became evident in 6 h after the addition of ISC to the cells, gradually increased with time, and then leveled off. Cytolytic activity of M-Ad ISC is specific for M-Ad, since ISC do not lyse mouse embryonic cells infected with human adenovirus type 12 and vice versa. Kinetic study of the development of cell-mediated immunity to M-Ad assayed by 51Cr release showed that cytolytic activity of ISC in infected mice became detectable 4 days postinfection, reached its peak level about 7 to 10 days postinfection, and fell to undetectable levels about 10 days thereafter. This is consistent with the data obtained by inhibition of intracellular viral antigen synthesis or by the macrophage migration inhibition test in our prevous reports.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler W. H., Rabinowitz S. G. Host defenses during primary Venezuelan equine encephalomyelitis virus infection in mice. II. In vitro methods for the measurement and qualitation of the immune response. J Immunol. 1973 May;110(5):1354–1362. [PubMed] [Google Scholar]
- Bhatnagar R. M., Zabriskie J. B., Rausen A. R. Cellular immune responses to methylcholanthrene-induced fibrosarcoma in BALB/c mice. J Exp Med. 1975 Oct 1;142(4):839–855. doi: 10.1084/jem.142.4.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanden R. V., Gardner I. D. The cell-mediated immune response to ectromelia virus infection. I. Kinetics and characteristics of the primary effector T cell response in vivo. Cell Immunol. 1976 Mar 15;22(2):271–282. doi: 10.1016/0008-8749(76)90029-0. [DOI] [PubMed] [Google Scholar]
- Brunner K. T., Mauel J., Rudolf H., Chapuis B. Studies of allograft immunity in mice. I. Induction, development and in vitro assay of cellular immunity. Immunology. 1970 Apr;18(4):501–515. [PMC free article] [PubMed] [Google Scholar]
- Cambridge G., Mackenzie J. S., Keast D. Cell-mediated immune response to influenza virus infections in mice. Infect Immun. 1976 Jan;13(1):36–43. doi: 10.1128/iai.13.1.36-43.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty P. C., Zinkernagel R. M. Specific immune lysis of paramyxovirus-infected cells by H-2-compatible thymus-derived lymphocytes. Immunology. 1976 Jul;31(1):27–32. [PMC free article] [PubMed] [Google Scholar]
- Gardner I., Bowern N. A., Blanden R. V. Cell-mediated cytotoxicity against ectromelia virus-infected target cells. I. Specificity and kinetics. Eur J Immunol. 1974 Feb;4(2):63–67. doi: 10.1002/eji.1830040202. [DOI] [PubMed] [Google Scholar]
- Gardner I., Bowern N. A., Blanden R. V. Cell-mediated cytotoxicity against ectromelia virus-infected target cells. II. Identification of effector cells and analysis of mechanisms. Eur J Immunol. 1974 Feb;4(2):68–72. doi: 10.1002/eji.1830040203. [DOI] [PubMed] [Google Scholar]
- Griffin D. E., Johnson R. T. Cellular immune response to viral infection: in vitro studies of lymphocytes from mice infected with Sindbis virus. Cell Immunol. 1973 Dec;9(3):426–434. doi: 10.1016/0008-8749(73)90057-9. [DOI] [PubMed] [Google Scholar]
- Hamada C., Uetake H. Mechanism of induction of cell-mediated immunity to virus infections: in vitro inhibition of intracellular multiplication of mouse adenovirus by immune spleen cells. Infect Immun. 1975 May;11(5):937–943. doi: 10.1128/iai.11.5.937-943.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellman A., Fowler A. K., Steinman H. G., Buzzerd P. M. Studies of the blastogenic response of murine lymphocyte. 3. Specific viral transformation. Proc Soc Exp Biol Med. 1972 Oct;141(1):106–109. doi: 10.3181/00379727-141-36726. [DOI] [PubMed] [Google Scholar]
- Häyry P., Defendi V. Mixed lymphocyte cultures produce effector cells: model in vitro for allograft rejection. Science. 1970 Apr 3;168(3927):133–135. doi: 10.1126/science.168.3927.133. [DOI] [PubMed] [Google Scholar]
- Inada T., Uetake H. Virus-Induced Specific Cell Surface Antigen(s) on Mouse Adenovirus-Infected Cells. Infect Immun. 1977 Oct;18(1):41–45. doi: 10.1128/iai.18.1.41-45.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koszinowski U., Thomssen R. Target cell-dependent T cell-mediated lysis of vaccinia virus-infected cells. Eur J Immunol. 1975 Apr;5(4):245–251. doi: 10.1002/eji.1830050405. [DOI] [PubMed] [Google Scholar]
- Rosenberg G. L., Farber P. A., Notkins A. L. In vitro stimulation of sensitized lymphocytes by herpes simplex virus and vaccinia virus. Proc Natl Acad Sci U S A. 1972 Mar;69(3):756–760. doi: 10.1073/pnas.69.3.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg G. L., Notkins A. L. Induction of cellular immunity to herpes simplex virus: relationship to the humoral immune response. J Immunol. 1974 Mar;112(3):1019–1025. [PubMed] [Google Scholar]
- Simons M. J., Fitzgerald M. G. Rubella virus and human lymphocytes in culture. Lancet. 1968 Nov 2;2(7575):937–940. doi: 10.1016/s0140-6736(68)91167-7. [DOI] [PubMed] [Google Scholar]
- Smith K. A., Chess L., Mardiney M. R., Jr The characteristics of lymphocyte tritiated thymidine incorporation in response to mumps virus. Cell Immunol. 1972 Dec;5(4):597–603. doi: 10.1016/0008-8749(72)90111-6. [DOI] [PubMed] [Google Scholar]


