Abstract
Background
Previous functional neuroimaging studies investigating the neuroanatomy of conversion disorder have yielded inconsistent results that may be attributed to small sample sizes and disparate methodologies. The objective of this study was to better define the functional neuroanatomical correlates of conversion disorder.
Methods
Ten subjects meeting clinical criteria for unilateral sensory conversion disorder underwent fMRI during which a vibrotactile stimulus was applied to anesthetic and sensate areas. A block design was used with 4 s of stimulation followed by 26 s of rest, the pattern repeated 10 times. Event-related group averages of the BOLD response were compared between conditions.
Results
All subjects were right-handed females, with a mean age of 41. Group analyses revealed 10 areas that had significantly greater activation (p < .05) when stimulation was applied to the anesthetic body part compared to the contralateral sensate mirror region. They included right paralimbic cortices (anterior cingulate cortex and insula), right temporoparietal junction (angular gyrus and inferior parietal lobule), bilateral dorsolateral prefrontal cortex (middle frontal gyri), right orbital frontal cortex (superior frontal gyrus), right caudate, right ventral-anterior thalamus and left angular gyrus. There was a trend for activation of the somatosensory cortex contralateral to the anesthetic region to be decreased relative to the sensate side.
Conclusions
Sensory conversion symptoms are associated with a pattern of abnormal cerebral activation comprising neural networks implicated in emotional processing and sensory integration. Further study of the roles and potential interplay of these networks may provide a basis for an underlying psychobiological mechanism of conversion disorder.
Keywords: Conversion disorder, Functional neuroimaging, Sensory
Highlights
-
•
fMRI was used to study subjects with unilateral sensory conversion disorder.
-
•
Sensory stimulation of anesthetic body part compared to sensate mirror region
-
•
10 brain regions, including right limbic cortices and TPJ, were abnormally active.
-
•
Implicated neural networks may provide a mechanism for conversion disorder.
1. Introduction
Conversion disorder is a controversial and challenging diagnosis that lies on the interface between neurology and psychiatry. It can manifest as a wide spectrum of symptoms, from deficit states such as paresis, blindness and anesthesia to hyperactive states such as tremor and non-epileptic seizures. There have been many recent studies trying to utilize neuroimaging in an attempt to understand the underlying functional neuroanatomical bases of conversion disorder (Carson et al., 2012). Motor conversion disorder has been the most frequently studied sub-type, however, there have been concerns that concurrent emotional and motivational responses generated during active motor tasks may complicate interpretation of results from such paradigms (Price and Friston, 2002).
Anesthesia has long been embedded at the core of conversion disorder and was described by Pierre Janet as “clear, easily appreciable and very characteristic… the typical symptom of hysteria” (Janet, 1901)”. With regard to functional neuroimaging, passive somatosensory stimulation is readily testable in scanners (Graham and Staines, 2001) and the resultant task-relevant somatosensory neural activations are well understood (Staines et al., 2002). This makes anesthesia a favorable and feasible sub-type for investigating conversion disorder. Similar to the field as a whole, previous sensory conversion neuroimaging studies have yielded inconsistent results that may be attributed to small sample sizes and disparate methodologies. This author group previously utilized fMRI to study a sample of three sensory conversion patients (Ghaffar et al., 2006). We observed increased activity in multiple brain regions outside of the primary somatosensory cortex and described them as “ancillary” areas of activation. However, due to individual participant differences and a small sample size that precluded an appropriate group analysis; we were limited in our ability to draw conclusions for such activations. Other studies investigating a range of conversion symptoms have also observed ancillary activation but the specific areas reported, which broadly include the anterior cingulate cortex (ACC), insula, frontal cortex, parietal cortex, basal ganglia and thalamus, have varied considerably between studies (Browning et al., 2011). As a result, attempts to reconcile findings and formulate common unifying mechanisms have posed challenging.
The purpose of the present study is to utilize fMRI to conduct a within-subject group analysis on 10 subjects with sensory conversion disorder in an attempt to better define the functional neuroanatomical correlates of sensory conversion symptomatology with a particular focus on the role of ancillary areas of activation. It should be noted that the raw data from the three subjects previously studied (Ghaffar et al., 2006) has been included in our ten subject group analysis.
2. Methods
2.1. Participants
Ten subjects meeting Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) criteria for unilateral conversion disorder, sensory subtype participated in this study. All subjects were right-handed and female with a mean age of 41 years (range 25–58 years). Conversion sensory loss was localized to the left side for seven subjects and the right side for three subjects. Three subjects had co-morbid depression with one of the three also diagnosed with PTSD. The other seven subjects had no known psychiatric comorbidity. Absence of neurological disease was ascertained by neurologists using clinical examination and investigations including structural brain MRI, EMG/NCS, and evoked potentials. Experimental procedures were approved by the Sunnybrook Health Sciences Centre Ethics Committee. After complete description of the study to the subjects, written informed consent was obtained from all subjects.
2.2. Data acquisition
Each subject underwent functional and anatomical imaging on a research-dedicated MRI scanner operating at 3.0 T (GE HealthCare, Milwaukee, WI) using a standard birdcage head coil. High-resolution T1-weighted anatomical images were acquired using axial 3D volume imaging (fast spoiled gradient echo pulse sequence; echo time (TE)/flip angle (θ) = 5.4 ms/35°, 124 slices 1.4 mm thick, 256 × 192 matrix, field of view (FOV) 22 cm anterior to posterior, 16 cm left to right, 6 min). Subsequent fMRI acquisitions utilized heavily T2*-weighted fast gradient echo imaging with single-shot spiral in–out readout (θ/TE/TR = 70°/30/2000 ms, 26 slices, 5 mm thick, 64 × 64 matrix, FOV 20 cm, 5 min) to obtain blood oxygen level dependent (BOLD) contrast. Total scan time was approximately 40 min.
2.3. Somatosensory stimulation
For each of the participants, somatosensory stimuli were presented during the acquisition of fMRI data. Vibrotactile stimulation was applied in a block design (4 s stimulation/26 s no-stimulation/10 repeats) in separate 5 minute scans under the following conditions: 1) unilateral stimulation of the symptomatic limb and 2) unilateral stimulation of the asymptomatic limb. Stimulation was targeted to the body part that had the greatest sensory loss reported by the patient (upper limb/hand or lower limb/foot). For the asymptomatic side, the mirror region was stimulated. Specific stimulation sites differed across participants with 6 having stimulation sites in the upper limb/hand and 4 in the lower limb/foot.
Somatosensory stimuli consisted of discrete vibrations at a constant frequency of 25 Hz delivered by a customized MRI-compatible device (Graham and Staines, 2001). Vibrotactile stimulation was controlled by converting digitally generated waveforms to an analog signal (DAQCard 6024E, National Instruments, Austin, Texas) and then amplifying the signal (Bryston 2B-LP, Peterborough, Ontario) using a custom program written in LabVIEW (National Instruments, Austin, Texas). Varying the amplitude of the driving voltage to the vibrotactile device produced proportional changes in vibration amplitude in the MR environment (Graham and Staines, 2001). Output from the computer was routed through a penetration panel to the magnet room using a filtered 9-pin D sub-connector and shielded cable to ensure that no perceptible torque was produced by currents induced by radio-frequency transmit pulses or time-varying magnetic field gradients during imaging. The proper functioning of the vibrotactile stimulation device was manually verified by two researchers at the beginning and end of each experiment to ensure accurate stimulus delivery. During each verification procedure, a researcher positioned at the scanning bed, applied the device to himself, while a second operator activated it from the control room. Further details concerning the ability of this device to activate somatosensory processing have been described in previous research (Graham and Staines, 2001; Staines et al., 2002), and occasionally, some individuals have not shown S1 activation.
2.4. Data analysis
Raw data was reconstructed offline and a time series of 154 images per slice was generated for each functional scan. The resulting time courses were analyzed using BrainVoyager QX software (Brain Innovation, Maastricht, Netherlands). The first 4 volumes of each time series were excluded to prevent artifact from transient signal changes as the brain reached a steady magnetized state. Prior to co-registration, the functional data was pre-processed by linear trend removal, temporal high pass filtering to remove non-linear low frequency drift, and 3-dimensional motion correction using trilinear interpolation to detect and correct for small head movements during the scan by spatially realigning all subsequent volumes to the fifth volume. Estimated translation and rotation measures were visually inspected and never exceeded 1 mm and 1°, respectively. The functional data sets were transformed into Talairach space (Talairach and Tournoux, 1988) by coregistering the functional data with the anatomical data for each subject. The resulting volume time courses were filtered using a 6 mm Gaussian kernel at full-width half-maximum.
In order to statistically evaluate the relative differences across the two main experimental conditions, stimulation to the symptomatic limb and stimulation to the asymptomatic limb, a multiple regression approach was employed using two predictors: 1) stimulation of the symptomatic side and 2) stimulation of the asymptomatic side, with the 26 s of no stimulation serving as a baseline. Two stimulation protocols using dummy-predictors (for those predictors not included in a given scan) were adopted and convolved with a boxcar hemodynamic response function (Boynton et al., 1996) to account for the expected shape and temporal delays of the physiological response. The resulting reference functions served as the model for the response time course functions used in the general linear model. A random effects analysis was used to investigate regions that were sensitive to the experimental manipulations. Contrast maps were created using a voxel-based approach to show relative changes for stimulation of the symptomatic versus the asymptomatic side. Activated voxels were considered significant if the threshold exceeded p < 0.001 uncorrected and formed a cluster of 14 contiguous voxels, based on a cluster size threshold estimator simulation BrainVoyager QX software (Brain Innovation, Mastricht, The Netherlands), corresponding to a corrected threshold of p < 0.05 (Forman et al., 1995). The center of gravity and t-statistics were extracted for each significant cluster. Event-related averaging was applied to each cluster to determine the BOLD response characteristics for each task condition and peak stimulus-related signal change was extracted and averaged across subjects. In addition, because of the variability of symptomatic areas of the body, activation of the primary somatosensory cortex (S1) was assessed in each individual as the volume (i.e., number of activated voxels within S1) of activation (mm3) with stimulation of the contralateral body part. S1 was defined by the central sulcus anteriorly and the post-central sulcus posteriorly.
3. Results
Group analysis of the fMRI data revealed 10 areas that had significantly greater activation (p < 0.05) when stimulation was applied to the anesthetic body part compared to the asymptomatic mirror region on the contralateral side of the body. They included right paralimbic cortices (insula and ACC), the right temporoparietal junction (TPJ) (angular gyrus and inferior parietal lobule), bilateral dorsolateral prefrontal cortex (middle frontal gyri), right orbital frontal cortex (superior frontal gyrus), right caudate, right ventral-anterior thalamus and left angular gyrus. A summary of these ancillary activations is provided in Table 1, with select regions shown on a contrast map in Fig. 1. Event-related group averages of the BOLD response time-locked to the onset of somatosensory stimulation are also shown for select ancillary regions in Fig. 2.
Table 1.
Ancillary brain regions that were significantly more active when vibrotactile stimuli were delivered to the symptomatic body part compared to the asymptomatic mirror region (n = 10; p < 0.05 corrected).
| Brain region | X | Y | Z | Volume (mm3) | T value |
|---|---|---|---|---|---|
| Right inferior parietal lobule (BA 40) | 54 | −38 | 26 | 227 | 8.57 |
| Right insula | 40 | −19 | 2 | 463 | 4.49 |
| Right middle frontal gyrus (BA 9) | 29 | 19 | 33 | 450 | 4.77 |
| Right superior frontal gyrus (BA 10) | 17 | 67 | 17 | 171 | 4.41 |
| Right caudate | 9 | 6 | 6 | 201 | 5.75 |
| Right ventral anterior thalamus | 14 | −4 | 12 | 113 | 4.28 |
| Right anterior cingulate (BA 32) | 2 | 45 | 7 | 876 | 6.37 |
| Right angular gyrus (BA39) | 39 | −57 | 32 | 327 | 6.16 |
| Left middle frontal gyrus (BA 8) | −36 | 15 | 44 | 544 | 5.56 |
| Left angular gyrus (BA 39) | −45 | −61 | 33 | 179 | 5.02 |
Legend: BA = Brodmann area.
Fig. 1.
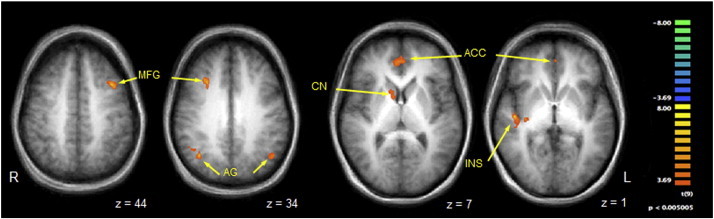
Contrast map of selected regions of BOLD activation that were significantly more active when vibrotactile stimuli were delivered to the symptomatic body part compared to the asymptomatic mirror region. Legend: anatomical images are derived from averages of T1-weighted scans from all participants and have been AC–PC aligned and transformed to Talairach space. MFG = middle frontal gyrus; AG = angular gyrus; CN = caudate nucleus; ACC = anterior cingulate cortex; INS = insula. See Table 1 for full list of activated regions. Per voxel cutoff of p < 0.005.
Fig. 2.
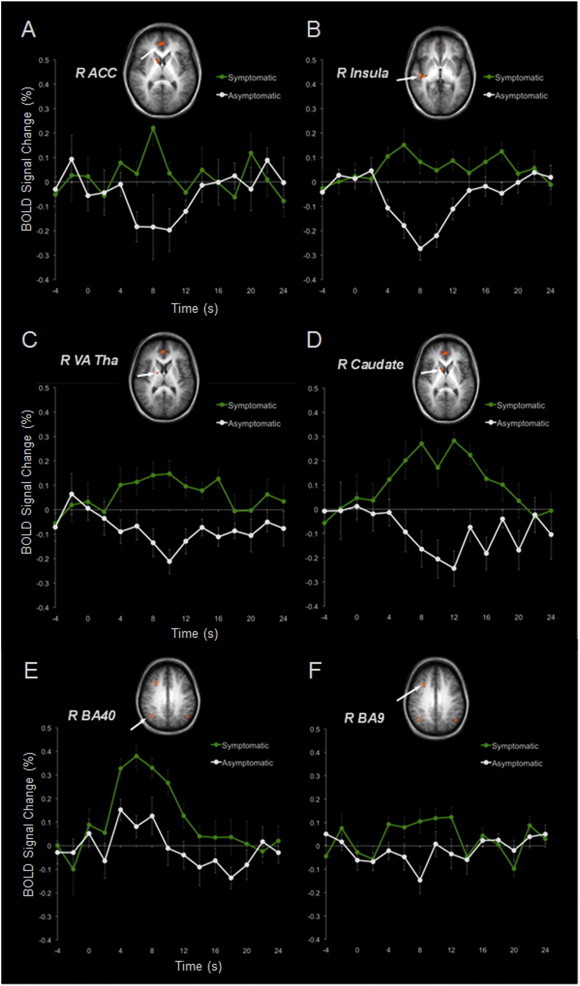
Event-related group averages of the BOLD response time-locked to the onset of somatosensory stimulation. Legend: ancillary brain regions A–F: A = right anterior cingulate cortex (R ACC), B = right insula (R insula), C = right ventral anterior nucleus of the thalamus (R Va Tha), D = right caudate (R caudate), E = right angular gyrus (R BA 40), and F = right dorsolateral prefrontal cortex (R BA 9). Stimulation was applied for a duration of 4 s. The green line represents stimulation applied to the symptomatic body part and the white line to the asymptomatic body part. Error bars represent the standard error.
The contralateral somatosensory cortex (S1) showed decreased activation when stimulation was applied to the anesthetic compared to the mirror asymptomatic region, but did not reach statistical significance (p = 0.11). This comparison is depicted in Fig. 3. It should be noted that three participants showed no S1 activation to either side of stimulation and thus were excluded from the S1 group analysis.
Fig. 3.
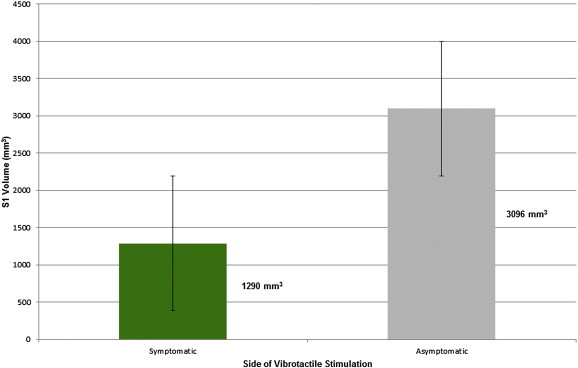
Activation of somatosensory cortex with stimulation of the symptomatic side or asymptomatic side. Legend: S1 = primary somatosensory cortex. S1 volume on the y-axis is referring to the volume of activation in cubic mm. Three participants showed no activation in S1 to either side of stimulation. Error bars represent the standard error.
4. Discussion
The main findings from this fMRI study of 10 sensory conversion disorder subjects were that paralimbic cortices and the right TPJ had significantly greater activation when somatosensory stimulation was applied to the anesthetic body part compared to the asymptomatic mirror region. Other predominately right-sided cortical and subcortical ancillary brain regions also had significantly increased activity. These findings of ancillary activation were present in the context of a trend towards decreased activation of the contralateral S1.
A few previous studies of sensory conversion disorder have reported a similar pattern of suppression of sensory cortical function coupled with concomitant increased activity in ancillary brain regions. In one of the first functional neuroimaging studies of conversion disorder, Tiihonen et al. (1995) reported a case study of a single patient with left-sided unilateral sensory deficit. During electrical sensory stimulation of the left side, SPECT revealed hypoperfusion of the right S1 and simultaneous hyperperfusion to the right frontal lobe areas. Two subsequent functional neuroimaging studies of sensory conversion disorder also follow this essential pattern. First, in a study of four patients with chronic pain and non-dermatomal sensory loss, deactivation of somatosensory cortex was observed along with increased activity in the right ACC (Mailis-Gagnon et al., 2003). Second, five patients with unexplained visual loss showed suppression of visual cortices paired with increased activity in the left inferior frontal cortex, insula, limbic structures and bilateral striatum (Werring et al., 2004). Though specific ancillary areas vary between studies, they have generally been proposed by the authors to be regions associated with emotional processing.
These studies are contrasted with a SPECT study of seven patients with unilateral sensorimotor loss that found hypoactivation of the contralateral basal ganglia and thalamus (Vuilleumier et al., 2001). However, it is difficult to compare findings from other studies with this one as subjects had motor as well as sensory loss of the affected body regions and simultaneous bilateral vibrational stimuli were applied during scanning. Furthermore, their method of probing brain activation focused on utilizing vibration as a means to passively examine motor control through the proprioceptive input of the stimuli. With regard to neuroimaging studies investigating isolated motor conversion weakness without sensory symptoms, some have followed the previously described pattern of functional cortical inhibition (but with respect to motor areas) and concomitant increases in ancillary brain regions (Kanaan, 2007; Burgmer et al., 2006; Marshall et al., 1997; Stone et al., 2007), while others have not (Spence et al., 2000; Van Beilen et al., 2011; Cojan et al., 2009).
Increased activity of the right TPJ is a novel finding amongst the limited literature devoted to functional neuroimaging of sensory conversion disorder. This region is thought to be implicated in multiple high-level cognitive functions such as self-agency (Farrer et al., 2003; Desmurget et al., 2009) and theory of mind (Scholz et al., 2009) but is most classically associated with sensory integration and attention (Downar et al., 2000; Mitchell, 2008; Tsakiris et al., 2008). Two neuroimaging studies of motor conversion symptomatology have observed hypoactivation in right TPJ areas. The first, a study of conversion paresis, proposed that this may reflect impaired interaction of bodily schema and environmental cues, resulting in an inability to initiate movement (Van Beilen et al., 2011). The second, a study of psychogenic tremor attributed their findings to a lack of self-agency related to absence of feed-forward sensory prediction signaling (Voon et al., 2010). Finally, one recent study by Aybek et al. (2014) asked conversion paresis patients to recall stressful life events during fMRI scanning and found increased right TPJ activity.
The literature of functional neuroimaging of conversion disorder has been dominated by small studies with marked heterogeneity regarding patient characteristics, task paradigms, controls/comparators and results. Our study sample size of 10 subjects represents the largest functional neuroimaging study of sensory conversion disorder to date. We believe that our findings have many implications towards understanding underlying psychobiological mechanisms of conversion symptomatology. The increased activation in paralimbic cortices taken together with the trend towards decreased activation of S1, supports a theory of suppression of the somatosensory system by emotion-based processing. This general concept whereby over-active emotional centers may inhibit sensorimotor processes was initially suggested by Pavlov (1941), in work that heavily drew on the ideas of Freud and Charcot. Subsequent functional neuroimaging case reports and smaller case series offered congruent findings (Tiihonen et al., 1995; Mailis-Gagnon et al., 2003; Werring et al., 2004; Kanaan, 2007; Burgmer et al., 2006; Marshall et al., 1997) and our results provide further strength for this fundamental model.
Explaining the increased activation of the right TPJ seen in our study is challenging as many of the previously described functions for this area, such as theory of mind, agency or sensory integration could be relevantly impaired in conversion disorder subjects. Interestingly, out-of-body experiences (OBEs), complex phenomena of failed sensory integration have been associated with hyperactivity of the right TPJ (Blanke et al., 2004). This specific localization has been supported by multiple lines of evidence, including neuroimaging studies, focal lesion case reports and electrical stimulation studies (De Ridder et al., 2007; Blanke and Arzy, 2005). OBEs are subjective episodes in which the self is perceived outside of the body combined with the impression of visualizing one's own body from a distant or elevated perspective (Blanke and Mohr, 2005). Blanke and Arzy (2005) have proposed that multisensory disintegration may lead to disruption of numerous aspects of self-processing and consequent illusory perceptions and agency. A role for processes similar to OBEs to be implicated in the neurobiology of conversion disorder was considered by Voon et al. (2010). However, this hypothesis fell out of favor as the authors noted that OBEs are specifically associated with hyperactivity of the right TPJ and their study found hypoactivity. We suggest that an altered/dissociated cognitive state akin to OBEs could indeed possibly be occurring in conversion patients. This may take the form of emotionally driven failed sensory integration that could either be operative in generation of symptoms or a consequence of an already established symptom process. Furthermore, the shared finding with Aybek et al. (2014) of right TPJ hyperactivity in the context of very different tasks suggests that this may be a common process intrinsic to conversion disorder subjects rather than a task-specific phenomenon.
The additional, predominately right-lateralized, ancillary regions of brain activation may be viewed either as part of neural networks involved in the postulated emotional suppression of sensory function or else as aberrant signs of sensory integration. The dorsolateral prefrontal cortex, for example, has previously been shown to be functionally coupled to S1 suppression in a study that asked motor conversion disorder subjects to imagine movements of their paralyzed hand (De Lange et al., 2010). While network-based theories have gained favor in the recent conversion disorder literature (Carson et al., 2012), the connections between the areas of frontal and temporoparietal multimodal cortex implicated in our study are complex and poorly understood. A recently proposed neurobiological model of ‘functional unawareness’ has attempted to link circuit disturbances in unilateral somatosensory conversion disorder. This model focuses on the overlap between pathways implicated in somatosensory neglect and dysfunction of attentional centers seen in functional neuroimaging studies of conversion disorder. Our findings of altered activity in regions including right-greater-than-left parietal cortex, ACC, striatum and thalamus align with/support this model (Perez et al., 2012). It is also possible that activation seen in these ancillary areas could be entirely independent of the proposed conversion processes; either activating in isolation or as part of other networks. Most notably, we cannot rule out the possibility that some of these areas may represent components of volitional cortico-striato-thalamic motor loops (Graybiel et al., 1994). What makes such an explanation unlikely in our study, however, is the passive sensory stimulation paradigm design.
Our study is not without limitations. The possibility of type 2 error cannot be excluded in relation to our failure to find statistically significant between group differences in S1 activation. Also, our study had an imbalanced lateralization of participants' symptoms: 7 left-sided and 3 right-sided. However, there were no significant clusters when the asymptomatic side was stimulated relative to the symptomatic side and thus we do not think our results are an artifact of lateralization. Finally, we cannot completely dismiss the possible influence of PTSD and depression, present in three of our subjects, on our imaging findings. Notwithstanding these points, our data add to a small but growing literature demonstrating that conversion disorder reflects the presence of dysfunctional brain networks. Such an explanation, rather than debunking long held psychoanalytic theory, complements Freudian ideas by demonstrating the association between abnormal sensory findings that defy conventional neurological explanation on the one hand, and extraneous limbic system activation as a marker of unconscious emotional dysregulation, on the other. Further research focused on understanding the roles of the implicated brain regions and their possible interplay is needed. Here, connectivity studies utilizing techniques such as MRI diffusion tensor imaging may prove informative. In addition, longitudinal studies that reassess functional brain changes over time, with or without symptomatic recovery, are needed to better characterize and appreciate postulated mechanisms underlying conversion disorder.
Conflicts of interest
Dr. Matthew J. Burke reports no disclosures. Dr. Omar Ghaffar reports no disclosures. Dr. W. Richard Staines reports no disclosures. Dr. Jonathan Downar reports no disclosures. Dr. Anthony Feinstein has received honoraria from Merck Serono, Bayer Healthcare Pharmaceuticals and Teva Neuroscience Canada.
Acknowledgements
An abstract for this manuscript was presented at the American Academy of Neurology Annual Meeting; Philadelphia, PA; April 26–May 2, 2014.
Contributor Information
Matthew J. Burke, Email: matt.burke@mail.utoronto.ca.
Omar Ghaffar, Email: omar.ghaffar@utoronto.ca.
W. Richard Staines, Email: rstaines@uwaterloo.ca.
Jonathan Downar, Email: Jonathan.Downar@uhn.ca.
Anthony Feinstein, Email: ant.feinstein@utoronto.ca.
References
- Aybek S., Nicholson T.R., Zelaya F., O'Daly O.G., Craig T.J., David A.S., Kanaan R.A. Neural correlates of recall of life events in conversion disorder. JAMA Psychiatry. 2014;71:52–60. doi: 10.1001/jamapsychiatry.2013.2842. 24258270 [DOI] [PubMed] [Google Scholar]
- Blanke O., Arzy S. The out-of-body experience: disturbed self-processing at the temporo-parietal junction. Neuroscientist: A Review Journal Bringing Neurobiology, Neurology and Psychiatry. 2005;11:16–24. doi: 10.1177/1073858404270885. 15632275 [DOI] [PubMed] [Google Scholar]
- Blanke O., Landis T., Spinelli L., Seeck M. Out-of-body experience and autoscopy of neurological origin. Brain: A Journal of Neurology. 2004;127:243–258. doi: 10.1093/brain/awh040. 14662516 [DOI] [PubMed] [Google Scholar]
- Blanke O., Mohr C. Out-of-body experience, heautoscopy, and autoscopic hallucination of neurological origin: implications for neurocognitive mechanisms of corporeal awareness and self-consciousness. Brain Research. Brain Research Reviews. 2005;50:184–199. doi: 10.1016/j.brainresrev.2005.05.008. 16019077 [DOI] [PubMed] [Google Scholar]
- Boynton G.M., Engel S.A., Glover G.H., Heeger D.J. Linear systems analysis of functional magnetic resonance imaging in human V1. Journal of Neuroscience: the Official Journal of the Society for Neuroscience. 1996;16:4207–4221. doi: 10.1523/JNEUROSCI.16-13-04207.1996. 8753882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning M., Fletcher P., Sharpe M. Can neuroimaging help us to understand and classify somatoform disorders? A systematic and critical review. Psychosomatic Medicine. 2011;73:173–184. doi: 10.1097/PSY.0b013e31820824f6. 21217095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgmer M., Konrad C., Jansen A., Kugel H., Sommer J., Heindel W. Abnormal brain activation during movement observation in patients with conversion paralysis. Neuroimage. 2006;29:1336–1343. doi: 10.1016/j.neuroimage.2005.08.033. 16213162 [DOI] [PubMed] [Google Scholar]
- Carson A.J., Brown R., David A.S., Duncan R., Edwards M.J., Goldstein L.H. Functional (conversion) neurological symptoms: research since the millennium. Journal of Neurology, Neurosurgery, and Psychiatry. 2012;83:842–850. doi: 10.1136/jnnp-2011-301860. 22661497 [DOI] [PubMed] [Google Scholar]
- Cojan Y., Waber L., Carruzzo A., Vuilleumier P. Motor inhibition in hysterical conversion paralysis. Neuroimage. 2009;47:1026–1037. doi: 10.1016/j.neuroimage.2009.05.023. 19450695 [DOI] [PubMed] [Google Scholar]
- De Lange F.P., Toni I., Roelofs K. Altered connectivity between prefrontal and sensorimotor cortex in conversion paralysis. Neuropsychologia. 2010;48:1782–1788. doi: 10.1016/j.neuropsychologia.2010.02.029. 20206641 [DOI] [PubMed] [Google Scholar]
- De Ridder D., Van Laere K., Dupont P., Menovsky T., Van de Heyning P. Visualizing out-of-body experience in the brain. New England Journal of Medicine. 2007;357:1829–1833. doi: 10.1056/NEJMoa070010. 17978291 [DOI] [PubMed] [Google Scholar]
- Desmurget M., Reilly K.T., Richard N., Szathmari A., Mottolese C., Sirigu A. Movement intention after parietal cortex stimulation in humans. Science (New York, N.Y.) 2009;324:811–813. doi: 10.1126/science.1169896. 19423830 [DOI] [PubMed] [Google Scholar]
- Downar J., Crawley A.P., Mikulis D.J., Davis K.D. A multimodal cortical network for the detection of changes in the sensory environment. Nature Neuroscience. 2000;3:277–283. doi: 10.1038/72991. 10700261 [DOI] [PubMed] [Google Scholar]
- Farrer C., Franck N., Georgieff N., Frith C.D., Decety J., Jeannerod M. Modulating the experience of agency: a positron emission tomography study. Neuroimage. 2003;18:324–333. doi: 10.1016/s1053-8119(02)00041-1. 12595186 [DOI] [PubMed] [Google Scholar]
- Forman S.D., Cohen J.D., Fitzgerald M., Eddy W.F., Mintun M.A., Noll D.C. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magnetic Resonance in Medicine: Official Journal of the Society of Magnetic Resonance in Medicine/Society of Magnetic Resonance in Medicine. 1995;33:636–647. doi: 10.1002/mrm.1910330508. 7596267 [DOI] [PubMed] [Google Scholar]
- Ghaffar O., Staines W.R., Feinstein A. Unexplained neurologic symptoms: an fMRI study of sensory conversion disorder. Neurology. 2006;67:2036–2038. doi: 10.1212/01.wnl.0000247275.68402.fc. 17159115 [DOI] [PubMed] [Google Scholar]
- Graham S.J., Staines W.R., Nelson A., Plewes D.B., McIlroy W.E. New devices to deliver somatosensory stimuli during functional MRI. Magnetic Resonance in Medicine: Official Journal of the Society of Magnetic Resonance in Medicine/Society of Magnetic Resonance in Medicine. 2001;46:436–442. doi: 10.1002/mrm.1211. 11550233 [DOI] [PubMed] [Google Scholar]
- Graybiel A.M., Aosaki T., Flaherty A.W., Kimura M. The basal ganglia and adaptive motor control. Science (New York, N.Y.) 1994;265:1826–1831. doi: 10.1126/science.8091209. 8091209 [DOI] [PubMed] [Google Scholar]
- Janet P. The Mental State of Hystericals. G. P. Putnam's Sons; New York: 1901. [Google Scholar]
- Kanaan R.A.A., Craig T.K.J., Wessely S.C., David A.S. Imaging repressed memories in motor conversion disorder. Psychosomatic Medicine. 2007;69:202–205. doi: 10.1097/PSY.0b013e31802e4297. 17327215 [DOI] [PubMed] [Google Scholar]
- Mailis-Gagnon A., Giannoylis I., Downar J., Kwan C.L., Mikulis D.J., Crawley A.P. Altered central somatosensory processing in chronic pain patients with “hysterical” anesthesia. Neurology. 2003;60:1501–1507. doi: 10.1212/wnl.60.9.1501. 12743239 [DOI] [PubMed] [Google Scholar]
- Marshall J.C., Halligan P.W., Fink G.R., Wade D.T., Frackowiak R.S. The functional anatomy of a hysterical paralysis. Cognition. 1997;64:B1–B8. doi: 10.1016/s0010-0277(97)00020-6. 9342933 [DOI] [PubMed] [Google Scholar]
- Mitchell J.P. Activity in right temporo-parietal junction is not selective for theory-of-mind. Cerebral Cortex (New York, N.Y.: 1991) 2008;18:262–271. doi: 10.1093/cercor/bhm051. 17551089 [DOI] [PubMed] [Google Scholar]
- Pavlov I. Conditioned Reflexes and Psychiatry. International Publishers; New York: 1941. [Google Scholar]
- Perez D.L., Barsky A.J., Daffner K., Silbersweig D.A. Motor and somatosensory conversion disorder: a functional unawareness syndrome? Journal of Neuropsychiatry and Clinical Neurosciences. 2012;24:141–151. doi: 10.1176/appi.neuropsych.11050110. 22772662 [DOI] [PubMed] [Google Scholar]
- Price C.J., Friston K.J. Functional imaging studies of neuropsychological patients: applications and limitations. Neurocase. 2002;8:345–354. doi: 10.1076/neur.8.4.345.16186. 12499409 [DOI] [PubMed] [Google Scholar]
- Scholz J., Triantafyllou C., Whitfield-Gabrieli S., Brown E.N., Saxe R. Distinct regions of right temporo-parietal junction are selective for theory of mind and exogenous attention. PloS One. 2009;4:e4869. doi: 10.1371/journal.pone.0004869. 19290043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spence S.A., Crimlisk H.L., Cope H., Ron M.A., Grasby P.M. Discrete neurophysiological correlates in prefrontal cortex during hysterical and feigned disorder of movement. Lancet. 2000;355:1243–1244. doi: 10.1016/S0140-6736(00)02096-1. 10770312 [DOI] [PubMed] [Google Scholar]
- Staines W.R., Graham S.J., Black S.E., McIlroy W.E. Task-relevant modulation of contralateral and ipsilateral primary somatosensory cortex and the role of a prefrontal-cortical sensory gating system. Neuroimage. 2002;15:190–199. doi: 10.1006/nimg.2001.0953. 11771988 [DOI] [PubMed] [Google Scholar]
- Stone J., Zeman A., Simonotto E., Meyer M., Azuma R., Flett S., Sharpe M. FMRI in patients with motor conversion symptoms and controls with simulated weakness. Psychosomatic Medicine. 2007;69:961–969. doi: 10.1097/PSY.0b013e31815b6c14. 17991812 [DOI] [PubMed] [Google Scholar]
- Talairach J., Tournoux P. Co-planar Stereotaxis Atlas of the Human Brain. Thieme; New York: 1988. [Google Scholar]
- Tiihonen J., Kuikka J., Viinamäki H., Lehtonen J., Partanen J. Altered cerebral blood flow during hysterical paresthesia. Biological Psychiatry. 1995;37:134–135. doi: 10.1016/0006-3223(94)00230-Z. 7536480 [DOI] [PubMed] [Google Scholar]
- Tsakiris M., Costantini M., Haggard P. The role of the right temporo-parietal junction in maintaining a coherent sense of one's body. Neuropsychologia. 2008;46:3014–3018. doi: 10.1016/j.neuropsychologia.2008.06.004. 18601939 [DOI] [PubMed] [Google Scholar]
- Van Beilen M., de Jong B.M., Gieteling E.W., Renken R., Leenders K.L. Abnormal parietal function in conversion paresis. PloS One. 2011;6:e25918. doi: 10.1371/journal.pone.0025918. 22039428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voon V., Gallea C., Hattori N., Bruno M., Ekanayake V., Hallett M. The involuntary nature of conversion disorder. Neurology. 2010;74:223–228. doi: 10.1212/WNL.0b013e3181ca00e9. 20083798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuilleumier P., Chicherio C., Assal F., Schwartz S., Slosman D., Landis T. Functional neuroanatomical correlates of hysterical sensorimotor loss. Brain: A Journal of Neurology. 2001;124:1077–1090. doi: 10.1093/brain/124.6.1077. 11353724 [DOI] [PubMed] [Google Scholar]
- Werring D.J., Weston L., Bullmore E.T., Plant G.T., Ron M.A. Functional magnetic resonance imaging of the cerebral response to visual stimulation in medically unexplained visual loss. Psychological Medicine. 2004;34:583–589. doi: 10.1017/S0033291703008985. 15099413 [DOI] [PubMed] [Google Scholar]


