Abstract
Background
A significant percentage of estrogen receptor (ER)–positive breast cancers are resistant to tamoxifen therapy. Seven in Absentia Homolog 2 (SIAH2), an E3 ubiquitin protein ligase, has been shown to be associated with resistance to antiestrogens. We sought to assess its role in the resistance of a breast cancer cell line, MCF-7, to the ER antagonist, tamoxifen.
Materials and methods
A bioinformatic approach was used for the analysis of SIAH2 expression in breast cancer. MCF-7 and MDA-MB-231, which are ER-positive and -negative breast cancer cell lines, respectively, were used for in vitro studies. SIAH2 and ER-α were selectively knocked down in these cell lines with small-interfering RNAs. Knockdowns were confirmed with Western blot analysis and quantitative real-time polymerase chain reaction. Cells with SIAH2 knockdown were treated with tamoxifen and compared with controls.
Results
Knockdown of SIAH2 followed by treatment with tamoxifen resulted in a significant decrease in the sensitivity of treated ER-positive cells. Of note, knockdown of SIAH2 resulted in downregulation of ER-α, whereas knockdown of ER-α had minimal effect on SIAH2. Consistent with this result, the bioinformatic analysis of clinical data revealed that SIAH2 expression is significantly correlated with ER positivity in human breast cancers, and low SIAH2 expression is associated with a poorer response to tamoxifen.
Conclusions
SIAH2 appears to be an important modulator of tamoxifen sensitivity in ER-positive MCF-7 cells, mediated, at least in part, through regulation of ER-α expression. Low expression of SIAH2 may be one of the mechanisms that contribute to tamoxifen resistance in human breast cancer.
Keywords: Breast cancer, Tamoxifen, Resistance, SIAH2, Estrogen receptor
1. Introduction
Breast cancer is the most common malignancy in females worldwide, excluding skin cancers, and the second leading cause of cancer death among women after lung cancer [1]. One of the most important biologic prognostic factors is estrogen receptor (ER) status, with high ER expression being associated with better outcome [2]. The proportion of ER-positive patients is approximately 70% and appears to increase with age from 51.4% in younger patients (<35 y) to 81.6% in the oldest age group [3]. Several studies have seen increasing trends of estrogen receptor (ER)–positive breast cancers and decreasing ER-negative breast cancers [4,5]. Treatment of ER-positive breast cancer with endocrine therapy has significantly altered the long-term survival of these patients; however, resistance to targeted therapy has become a significant clinical problem [6]. Drugs targeting the estrogen receptor or estrogen-activated pathways have become critical in the treatment of breast cancer. Treatment with selective estrogen receptor modulators like tamoxifen and raloxifene have become the mainstay of this approach, with the optimal duration of adjuvant endocrine therapy currently at 5 y. However, this therapy is limited by the development of resistance; many groups are trying to understand the mechanisms behind this [7–11].
Seven In Absentia Homolog 2 (SIAH2) protein is an E3 ubiquitin ligase involved in ubiquitination and proteasome-mediated degradation of other proteins. For example, SIAH2 plays a critical role in controlling the abundance of hypoxia-inducible factor-1α through the prolyl hydroxylases [12]. SIAH2 is also involved in many cancers such as leukemia and prostate cancer [13–15]. Two recent genome-wide association studies identified a genetic variant in the SIAH2 locus associated with ER-positive breast cancer [16,17], suggesting that the expression of SIAH2 might be affected by this genetic variant and consequently correlated with ER-related tumor progression or hormone therapy response. In addition, Jansen et al. [18] noted that SIAH2 significantly predicted first-line tamoxifen treatment failure in breast cancer. In particular, they noted that ICI 164,384, a selective ER degrader, appears to downregulate SIAH2. However, the role of SIAH2 in tamoxifen response/resistance has not been studied. Here we examined the role of SIAH2 in endocrine therapy resistance in the breast cancer cell line, MCF-7. Our findings suggests that SIAH2 is an important mediator of tamoxifen sensitivity through ER regulation and that assessing the level of expression of SIAH2 in ER-positive breast cancers may help direct appropriate use of hormonal therapy.
2. Materials and methods
2.1. Cell culture, media, and small-interfering RNA knockdown
MCF-7 and MDA-MB-231 cells were obtained from the American Type Culture Collection in Manassas, VA. Both cell lines were cultured in Dulbecco’s modified Eagle medium with 10% calf serum, 1% antibiotics (penicillin-streptomycin), and 1% glutamine. Small-interfering RNA (siRNA) knockdown was performed with the following oligos obtained from Thermo Scientific Dharmacon in Waltham, MA: Control siRNA, siSIAH2#1, siSIAH#2, siESR1#1, and siESR1#2. The siRNA oligo sequences are the following: ON-TARGET plus Control siRNA (Cat#D-001810-02-05), siSIAH2#1 (GCUAA UAAACCCUGCAGCAdTdT), siSIAH2#2 (CGCCAGAAGUUGA GCUGCUdTdT), siESR1#1 (GAGAAGUAUUCAAGGACAUdTdT), and siESR1#2 (AAUGAUGAAAGGUGGGAUAdTdT). siRNA control (CTTACGCTGAGTACTTCGA).
The siRNA transfection was performed using RNAiMAX reagent (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol. Briefly, siRNA oligo was added to 500 µL of OptiMEM in six-well plates, followed by the addition of 6 µL of RNAiMAX. The siRNA oligo precipitates were incubated for 10 min at room temperature. Then, 2 × 105 cells resuspended in 2 mL of antibiotic-free Dulbecco’s modified Eagle medium media were added, with the resultant concentration of siRNA being 30 nM. Three days later, cells were harvested for Western blotting or subject to RNA extraction for quantitative real-time polymerase chain reaction (qRT-PCR).
2.2. Antibodies and chemical reagents
Anti-ER-α (sc-543; Santa Cruz Biotechnology, Santa Cruz, CA), anti-SIAH2 (SAB2102142; Sigma-Aldrich, St. Louis, MO), and anti-β-actin (Sigma-Aldrich) antibodies were purchased from the respective companies. Tamoxifen and 17-β-estradiol were purchased from Sigma.
2.3. Western blotting
Transfected cells were lysed in lysis buffer (Cell Signaling Technology, Danvers, MA), with protein quantification being performed using the Pierce (Rockford, IL) Protein BCA assay system. Lysates were then subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis 4%–12% gels (Invitrogen) and transferred onto Immuno-Blot PVDF membranes (Bio-Rad, Hercules, CA). The membranes were then probed with primary antibodies as noted above followed by a secondary antibody conjugated with horseradish peroxidase and detected by the ECL system (Thermo Scientific, Waltham, MA).
2.4. Data mining
The Oncomine program (www.oncomine.com) was used for analysis of SIAH2 expression. SIAH2 expression data from the following data sets were used for analysis including Curtis breast data set (EGAS00000000083 from the European Genome-Phenome Archive) [19], The Cancer Genome Atlas breast data set (Data Link: http://tcga-data.nci.nih.gov/tcga/) and Zhao breast data set (GSE3971) [20]. The differential expression between cancer versus normal tissue or ER-positive versus ER-negative patients was compared by a Student t-test.
Kaplan-Meier curves were generated using the Internet program, http://kmplot.com/analysis [21]. Only ER-positive breast cancer patients with systemic treatment with tamoxifen were used for plotting the Kaplan-Meier curves, regardless of status of progesterone receptor, lymph node, or grade. For relapse-free survival analysis, the expression of SIAH2 probe (Affymetrix ID:209339_at, with values ranging from 52 to 10,934) was split at 786 as a cutoff value to define low SIAH2 expression patients (the lower quartile [25%] with expression <786) and high SIAH2 expression patients (higher three quartiles [75%] with expression values >786). For overall survival analysis, the same parameters were used, except the cutoff value was set at 638 (range from 58 to 5899).
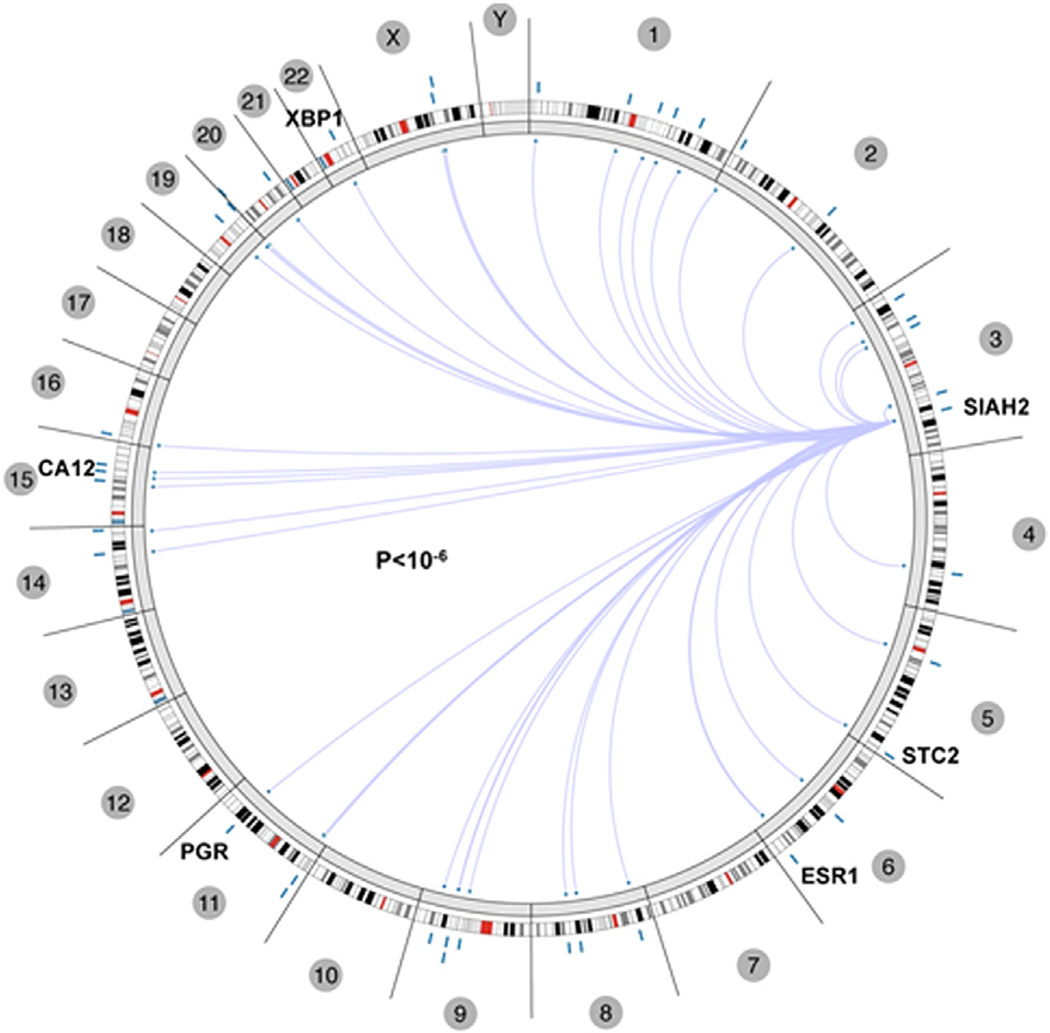
Assessment of the correlation of SIAH2 expression with ER status in Circos graph was made with the online program, http://explorer.cancerregulome.org. Spearman correlation showed pairwise correlation between two genes. Only the top 40 with P value > −log10 were shown in Circos.
3. Results
3.1. SIAH2 expression correlates with ER positivity
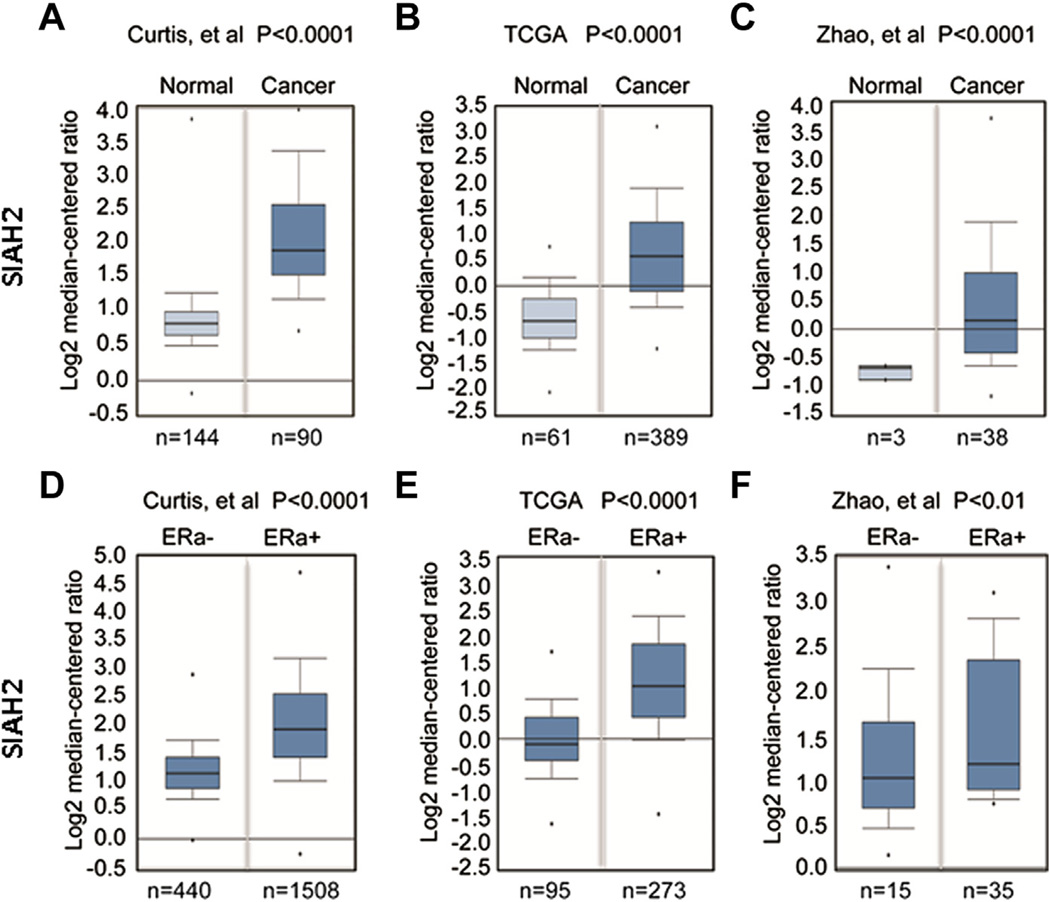
The genetic association of SIAH2 in ER-positive breast cancers prompted us to assess whether SIAH2 is highly expressed in breast cancer. We mined databases using the Oncomine program and found that SIAH2 expression is significantly higher (P < 0.0001) in breast cancers in comparison with normal breast tissue (Fig. 1A and Fig. 2). The data set from Curtis et al. compared normal breast tissue with both invasive ductal and invasive lobular carcinoma. The TCGA and Zhao et al. data sets compared normal breast tissue with invasive ductal carcinoma only. We then compared SIAH2 expression in ER-positive and ER-negative breast cancer in these samples and found a strong association of high expression of SIAH2 with ER-positive breast cancers (P < 0.01) (Fig. 1B). These data, together with the genome-wide association of SIAH2 and ER-positive breast cancers, suggest that SIAH2 may play an important role in ER-driven breast cancer.
Fig. 1.
Expression of SIAH2 in breast cancer specimens and cell lines. (A) Data mining using the Oncomine program showing that SIAH2 is highly expressed in breast cancer specimens as compared with normal breast tissue. (B) SIAH2 expression also correlates with ER positivity in breast cancer. Each box represents multiple samples from one class. The whiskers indicate the normalized expression values including the maximum value, the 90th, 75th, median, 25th and 10th percentiles, and minimum values. The indicated numbers are cases of patient samples or normal tissue.
Fig. 2.
The correlation of gene expression of SIAH2 and other genes from TCGA breast cancer data, as analyzed by Regulome program. Shown are the 23 chromosomes (including the X/Y chromosomes). The well-known ER target genes, including ER (ESR1) itself, with genomic location are shown in the Circos plot. The association arcs in the center indicate significant associations between two molecular features with the ends of the arcs positioned according to the genomic coordinates of each of the two features.
3.2. SIAH2 effect on tamoxifen sensitivity
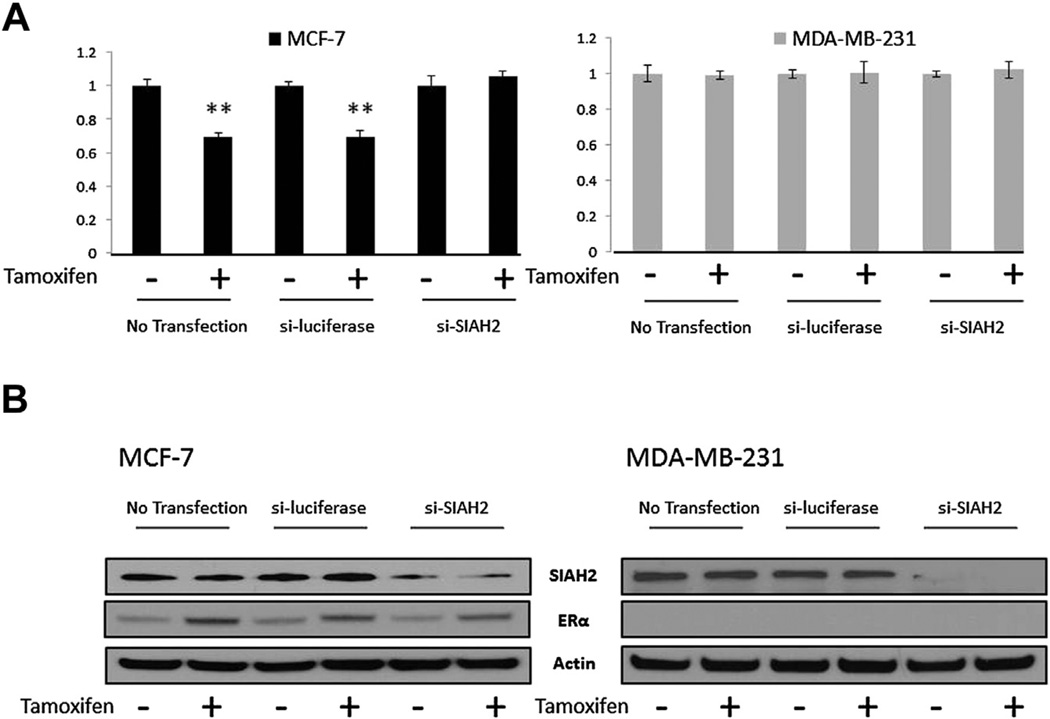
We next sought to assess the role of SIAH2 in tumor cell sensitivity to tamoxifen, a competitive inhibitor of the ER ligand. Transient knockdown of SIAH2 was achieved in MCF-7 and MDA-MB-231 cells by transfecting the cells with SIAH2 siRNA or an siRNA control. Although MDA-MB-231 has higher baseline levels of SIAH2 expression as compared with MCF-7, by Western blot (data not shown), MDA-MB-231 is inherently resistant to tamoxifen due to the lack of ER expression. After 24 h of incubation, cells were treated with 1 µM of tamoxifen for 48 h and then harvested for cell counting and Western blot analysis. When comparing untreated cells to those treated with tamoxifen, we observed an approximately 40% decrease in absolute cell number in the MCF-7 cells (Fig. 3A). Similarly, we observed an approximately 35%–40% decrease in cell count when MCF-7 cells were transfected with control siRNA and then were treated with tamoxifen. However, when SIAH2 was knocked down in MCF-7 cells, which were then treated with tamoxifen, there was no significant decrease in cell count, as compared with cells not treated with tamoxifen, indicating a decrease in sensitivity of the MCF-7 cells to tamoxifen. Knockdown of SIAH2 in the MCF-7 cells was confirmed by Western blot analysis (Fig. 3B). In comparison, MDA-MB-231 (ER-negative) cells, when treated with tamoxifen after SIAH2 knockdown, did not demonstrate any appreciable difference in cell number compared with those not treated with tamoxifen.
Fig. 3.
Effect of SIAH2 knockdown on tamoxifen sensitivity. (A) MCF-7 and MDA-MB-231 cell counts with and without SIAH2 knockdown shown as ratios following treatment with tamoxifen 1 µM. siSIAH2 knockdown followed by tamoxifen treatment showed decreased sensitivity. (B) Western blot confirming knockdown of SIAH2 and subsequent effect on ER-α.
3.3. SIAH2 mediates ER-α expression in an ER-positive breast cancer cell line
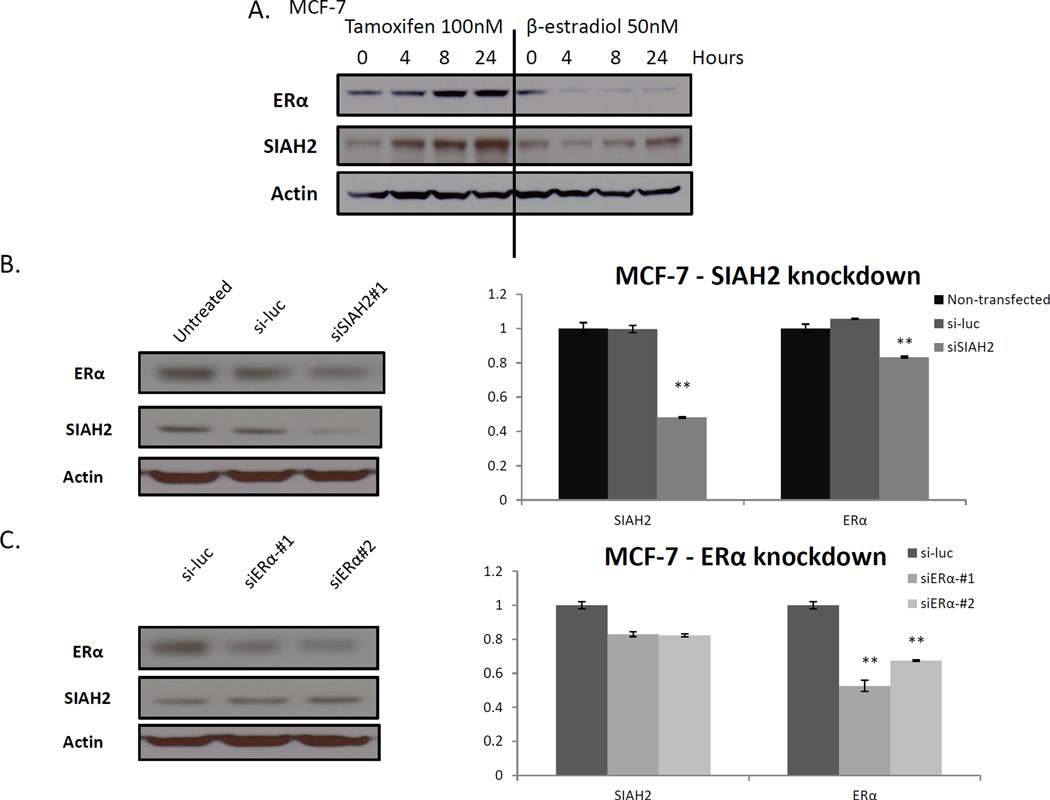
Because of the association of SIAH2 expression with ER status, we evaluated the effect of siRNA-mediated knockdown of SIAH2 on ER-α expression. First, the effect of tamoxifen treatment on ER-α in MCF-7 cells was assessed. Cells were hormone-starved with charcoal-stripped media for 48 h and then treated with tamoxifen (100 nM) or βestradiol (50 nM) for 0, 4, 8, and 24 h. We observed the expected induction of ER-α, as well as in SIAH2, after treatment with tamoxifen (Fig. 4A). Conversely, when MCF-7 cells were treated with β-estradiol, we observed a downregulation of ER-α with an induction of SIAH2 at 24 h (Fig. 4A). Next, we performed selective knockdown of SIAH2 in ER-positive MCF-7 cells and found that this resulted in downregulation of ER-α by Western blot analysis and qRT-PCR (P < 0.01) (Fig. 4B). Knockdown of SIAH2 was confirmed by Western blot analysis. Knockdown of ER-α, however, had minimal effect on SIAH2, as also confirmed by qRT-PCR (P < 0.05) (Fig. 4C).
Fig. 4.
Effect of SIAH2 knockdown on ER-α expression. (A) MCF-7 cells treated with tamoxifen had induction of ER-α and SIAH2. Treatment with β-estradiol resulted in downregulation of ER-α, with little change in SIAH2 expression. (B) Selective SIAH2 knockdown with siRNA in MCF-7 cells resulted in downregulation of ER-α by Western blot analysis and quantitative real-time polymerase chain reaction. (C) Selective knockdown of ER-α resulted in downregulation of ER-α, but with minimal change in SIAH2 (**P < 0.005).
3.4. Low expression of SIAH2 is associated with a worse prognosis in ER-positive breast cancer patients treated with tamoxifen
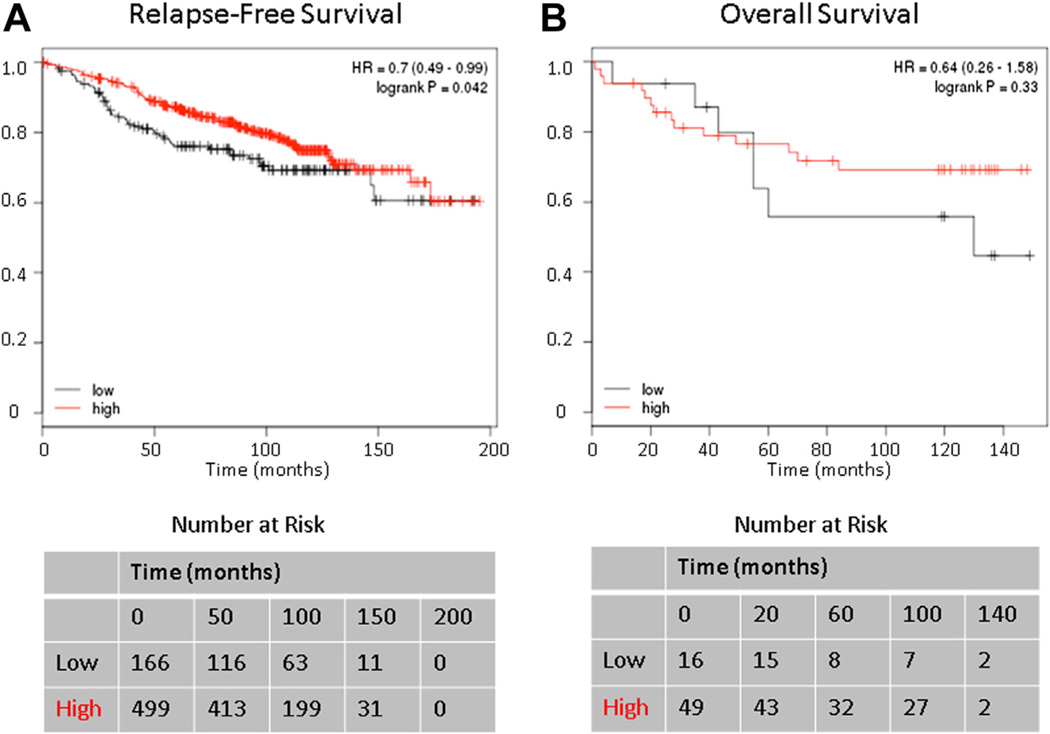
To investigate the clinical relevance of levels of SIAH2 expression in breast cancers, we mined the available data and found that when analyzing outcomes for patients with ER-positive tumors whose treatment included tamoxifen, there was a lower relapse-free survival in patients with low expression of SIAH2 as compared with patients with high expression (P < 0.05) (Fig. 5A). There was no difference in overall survival, however, based on the available data (P = 0.33) (Fig. 5B).
Fig. 5.
The effect of SIAH2 expression on the Kaplan-Meier survival curve for (A) relapse-free survival and (B) overall survival in patients with ER-positive breast cancer treated with tamoxifen. The lower quartile (25%) of SIAH2 expression in patients with ER-positive breast cancer treated with tamoxifen had a poorer relapse-free survival as compared with patients with high SIAH2 expression (the rest of 75%) (P < 0.05). Overall survival, however, did not reach statistical significance (P = 0.33).
4. Discussion
Prophylactic hormone therapy with tamoxifen is a mainstay of treatment for ER-positive breast cancer. Tamoxifen was first approved for the treatment of metastatic breast cancer in postmenopausal women in the 1970s; however, since the 1990s, its role as an agent for prophylaxis has been well established [22]. Recently, den Hollander et al. [23] summarized the wide range of targeted therapeutics for breast cancer prevention with tamoxifen playing a key role in prophylaxis for high-risk patients. Recurrence of breast cancer, however, due to de novo resistance or acquired resistance against these drugs can lead to worsening prognosis.
Our data suggest that SIAH2 may facilitate the sensitivity to tamoxifen in ER-positive breast cancer. In 2009, Jansen et al. [18] showed similar findings in patient samples, noting that downregulation of SIAH2 was associated with resistance to endocrine therapy. However, the focus of their in vitro experiments was ICI 164,384 treatment—an ER antagonist that functions by decreasing the levels of ER by diminishing the protein half-life. Other groups have identified single nucleotide polymorphisms in this critical protein in the development of hormonal receptor–positive breast cancer [16]. Zhang et al. [17] also recently identified a common variant in the SIAH2 locus in ER-positive breast cancer. Although the functional role of this variant is not defined, these data further confirm the direct relationship of SIAH2 and ER status. We sought to test the relationship of SIAH2 with tamoxifen, a clinically relevant drug that acts through a different mechanism from ICI 164,384, serving as a competitive inhibitor of the ER ligand. The effects of tamoxifen on SIAH2 have also not been well studied.
In our experiments, we observed induction of ER-α and SIAH2 by Western blot and qRT-PCR in an ER-positive cell line, MCF-7 treated with tamoxifen. We noted this induction to be time dependent. However, when we performed the knockdown of SIAH2 and treated with tamoxifen, we observed a blunted effect on the induction of ER-α, which we believe leads to resistance to tamoxifen therapy. This suggests that loss of SIAH2 may play an important role in the development of resistance, whereby tumors with decreased SIAH2 acquire the ability to proliferate in the setting of tamoxifen. The survival data that we evaluated also provides some support to the importance of SIAH2 in the treatment of patients with breast cancer in relapse-free survival, although overall survival was not statistically different. The benefit of using this RNA-based microarray data is the quantity of data provided by these population-based genomic analyses. They help to elucidate genomic variants in breast cancer as well as give other groups the ability to assess subgroups; for example, as we have done with analyzing SIAH2 expression in ER-positive versus ER-negative breast cancer. This is, however, based on quantitative messenger RNA and DNA analyses and further studies are needed to understand these complex pathways.
These findings are based on in vitro experiments, and similar in vivo studies are required to show that downregulation of SIAH2 leads to resistance to tamoxifen treatment. Upregulation of SIAH2 may also have a sensitizing effect on this hormone therapy. Further studies should also be done to assess the effect of other selective estrogen receptor modulators on SIAH2. Future studies can also focus on SIAH2 as a predictive biomarker in choosing the appropriate prophylactic therapy for breast cancer. Moreover, patient samples could be further analyzed to assess the impact that targeting this protein may have on therapy.
Footnotes
Presented at the Academic Surgical Congress in San Diego, CA -and awarded the AAS Outstanding Resident Research Award on February 2014.
Author contributions: Conception and design: R.B.I., J.Y., A.L.H., A.M.D.; Analysis and interpretation: R.B.I., J.Y., A.M.D.; Data collection: R.B.I.; Writing the article: R.B.I., J.Y.; Critical revision of article and obtaining of funding: A.M.D.
Disclosure
The authors reported no proprietary or commercial interest in any product mentioned or concept discussed in this article.
REFERENCES
- 1.Desantis C, Ma J, Bryan L, Jemal A. Breast cancer statistics, 2013. CA Cancer J Clin. 2014;64:52. doi: 10.3322/caac.21203. [DOI] [PubMed] [Google Scholar]
- 2.Goldhirsch A, Colleoni M, Gelber RD. Endocrine therapy of breast cancer. Ann Oncol. 2002;13(Suppl 4):61. doi: 10.1093/annonc/mdf640. [DOI] [PubMed] [Google Scholar]
- 3.Hou N, Huo D. A trend analysis of breast cancer incidence rates in the United States from 2000 to 2009 shows a recent increase. Breast Cancer Res Treat. 2013;138:633. doi: 10.1007/s10549-013-2434-0. [DOI] [PubMed] [Google Scholar]
- 4.Anderson WF, Katki HA, Rosenberg PS. Incidence of breast cancer in the United States: current and future trends. J Natl Cancer Inst. 2011;103:1397. doi: 10.1093/jnci/djr257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bentzon N, During M, Rasmussen BB, Mouridsen H, Kroman N. Prognostic effect of estrogen receptor status across age in primary breast cancer. Int J Cancer. 2008;122:1089. doi: 10.1002/ijc.22892. [DOI] [PubMed] [Google Scholar]
- 6.Early Breast Cancer Trialists’ Collaborative G. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;365:1687. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 7.Williams KE, Anderton DL, Lee MP, Pentecost BT, Arcaro KF. High-density array analysis of DNA methylation in tamoxifen-resistant breast cancer cell lines. Epigenetics. 2013;9 doi: 10.4161/epi.27111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Palmieri C, Patten DK, Januszewski A, Zucchini G, Howell SJ. Breast cancer: current and future endocrine therapies. Mol Cell Endocrinol. 2014;382:695. doi: 10.1016/j.mce.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Droog M, Beelen K, Linn S, Zwart W. Tamoxifen resistance: from bench to bedside. Eur J Pharmacol. 2013;717:47. doi: 10.1016/j.ejphar.2012.11.071. [DOI] [PubMed] [Google Scholar]
- 10.Zhou J, Teng R, Wang Q, et al. Endocrine resistance in breast cancer: current status and a perspective on the roles of miRNAs (Review) Oncol Lett. 2013;6:295. doi: 10.3892/ol.2013.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huber-Keener KJ, Liu X, Wang Z, et al. Differential gene expression in tamoxifen-resistant breast cancer cells revealed by a new analytical model of RNA-Seq data. PLoS One. 2012;7:e41333. doi: 10.1371/journal.pone.0041333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakayama K, Frew IJ, Hagensen M, et al. Siah2 regulates stability of prolyl-hydroxylases, controls HIF1alpha abundance, and modulates physiological responses to hypoxia. Cell. 2004;117:941. doi: 10.1016/j.cell.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 13.Kramer OH, Stauber RH, Bug G, Hartkamp J, Knauer SK. SIAH proteins: critical roles in leukemogenesis. Leukemia. 2013;27:792. doi: 10.1038/leu.2012.284. [DOI] [PubMed] [Google Scholar]
- 14.Qi J, Nakayama K, Cardiff RD, et al. Siah2-dependent concerted activity of HIF and FoxA2 regulates formation of neuroendocrine phenotype and neuroendocrine prostate tumors. Cancer Cell. 2010;18:23. doi: 10.1016/j.ccr.2010.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qi J, Tripathi M, Mishra R, et al. The E3 ubiquitin ligase Siah2 contributes to castration-resistant prostate cancer by regulation of androgen receptor transcriptional activity. Cancer Cell. 2013;23:332. doi: 10.1016/j.ccr.2013.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elgazzar S, Zembutsu H, Takahashi A, et al. A genome-wide association study identifies a genetic variant in the SIAH2 locus associated with hormonal receptor-positive breast cancer in Japanese. J Hum Genet. 2012;57:766. doi: 10.1038/jhg.2012.108. [DOI] [PubMed] [Google Scholar]
- 17.Zhang B, Li Y, Zheng X, et al. A common variant in the SIAH2 locus is associated with estrogen receptor-positive breast cancer in the Chinese Han population. PLoS One. 2013;8:e79365. doi: 10.1371/journal.pone.0079365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jansen MP, Ruigrok-Ritstier K, Dorssers LC, et al. Downregulation of SIAH2, an ubiquitin E3 ligase, is associated with resistance to endocrine therapy in breast cancer. Breast Cancer Res Treat. 2009;116:263. doi: 10.1007/s10549-008-0125-z. [DOI] [PubMed] [Google Scholar]
- 19.Curtis C, Shah SP, Chin SF, et al. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature. 2012;486:346. doi: 10.1038/nature10983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao H, Langerod A, Ji Y, et al. Different gene expression patterns in invasive lobular and ductal carcinomas of the breast. Mol Biol Cell. 2004;15:2523. doi: 10.1091/mbc.E03-11-0786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gyorffy B, Lanczky A, Eklund AC, et al. An online survival analysis tool to rapidly assess the effect of 22,277 genes on breast cancer prognosis using microarray data of 1,809 patients. Breast Cancer Res Treat. 2010;123:725. doi: 10.1007/s10549-009-0674-9. [DOI] [PubMed] [Google Scholar]
- 22.Sainsbury R. The development of endocrine therapy for women with breast cancer. Cancer Treat Rev. 2013;39:507. doi: 10.1016/j.ctrv.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 23.den Hollander P, Savage MI, Brown PH. Targeted therapy for breast cancer prevention. Front Oncol. 2013;3:250. doi: 10.3389/fonc.2013.00250. [DOI] [PMC free article] [PubMed] [Google Scholar]