Abstract
Background and Aims
Cardiovascular complications are major causes of morbidity and mortality in patients with autosomal dominant polycystic kidney disease (ADPKD). In particular, hypertension is insidious and remains a continuous problem that evolves during the course of the disease. Hypertension in ADPKD has been associated with abnormality in the renin-angiotensin-aldosterone system (RAAS). Early vascular changes have also been reported in young ADPKD patients. In addition, the cellular functions of mechanosensory cilia within vascular system have emerged recently. The basic and clinical perspectives of RAAS, vascular remodeling and sensory cilia are reviewed with regard to hypertension in ADPKD.
Keywords: Angiotensin, Blood Pressure, Cardiovascular, Cilia Biology, Hypertension, ADPKD
Introduction
Autosomal dominant polycystic kidney disease (ADPKD) is a systemic hereditary disorder that affects more than 12.5 million individuals worldwide. Thus, ADPKD is the most common life-threatening hereditary genetic disease, compared to the numbers of individuals affected by cystic fibrosis, Downs syndrome, hemophilia, muscular dystrophy, and sickle cell anemia, combined (1, 2). Approximately 600,000 Americans have the disease, and the prevalence of the disease varies between 1:400 and 1: 1000 in the general population (3, 4).
ADPKD is characterized by a long course of normal glomerular filtration rate (GFR), micro-albuminuria and early development of hypertension, followed by a persistent decrease in GFR in the fifth to sixth decades. This eventually leads to end stage renal disease in approximately 50% of patients (5, 6). Although the kidneys are the major sites of clinical disease (Figure 1), the prevalence of extrarenal manifestations in ADPKD is high. These extrarenal manifestations include cyst formation in other ductal organs and various cardiovascular abnormalities (7-11). Cardiovascular abnormalities may include hypertension, cerebral and coronary artery aneurysms, mitral valve prolapse, aortic root dilation, dissection of the thoracic aorta, aneurysm formation in the abdominal aorta, vascular ectasia, and abnormal function of the microvascular bed (12-15).
Figure 1. Autosomal dominant polycystic kidney.
a. Polycystic kidney is characterized by the presence of numerous fluid-filled cysts. An ADPKD kidney can reach over 10 inches in length, about 3-4 times larger than a normal four-inch long kidney. b. Fluid accumulation in the cysts contributes to the weight and size of an ADPKD kidney. c. In contrast to heavier tissue mass in renal carcinoma, an ADPKD kidney could lose about 7-28 ounces of weight after fluid cyst removal. This is an important phenotypic characteristic that despite its large size and volume, an ADPKD kidney actually contains equal or less tissue mass than a normal kidney.
The frequencies of extrarenal manifestations of ADPKD include hypertension (78%), hepatic cysts (75%), diverticulosis (70%), ovarian cysts (40%), cardiac valve disorders (25%), inguinal hernias (15%) and intracranial aneurysms (10%) (16). Prevalence of intracranial aneurysms in ADPKD individuals is increased several folds compared to the general population (4.0-11.7% versus 1.0%) and intracranial aneurysm rupture remains a devastating complication in ADPKD (17). Screening ADPKD individuals with a family history of sub-arachnoid hemorrhage is thus essential, because intracranial aneurysms appears to cluster in ADPKD families with history of cerebral hemorrhage (18). A retrospective study of 77 ADPKD individuals with aneurysms showed that only 29% were normotensive within one year prior to rupture (19). In particular, renal function was normal in half of these patients. Therefore, hypertension is an associated finding in these individuals. More important, hypertension has been a continual risk factor for cardiovascular diseases in ADPKD (15). It occurs early in ADPKD individuals, compared to their age-matched cohorts and remains the most frequent cause of mortality (12).
The discovery of the PKD1 and PKD2 genes has opened up avenues of research in molecular biology to further understand the role of genetic mutations in the pathogenesis of this devastating disease. PKD1 encodes for polycystin-1, which is involved in the mechanosensory function, and polycystin-2 encoded by PKD2 is a sensory calcium channel (20-29). The PKD1 mutation affects nearly 85% of the patients with ADPKD, and they have a shorter and more severe disease progressions than patients with PKD2 mutation; i.e. 54 years compared to 74 years, respectively (30).
While numerous progresses have been reported about the pathology and pathogenesis of cystic kidneys (31), this article is intended to review the hypertension aspect of ADPKD. In particular, several ideas of “cilia hypothesis” have been discussed regarding polycystic kidneys (32-36). These ideas will be revisited to understand the constituents of the cardiovascular complications, such as hypertension, with respect to the new clinical and basic science development in ADPKD.
Pathogenesis of hypertension in ADPKD
Progressive enlargement of cysts by magnetic resonance imaging, as studied in the recent CRISP (The consortium for radiologic imaging studies of polycystic kidney disease) trial and other studies, has been linked to deterioration of renal function in ADPKD (6, 37, 38). These studies have shown that renal function remains relatively stable until the renal volume reaches 1500 cm3, at which point there is a rapid decline that necessitates renal replacement therapy (39). It is believed that distortion of the renal architecture leads to structural damage and tubular dysfunction and results in activation of the reninangiotensin-aldosterone system (RAAS). These findings have also been reported in hypertensive animal models of polycystic kidney disease (40, 41). This mechanism, which is well documented in the clinical course of this disease (42), has also been proposed to contribute to the increased blood pressure seen early in ADPKD patients. Changes in the vasculatures during the course of the disease progression have also been observed in both human (43-47) and animal (48-52) studies. Most recently, ciliary defects have also been proposed to cause the complication of hypertension in ADPKD (20, 25). We will therefore look at these possibilities as the origins of hypertension in ADPKD.
Renin-angiotensin system
Compared with age-matched healthy control subjects, an increase in intrarenal RAAS activity is seen in ADPKD patients, regardless of their blood pressure and renal function (53). Angiotensin II has been implicated in the development of vascular hypertrophy, resulting in structural remodeling (54). Angiotensin II generation via ACE-independent chymase-like pathways has been shown in ADPKD kidneys, making it an attractive target for pharmacological intervention (Figure 2). Control of blood pressure in most renal diseases has shown benefit to slow the decline in renal function. In particular, the use of angiotensin converting enzyme inhibitors (ACE-I) and angiotensin receptor blockers (ARB) to block the RAAS has shown additional benefits (55, 56). Although many clinical trials have been undertaken to halt the progression of renal failure by optimal control of blood pressure and micro-albuminuria with RAAS blockade, there has been no data to show significant decrease in the inevitable progression to renal failure (57, 58). However, there is a general consensus that early identification of ADPKD individuals and achieving optimal blood pressure control with ACE-I and other agents remain crucial in preventing the cardiovascular morbidity and mortality associated with this disease (59).
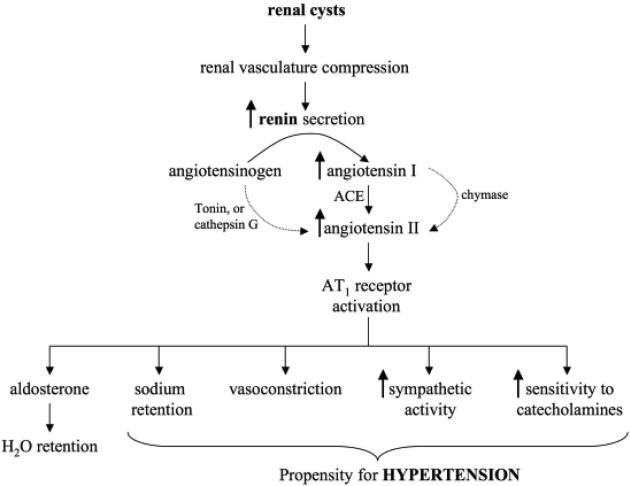
Figure 2. Hypertension in polycystic kidney disease.
Renal cysts are thought to compress and disrupt the vascular network in the kidney. The kidney would then become ischemic, which would induce renin release from the juxtaglomerular apparatus. The increase in renin secretion could accelerate the conversion of angiotensinogen to angiotensin I, which is converted by angiotensin converting enzyme (ACE) to angiotension II. Activation of angiotensin receptor (AT1) would initiate cascades of physiological responses that would lead to hypertension. Additional secondary pathways exist in converting angiotensinogen to angitotensin II. As indicated by the light arrows, these secondary pathways involve chymase, tonin and/or cathepsin G.
Studies which included ambulatory blood pressure monitoring have shown that in children and young adults with ADPKD, increases in left ventricular mass index occurred earlier than changes in renal volume in the hypertensive and borderline hyper-tensive group (60). In addition, the most significant finding was that even among the normotensive children, higher indices were noted in those within the upper quartile of the normal blood pressure. This suggests that cardiovascular organs remain vulnerable targets in ADPKD patients, even in the early stages of the disease.
Interestingly, the likelihood of hypertension in ADPKD patients is significantly greater when the affected parent was hypertensive (61). Thus, the family history is especially critical in this case, although it is not immediately understood whether this association is related to genetic modifiers or polymorphisms with regards to RAAS (62). One caveat is that pharmacological agents prescribed to inhibit RAAS result in incomplete inhibition of the RAAS. Angiotensin II, for example, can be generated via multiple pathways (Figure 2). Consistent with this view, the inhibition of RAAS activation by ACE inhibitors has not been always proven to retard the course of the disease, compared to results seen in renal diseases with primary glomerular injury (63). There are also other factors contributing to the increase in blood pressure. Tubular dysfunction caused by structural changes leading to retention of sodium and water and increased sympathetic activity, for example, is seen in ADPKD patients (64). Nonetheless, inhibition of RAAS activation might provide a logical way to reduce factors that contribute to the propensity for high blood pressure in ADPKD (Figure 2).
Vascular remodeling
There is growing evidence in literature that once a change in GFR is observed, extensive vascular remodeling has already occurred (43-47). Scientists and clinicians have been focusing their efforts on characterizing early biomarkers of injury. Increased urinary excretion of MCP-1 (monocyte chemoattractant protein-1) in adult patients with ADPKD has been reported, and microalbuminuria has been established as a frequent sign of progression of the disease (65, 66). Recently, changes in vascular remodeling with altered intima-media thickness of carotid arteries (CIMT), impaired endothelial-dependent vascular relaxation (EDVR) and vascular ultrasound thickness have been reported in ADPKD patients (15, 45, 47, 67-70). CIMT in hypertensive ADPKD patients and patients with essential hypertension was significantly greater than that of normotensive ADPKD patients and healthy subjects. In addition, CIMP in normotensive ADPKD patients is significantly greater than in healthy subjects.
Polycystin-1 and polycystin-2 are expressed in vascular smooth muscle cells (71, 72). These proteins have an important role in maintaining the integrity of dense plaques of the arterial wall (73). Aortic root dilatation is a known finding in patients with ADPKD, and aortic dissection is a known extra renal complication in these patients (7). Altered expression of polycystins in arterial smooth muscle cells, together with collagen disruption and cystic degeneration, has been seen in histological specimens of aortic dissections and cerebral aneurysms from patients with ADPKD. It has further been reported that altered functional polycystins would result in various degrees of cardiovascular abnormalities (49-52). In addition to focal hemorrhage, altered polycystin function in the mouse model exhibits progressive total body edema, a feature of cardiac failure (52).
An important question that remains unanswered is whether or not changes in vasculature is a direct result of the enlargement of the kidney. Nonetheless it is known that the remodeling of vasculature is a counter beneficiary that can result in a more severe hypertension (74-76). Vascular remodeling in the arteries may develop through inward/outward hypotrophic or hypertrophic mechanisms (77). In either instance, vascular remodeling will place greater stress on the cardiovascular system. In light of this view, several anti-proliferative therapies have been proposed as potential treatments in ADPKD. At present, however, the immediate impact of this therapy on blood pressure is still unknown.
Other contributing factors
Other factors that may contribute to cardiovascular hypertension in ADPKD include insulin resistance, asymmetric dimethylarginine (ADMA), and vascular cilia dysfunction.
Numerous studies have shown a causal association between insulin resistance or hyperinsulinemia and hypertension. Insulin resistance and concomitant hyperinsulinemia are present very early in the course of renal diseases including in ADPKD (78, 79). Increased sympathetic activity present in individuals with chronic renal disease can contribute to derangements of glucose metabolism (80). Although the mechanistic relationship between insulin resistance and polycystic kidney disease is not clear, the correlation between insulin resistance and left ventricular mass index in individuals with polycystic kidney disease has been shown to be independent of age, weight, systolic blood pressure and albuminuria (79). Thus, ADPKD patients with insulin resistance could have a more complex outcome on cardiovascular complications. Insulin-mediated sodium retention, increased sympathetic activity, impairment of endothelial nitric oxide production and increased endothelin-1 secretion have been suggested to worsen vascular hypertension due to insulin resistance (81). However, their relevance with regards to polycystic kidney remains to be explored further.
To further examine endothelial function in ADPKD, the plasma concentrations of vasodilator nitric oxide were measured in twenty seven ADPKD patients and twenty seven healthy controls. In this study, the plasma concentration of nitric oxide was shown to be reduced in ADPKD patients, confirming an association between ADPKD and endothelial dys-function (82). To further substantiate the endothelial dysfunction in ADPKD, levels of ADMA (a marker of an inhibitor of nitric oxide synthase) were significantly increased in patients with early ADPKD compared to healthy age-matched individuals (83). Although the significance of ADMA in ADPKD is not immediately understood, endothelia-dependent vasodilation offers substantial evidence which is too important to ignore.
Cilia and their functions have been reported to play essential roles in renal epithelial cells and in cystic kidney diseases. Vascular cells, too, have cilia (Figure 3). Endovascular cilia are thought to function as local regulators of blood vessel (20, 25, 84-87). Local regulation of blood vessels, also known as autoregulation, is a necessary mechanism to achieve immediate control of blood pressure within a region. Autoregulation is an effective and efficient way to control the amount of blood flow locally without altering the neighboring systems significantly (88).
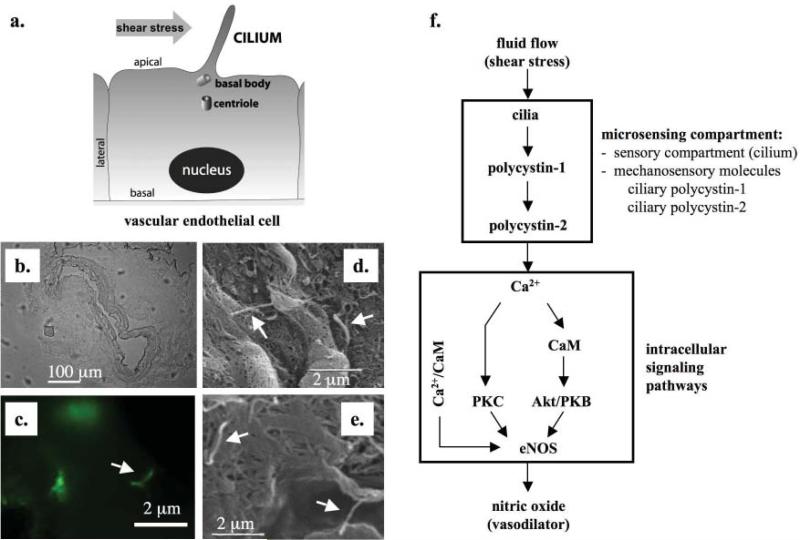
Figure 3. Primary cilia and hypertension.
Primary cilia are believed to play significant roles in the formation of renal cysts and the development of hypertension in ADPKD. a. Within the lumen of a blood vessel, each vascular endothelial cell possesses one primary cilium, originated from the apical cell membrane. Cilium is projected from “mother” centriole, which is also known as basal body. Similar to their function in the renal tubule which is to sense urine flow, cilia in the endothelial lining of a vessel are hypothesized to sense blood flow (shear stress). b. Projecting into the lumen, cilia function like antennae to sense changes in blood flow or pressure. Femoral arteries from adult rodents were isolated and prepared for fluorescence imaging studies. A segment of the artery was embedded in OCT solution, and sectioned at a thickness of 5 μm. The image was taken at 10× to outline the embedded tissue. c. The embedded tissue was fixed and labeled with antibody against polaris, a structural protein for cilia. The presence of cilia was observed using florescence imaging. d, e. Arterial segments from femoral arteries of adult rodents were cut open and coated with 10 μm of gold particles and analyzed with scanning electron microscopy. White arrows indicate the presence of cilia. f. Upon sensing these changes, mechanosensory polycystins complex is activated. This results in the influx of extracellular calcium that would initiate biochemical cascades that lead to production of NO vasodilator through endothelial nitric oxide (eNOS). Activation of eNOS also depends on the contribution of calmodulin (CaM), phosphoinositide kinase-3 (PI3K) Akt/protein kinase B (PKB) in addition to calcium-dependent protein kinase (PKC).
In the most recent studies within the cardiovascular field (20, 25), it is shown that each endothelial cell has one primary cilium, and shear stress that produces enough drag force on the cell surface is able to bend and “activate” the primary cilium (Figure 3). Although the biophysical properties of vascular cilia are less studied and not well known, it has been proposed that endothelial cilia are shear stress sensors (20, 25). In addition, when non ciliated cells are challenged with fluid-shear stress, they show significantly less induction of the shear marker Krüppel-Like Factor-2, as compared to ciliated endothelial cells (84, 86). Because primary cilia sense only a low range of fluid-shear stress, endothelial cells in vivo most likely project the cilia in the areas with more subtle shear stress. Consistent with this idea, cilia in vascular endothelial cells disassemble after a long exposure to high fluid shear stress (89). Because atherosclerosis also develops in the arterial system at sites of low shear stress, cilia have been further proposed to play an important role in atherogenesis, a process of forming atheromas in the inner lining of arteries (87).
As micro-sensory compartments, ciliary functions depend on mechano-proteins such as polycystin-1 and polycystin-2 (Figure 3). Thus, the overall functions of these sensory compartments depend on both functional and structural cilia proteins. Within a blood vessel, an abrupt increase in blood pressure or shear stress can be detected by these sensory proteins localized in the cilia (20, 25). With cilia as a local regulatory mechanism, the extracellular fluid mechanics can then be transduced and translated into a complex of intracellular signaling, which in turn would activate eNOS - an endothelial enzyme that synthesizes nitric oxide (NO) gas. The release of NO from endothelial cells will diffuse to the neighboring smooth muscle cells, thereby, promoting vasodilation (90-92). The overall cilia dysfunction in ADPKD may thus result in an increased total peripheral resistance, thereby increasing blood pressure.
Treatment of hypertension in ADPKD
Mutations in PKD1 and PKD2 have been suggested to contribute to vascular hypertension (30, 93, 94). This is probably due to failure to convert an increase in mechanical blood flow into cellular NO biosynthesis in order to control the vascular tone, i.e. blood pressure (20, 25). Of note is that hypertension occurs at a much earlier age in patients with ADPKD than in the general population (95). The median age of hypertension in ADPKD is about 30 years, compared with a median age of 45-55 years in patients with essential hypertension (61). Hypertension occurs in children even before they are diagnosed with ADPKD (96-99) or before any substantial reduction in glomerular filtration rate is observed (100, 101).
Uncontrolled hypertension can, in fact, deteriorate kidney function much faster. In ADPKD, some studies have further suggested that high blood pressure would promote faster cyst growth (102, 103). Early and effective treatments of hypertension become very important to decrease the morbidity and mortality of patients with ADPKD, including the dreaded complication of intracranial aneurysm ruptures (12, 97). Therefore, it makes sense to control the blood pressure in young ADPKD patients aggressively.
Antihypertensives
Studies in experimental models of polycystic kidney disease have shown that the ACE-I enalapril or the ARB losartan are more effective in lowering blood pressure, maintaining renal volume and preserving renal function than hydralazine (104). Moreover, in a seven-year prospective study comparing rigorous versus standard blood pressure control, enalapril was found to be more effective than calcium channel blocker amlodipine in reducing left ventricular mass index (LVMI). However, none of these agents affects the rate of decline in renal function (105). Similarly, an atenolol-based regimen, compared to enalapril, showed no difference in decline of renal function over a three-year period (106). In a six months of follow-up study, ARB candesartan shows little change in blood pressure or renal progression in ADPKD patients (107). However, candesartan reduced excretion of urinary fatty acid binding protein, which has been reported to be higher in ADPKD patients.
It was also reported that muscle sympathetic nerve activity is increased in hypertensive patients with ADPKD, regardless of renal function (64, 108). This suggests that sympathetic hyperactivity could contribute to the pathogenesis of hypertension in ADPKD. However, it is not immediately understood whether the sensitivity to sympathetic nerve activity is a secondary effect due to an increase in RAAS system (Figure 2). Of apparent complexity is that angiotensin can stimulate the sympathetic nervous system and that sympathetic nerve activity can also stimulate RAAS (109, 110). At least in murine models of PKD, bilateral renal denervation could reduce cystic kidney size, cyst volume and most importantly, systolic blood pressure (111). It is therefore very likely that sympathetic nerve activity would activate RAAS system, which would increase blood pressure.
Regardless, in a 3-year prospective randomized double-blind study that compared ACE-I ramipril and the beta-blocker metoprolol as first line therapy in hypertensive ADPKD patients, no differences in renal function or LVMI were detected (112). However, LVMI increased in patients with standard blood pressure control while it remained stable or improved in patients with rigorous blood pressure control, suggesting that aggressive blood pressure control is necessary in ADPKD patients (113).
Aside from ACE-I, calcium channel blockers have also been reported to reduce blood pressure effectively and preserve renal function in ADPKD patients (114). A different study, however, showed that the renoprotective effect of ARB was considerably more favorable than calcium channel blocker in the treatment of ADPKD (115). The use of diuretics in hypertensive ADPKD patients also results in a similar decrease in blood pressure, although they may promote a faster loss of renal function compared to ACE-I therapy (57). In addition, vasopressin V2 receptor antagonists have been proposed to have anti-hypertensive effect (116), probably by reducing renal sodium reabsorption.
Although a definitive study to demonstrate efficacy of ACE-I on renal progression in ADPKD is not available, there is a wealth of evidence that suggest ACE-I are beneficial in slowing renal progression in non-diabetic kidney disease. Clinical practice guidelines from the National Kidney Foundation and the Joint National Committee on Prevention Detection Evaluation and Treatment of High Blood Pressure call for ACE-I as the first line of agent for treatment of hypertension in patients with chronic kidney disease (117-119). Although ACE-I and ARB are considered first line agents to treat hypertension in ADPKD, caution needs to be used in pregnant women due to known birth defects and in advanced renal failure due to risk of life-threatening hyperkalemia. Overall, early and effective treatment of hypertension is very important to decrease the morbidity and mortality of patients with ADPKD.
Transplantation
Activation of the RAAS has been found in normotensive and hypertensive ADPKD subjects. As cysts enlarge, they compress the renal vasculature and attenuate the renal vessels, causing intrarenal ischemia and activation of the RAAS (120-122). Since cardiovascular abnormalities are thought to initiate from the cystic kidneys in ADPKD, there has been a great interest in studying the outcomes after renal transplantation in these individuals. Earlier outcome studies in renal transplant recipients with ADPKD on azathiprine or cyclosporine immunosuppression have shown an increase in cardiovascular morbidity compared to non-ADPKD transplant recipients (123). However, cardiovascular events, graft and patient survival up to 10 years of follow-up were not found to be significantly different between transplant recipients with ADPKD and non-diabetic transplant recipients (124). The severity, rather than occurrence, of urinary tract infections are more significant in post-transplant recipients with ADPKD compared to their non-diabetic cohorts (124).
In a different report of eleven transplant cases in hypertensive ADPKD, six patients showed improved blood pressure after transplantation (125). Improved blood pressure was defined as the ability to reduce antihypertensive drug treatment after renal transplantation. These results suggest that while renal transplantation seems to have some beneficial outcomes, it is not sufficient to eradicate the hypertension in ADPKD patients. In an observational study on 312 children with ADPKD, it is further reported that high blood pressure promotes a faster renal volume growth (126). ADPKD children with high blood pressure have faster renal growth than those with lower blood pressure. This suggests that hypertension is a risk factor independent from kidney function in ADPKD. Hence, therapeutic interventions in controlling blood pressure remain necessary and beneficial for ADPKD patients, regardless of kidney function or transplant.
Novel therapy
Although ciliary therapy does not exist today, it is appealing and tempting to speculate the possibility of treatment for localized blood vessel injuries such as aneurysm, atherosclerosis, dissection, edema, hemorrhage, and vascular ectasia, among others in ADPKD. In particular, endothelium-dependent relaxation is impaired, and endothelial nitric oxide synthase activity is decreased in patients with ADPKD (63, 69, 127). The endothelial dysfunction due to impaired release of NO in ADPKD patients becomes a crucial pathogenesis of hypertension. The imbalance in endothelium-derived vasoactive mediators might therefore need to be considered seriously in ADPKD patients (70, 128).
Of particular interest is a high level of polycystin expression in the vascular system, which is required for the structural integrity of blood vessels (72, 129-131). The expression of polycystins in human endothelial cilia further provides a critical link between cilia and the vasculature (20, 25, 89). Although there are only limited reports on ciliary polycystins in vascular endothelial cells, abnormal expressions of polycystins are known to prevent normal cilia function that converts changes in fluid flow into biosynthesis of NO in interlobar arteries obtained from ADPKD patients (20).
Interestingly, changes in extracellular fluid flow can either activate or inactivate cilia function through polycystin-1 and polycystin-2 complex (25). A sudden increase in blood flow, as seen in daily fluctuation of blood pressure, would activate cilia through polycystin complex. This, in turn, would allow calcium influx and initiate a series of biochemical cascades that lead to NO production (Figure 3). NO is a very potent physiological vasodilator that would decrease the baseline tension (contraction) of the vessel, and thus decrease vascular pressure. Subsequently, the dilation of the vessel during a sudden increase in blood pressure is a necessary local regulation to avoid focal injury of the blood vessel.
On the other hand, prolonged exposure to higher blood flow as seen in chronic hypertension would inactivate cilia by down-regulating functional polycystin-1 (25). Functional polycystin-1 as a mechanosensory molecule can be inactivated by proteolytic cleavage after exposure to high fluid-shear stress. This may further suggest that in patients with high blood pressure, cilia would very unlikely sense minute changes in blood pressure, which might result in failure to provide a mechanism to autoregulate the local circulatory system. In addition, the presence of cilia in vascular smooth muscle cells has also been reported, and sensory polycystin-1 and polycystin-2 complex is localized in the cilia (132). Although their roles are not clear at present, the vascular cilia are positioned in such a way that they maintain a specific alignment with respect to the lumen of the artery. Further studies of the role of this alignment may be necessary to shed light on their possible functions with regard to vascular hypertension.
Current clinical trial in hypertensive ADPKD
There is a wealth of evidence to indicate that aggressive blood pressure control in ADPKD patients is a clinical necessity, regardless of kidney function. Drugs that inhibit the RAAS might be beneficial in this context. However, more robust clinical studies are essential to improve clinical outcomes with greater certainty. In particular, we need a more comprehensive clinical study to provide a greater number of subjects, longer follow-up time with clear primary endpoints, and assessment of PKD1 or PKD2 mutation either through sequence analysis or radiological imaging studies (133). An example of such a comprehensive study that is currently underway is HALT Progression of Polycystic Kidney Disease (HALT PKD), which is a clinical trial evaluating the effectiveness of inhibiting RAAS in blood pressure control. The study will also determine whether a combined therapy of an ACE-I and an ARB would be superior than monotherapy ACE-I alone to delay the progression of renal disease and whether a more “rigorous” blood pressure (<110/75) would be more effective than standard blood pressure target (<130/80) in preserving renal function (5). There is certainly much work remaining, and we are on the verge of conjuring and applying our concept and understanding of ADPKD for a better clinical practice and patient outcomes.
Acknowledgements
The work from our laboratory that is cited in this review has been provided by grants from the NIH, AHA, and The University of Toledo research programs. The human tissue collection has been supported by The University of Toledo Medical Center (http://utmc.utoledo.edu), PKD Foundation (http://www.pkdcure.org) and assistance from Maki Takahashi and Christine M. Horvat. We thank Charisse Montgomery for her editorial review of the manuscript. Due to the space limitation, we apologize to those whose work is not described in this review manuscript.
Footnotes
Conflict of Interest
None declared.
References
- 1.US Renal Data System: USRDS 2006 Annual Data Report. The National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Disease; Bethesda, MD.: 2006. [Google Scholar]
- 2.Contemporary Dialysis & Nephrology Magazine. Ashlee Publishing Co., Inc.; 18 East 41st Street, New York, NY: Oct, 1998. [Google Scholar]
- 3.US Renal Data System: USRDS 2008 Annual Data Report. The National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Disease; Bethesda, MD.: 2008. [Google Scholar]
- 4.Braun WE. Autosomal dominant polycystic kidney disease: emerging concepts of pathogenesis and new treatments. Cleve Clin J Med. 2009;76:97–104. doi: 10.3949/ccjm.76a.gr001. [DOI] [PubMed] [Google Scholar]
- 5.Chapman AB. Approaches to testing new treatments in autosomal dominant polycystic kidney disease: insights from the CRISP and HALT-PKD studies. Clin J Am Soc Nephrol. 2008;3:1197–1204. doi: 10.2215/CJN.00060108. [DOI] [PubMed] [Google Scholar]
- 6.Chapman AB, Guay-Woodford LM, Grantham JJ, et al. Renal structure in early autosomal-dominant polycystic kidney disease (ADPKD): The Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease (CRISP) cohort. Kidney Int. 2003;64:1035–45. doi: 10.1046/j.1523-1755.2003.00185.x. [DOI] [PubMed] [Google Scholar]
- 7.Biagini A, Maffei S, Baroni M, et al. Familiar clustering of aortic dissection in polycystic kidney disease. Am J Cardiol. 1993;72:741–2. doi: 10.1016/0002-9149(93)90896-k. [DOI] [PubMed] [Google Scholar]
- 8.Capisonda R, Phan V, Traubuci J, Daneman A, Balfe JW, Guay-Woodford LM. Autosomal recessive polycystic kidney disease: outcomes from a single-center experience. Pediatr Nephrol. 2003;18:119–26. doi: 10.1007/s00467-002-1021-0. [DOI] [PubMed] [Google Scholar]
- 9.Gabow PA. Autosomal dominant polycystic kidney disease. N Engl J Med. 1993;329:332–42. doi: 10.1056/NEJM199307293290508. [DOI] [PubMed] [Google Scholar]
- 10.Roy S, Dillon MJ, Trompeter RS, Barratt TM. Autosomal recessive polycystic kidney disease: long-term outcome of neonatal survivors. Pediatr Nephrol. 1997;11:302–6. doi: 10.1007/s004670050281. [DOI] [PubMed] [Google Scholar]
- 11.Torres VE, Harris PC. Mechanisms of Disease: autosomal dominant and recessive polycystic kidney diseases. Nat Clin Pract Nephrol. 2006;2:40–55. doi: 10.1038/ncpneph0070. quiz 55. [DOI] [PubMed] [Google Scholar]
- 12.Fick GM, Johnson AM, Hammond WS, Gabow PA. Causes of death in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 1995;5:2048–56. doi: 10.1681/ASN.V5122048. [DOI] [PubMed] [Google Scholar]
- 13.Guay-Woodford LM. Renal cystic diseases: diverse pheno-types converge on the cilium/centrosome complex. Pediatr Nephrol. 2006;21:1369–76. doi: 10.1007/s00467-006-0164-9. [DOI] [PubMed] [Google Scholar]
- 14.Lilova MI, Petkov DL. Intracranial aneurysms in a child with autosomal recessive polycystic kidney disease. Pediatr Nephrol. 2001;16:1030–2. doi: 10.1007/s004670100019. [DOI] [PubMed] [Google Scholar]
- 15.Ecder T, Schrier RW. Cardiovascular abnormalities in autosomal-dominant polycystic kidney disease. Nat Rev Nephrol. 2009;5:221–8. doi: 10.1038/nrneph.2009.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perrone RD. Extrarenal manifestations of ADPKD. Kidney Int. 1997;51:2022–36. doi: 10.1038/ki.1997.276. [DOI] [PubMed] [Google Scholar]
- 17.Chapman AB, Rubinstein D, Hughes R, et al. Intracranial aneurysms in autosomal dominant polycystic kidney disease. N Engl J Med. 1992;327:916–20. doi: 10.1056/NEJM199209243271303. [DOI] [PubMed] [Google Scholar]
- 18.Belz MM, Hughes RL, Kaehny WD, et al. Familial clustering of ruptured intracranial aneurysms in autosomal dominant polycystic kidney disease. Am J Kidney Dis. 2001;38:770–6. doi: 10.1053/ajkd.2001.27694. [DOI] [PubMed] [Google Scholar]
- 19.Chauveau D, Pirson Y, Verellen-Dumoulin C, Macnicol A, Gonzalo A, Grunfeld JP. Intracranial aneurysms in autosomal dominant polycystic kidney disease. Kidney Int. 1994;45:1140–6. doi: 10.1038/ki.1994.151. [DOI] [PubMed] [Google Scholar]
- 20.AbouAlaiwi WA, Takahashi M, Mell BR, et al. Ciliary polycystin-2 is a mechanosensitive calcium channel involved in nitric oxide signaling cascades. Circ Res. 2009;104:860–9. doi: 10.1161/CIRCRESAHA.108.192765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hou B, Kolpakova-Hart E, Fukai N, Wu K, Olsen BR. The polycystic kidney disease 1 (Pkd1) gene is required for the responses of osteochondroprogenitor cells to midpalatal suture expansion in mice. Bone. 2009;44:1121–33. doi: 10.1016/j.bone.2009.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Masyuk AI, Masyuk TV, Splinter PL, Huang BQ, Stroope AJ, LaRusso NF. Cholangiocyte cilia detect changes in luminal fluid flow and transmit them into intracellular Ca2+ and cAMP signaling. Gastroenterology. 2006;131:911–20. doi: 10.1053/j.gastro.2006.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McGrath J, Somlo S, Makova S, Tian X, Brueckner M. Two populations of node monocilia initiate left-right asymmetry in the mouse. Cell. 2003;114:61–73. doi: 10.1016/s0092-8674(03)00511-7. [DOI] [PubMed] [Google Scholar]
- 24.Nauli SM, Alenghat FJ, Luo Y, et al. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet. 2003;33:129–37. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- 25.Nauli SM, Kawanabe Y, Kaminski JJ, Pearce WJ, Ingber DE, Zhou J. Endothelial cilia are fluid shear sensors that regulate calcium signaling and nitric oxide production through polycystin-1. Circulation. 2008;117:1161–71. doi: 10.1161/CIRCULATIONAHA.107.710111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nauli SM, Rossetti S, Kolb RJ, et al. Loss of polycystin-1 in human cyst-lining epithelia leads to ciliary dysfunction. J Am Soc Nephrol. 2006;17:1015–25. doi: 10.1681/ASN.2005080830. [DOI] [PubMed] [Google Scholar]
- 27.Xiao Z, Zhang S, Mahlios J, Zhou G, et al. Cilia-like structures and polycystin-1 in osteoblasts/osteocytes and associated abnormalities in skeletogenesis and Runx2 expression. J Biol Chem. 2006;281:30884–95. doi: 10.1074/jbc.M604772200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu C, Rossetti S, Jiang L, et al. Human ADPKD primary cyst epithelial cells with a novel, single codon deletion in the PKD1 gene exhibit defective ciliary polycystin localization and loss of flow-induced Ca2+ signaling. Am J Physiol Renal Physiol. 2007;292:F930–45. doi: 10.1152/ajprenal.00285.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu C, Shmukler BE, Nishimura K, et al. Attenuated, flow-induced ATP release contributes to absence of flow-sensitive, purinergic Cai2+ signaling in human ADPKD cyst epithelial cells. Am J Physiol Renal Physiol. 2009;296:F1464–76. doi: 10.1152/ajprenal.90542.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hateboer N, v Dijk MA, Bogdanova N, et al. Comparison of phenotypes of polycystic kidney disease types 1 and 2. European PKD1-PKD2 Study Group. Lancet. 1999;353:103–7. doi: 10.1016/s0140-6736(98)03495-3. [DOI] [PubMed] [Google Scholar]
- 31.Torres VE, Harris PC. Autosomal dominant polycystic kidney disease: the last 3 years. Kidney Int. 2009;76:149–68. doi: 10.1038/ki.2009.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hildebrandt F, Otto E. Cilia and centrosomes: a unifying pathogenic concept for cystic kidney disease? Nat Rev Genet. 2005;6:928–40. doi: 10.1038/nrg1727. [DOI] [PubMed] [Google Scholar]
- 33.Kolb RJ, Nauli SM. Ciliary dysfunction in polycystic kidney disease: an emerging model with polarizing potential. Front Biosci. 2008;13:4451–66. doi: 10.2741/3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nauli SM, Zhou J. Polycystins and mechanosensation in renal and nodal cilia. Bioessays. 2004;26:844–56. doi: 10.1002/bies.20069. [DOI] [PubMed] [Google Scholar]
- 35.Pazour GJ. Intraflagellar transport and cilia-dependent renal disease: the ciliary hypothesis of polycystic kidney disease. J Am Soc Nephrol. 2004;15:2528–36. doi: 10.1097/01.ASN.0000141055.57643.E0. [DOI] [PubMed] [Google Scholar]
- 36.Zhou J. Polycystins and primary cilia: primers for cell cycle progression. Annu Rev Physiol. 2009;71:83–113. doi: 10.1146/annurev.physiol.70.113006.100621. [DOI] [PubMed] [Google Scholar]
- 37.Lee YR, Lee KB. Reliability of magnetic resonance imaging for measuring the volumetric indices in autosomal-dominant polycystic kidney disease: correlation with hypertension and renal function. Nephron Clin Pract. 2006;103:c173–80. doi: 10.1159/000092915. [DOI] [PubMed] [Google Scholar]
- 38.Grantham JJ, Cook LT, Torres VE, et al. Determinants of renal volume in autosomal-dominant polycystic kidney disease. Kidney Int. 2008;73:108–16. doi: 10.1038/sj.ki.5002624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grantham JJ, Torres VE, Chapman AB, et al. Volume progression in polycystic kidney disease. N Engl J Med. 2006;354:2122–30. doi: 10.1056/NEJMoa054341. [DOI] [PubMed] [Google Scholar]
- 40.Kaspareit-Rittinghausen J, Deerberg F, Rapp KG, Wcislo A. Renal hypertension in rats with hereditary polycystic kidney disease. Z Versuchstierkd. 1990;33:201–4. [PubMed] [Google Scholar]
- 41.Lavoie JL, Lake-Bruse KD, Sigmund CD. Increased blood pressure in transgenic mice expressing both human renin and angiotensinogen in the renal proximal tubule. Am J Physiol Renal Physiol. 2004;286:F965–71. doi: 10.1152/ajprenal.00402.2003. [DOI] [PubMed] [Google Scholar]
- 42.Ramunni A, Saracino A, Esposito T, Saliani MT, Coratelli P. Renal vascular resistance and renin-angiotensin system in the pathogenesis of early hypertension in auto-somal dominant polycystic kidney disease. Hypertens Res. 2004;27:221–5. doi: 10.1291/hypres.27.221. [DOI] [PubMed] [Google Scholar]
- 43.Azurmendi PJ, Fraga AR, Galan FM. Early renal and vascular changes in ADPKD patients with low-grade albumin excretion and normal renal function. Nephrol Dial Transplant. 2009;24:2458–63. doi: 10.1093/ndt/gfp136. [DOI] [PubMed] [Google Scholar]
- 44.Itty CT, Farshid A, Talaulikar G. Spontaneous coronary artery dissection in a woman with polycystic kidney disease. Am J Kidney Dis. 2009;53:518–21. doi: 10.1053/j.ajkd.2008.08.027. [DOI] [PubMed] [Google Scholar]
- 45.Rong S, Jin X, Ye C, Chen J, Mei C. Carotid vascular remodelling in patients with autosomal dominant polycystic kidney disease. Nephrology (Carlton) 2009;14:113–7. doi: 10.1111/j.1440-1797.2008.01049.x. [DOI] [PubMed] [Google Scholar]
- 46.Sawicki M, Walecka A, Rozanski J, Safranow K, Ciechanowski K. Doppler sonography measurements of renal vascular resistance in autosomal-dominant polycystic kidney disease. Med Sci Monit. 2009;15:MT101–4. [PubMed] [Google Scholar]
- 47.Turkmen K, Oflaz H, Uslu B, et al. Coronary flow velocity reserve and carotid intima media thickness in patients with autosomal dominant polycystic kidney disease: from impaired tubules to impaired carotid and coronary arteries. Clin J Am Soc Nephrol. 2008;3:986–91. doi: 10.2215/CJN.02330607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arnaout MA. Molecular genetics and pathogenesis of autosomal dominant polycystic kidney disease. Annu Rev Med. 2001;52:93–123. doi: 10.1146/annurev.med.52.1.93. [DOI] [PubMed] [Google Scholar]
- 49.Boulter C, Mulroy S, Webb S, Fleming S, Brindle K, Sandford R. Cardiovascular, skeletal, and renal defects in mice with a targeted disruption of the Pkd1 gene. Proc Natl Acad Sci U S A. 2001;98:12174–9. doi: 10.1073/pnas.211191098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim K, Drummond I, Ibraghimov-Beskrovnaya O, Klinger K, Arnaout MA. Polycystin 1 is required for the structural integrity of blood vessels. Proc Natl Acad Sci U S A. 2000;97:1731–6. doi: 10.1073/pnas.040550097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Muto S, Aiba A, Saito Y, et al. Pioglitazone improves the phenotype and molecular defects of a targeted Pkd1 mutant. Hum Mol Genet. 2002;11:1731–42. doi: 10.1093/hmg/11.15.1731. [DOI] [PubMed] [Google Scholar]
- 52.Wu G, Markowitz GS, Li L, et al. Cardiac defects and renal failure in mice with targeted mutations in Pkd2. Nat Genet. 2000;24:75–8. doi: 10.1038/71724. [DOI] [PubMed] [Google Scholar]
- 53.Chapman AB, Gabow PA. Hypertension in autosomal dominant polycystic kidney disease. Kidney Int Suppl. 1997;61:S71–3. [PubMed] [Google Scholar]
- 54.McPherson EA, Luo Z, Brown RA, et al. Chymase-like angiotensin II-generating activity in end-stage human autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2004;15:493–500. doi: 10.1097/01.asn.0000109782.28991.26. [DOI] [PubMed] [Google Scholar]
- 55.Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med. 1993;329:1456–62. doi: 10.1056/NEJM199311113292004. [DOI] [PubMed] [Google Scholar]
- 56.Brenner BM, Cooper ME, de Zeeuw D, et al. Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345:861–9. doi: 10.1056/NEJMoa011161. [DOI] [PubMed] [Google Scholar]
- 57.Ecder T, Edelstein CL, Fick-Brosnahan GM, et al. Diuretics versus angiotensin-converting enzyme inhibitors in autosomal dominant polycystic kidney disease. Am J Nephrol. 2001;21:98–103. doi: 10.1159/000046231. [DOI] [PubMed] [Google Scholar]
- 58.Jafar TH, Stark PC, Schmid CH, et al. The effect of angiotensin-converting-enzyme inhibitors on progression of advanced polycystic kidney disease. Kidney Int. 2005;67:265–71. doi: 10.1111/j.1523-1755.2005.00077.x. [DOI] [PubMed] [Google Scholar]
- 59.Lawson CR, Doulton TW, MacGregor GA. Autosomal dominant polycystic kidney disease: role of the reninangiotensin system in raised blood pressure in progression of renal and cardiovascular disease. J Renin Angiotensin Aldosterone Syst. 2006;7:139–45. doi: 10.3317/jraas.2006.023. [DOI] [PubMed] [Google Scholar]
- 60.Cadnapaphornchai MA, McFann K, Strain JD, Masoumi A, Schrier RW. Increased left ventricular mass in children with autosomal dominant polycystic kidney disease and borderline hypertension. Kidney Int. 2008;74:1192–6. doi: 10.1038/ki.2008.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schrier RW, Johnson AM, McFann K, Chapman AB. The role of parental hypertension in the frequency and age of diagnosis of hypertension in offspring with autosomal-dominant polycystic kidney disease. Kidney Int. 2003;64:1792–9. doi: 10.1046/j.1523-1755.2003.00264.x. [DOI] [PubMed] [Google Scholar]
- 62.Giner V, Poch E, Bragulat E, Oriola J, Gonzalez D, Coca A, De La Sierra A. Renin-angiotensin system genetic polymorphisms and salt sensitivity in essential hypertension. Hypertension. 2000;35:512–7. doi: 10.1161/01.hyp.35.1.512. [DOI] [PubMed] [Google Scholar]
- 63.Wang D, Strandgaard S. The pathogenesis of hypertension in autosomal dominant polycystic kidney disease. J Hypertens. 1997;15:925–33. doi: 10.1097/00004872-199715090-00002. [DOI] [PubMed] [Google Scholar]
- 64.Klein IH, Ligtenberg G, Oey PL, Koomans HA, Blankestijn PJ. Sympathetic activity is increased in polycystic kidney disease and is associated with hypertension. J Am Soc Nephrol. 2001;12:2427–33. doi: 10.1681/ASN.V12112427. [DOI] [PubMed] [Google Scholar]
- 65.Cowley BD, Jr., Ricardo SD, Nagao S, Diamond JR. Increased renal expression of monocyte chemoattractant protein-1 and osteopontin in ADPKD in rats. Kidney Int. 2001;60:2087–96. doi: 10.1046/j.1523-1755.2001.00065.x. [DOI] [PubMed] [Google Scholar]
- 66.Zheng D, Wolfe M, Cowley BD, Jr., Wallace DP, Yamaguchi T, Grantham JJ. Urinary excretion of monocyte chemoattractant protein-1 in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2003;14:2588–95. doi: 10.1097/01.asn.0000088720.61783.19. [DOI] [PubMed] [Google Scholar]
- 67.Kocaman O, Oflaz H, Yekeler E, et al. Endothelial dys-function and increased carotid intima-media thickness in patients with autosomal dominant polycystic kidney disease. Am J Kidney Dis. 2004;43:854–60. doi: 10.1053/j.ajkd.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 68.Persu A, Stoenoiu MS, Messiaen T, et al. Modifier effect of ENOS in autosomal dominant polycystic kidney disease. Hum Mol Genet. 2002;11:229–41. doi: 10.1093/hmg/11.3.229. [DOI] [PubMed] [Google Scholar]
- 69.Wang D, Iversen J, Strandgaard S. Endothelium-dependent relaxation of small resistance vessels is impaired in patients with autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2000;11:1371–6. doi: 10.1681/ASN.V1181371. [DOI] [PubMed] [Google Scholar]
- 70.Al-Nimri MA, Komers R, Oyama TT, Subramanya AR, Lindsley JN, Anderson S. Endothelial-derived vasoactive mediators in polycystic kidney disease. Kidney Int. 2003;63:1776–84. doi: 10.1046/j.1523-1755.2003.00913.x. [DOI] [PubMed] [Google Scholar]
- 71.Griffin MD, Torres VE, Grande JP, Kumar R. Vascular expression of polycystin. J Am Soc Nephrol. 1997;8:616–26. doi: 10.1681/ASN.V84616. [DOI] [PubMed] [Google Scholar]
- 72.Torres VE, Cai Y, Chen X, et al. Vascular expression of polycystin-2. J Am Soc Nephrol. 2001;12:1–9. doi: 10.1681/ASN.V1211. [DOI] [PubMed] [Google Scholar]
- 73.Qian Q, Li M, Cai Y, et al. Analysis of the polycystins in aortic vascular smooth muscle cells. J Am Soc Nephrol. 2003;14:2280–7. doi: 10.1097/01.asn.0000080185.38113.a3. [DOI] [PubMed] [Google Scholar]
- 74.Arribas SM, Hillier C, Gonzalez C, McGrory S, Dominic-zak AF, McGrath JC. Cellular aspects of vascular remodeling in hypertension revealed by confocal microscopy. Hypertension. 1997;30:1455–64. doi: 10.1161/01.hyp.30.6.1455. [DOI] [PubMed] [Google Scholar]
- 75.Heerkens EH, Izzard AS, Heagerty AM. Integrins, vascular remodeling, and hypertension. Hypertension. 2007;49:1–4. doi: 10.1161/01.HYP.0000252753.63224.3b. [DOI] [PubMed] [Google Scholar]
- 76.Madeddu P, Emanueli C, El-Dahr S. Mechanisms of disease: the tissue kallikrein-kinin system in hypertension and vascular remodeling. Nat Clin Pract Nep. 2007;3:208–21. doi: 10.1038/ncpneph0444. [DOI] [PubMed] [Google Scholar]
- 77.Johns C, Gavras I, Handy DE, Salomao A, Gavras H. Models of experimental hypertension in mice. Hypertension. 1996;28:1064–9. doi: 10.1161/01.hyp.28.6.1064. [DOI] [PubMed] [Google Scholar]
- 78.Fliser D, Pacini G, Engelleiter R, et al. Insulin resistance and hyperinsulinemia are already present in patients with incipient renal disease. Kidney Int. 1998;53:1343–7. doi: 10.1046/j.1523-1755.1998.00898.x. [DOI] [PubMed] [Google Scholar]
- 79.Lumiaho A, Pihlajamaki J, Hartikainen J, et al. Insulin resistance is related to left ventricular hypertrophy in patients with polycystic kidney disease type 1. Am J Kidney Dis. 2003;41:1219–24. doi: 10.1016/s0272-6386(03)00354-8. [DOI] [PubMed] [Google Scholar]
- 80.Ishii M, Ikeda T, Takagi M, et al. Elevated plasma catecholamines in hypertensives with primary glomerular diseases. Hypertension. 1983;5:545–51. doi: 10.1161/01.hyp.5.4.545. [DOI] [PubMed] [Google Scholar]
- 81.Sarafidis PA, Bakris GL. Review: Insulin and endothelin: an interplay contributing to hypertension development? J Clin Endocrinol Metab. 2007;92:379–85. doi: 10.1210/jc.2006-1819. [DOI] [PubMed] [Google Scholar]
- 82.Clausen P, Feldt-Rasmussen B, Iversen J, Lange M, Eidemak I, Strandgaard S. Flow-associated dilatory capacity of the brachial artery is intact in early autosomal dominant polycystic kidney disease. Am J Nephrol. 2006;26:335–9. doi: 10.1159/000094402. [DOI] [PubMed] [Google Scholar]
- 83.Wang D, Strandgaard S, Borresen ML, et al. Asymmetric dimethylarginine and lipid peroxidation products in early autosomal dominant polycystic kidney disease. Am J Kidney Dis. 2008;51:184–91. doi: 10.1053/j.ajkd.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 84.Hierck BP, Van der Heiden K, Alkemade FE, et al. Primary cilia sensitize endothelial cells for fluid shear stress. Dev Dyn. 2008;237:725–35. doi: 10.1002/dvdy.21472. [DOI] [PubMed] [Google Scholar]
- 85.Poelmann RE, Van der Heiden K, Gittenberger-de Groot A, Hierck BP. Deciphering the endothelial shear stress sensor. Circulation. 2008;117:1124–6. doi: 10.1161/CIRCULATIONAHA.107.753889. [DOI] [PubMed] [Google Scholar]
- 86.Van der Heiden K, Groenendijk BC, Hierck BP, et al. Monocilia on chicken embryonic endocardium in low shear stress areas. Dev Dyn. 2006;235:19–28. doi: 10.1002/dvdy.20557. [DOI] [PubMed] [Google Scholar]
- 87.Van der Heiden K, Hierck BP, Krams R, et al. Endothelial primary cilia in areas of disturbed flow are at the base of atherosclerosis. Atherosclerosis. 2008;196:542–50. doi: 10.1016/j.atherosclerosis.2007.05.030. [DOI] [PubMed] [Google Scholar]
- 88.Greisen G. Autoregulation of cerebral blood flow in newborn babies. Early Hum Dev. 2005;81:423–8. doi: 10.1016/j.earlhumdev.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 89.Iomini C, Tejada K, Mo W, Vaananen H, Piperno G. Primary cilia of human endothelial cells disassemble under laminar shear stress. J Cell Biol. 2004;164:811–7. doi: 10.1083/jcb.200312133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Feletou M, Vanhoutte PM. Endothelium-dependent hyperpolarizations: past beliefs and present facts. Ann Med. 2007;39:495–516. doi: 10.1080/07853890701491000. [DOI] [PubMed] [Google Scholar]
- 91.Ignarro LJ, Cirino G, Casini A, Napoli C. Nitric oxide as a signaling molecule in the vascular system: an overview. J Cardiovasc Pharmacol. 1999;34:879–86. doi: 10.1097/00005344-199912000-00016. [DOI] [PubMed] [Google Scholar]
- 92.Nakane M. Soluble guanylyl cyclase: physiological role as an NO receptor and the potential molecular target for therapeutic application. Clin Chem Lab Med. 2003;41:865–70. doi: 10.1515/CCLM.2003.131. [DOI] [PubMed] [Google Scholar]
- 93.Hateboer N, Veldhuisen B, Peters D, et al. Location of mutations within the PKD2 gene influences clinical outcome. Kidney Int. 2000;57:1444–51. doi: 10.1046/j.1523-1755.2000.00989.x. [DOI] [PubMed] [Google Scholar]
- 94.Rossetti S, Harris PC. Genotype-phenotype correlations in autosomal dominant and autosomal recessive polycystic kidney disease. J Am Soc Nephrol. 2007;18:1374–80. doi: 10.1681/ASN.2007010125. [DOI] [PubMed] [Google Scholar]
- 95.Kelleher CL, McFann KK, Johnson AM, Schrier RW. Characteristics of hypertension in young adults with autosomal dominant polycystic kidney disease compared with the general U.S. population. Am J Hypertens. 2004;17:1029–34. doi: 10.1016/j.amjhyper.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 96.Sedman A, Bell P, Manco-Johnson M, et al. Auto-somal dominant polycystic kidney disease in childhood: a longitudinal study. Kidney Int. 1987;31:1000–5. doi: 10.1038/ki.1987.98. [DOI] [PubMed] [Google Scholar]
- 97.Fick GM, Duley IT, Johnson AM, Strain JD, Manco-Johnson ML, Gabow PA. The spectrum of autosomal dominant polycystic kidney disease in children. J Am Soc Nephrol. 1994;4:1654–60. doi: 10.1681/ASN.V491654. [DOI] [PubMed] [Google Scholar]
- 98.Ivy DD, Shaffer EM, Johnson AM, Kimberling WJ, Dobin A, Gabow PA. Cardiovascular abnormalities in children with autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 1995;5:2032–6. doi: 10.1681/ASN.V5122032. [DOI] [PubMed] [Google Scholar]
- 99.Shamshirsaz AA, Reza Bekheirnia M, Kamgar M, et al. Autosomal-dominant polycystic kidney disease in infancy and childhood: progression and outcome. Kidney Int. 2005;68:2218–24. doi: 10.1111/j.1523-1755.2005.00678.x. [DOI] [PubMed] [Google Scholar]
- 100.Chapman AB, Schrier RW. Pathogenesis of hypertension in autosomal dominant polycystic kidney disease. Semin Nephrol. 1991;11:653–60. [PubMed] [Google Scholar]
- 101.Ecder T, Schrier RW. Hypertension in autosomal-dominant polycystic kidney disease: early occurrence and unique aspects. J Am Soc Nephrol. 2001;12:194–200. doi: 10.1681/ASN.V121194. [DOI] [PubMed] [Google Scholar]
- 102.Gabow PA, Chapman AB, Johnson AM, et al. Renal structure and hypertension in autosomal dominant polycystic kidney disease. Kidney Int. 1990;38:1177–80. doi: 10.1038/ki.1990.330. [DOI] [PubMed] [Google Scholar]
- 103.Gonzalo A, Gallego A, Rivera M, Orte L, Ortuno J. Influence of hypertension on early renal insufficiency in autosomal dominant polycystic kidney disease. Nephron. 1996;72:225–30. doi: 10.1159/000188846. [DOI] [PubMed] [Google Scholar]
- 104.Keith DS, Torres VE, Johnson CM, Holley KE. Effect of sodium chloride, enalapril, and losartan on the development of polycystic kidney disease in Han:SPRD rats. Am J Kidney Dis. 1994;24:491–8. doi: 10.1016/s0272-6386(12)80907-3. [DOI] [PubMed] [Google Scholar]
- 105.Schrier R, McFann K, Johnson A, et al. Cardiac and renal effects of standard versus rigorous blood pressure control in autosomal-dominant polycystic kidney disease: results of a seven-year prospective randomized study. J Am Soc Nephrol. 2002;13:1733–9. doi: 10.1097/01.asn.0000018407.60002.b9. [DOI] [PubMed] [Google Scholar]
- 106.van Dijk MA, Breuning MH, Duiser R, van Es LA, Westendorp RG. No effect of enalapril on progression in autosomal dominant polycystic kidney disease. Nephrol Dial Transplant. 2003;18:2314–20. doi: 10.1093/ndt/gfg417. [DOI] [PubMed] [Google Scholar]
- 107.Nakamura T, Sugaya T, Kawagoe Y, Ueda Y, Osada S, Koide H. Candesartan reduces urinary fatty acid-binding protein excretion in patients with autosomal dominant polycystic kidney disease. Am j Med Sci. 2005;330:161–5. doi: 10.1097/00000441-200510000-00002. [DOI] [PubMed] [Google Scholar]
- 108.Neumann J, Ligtenberg G, Klein IH, Blankestijn PJ. Pathogenesis and treatment of hypertension in polycystic kidney disease. Curr Opin Nephrol Hypertens. 2002;11:517–21. doi: 10.1097/00041552-200209000-00007. [DOI] [PubMed] [Google Scholar]
- 109.Mancia G, Dell'Oro R, Quarti-Trevano F, Scopelliti F, Grassi G. Angiotensin-sympathetic system interactions in cardiovascular and metabolic disease. J Hypertens Suppl. 2006;24:S51–6. doi: 10.1097/01.hjh.0000220407.84363.fb. [DOI] [PubMed] [Google Scholar]
- 110.van den Meiracker AH, Boomsma F. The angiotensin II-sympathetic nervous system connection. J Hypertens. 2003;21:1453–4. doi: 10.1097/00004872-200308000-00005. [DOI] [PubMed] [Google Scholar]
- 111.Gattone VH, 2nd, Siqueira TM, Jr, Powell CR, Trambaugh CM, Lingeman JE, Shalhav AL. Contribution of renal innervation to hypertension in rat autosomal dominant polycystic kidney disease. Exp Biol Med (Maywood) 2008;233:952–7. doi: 10.3181/0802-RM-54. [DOI] [PubMed] [Google Scholar]
- 112.Zeltner R, Poliak R, Stiasny B, Schmieder RE, Schulze BD. Renal and cardiac effects of antihypertensive treatment with ramipril vs metoprolol in autosomal dominant polycystic kidney disease. Nephrol Dial Transplant. 2008;23:573–9. doi: 10.1093/ndt/gfm731. [DOI] [PubMed] [Google Scholar]
- 113.Masoumi A, Reed-Gitomer B, Kelleher C, Bekheirnia MR, Schrier RW. Developments in the management of autosomal dominant polycystic kidney disease. Ther Clin Risk Manag. 2008;4:393–407. doi: 10.2147/tcrm.s1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kanno Y, Suzuki H, Okada H, Takenaka T, Saruta T. Calcium channel blockers versus ACE inhibitors as anti-hypertensives in polycystic kidney disease. QJM. 1996;89:65–70. doi: 10.1093/oxfordjournals.qjmed.a030139. [DOI] [PubMed] [Google Scholar]
- 115.Nutahara K, Higashihara E, Horie S, et al. Calcium channel blocker versus angiotensin II receptor blocker in autosomal dominant polycystic kidney disease. Nephron Clin Pract. 2005;99:c18–23. doi: 10.1159/000081790. [DOI] [PubMed] [Google Scholar]
- 116.Torres VE. Role of vasopressin antagonists. Clin J Am Soc Nephrol. 2008;3:1212–8. doi: 10.2215/CJN.05281107. [DOI] [PubMed] [Google Scholar]
- 117.K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–266. [PubMed] [Google Scholar]
- 118.K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis. 2004;43:S1–290. [PubMed] [Google Scholar]
- 119.Chobanian AV, Bakris GL, Black HR, et al. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA. 2003;289:2560–72. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 120.Chapman AB, Johnson A, Gabow PA, Schrier RW. The renin-angiotensin-aldosterone system and auto-somal dominant polycystic kidney disease. N Engl J Med. 1990;323:1091–6. doi: 10.1056/NEJM199010183231602. [DOI] [PubMed] [Google Scholar]
- 121.Ecder T, Chapman AB, Brosnahan GM, Edelstein CL, Johnson AM, Schrier RW. Effect of antihypertensive therapy on renal function and urinary albumin excretion in hypertensive patients with autosomal dominant polycystic kidney disease. Am J Kidney Dis. 2000;35:427–32. doi: 10.1016/s0272-6386(00)70195-8. [DOI] [PubMed] [Google Scholar]
- 122.Graham PC, Lindop GB. The anatomy of the renin-secreting cell in adult polycystic kidney disease. Kidney Int. 1988;33:1084–90. doi: 10.1038/ki.1988.115. [DOI] [PubMed] [Google Scholar]
- 123.Florijn KW, Chang PC, van der Woude FJ, van Bockel JH, van Saase JL. Long-term cardiovascular morbidity and mortality in autosomal dominant polycystic kidney disease patients after renal transplantation. Transplantation. 1994;57:73–81. doi: 10.1097/00007890-199401000-00014. [DOI] [PubMed] [Google Scholar]
- 124.Stiasny B, Ziebell D, Graf S, Hauser IA, Schulze BD. Clinical aspects of renal transplantation in polycystic kidney disease. Clin Nephrol. 2002;58:16–24. doi: 10.5414/cnp58016. [DOI] [PubMed] [Google Scholar]
- 125.Shiroyanagi Y, Tanabe K, Hashimoto Y, et al. Kidney transplantation in the recipient with autosomal-dominant polycystic kidney disease: a single center experience. Transplant Proc. 2000;32:1841–3. doi: 10.1016/s0041-1345(00)01457-3. [DOI] [PubMed] [Google Scholar]
- 126.Fick-Brosnahan GM, Tran ZV, Johnson AM, Strain JD, Gabow PA. Progression of autosomal-dominant polycystic kidney disease in children. Kidney Int. 2001;59:1654–62. doi: 10.1046/j.1523-1755.2001.0590051654.x. [DOI] [PubMed] [Google Scholar]
- 127.Wang D, Iversen J, Wilcox CS, Strandgaard S. Endothelial dysfunction and reduced nitric oxide in resistance arteries in autosomal-dominant polycystic kidney disease. Kidney Int. 2003;64:1381–8. doi: 10.1046/j.1523-1755.2003.00236.x. [DOI] [PubMed] [Google Scholar]
- 128.Merta M, Reiterova J, Rysava R, et al. Role of endothelin and nitric oxide in the pathogenesis of arterial hypertension in autosomal dominant polycystic kidney disease. Physiol Res. 2003;52:433–7. [PubMed] [Google Scholar]
- 129.Chauvet V, Qian F, Boute N, et al. Expression of PKD1 and PKD2 transcripts and proteins in human embryo and during normal kidney development. American J Pathol. 2002;160:973–83. doi: 10.1016/S0002-9440(10)64919-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ibraghimov-Beskrovnaya O, Dackowski WR, Foggen-steiner L, et al. Polycystin: in vitro synthesis, in vivo tissue expression, and subcellular localization identifies a large membrane-associated protein. Proc Natl Acad Sci U S A. 1997;94:6397–402. doi: 10.1073/pnas.94.12.6397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ong AC, Harris PC, Biddolph S, Bowker C, Ward CJ. Characterisation and expression of the PKD-1 protein, polycystin, in renal and extrarenal tissues. Kidney Int. 1999;55:2091–116. doi: 10.1046/j.1523-1755.1999.00404.x. [DOI] [PubMed] [Google Scholar]
- 132.Lu CJ, Du H, Wu J, et al. Non-random distribution and sensory functions of primary cilia in vascular smooth muscle cells. Kidney Blood Press Res. 2008;31:171–84. doi: 10.1159/000132462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Steinman TI. Renal and cardiac effects of antihypertensive treatment with ramipril versus metoprolol in autosomal dominant polycystic kidney disease. Nephrol Dial Transplant. 2008;23:431–3. doi: 10.1093/ndt/gfm865. [DOI] [PubMed] [Google Scholar]