Abstract
Aim: Cell-free hemoglobin-based oxygen carriers (HBOCs) may increase the risk of myocardial infarction and death. We studied the effect of an angiotensin-converting enzyme (ACE) inhibitor on HBOC-induced adverse cardiovascular outcomes and elucidated the underlying mechanisms. Results: With a dog cardiopulmonary bypass model, we demonstrated that a high-dose HBOC (3%, w/v) did not reduce—but aggravated—cardiac ischemia/reperfusion injury. Animals administered a high-dose HBOC experienced coronary artery constriction and depression of cardiac function. Exposure of isolated coronary arteries or human umbilical vein endothelial cells to high-dose HBOC caused impaired endothelium-dependent relaxation, increased endothelial cell necrosis/apoptosis, and elevated NAD(P)H oxidase expression (gp91phox, p47phox, p67phox, and Nox1) and reactive oxygen species (ROS) production. All observed adverse outcomes could be suppressed by the ACE inhibitor captopril (100 μM). Co-incubation with free radical scavenger tempol or NAD(P)H oxidase inhibitor apocynin had no effect on captopril action, suggesting that the positive effects of captopril are ROS- and NAD(P)H oxidase dependent. ACE inhibition by captopril also contributed to these effects. In addition, bioavailable nitrite oxide (NO) reduced by high-dose HBOC was preserved by captopril. Furthermore, HBOC, at concentrations greater than 0.5%, inhibited large conductance Ca2+-activated K+ channel currents in vascular smooth muscle cells in a dose-dependent manner, although captopril failed to improve current activity, providing additional evidence that captopril's effects are mediated by the endothelium, but not by the smooth muscle. Innovation and Conclusion: Captopril alleviates high-dose HBOC-induced endothelial dysfunction and myocardial toxicity, which is mediated by synergistic depression of NAD(P)H oxidase subunit overproduction and increases in vascular NO bioavailability. Antioxid. Redox Signal. 21, 2095–2108.
Introduction
Hemoglobin-based oxygen carriers (HBOCs) were initially developed as blood substitutes for surgical and trauma patients with hemorrhagic shock, especially in emergency and military settings (8). Although HBOCs possess inherent advantages compared with stored erythrocytes, significant problems associated with their infusion hindered clinical applications. These include brief circulating half-life, renal toxicity and vasoactivity (9). HBOC half-life and renal issues have been addressed by increasing the molecular size of the drug via chemical cross-linkage, polymerization, pegylation, or liposome encapsulation, but HBOC vasoactivity remains unsolved. Importantly, most complications observed in clinical trials, such as gastrointestinal side effects, hypertension, chest pain, and myocardial infarction, can be attributed to this vasoactivity (1, 32). Among those complications, myocardial infarction is believed to be the most serious: The patient who receives an HBOC likely has systemic ischemia or shock, and impaired cardiac function will, undoubtedly, aggravate the clinical picture (27).
Innovation.
This study provides evidence that the vasoactivity and myocardial toxicity of hemoglobin-based oxygen carriers (HBOCs) are mainly mediated by endothelial dysfunction. The inhibition of angiotensin-converting enzyme by captopril alleviates these damage via synergistic reduction of NAD(P)H oxidase-induced reactive oxygen species overproduction and increased NO bioavailability. These findings may be helpful in improving the clinical safety of present HBOCs and developing novel HBOCs without adverse cardiovascular effects.
To date, the mechanisms responsible for HBOC-induced myocardial toxicity have not been completely understood. A widely accepted explanation takes into account vasoactivity caused by the scavenging of endothelium-derived nitric oxide (NO) as a key factor (16). Usually, hemoglobin molecules used to manufacture HBOCs are not contained by the erythrocyte membrane and can rapidly inactivate NO. Consequences of NO inactivation include pulmonary hypertension, elevated systemic vascular resistance, and increased release of pro-inflammatory mediators, which can reduce myocardial oxygen (O2) supplies, imposing another cardiac burden. However, direct evidence to support this hypothesis is lacking, and the exact mechanism as well as effective therapeutic targets is also elusive.
As an impairment of endothelium-dependent vasorelaxation, endothelial dysfunction is responsible for diverse disorders such as hypertension, hypercholesterolemia, aging, cigarette smoking, diabetes, and heart failure (7). Endothelial dysfunction harms the cardiovascular system by destroying vascular homeostasis, reducing the myocardial O2 supply, and, ultimately, damaging the myocardium, which manifests in a manner similar to HBOC-induced cardiovascular side effects. Indeed, HBOC is suspected to cause endothelial dysfunction through its nitric oxide (NO) scavenging properties and auto-oxidation-induced reactive oxygen species (ROS) generation. In support of this mechanism, research suggests that a hemolytic state with abundant free and circulating hemoglobin can cause endothelial dysfunction (26). Furthermore, HBOCs have been reported to extravasate to the interstitial vessel space by the induction of gaps between endothelial cells, regardless of the molecular size of the drug (13). In addition, compromised endothelial function, as with diabetes mellitus, can increase the risk of HBOC extravasation and vasoconstriction (37).
Collectively, these findings suggest that endothelial damage contributes to the adverse cardiovascular effects induced by HBOC. As the earliest angiotensin-converting enzyme (ACE) inhibitor, captopril is reported to exhibit the ability to scavenge free radicals and provide protection against free radical-mediated oxidative damage. In addition, accumulated evidence indicated that captopril treatment causes an increase in plasma bradykinin levels, followed by an increase in the activation of NO synthase and NO production in the vascular system. These benefits of captopril may help reduce cardiovascular events via improving endothelial function, which have been observed in the coronary and peripheral circulation (35). Therefore, we tested the hypothesis that an ACE inhibitor—specifically captopril—could attenuate HBOC-induced myocardial toxicity by preventing endothelial dysfunction.
Results
Cardiac ischemia/reperfusion injury exacerbated by high-dose HBOC is reduced by captopril
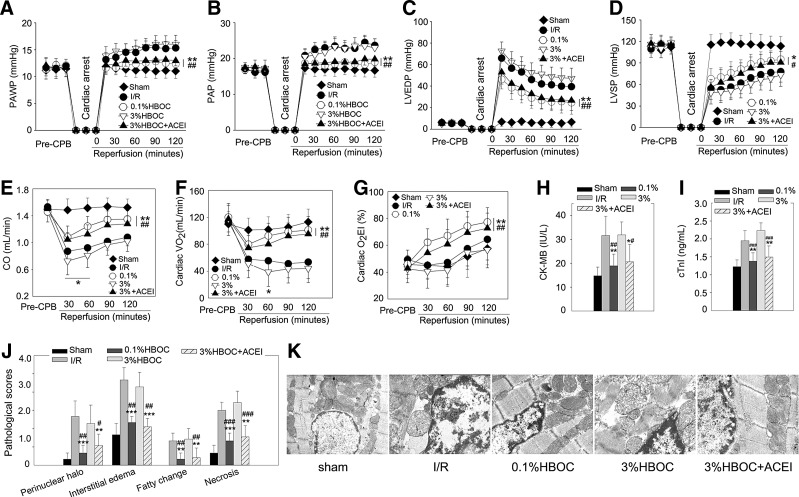
A dog CPB model was used to assess the effect of an HBOC on in vivo cardiac ischemia/reperfusion (I/R) injury. The 0.1%HBOC caused a clear cardioprotective effect, whereas increasing the concentration to 3% did not enhance this effect, as evidenced by increased pulmonary artery wedge pressure, pulmonary arterial pressure and left ventricular (LV) end-diastolic pressure, and reduced LV systolic pressure and cardiac output (CO) (all p<0.001 vs. the sham group; Fig. 1A–E). The recovery of CO, especially during the first 60 min after declamping, was even worse compared with the I/R group (p<0.05; Fig. 1E). Besides, heart rate, central venous pressure, and mean arterial pressure were not different among groups (data not shown). Finally, myocardial injury was accompanied by a reduction in cardiac O2 utilization. We confirmed that reductions of both cardiac O2 consumption (VO2) and O2 extraction index (O2EI) were alleviated by the 0.1%HBOC (both p<0.01 vs. the I/R group; Fig. 1F, G). However, the 3%HBOC failed to improve these parameters and further decreased cardiac VO2 at 60 min after declamping (p<0.05 vs. the I/R group). In addition, releases of creatine kinase-MB (CK-MB) and cardiac troponin-I (cTnI) were high in the 3%HBOC group, and cTnI release was even greater than that of the I/R group (p<0.05; Fig. 1H, I). H&E staining indicated that the 1%HBOC limited myocardial histopathological changes. Conversely, the 3%HBOC did not improve these parameters and further increased myocardial necrosis (p<0.05 vs. the I/R group; Fig. 1J). Moreover, after the 3%HBOC treatment, the mitochondrial swelling caused by I/R injury persisted, and mitochondria displayed disorganized cristae and reduced matrix density (Fig. 1K). Interestingly, the adverse effects of the 3%HBOC were suppressed by pretreatment with an endothelial protective agent captopril (100 μM). Collectively, these results suggest that the high-dose HBOC aggravates in vivo cardiac I/R injury and that captopril is able to attenuate this damage.
FIG. 1.
Cardiac I/R injury exacerbated by high-dose HBOC is reduced by captopril. The cardiac functional parameters, including PAWP (A), PAP (B), LVEDP (C), LVSP (D), CO (E), and cardiac VO2 (F) and O2EI (G), of dog hearts during CPB study (n=6). Total CK-MB (H) and cTnI (I) releases after 2-h reperfusion (n=6). (J) Pathological scores for acute myocardial necrosis, interstitial edema, perinuclear halo, and fatty changes (n=5). (K) Structural mitochondrial changes of dog LV tissues assessed by transmission electron microscopy (n=5, five fields for each specimen). Original magnification×12,000. Values are presented as mean±SD. *p<0.05, **p<0.01, and ***p<0.001 versus the I/R group; #p<0.05, ##p<0.01, and ###p<0.001 versus the 3%HBOC group. ACEI in the figure indicates ACE inhibitor captopril. CO, cardiac output; CK-MB, creatine kinase-MB; CPB, cardiopulmonary bypass; HBOC, hemoglobin-based oxygen carriers; I/R, ischemia/reperfusion; LVEDP, left ventricular end-diastolic pressure; LVSP, left ventricular systolic pressure; O2EI, O2 extraction index; PAP, pulmonary arterial pressure; PAWP, pulmonary artery wedge pressure; VO2, cardiac O2 consumption.
Captopril attenuates high-dose HBOC-induced vasospasm and cardiac function impairment
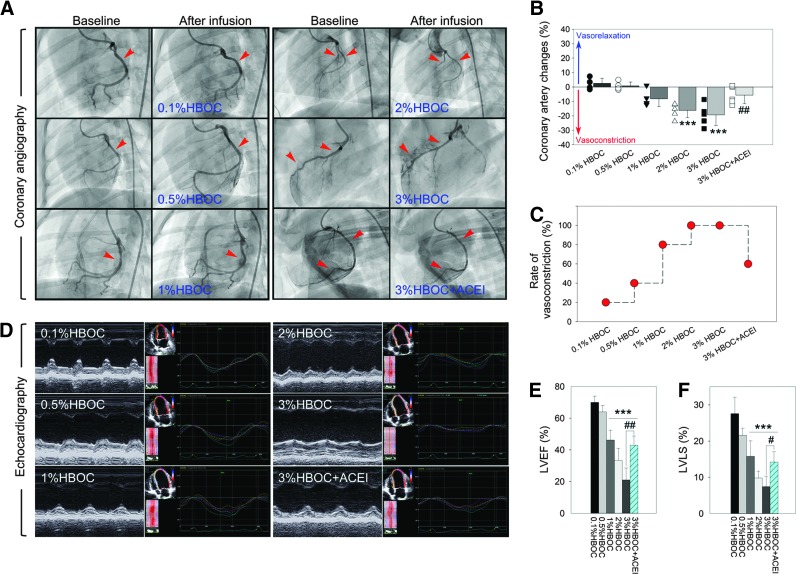
Next, we visualized acute effects of an HBOC on the cardiovascular system using invasive coronary angiography and echocardiography. Data show that intracoronary infusion of the 0.1%HBOC caused slight vasoconstriction in two of the five dogs, and changes in coronary artery diameter were negligible (2.54%±3.56%; Fig. 2A–C). Increasing the HBOC to 1% enhanced vascular constriction (−8.15%±5.45%, p=0.053 vs. the 0.1%HBOC group) in 80% of the experimental animals (four of five dogs). After infusion of the 3%HBOC, all animals were vasoconstricted and had vasospasms. The change in the coronary artery diameter was greatest with the 3%HBOC (−19.29%±7.53%, p<0.001 vs. the 0.1%HBOC group). Along with coronary artery constriction, the HBOC also dose dependently impaired cardiac function (Fig. 2D). Both the LV ejection fraction (LVEF) and the LV longitudinal strain (LVLS) were greatly reduced when the HBOC exceeded 1% (Fig. 2E, F). Of note, in the 3%HBOC group, two animals died from vasospasm-induced ventricular fibrillation and cardiac arrest. Thus, an additional two dogs were included in the study. This event also suggested that 3% was the highest dose that could be used experimentally in our investigation. Pretreatment with captopril reversed vasoactive effects of the 3%HBOC, reducing the rate of vasoconstriction from 100% to 60% (three of five dogs). Coronary artery diameter changes also decreased to −5.54%±5.77% (p<0.01 vs. the 3%HBOC group). These data clearly indicate that the adverse effects of HBOC in the cardiovascular system are dose dependent, which appears when HBOC reaches a threshold and can be attenuated by captopril.
FIG. 2.
Captopril attenuates high-dose HBOC-induced vasospasm and cardiac function impairment. (A) Representative coronary angiograms acquired at baseline and after intracoronary infusion of HBOC. Red arrow heads indicate constricted segments. (B, C) The percentage of change in coronary artery luminal diameter and rate of vasoconstriction. Negative values for coronary artery changes indicate vasoconstriction. (D) Representative M-Mode echocardiogram and speckle tracking images after intracoronary infusion of HBOC. (E, F) Quantitative analysis of LVEF and LVLS. Values are presented as mean±SD (n=5 per group). ***p<0.001 versus the 0.1%HBOC group; #p<0.05 and ##p<0.01 versus the 3%HBOC group. ACEI in the figure indicates ACE inhibitor captopril. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Captopril recovers endothelium-dependent vasorelaxation impaired by high-dose HBOC
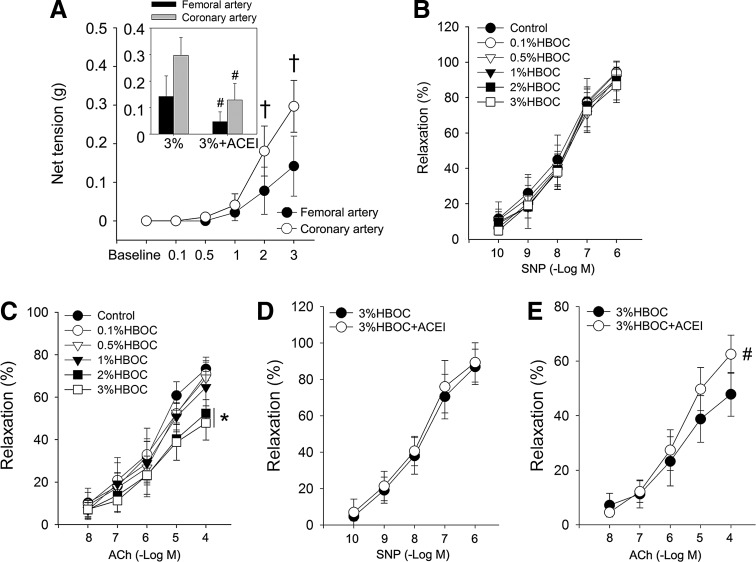
The endothelium is a critical biological barrier for the maintenance of vascular function and homeostasis, so it influences cardiac performance (6). We tested the effect of HBOC on the endothelium using an in vitro organ bath study. Isolated coronary and femoral arteries from dogs were incubated with increasing concentrations of an HBOC at 37°C for 10 min. Data indicate that the net tensions of both arterial rings were dramatically elevated with increased HBOC, and this was more prominent in the coronary artery compared with the femoral artery (0.30±0.06 g vs. 0.14±0.05 g, p<0.05, Fig. 3A). Next, dog coronary arteries preconstricted with phenylephrine (10−7 M) were relaxed with the addition of acetylcholine (ACh, 1×10−8 to 1×10−4 M) or sodium nitroprusside (SNP, 1×10−10 to 1×10−6 M). The endothelium-independent vasorelaxation induced by SNP did not differ among groups (Fig. 3B). However, the maximal endothelium-dependent relaxation induced by ACh (1×10−4 M) was much less in 3%HBOC-treated vessels (47.86%±8.03%) than in 0.1%HBOC-treated vessels (70.86%±5.66%, p<0.05; Fig. 3C). Further experiments indicated that the impaired endothelium-dependent relaxation induced by the 3%HBOC was prevented with captopril pretreatment (62.53%±6.96%, p<0.05 vs. the 3%HBOC group; Fig. 3D, E). These findings support our hypothesis that the adverse cardiovascular outcomes caused by high-dose HBOC are mediated by endothelial dysfunction and that captopril can reverse this deleterious effect on the endothelium.
FIG. 3.
Captopril reduces high-dose HBOC-induced endothelial dysfunction. (A) The net tension of coronary artery and femoral artery after incubation with HBOC. (B–E) SNP-induced endothelium-independent relaxation and ACh-induced endothelium-dependent relaxation in coronary arteries after incubation with HBOC or HBOC+ACEI. Control vessels were treated with KH solution alone. Values are presented as mean±SD (n=5 to 6 per group). *p<0.05 versus the 0.1%HBOC group; #p<0.05 versus the 3%HBOC group; †p<0.05 versus the femoral artery. ACEI in the figure indicates ACE inhibitor captopril.
Captopril limits endothelial cell damage caused by high-dose HBOC
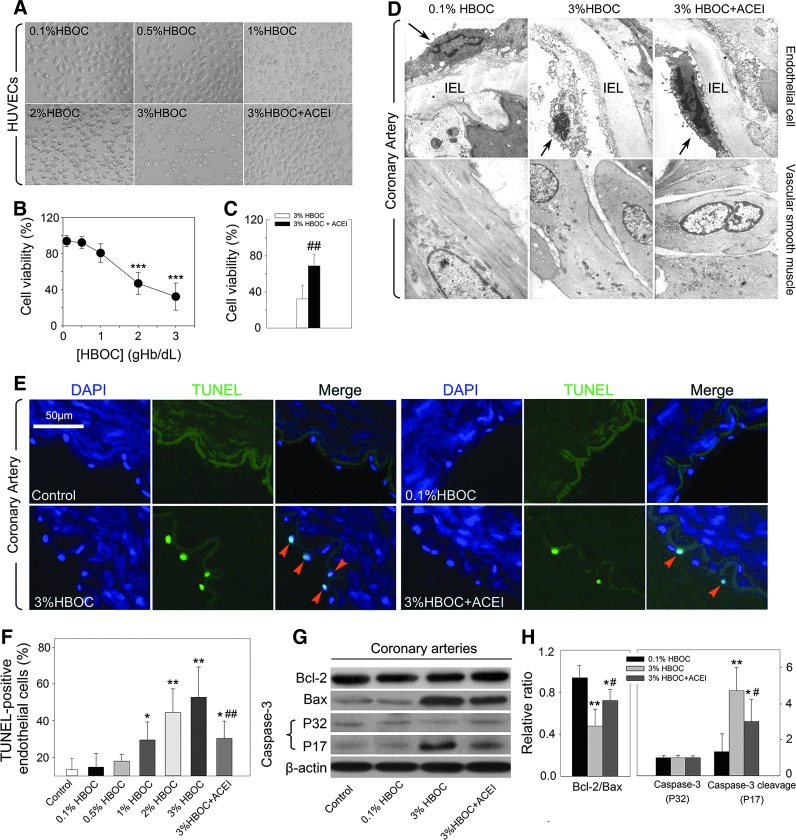
The effect of HBOC on endothelial cell structure and viability was tested by incubating it with human umbilical vein endothelial cells (HUVECs). After 2 h of co-incubation, the 0.1%HBOC did not remarkably alter the structure or viability of HUVECs. However, with increasing HBOC concentrations, HUVECs structures began to collapse and rupture (Fig. 4A–C). Endothelial cell viability detected by MTT assay was greatly depressed after exposure to either the 2%HBOC or the 3%HBOC (p<0.001 and p<0.001 vs. the 0.1%HBOC, respectively). The addition of captopril (100 μM) protected HUVEC structure and significantly increased cell viability (p<0.01 vs. the 3%HBOC group). Next, we studied the ultrastructural changes of the endothelium in the coronary artery by transmission electron microscopy. As shown in Figure 4D (upper panel), the endothelial cells of the 0.1%HBOC group were attached to the basal membrane and appeared flattened and elongated, with a prominent glycocalyx on the luminal side and smooth contours. Conversely, the 3%HBOC group exhibited signs of endothelial cell necrosis with condensed nuclei, clear perinuclear cytoplasm, and desquamation. Denudated regions and exposure of the subendothelial space were also observed. Captopril treatment preserved the endothelial lining and alleviated the adverse effect of the 3%HBOC on the endothelium morphology. Besides, no differences in the smooth muscle layer were observed among groups (Fig. 4D, lower panel). These results support our findings in the organ bath study, suggesting that HBOC impaired the vascular endothelium rather than the smooth muscle.
FIG. 4.
Captopril limits endothelial cell damage and apoptosis caused by high-dose HBOC. (A) Representative images of HUVECs after exposure to HBOC with or without 100 μM captopril pretreatment (n=3). (B, C) Cell viability measured by MTT test (n=5). (D) Representative images of the ultrastructure of the coronary artery endothelial cell and smooth muscle (n=3). Original magnification×12,000. Black arrows indicate endothelial cells; IEL indicates internal elastic lamina. (E, F) TUNEL assay. Nuclei stained by DAPI appear light blue; TUNEL-positive nuclei stained by FITC appear green. Scale bar: 50 μm (n=5 per group, five fields for each specimen). Red arrow heads indicate apoptotic cells. (G, H) Western blot analysis of expressions of Bcl-2, Bax, total caspase-3 (P32), and caspase-3 cleavage (P17) in coronary arteries. The blot is a representative of blots obtained from five independent experiments. Values are presented as mean±SD. *p<0.05, **p<0.01, and ***p<0.001 versus the 0.1%HBOC group; #p<0.05 and ##p<0.01 versus the 3%HBOC group. ACEI in the figure indicates ACE inhibitor captopril. HUVECs, human umbilical vein endothelial cells. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Captopril reduces endothelial cell apoptosis caused by high-dose HBOC
Endothelial cell apoptosis is reported to be coincident with early endothelial dysfunction (14). Thus, we next investigated whether HBOC leads to endothelial cell apoptosis. After incubation with HBOC, the number of TUNEL-positive endothelial cells in coronary arteries increased in a dose-related manner (from 14.79%±7.34% in the 0.1%HBOC group to 59.85%±16.40% in the 3%HBOC group; p<0.01), whereas protection of the endothelium by captopril partially abolished this pro-apoptotic effect (30.26%±9.45%, p<0.05 vs. the 3%HBOC group; Fig. 4E, F). To determine whether apoptosis-related proteins were involved in this phenomenon, we incubated coronary arteries with HBOC and measured Bcl-2, Bax, total caspase-3, and caspase-3 cleavage expressions. Compared with 0.1%HBOC, the 3%HBOC reduced the relative ratio of Bcl-2/Bax (p<0.01; Fig. 4G, H) and greatly up-regulated caspase-3 cleavage (p<0.01). Consistent with data from TUNEL staining, pretreatment with captopril (100 μM) markedly increased the Bcl-2/Bax ratio (p<0.05 vs. the 3%HBOC group) and down-regulated caspase-3 cleavage (p<0.05 vs. the 3%HBOC group), thereby reducing HBOC-induced endothelial cell apoptosis.
Captopril alleviates HBOC-induced endothelial dysfunction by attenuation of ROS production
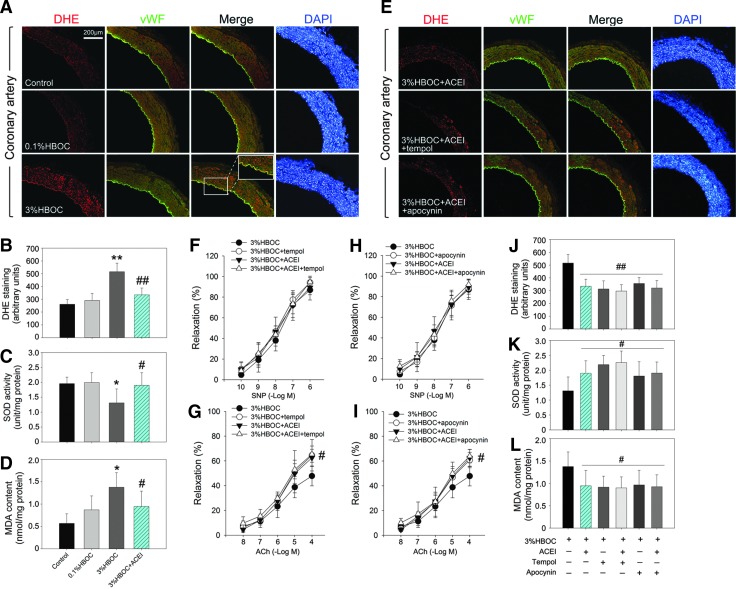
The results of dihydroethidium/von Willebrand factor (DHE/vWF) double staining proved that the 3%HBOC increased ROS production in coronary arteries, especially within the endothelium (Fig. 5A). Therefore, we quantified ROS production by the incubation of HUVECs with DHE. Our data confirmed that the 3%HBOC treatment resulted in increased ROS production (p<0.01 vs. the 0.1%HBOC group; Fig. 5B), inhibited superoxide dismutase (SOD) activity, and elevated malonaldehyde (MDA) formation (Fig. 5C, D). Moreover, captopril incubation reduced DHE staining of coronary arteries after exposure to the 3%HBOC. In addition, ROS production and MDA formation were decreased and SOD activity was increased, suggesting an anti-oxidative effect of captopril in HBOC-treated vessels. Pretreating dog coronary arteries with tempol (10 μM, 37°C for 2 h), an SOD mimetic, did not alter SNP-induced endothelium-independent vasorelaxation, but significantly reduced DHE staining of arteries and increased ACh-induced relaxation in vessels treated with the 3%HBOC (Fig. 5E–G). More importantly, the positive effect of captopril on endothelial function and redox status could not be further enhanced after tempol pretreatment (Fig. 5E–G, J–L). Collectively, these results suggest the following: (1) HBOC-induced endothelial dysfunction in coronary arteries is ROS dependent; (2) captopril and tempol may have similar mechanisms for normalizing endothelium-dependent vasorelaxation via ROS scavenging.
FIG. 5.
Effect of captopril on HBOC-induced endothelial dysfunction is ROS- and NAD(P)H oxidase dependent. (A, E) Representative immunohistochemical staining of coronary arteries for DHE and vWF. Scale bar: 200 μm. (B–D) The ROS production, SOD activity, and MDA formation in HUVECs after indicated treatment. One unit of SOD activity corresponded to 50% reduction of absorbance at 550 nm. (F–I) SNP-induced endothelium-independent relaxation and ACh-induced endothelium-dependent relaxation in coronary arteries after indicated treatment. Control vessels were incubated with KH solution alone. (J–L) ROS production, SOD activity, and MDA formation in HUVECs after indicated pretreatment and exposure to the 3%HBOC. Values are presented as mean±SD (n=5 to 6 per group). *p<0.05 and **p<0.01 versus the 0.1%HBOC group; #p<0.05 and ##p<0.01 versus the 3%HBOC group. ACEI in the figure indicates ACE inhibitor captopril. ACE, angiotensin-converting enzyme; DHE, dihydroethidium; MDA, malonaldehyde; ROS, reactive oxygen species; SOD, superoxide dismutase; vWF, von Willebrand factor. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Captopril ameliorates heme-mediated oxidative toxicity on coronary artery endothelium
To investigate whether heme-mediated oxidative toxicity is involved in these adverse cardiovascular effects, the HBOC in oxidative form (met-HBOC) was used. It is oxidatively more damaging and loses heme more readily than ferrous HBOC. Data indicate that 3% (w/v) met-HBOC did not affect the tension of coronary artery rings but induced an impairment in endothelium-dependent relaxation, which could be greatly attenuated by captopril pretreatment (Supplementary Fig. S2C–E; Supplementary Data are available online at www.liebertpub.com/ars). A similar phenomenon can also be observed after treatment of the coronary artery rings with hemin, the oxidized prosthetic heme moiety of hemoglobin (Supplementary Fig. S2C, F, G). These findings provide additional support for oxidative toxicity on vascular endothelium mediating the adverse cardiovascular outcomes and can be counteracted by captopril.
Inhibition of NAD(P)H oxidase by captopril abolishes HBOC-induced endothelial dysfunction
To determine whether NAD(P)H oxidase is a therapeutic target of captopril, with regard to HBOC-induced ROS overproduction and endothelial dysfunction, vascular rings from dog coronary arteries were incubated with apocynin (100 μM, 37°C for 2 h), a selective NAD(P)H oxidase inhibitor. This partially, but significantly, normalized ACh-induced endothelium-dependent relaxation in vessels treated with the 3%HBOC (Fig. 5H, I). Furthermore, pretreatment of HUVECs with apocynin significantly increased SOD activity and down-regulated ROS and MDA production (Fig. 5J–L). These results indicate that NAD(P)H oxidase is a source of ROS and contributes to HBOC-induced endothelial dysfunction. The inhibition of NAD(P)H oxidase by apocynin did not further improve the endothelial protective and anti-oxidative effects of captopril, indicating that these effects of captopril are mainly mediated by the depression of NAD(P)H oxidase.
Captopril attenuates HBOC-induced NAD(P)H oxidase subunit overexpression and activity
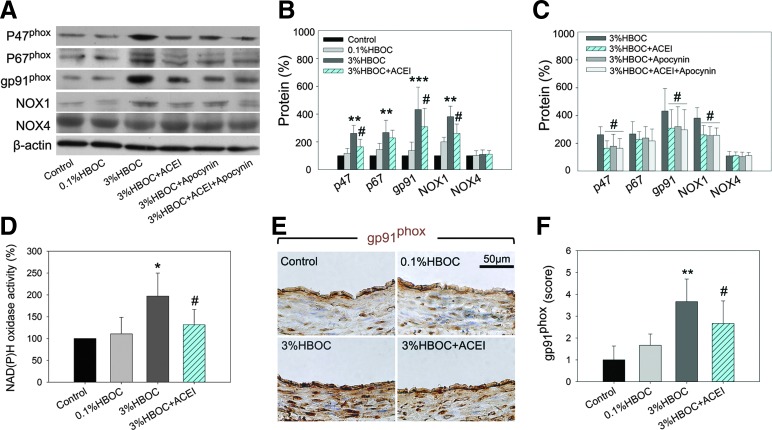
Next, we measured the expressions of NAD(P)H oxidase subunits in HBOC-treated coronary arteries using Western blot. Accompanied with up-regulated NAD(P)H oxidase activity, vascular expression of the essential subunits, p47phox, p67phox, and gp91phox, as well as the catalytic subunit Nox1, were markedly increased by the 3%HBOC compared with control and vessels treated with the 0.1%HBOC (Fig. 6A–D). Captopril, apocynin, and a combination of both agents could similarly depress expression of these proteins. Of note, gp91phox was increased nearly four-fold by the 3%HBOC, and this was the most up-regulated subunit. Data from immunohistochemistry staining confirmed that gp91phox expression was elevated by the 3%HBOC and that captopril inhibited this up-regulation (Fig. 6E, F). No differences in Nox4 expression were observed among the treatment groups.
FIG. 6.
Captopril attenuates HBOC-induced NAD(P)H oxidase subunit overexpression and activity. (A–C) Western blot analysis of NAD(P)H oxidase subunits expressions (p47phox, p67phox, gp91phox, Nox1, and Nox4) in coronary arteries. The blot is a representative of blots obtained from five independent experiments. (D) NAD(P)H oxidase activity in HUVECs. (E, F) Representative of immunohistochemical staining and quantification analysis for gp91phox-positive protein in coronary arteries. Scale bar: 50 μm. Values are presented as mean±SD. *p<0.05, **p<0.01, and ***p<0.001 versus the 0.1%HBOC group; #p<0.05 versus the 3%HBOC group. ACEI in the figure indicates ACE inhibitor captopril. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
ACE inhibition contributes to the positive effects of captopril
Captopril possesses free sulfhydryl (-SH) groups and, thus, may be active as the direct antioxidant. To confirm that ACE blockade contributes to the action of captopril, enalaprilat, an active ACE inhibitor without -SH groups was used. The results clearly show that enalaprilat is also capable of increasing the viability of HUVECs and reverse endothelial dysfunction in coronary artery after exposure to 3%HBOC (Supplementary Fig. S3A–C). In addition, pretreatment with either captopril or enalaprilat can greatly down-regulate the ACE levels in coronary artery homogenates or cell-free supernatants of HUVECs (Supplementary Fig. S3D, E). Therefore, these results provide evidence that the inhibition of ACE contributes to the action of captopril against HBOC-related damage.
Preservation of bioavailable NO by captopril contributes to amelioration of HBOC-induced endothelial dysfunction
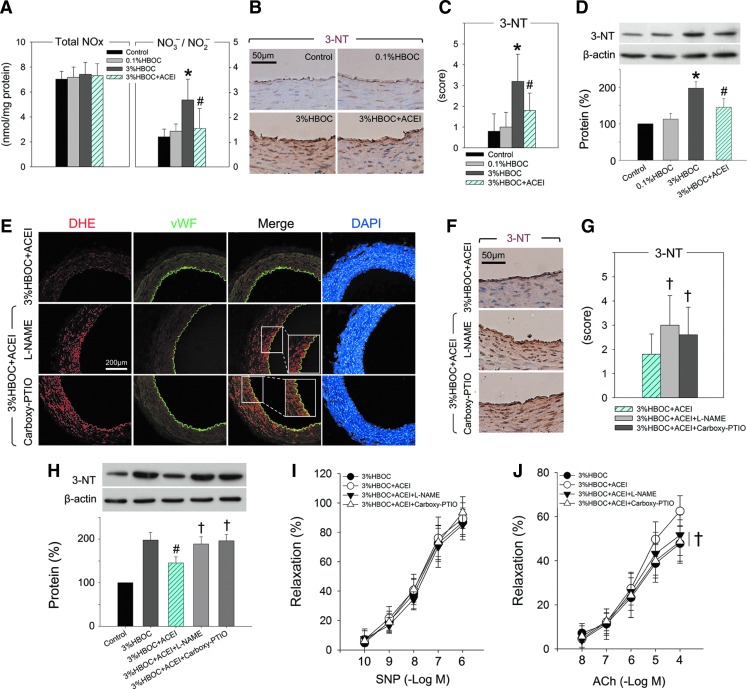
Bioavailable NO is the key endothelium-derived relaxing factor that is pivotal in the maintenance of vascular tone and reactivity (10). Our data indicated that the expression of eNOS, peNOS, and iNOS in the coronary arteries was not altered after exposure to either the 0.1%HBOC or the 3%HBOC. Pretreatment with captopril did not change the protein expressions either, indicating that both an HBOC and captopril could not influence major sources of NO (Supplementary Fig. S4A, B). On the other hand, total NOx (NO2− and NO3−) increased modestly after treatment with the 3%HBOC (p=0.69 vs. the 0.1%HBOC group). However, since increased NOx was chiefly caused by increased formation of NO3−, a breakdown product of peroxynitrite (ONOO−), the NO3−/NO2− ratio in the 3%HBOC group was significantly increased (p<0.05 vs. 0.1%HBOC; Fig. 7A). Expression of 3-nitrotyrosine (3-NT), a stable product of ONOO− and protein, was also up-regulated by the 3%HBOC (p<0.05 vs. the 0.1%HBOC group; Fig. 7B–D). These results suggest that more NO was inactivated to form ONOO− and that bioavailable NO was reduced by the 3%HBOC. Interestingly, elevations of both ONOO− (reflected by NO3−/NO2− ratio) and 3-NT were significantly limited by captopril (p<0.05 vs. the 3%HBOC group; Fig. 7A–D). In contrast, inhibition of NOS by L-NAME or exhaustion of NO by carboxy-PTIO totally reversed the anti-oxidative and endothelial protective effects of captopril (Fig. 7E–J and Supplementary Fig. S4C–E). This finding is consistent with a previous clinical report which suggested that improved endothelial function due to an ACE inhibitor was partially mediated by increasing NO bioavailability (18). Our study further demonstrated that tempol reduced both ONOO− and 3-NT which were elevated by the 3%HBOC, although tempol had no effect on vessels treated with the 0.1%HBOC (Supplementary Fig. S5). This indicates that overproduced ROS, induced by the 3%HBOC, may be responsible for reduced bioavailable NO.
FIG. 7.
Preservation of bioavailable NO by captopril contributes to amelioration of HBOC-induced endothelial dysfunction. (A) Total NOx and NO3−/NO2− ratio measured in coronary arteries after indicated treatment. NO3− was first converted into NO2− by nitrate reductase, and then NO2− was assayed. (B, C, F, G) Representative of immunohistochemical staining and quantification analysis for 3-NT-positive protein in coronary arteries. Scale bar: 50 μm. (D, H) The levels of 3-NT measured by Western blotting. (E) Representative immunohistochemical staining of coronary arteries for DHE and vWF. Scale bar: 200 μm. (I, J) SNP-induced endothelium-independent relaxation and ACh-induced endothelium-dependent relaxation in coronary arteries after indicated pretreatment and incubation with the 3%HBOC. Values are presented as mean±SD (n=5 to 6 per group). *p<0.05 versus the 0.1%HBOC group; #p<0.05 versus the 3%HBOC group; †p<0.05 versus the 3%HBOC+ACEI group. ACEI in the figure indicates ACE inhibitor captopril. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
HBOC-induced endothelial dysfunction is unlikely to be caused by macrophage infiltration and inflammation
We investigated whether HBOC-induced endothelial dysfunction is attributed to macrophage infiltration and inflammation by immunohistochemical staining of CD68, E-selectin, and ICAM-1. Weak staining of CD68, a well-characterized macrophage marker, was observed in vessels treated with either the 0.1%HBOC or the 3%HBOC (Supplementary Fig. S6A). There were also no differences in E-selectin and ICAM-1-positive staining and expression among groups, indicating that the HBOC did not evoke vascular inflammation (Supplementary Fig. S6B–G). Our results, therefore, suggest that HBOC-induced endothelial dysfunction is unlikely to be caused by increased infiltration of leukocytes and vascular inflammation.
Captopril fails to improve BKCa channel currents inhibited by high-dose HBOC
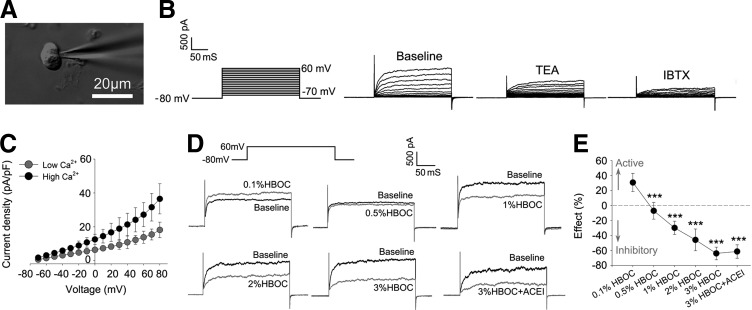
Finally, vascular smooth muscle cells were isolated from dog coronary arteries, and the effects of an HBOC on large-conductance Ca2+-activated K+ (BKCa) channels were evaluated using a whole-cell patch-clamp technique (Fig. 8A). The properties of the BKCa channel were identified by the fact that the recorded voltage-dependent outward currents were significantly blocked by 1 mM tetraethylammonium (TEA, a nonselective BKCa inhibitor) or 100 nM iberiotoxin (IBTX, a specific BKCa inhibitor) and enhanced by high Ca2+ (Fig. 8B, C). Experiments were then undertaken to determine the effect of HBOC on BKCa channel currents. As shown in Figure 8D, acute addition of 0.1%HBOC to the bath solution significantly increased whole-cell outward currents, a finding that may explain why three of five dogs had vasodilation in the coronary angiography study. At concentrations greater than 0.5%, HBOC inhibited whole-cell currents in a dose-dependent fashion. BKCa channel currents were reduced by 63.93%±8.43% after exposure to the 3%HBOC, and the addition of captopril (100 μM) failed to improve blockade (61.39%±9.01%; Fig. 8E). These data not only confirm that captopril is endothelium dependent, but also provide mechanistic evidence that inhibition of the BKCa channel may contribute to HBOC-induced hypertension.
FIG. 8.
Captopril fails to improve BKCa channel currents inhibited by high-dose HBOC. (A) Representative image of whole-cell outward current recording by a patch-clamp technique. Scale bar: 25 μm. (B) Whole-cell BKCa currents recorded from isolated VSMCs before (baseline) and after inhibition with 1 mM TEA or 100 nM IBTX. (C) Current–voltage relationship of BKCa currents with low Ca2+ or high Ca2+. (D) Representative BKCa currents recorded before (baseline) and after presence of 0.1%HBOC, 0.5%HBOC, 1%HBOC, 2%HBOC, 3%HBOC, or 3%HBOC+ACEI. (E) Quantification analysis of the effect of HBOC and ACE inhibitor on BKCa currents. Values are presented as mean±SD (n=6 to 8 per group). ***p<0.001 versus the 0.1%HBOC group. ACEI in the figure indicates ACE inhibitor captopril.
Discussion
In theory, HBOCs enable the delivery of more O2 to hypoxic tissues due to its higher O2 affinity, lower viscosity, and smaller mean diameter than human erythrocytes. These features may provide sufficient perfusion of the microcirculation, thereby alleviating cardiac I/R injury. Our previous work and other studies indicate that HBOCs protect the heart against I/R injury, and the underlying molecular mechanisms of this protection are implicated in the attenuation of myocardial apoptosis, quenching of myocardial oxidative stress, and restoration of the nitroso-redox balance (15, 19, 22–24). This protection, however, was not observed in clinical settings, as many clinical studies indicated that HBOCs increase cardiovascular complications such as myocardial infarction (27). This discrepancy is probably associated with the dosage of HBOCs. In clinical studies, HBOCs were mostly used as red blood cell substitutes for patients with hemorrhagic shock. Approximately 750–1000 ml HBOC was infused, and the estimated HBOC level in circulation was above 2% (27). From the data of this study, this dosage is high enough to induce vasoconstriction and bring damage to the heart. In addition, ascorbic acid is a strong antioxidant that is capable of reducing methemoglobin (MetHb) into oxyHb. This may explain why the MetHb formation was depressed under a physiological condition of rats in our study. However, unlike rats, humans cannot synthesize ascorbic acid at all, so the HBOC is more suspected to auto-oxidation in human body, which should also be taken into account for the adverse clinical outcomes of patients. Our current studies confirm that the vasoactivity and adverse cardiac effects of HBOCs are dose dependent and mainly induced by coronary artery endothelial dysfunction and damage. Vasoconstriction is a major obstacle hindering clinical development of HBOCs (16). Inhalation of NO or intravenous administration of an NO donor, such as sodium nitrite and nitroglycerin, has been reported to prevent systemic hypertension induced by HBOCs (22, 23). However, NO-based treatment may not be optimal, because hemoglobin is vulnerable and easily converted to MetHb by NO, disabling its O2-carrying capacity (36). Combining NO with ROS can also form ONOO−, which can react with DNA, proteins, and lipids, potentially leading to cellular damage and cytotoxicity (38). In addition to NO, haptoglobin is reported to suppress hemoglobin-mediated hemodynamic imbalance and heme-mediated oxidative toxicity in vivo, and this may counteract the vasoconstrictive and oxidative toxicities of HBOCs (2, 4). Unfortunately, the current expense and scarcity of haptoglobin make routine use impossible. Data from our study support that captopril, as an alternative, is also effective in reducing the adverse cardiovascular effects induced by HBOC, which include endothelial and myocardial injuries.
Accumulating evidence suggests that ACE inhibitor confers acute and clinically relevant vascular protection via improvement of endothelial dysfunction (3, 30). A recent multicenter, multinational study suggests that for patients undergoing coronary artery bypass graft surgery, the administration of an ACE inhibitor markedly improves cardiovascular and renal outcomes (12). In agreement with these reports, our study suggests that endothelial dysfunction and myocardial injury caused by high-dose HBOC can be greatly ameliorated by an ACE inhibitor. Mechanistic studies indicate that this effect is mediated by synergistic reduction of NAD(P)H oxidase–induced ROS overproduction and increased NO bioavailability. Specific mechanisms of action are unclear, but may be explained by the following details. First, captopril can prolong the half life of potent stimulators of NO such as bradykinin, thereby increasing NO bioavailability in the endothelium. Second, after exposure to acellular hemoglobin or I/R injury, the level of potent vasoconstrictor angiotensin II increases, and this has been implicated in stimulating production of other vasoconstrictors such as endothelin-1 and prostanoid PGH2 (5, 33). Captopril can reduce these vasoconstrictors and improve vasomotor function. In addition, the angiotensin II blocking effects of captopril can limit vascular NAD(P)H oxidase activity and reduce oxidative stress in vessels (25).
Another important finding of this study is that HBOC induces endothelial dysfunction by increasing ROS generation in the vascular endothelium. We identified NAD(P)H oxidase as the major source of ROS and showed that high-dose HBOC increases the expression of NAD(P)H oxidase subunits, including P47phox, P67phox, gp91phox, and Nox1. Accordingly, the inhibition of NAD(P)H oxidase partially abolished ROS production and endothelial dysfunction in vessels treated with HBOC. Furthermore, ROS scavenging with tempol significantly reversed impairments in endothelium-dependent relaxation resulting from high-dose HBOC. Although our data suggest that NAD(P)H oxidase is important to endothelial oxidative stress, we believe that this damage is multifactorial. Both excessive O2 delivered by HBOC and heme-auto-oxidation are capable of producing ROS and accelerating oxidative stress. This explains why the inhibition of NAD(P)H oxidase only partially abolished HBOC-induced endothelial dysfunction. Of note, Nox4 was the only NAD(P)H oxidase subunit not altered by high-dose HBOC. Nox4 has a high constitutive activity, is highly expressed in some cells, and differs from other Nox proteins in that it predominantly releases H2O2, which cannot scavenge NO (11). Moreover, endogenous vascular Nox4 has been reported to potentially protect vascular function (31). Thus, it is reasonable that Nox4 is not involved in HBOC-induced vascular redox imbalances.
In addition to oxidative stress, we measured HBOC's effect on bioavailable NO. Footprint markers of ONOO−, NO3−, and 3-NT formations in vessels and endothelial cells were greatly increased after treatment with high-dose HBOC, indicating that more NO was quenched. However, the environment responsible for NO generation was not changed, as evidenced by similar eNOS/peNOS, and iNOS expression. These data suggest that bioavailable NO in vessels is dramatically reduced by high-dose HBOC. We, therefore, conclude that the reduction of bioavailable NO also contributes to HBOC-induced endothelial dysfunction. It has been proposed that the scavenging of endothelium-derived NO (synthesized by NOS3) by cell-free hemoglobin is responsible for the HBOC-induced vasoconstriction (36). Reducing NO affinity has long been regarded as a solution for limitation of vasoconstriction after HBOC administration. The strategies included genetic modification of the heme pocket in hemoglobin, and attenuation of HBOC extravasation through endothelial junctions by producing larger hemoglobin molecules (28, 34). However, our study suggests that an increase of the size of hemoglobin cannot effectively avoid this extravasation, as NO scavenging was still observed after the use of polymerized hemoglobin. We speculate that the reason is probably related to HBOC-induced endothelial damage and, in turn, increase of its extravasation.
Recent investigations suggest that the vasoactivity and clinically significant adverse outcomes of HBOCs can be synergized or amplified by the presence of chronic comorbidities such as hypertension, atherosclerosis, and diabetes (29, 37). We agree with these reports and confirm that HBOC directly impairs the endothelium and causes endothelial dysfunction in a dose-dependent manner. As mentioned earlier, HBOC has properties that may contribute to endothelial dysfunction, including scavenging NO, generating excessive ROS, inhibiting nitric oxide synthase (NOS), and possibly stimulating the release of vasoconstrictors such as endothelin.6,35 We confirmed the vasoactivity of high-dose HBOC and revealed that endothelial damage and dysfunction were involved in this effect, a finding consistent with a previous report that HBOCs induced prolonged systemic vasoconstriction in wild-type mice but not in mice congenitally deficient in eNOS (36).
Patch-clamp data further indicate that HBOC, at concentrations greater than 0.5%, can dose dependently inhibit BKCa channel currents. This provides a novel mechanism for HBOC-induced vasoactivity and hypertension. It is well known that BKCa channels contribute to the regulation of vascular tone in a negative feedback manner (17). The activation of BKCa channels hyperpolarizes the membrane potential and leads to vasodilation, whereas the inhibition of BKCa channels promotes depolarization and causes vasospasm. In a normal vascular system, HBOC cannot easily interact with vascular smooth muscle due to the endothelial barrier. In pathological conditions, in which endothelial damage is a component, the likelihood of such interactions is greatly increased. That such vasoactivity and adverse outcomes of HBOCs can be synergized by hypertension, atherosclerosis, and diabetes is, therefore, expected. In the present study, captopril attenuated high-dose HBOC-induced vasoconstriction, but did not improve inhibited BKCa currents, so the effect of captopril is endothelium dependent, but not directly in smooth muscle. Thus, protection of the vascular endothelium and stabilization of endothelial function by captopril may offer a promising and rational method for mitigating HBOC-related adverse cardiovascular effects.
In summary, we report that ACE inhibitor captopril alleviates high-dose HBOC-induced endothelial dysfunction and myocardial toxicity, which is mediated by synergistic reduction of NAD(P)H oxidase-induced ROS overproduction and increased NO bioavailability. In addition, our work suggests that, before significant improvements in HBOCs are achieved, their use in patients with or vulnerable to endothelial dysfunction should be cautious.
Materials and Methods
All animal experimental procedures were performed in accordance with the policies of the Animal Care and Use Committee of Sichuan University, and conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996). The ACE inhibitor in this study was captopril (Sigma-Aldrich, St. Louis, MO).
Dog cardiopulmonary bypass model
A beagle dog cardiopulmonary bypass (CPB) model was previously established as described in the literature (20). In brief, after heart exposure through a mid-sternal incision and heparinization (3 mg/kg), the ascending aorta and the right atrial appendage were cannulated to establish a CPB circuit (StÖckert II, Munich, Germany). Except for the sham group, hearts were arrested by intra-aortic infusion of 40 ml/kg St.Thomas' solution (STS) alone (I/R group), STS with 0.1% HBOC (0.1%HBOC group), or STS with 3% HBOC (3%HBOC group). For 3%HBOC+ACEI group, the dogs were pretreated with an intravenous injection of captopril (3 mg/kg) for 10 min, then cardiac arrest was achieved via infusion of STS with 3%HBOC. After 2 h of cardiac arrest, the hearts were reperfused by aortic declamping.
Preparation of HBOC
The HBOC used in this study was glutaraldehyde-polymerized human placenta hemoglobin (PolyPHb, Supplementary Table 1), which was prepared as previously reported (21). Briefly, purified and viral inactivated fresh human placenta hemoglobin (Tianjin Union Stem Cell Genetic Engineering Ltd., Tianjin, China) was modified with bis(3,5-dibromosalicyl) fumarate to achieve optimal O2 affinity. After cross-linkage with glutaraldehyde, the mixture was subjected to ultrafiltration and molecular sieve chromatography. The final product had a well-preserved O2 carrying capacity and a low auto-oxidation rate under physiological condition (Supplementary Fig. S1).
An expanded Materials and Methods is provided in the online-only Data Supplement, including reagents, measurement of MetHb formation, preparation of MetHb form of HBOC, dog CPB model, effect of HBOC on dog heart during CPB, determination of myocardial necrosis, coronary angiography, echocardiography, measurement of vascular reactivity on isolated vessel rings, TUNEL staining, transmission electron microscopy, immunohistochemistry, cell culture, measurement of ACE levels in HUVECs and coronary artery, Western blot analysis, DHE-derived fluorescence assay of ROS production and NAD(P)H oxidase activity, SOD activity and MDA formation assays, determination of Nox, electrophysiological recording, and statistical analysis.
Supplementary Material
Abbreviations Used
- 3-NT
3-nitrotyrosine
- ACE
angiotensin-converting enzyme
- Ach
acetylcholine
- BKCa
Ca2+-activated K+ channel
- CK-MB
creatine kinase-MB
- CO
cardiac output
- CPB
cardiopulmonary bypass
- cTnI
cardiac troponin-I
- CVP
central venous pressure
- DHE
dihydroethidium
- HBOCs
hemoglobin-based oxygen carriers
- HUVECs
human umbilical vein endothelial cells
- I/R
ischemia/reperfusion
- IBTX
iberiotoxin
- LV
left ventricular
- LVEDP
left ventricular end-diastolic pressure
- LVEF
left ventricular ejection fraction
- LVLS
left ventricular longitudinal strain
- LVSP
left ventricular systolic pressure
- MAP
mean arterial pressure
- MDA
malonaldehyde
- MetHb
methemoglobin
- NO
nitrite oxide
- O2
oxygen
- O2EI
O2 extraction index
- ONOO−
peroxynitrite
- PAP
pulmonary arterial pressure
- PAWP
pulmonary artery wedge pressure
- ROS
reactive oxygen species
- SNP
sodium nitroprusside
- SOD
superoxide dismutase
- STS
St.Thomas' solution
- TEA
tetraethylammonium
- VO2
cardiac O2 consumption
- VSMCs
vascular smooth muscle cells
- vWF
von Willebrand factor
Acknowledgments
This study was supported by grants from the National Nature Science Foundation of China (81100180 and 81471042), the China Postdoctoral Specialized Science Foundation (201003700), the Specialized Research Fund for the Doctoral Program of Higher Education (20100181120090), and the Major Program of the Clinical High and New Technology of PLA (2010gxjs039). The authors thank Chengmin Yang and Shen Li for their technical assistance.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Alayash AI. Oxygen therapeutics: can we tame haemoglobin? Nat Rev Drug Discov 3: 152–159, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Baek JH, D'Agnillo F, Vallelian F, Pereira CP, Williams MC, Jia Y, Schaer DJ, and Buehler PW. Hemoglobin-driven pathophysiology is an in vivo consequence of the red blood cell storage lesion that can be attenuated in guinea pigs by haptoglobin therapy. J Clin Invest 122: 1444–1458, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonetti PO, Lerman LO, and Lerman A. Endothelial dysfunction a marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol 23: 168–175, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Boretti FS, Buehler PW, D'Agnillo F, Kluge K, Glaus T, Butt OI, Jia Y, Goede J, Pereira CP, and Maggiorini M. Sequestration of extracellular hemoglobin within a haptoglobin complex decreases its hypertensive and oxidative effects in dogs and guinea pigs. J Clin Invest 119: 2271–2280, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brocq ML, Leslie SJ, Milliken P, and Megson IL. Endothelial dysfunction: from molecular mechanisms to measurement, clinical implications, and therapeutic opportunities. Antioxid Redox Signal 10: 1631–1674, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Brutsaert DL. Cardiac endothelial-myocardial signaling: its role in cardiac growth, contractile performance, and rhythmicity. Physiol Rev 83: 59–115, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Cai H. and Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res 87: 840–844, 2000 [DOI] [PubMed] [Google Scholar]
- 8.Chang TMS. Therapeutic applications of polymeric artificial cells. Nat Rev Drug Discov 4: 221–235, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Chen JY, Scerbo M, and Kramer G. A review of blood substitutes: examining the history, clinical trial results, and ethics of hemoglobin-based oxygen carriers. Clinics 64: 803–813, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deanfield JE, Halcox JP, and Rabelink TJ. Endothelial function and dysfunction testing and clinical relevance. Circulation 115: 1285–1295, 2007 [DOI] [PubMed] [Google Scholar]
- 11.Dikalov SI, Dikalova AE, Bikineyeva AT, Schmidt HH, Harrison DG, and Griendling KK. Distinct roles of Nox1 and Nox4 in basal and angiotensin II-stimulated superoxide and hydrogen peroxide production. Free Radic Biol Med 45: 1340–1351, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drenger B, Fontes ML, Miao Y, Mathew JP, Gozal Y, Aronson S, Dietzel C, and Mangano DT. Patterns of use of perioperative angiotensin-converting enzyme inhibitors in coronary artery bypass graft surgery with cardiopulmonary bypassclinical perspective effects on in-hospital morbidity and mortality. Circulation 126: 261–269, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Dull RO, DeWitt BJ, Dinavahi R, Schwartz L, Hubert C, Pace N, and Fronticelli C. Quantitative assessment of hemoglobin-induced endothelial barrier dysfunction. Journal of Appl Physiol 97: 1930–1937, 2004 [DOI] [PubMed] [Google Scholar]
- 14.El Solh AA, Akinnusi ME, Baddoura FH, and Mankowski CR. Endothelial cell apoptosis in obstructive sleep apnea: a link to endothelial dysfunction. Am J Respir Crit Care Med 175: 1186–1191, 2007 [DOI] [PubMed] [Google Scholar]
- 15.George I, Yi GH, Schulman AR, Morrow BT, Cheng Y, Gu A, Zhang G, Oz MC, Burkhoff D, and Wang J. A polymerized bovine hemoglobin oxygen carrier preserves regional myocardial function and reduces infarct size after acute myocardial ischemia. Am J Physiol Heart Circ Physiol 291: 1126–1137, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Hai CM. Systems biology of HBOC-induced vasoconstriction. Curr Drug Discov Technol 9: 204–211, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hill MA, Yang Y, Ella SR, Davis MJ, and Braun AP. Large conductance, Ca2+-activated K+ channels (BKCa) and arteriolar myogenic signaling. FEBS Lett 584: 2033–2042, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hornig B, Landmesser U, Kohler C, Ahlersmann D, Spiekermann S, Christoph A, Tatge H, and Drexler H. Comparative effect of ACE inhibition and angiotensin II type 1 receptor antagonism on bioavailability of nitric oxide in patients with coronary artery disease role of superoxide dismutase. Circulation 103: 799–805, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Li T, Li J, Liu J, Zhang P, Wu W, Zhou R, Li G, Zhang W, Yi M, and Huang H. Polymerized placenta hemoglobin attenuates ischemia/reperfusion injury and restores the nitroso-redox balance in isolated rat heart. Free Radic Biol Med 46: 397–405, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Li T, Wu W, You Z, Zhou R, Li Q, Zhu D, Li H, Xiang X, Irwin MG, and Xia Z. Alternative use of isoflurane and propofol confers superior cardioprotection than using one of them alone in a dog model of cardiopulmonary bypass. Eur J Pharmacol 677: 138–146, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Li T, Yu R, Zhang HH, Wang H, Liang WG, Yang XM, and Yang CM. A method for purification and viral inactivation of human placenta hemoglobin. Artif Cells Blood Substit Immobil Biotechnol 34: 175–188, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Li T, Zhang P, Liu J, Zhou R, Li Q, You Z, and Dian K. Protective effects of hemoglobin-based oxygen carrier given to isolated heart during ischemia via attenuation of mitochondrial oxidative damage. Free Radic Biol Med 48: 1079–1089, 2010 [DOI] [PubMed] [Google Scholar]
- 23.Li T, Zhou R, Xiang X, Zhu D, Li Y, Liu J, Wu W, and Yang C. Polymerized human placenta hemoglobin given before ischemia protects rat heart from ischemia reperfusion injury. Artif Cells Blood Substit Biotechnol 39: 392–397, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Li T, Zhu D, Zhou R, Wu W, Li Q, and Liu J. HBOC attenuates intense exercise-induced cardiac dysfunction. Int J Sports Med 33: 338–345, 2012 [DOI] [PubMed] [Google Scholar]
- 25.Münzel T. and Keaney JF. Are ACE inhibitors a “magic bullet” against oxidative stress? Circulation 104: 1571–1574, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Minneci PC, Deans KJ, Zhi H, Yuen PS, Star RA, Banks SM, Schechter AN, Natanson C, Gladwin MT, and Solomon SB. Hemolysis-associated endothelial dysfunction mediated by accelerated NO inactivation by decompartmentalized oxyhemoglobin. J Clin Invest 115: 3409–3417, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Natanson C, Kern SJ, Lurie P, Banks SM, and Wolfe SM. Cell-free hemoglobin-based blood substitutes and risk of myocardial infarction and death: a meta-analysis. JAMA 299: 2304–2312, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olson JS, Foley EW, Rogge C, Tsai A-L, Doyle MP, and Lemon DD. NO scavenging and the hypertensive effect of hemoglobin-based blood substitutes. Free Radic Biol Med 36: 685–697, 2004 [DOI] [PubMed] [Google Scholar]
- 29.P Biro G. Adverse HBOC-Endothelial dysfunction synergism: a possible contributor to adverse clinical outcomes? Curr Drug Discov Technol 9: 194–203, 2012 [DOI] [PubMed] [Google Scholar]
- 30.Rajagopalan S. and Harrison DG. Reversing endothelial dysfunction with ace inhibitors: a new TREND? Circulation 94: 240–243, 1996 [DOI] [PubMed] [Google Scholar]
- 31.Schröder K, Zhang M, Benkhoff S, Mieth A, Pliquett R, Kosowski J, Kruse C, Lüdike P, Michaelis UR, and Weissmann N. Nox4 is a protective reactive oxygen species generating vascular NADPH oxidase. Circ Res 110: 1217–1225, 2012 [DOI] [PubMed] [Google Scholar]
- 32.Silverman TA. and Weiskopf RB. Hemoglobin-based oxygen carriers: current status and future directions. Transfusion 49: 2495–2515, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Simoni J, Simoni G, Moeller JF, Tsikouris JP, and Wesson DE. Evaluation of angiotensin converting enzyme (ACE)-like activity of acellular hemoglobin. Artif Cells Blood Substit Biotechnol 35: 191–210, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Varnado CL, Mollan TL, Birukou I, Smith BJ, Henderson DP, and Olson JS. Development of recombinant hemoglobin-based oxygen carriers. Antioxid Redox Signal 18: 2314–2328, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Verma S. and Anderson TJ. Fundamentals of endothelial function for the clinical cardiologist. Circulation 105: 546–549, 2002 [DOI] [PubMed] [Google Scholar]
- 36.Yu B, Raher MJ, Volpato GP, Bloch KD, Ichinose F, and Zapol WM. Inhaled nitric oxide enables artificial blood transfusion without hypertension. Circulation 117: 1982–1990, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu B, Shahid M, Egorina EM, Sovershaev MA, Raher MJ, Lei C, Wu MX, Bloch KD, and Zapol WM. Endothelial Dysfunction Enhances Vasoconstriction Due to Scavenging of Nitric Oxide by a Hemoglobin-based Oxygen Carrier. Anesthesiology 112: 586–594, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zimmet JM. and Hare JM. Nitroso–redox interactions in the cardiovascular system. Circulation 114: 1531–1544, 2006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.