Abstract
Background/Aims
Superficial esophageal squamous cell carcinoma (SESCC) is being increasingly detected during screening endoscopy. Endoscopic submucosal dissection (ESD) allows for en bloc and histologically complete resection of lesions. This study assessed the technical feasibility and long-term outcomes of ESD for SESCCs.
Methods
Between January 2005 and August 2012, 27 patients with 28 SESCCs underwent ESD at Pusan National University Hospital. The en bloc and pathologically complete resection rates, complication (perforation and bleeding) rate, incidence of esophageal stricture after ESD, and overall and disease-specific survival rates were evaluated.
Results
The en bloc and pathologically complete resection rates were 93% and 83%, respectively. No significant bleeding occurred, and perforation with mediastinal emphysema was observed in two patients (7%). Post-ESD stricture occurred in two patients (7%) who had mucosal defects involving more than three-fourths of the esophageal circumference. During a mean follow-up of 23 months, local tumor recurrence was seen in two of four lesions with pathologically incomplete resection; one was treated by re-ESD, and the other was treated by surgical esophagectomy. The 5-year overall and disease-specific survival rates were 84% and 100%, respectively.
Conclusions
ESD seems to be a feasible, effective curative treatment for SESCCs. All patients should be closely followed after ESD.
Keywords: Esophageal neoplasms, Squamous cell carcinoma, Endoscopic submucosal dissection, Outcome
INTRODUCTION
Recently, in Korea, the frequency of detecting superficial esophageal squamous cell carcinoma (SESCC) during screening endoscopy has been slowly increasing with the recent innovations in endoscopic technology, such as image-enhanced endoscopy and magnifying endoscopy.1–3 Even when the disease is detected at an early stage, surgical esophagectomy with lymph node dissection has been the best treatment for SESCCs.4,5 After esophagectomy, however, most patients experience disabling symptoms, such as regurgitation and dysphagia. Esophagectomy also has high morbidity and mortality rates.4,5 In contrast, endoscopic resection has some merits over esophagectomy, such as organ preservation, maintenance of the quality of life, and lower mortality and morbidity. Because of these merits, endoscopic resection, especially endoscopic mucosal resection (EMR), has been widely used for the treatment of SESCCs. In addition, the long-term survival outcomes after EMR of SESCCs suggest a favorable prognosis.6
However, conventional EMR techniques present limitations in terms of resectable lesion size, and large lesions should be resected in a piecemeal manner.7,8 Furthermore, EMR caused intense thermal denaturation of the lesion, which makes it difficult to perform precise histopathologic evaluation of the piecemeal-resected specimen, needed to determine the risk of lymph node metastasis.9 Moreover, local recurrence rates are reported to be higher after piecemeal resection than after en bloc resection.10
Endoscopic submucosal dissection (ESD) has the advantage of permitting en bloc and histologically complete resection of lesions. This technique results in a lower recurrence rate than that with EMR in early gastric neoplasms.11–13 On the basis of these results, ESD has been used for lesions of the esophagus mainly in Japan.14–16 However, because the lumen of the esophagus is narrow and its thin wall constantly moves with respiration and heartbeat, it is not easy to perform ESD. Because of technical difficulties and postoperative esophageal stricture, endoscopic treatment is principally limited to lesions that do not exceed three-fourths of the luminal circumference.17 Even though the short-term outcomes are remarkable,7,14,15 the long-term prognosis of SESCCs after ESD remains insufficient, especially in Korea. Therefore, the aim of this study was to assess the technical feasibility and long-term outcomes of ESD for the treatment of SESCCs.
MATERIALS AND METHODS
1. Patients
We retrospectively analyzed our database of all patients who underwent endoscopic resection at the Pusan National University Hospital, Busan, Korea, from January 2005 to August 2012. We identified a total of 27 patients with 28 SESCCs underwent ESD. All patients met the following eligibility criteria: histologically proven squamous cell carcinoma, tumor invasion depth of m1 (carcinoma in situ) to m3 (limited to muscularis mucosa) as measured by endoscopic ultrasonography (EUS), no lymph node and distant metastases by computed tomography (CT) and EUS, and no prior chemotherapy or radiation treatment. This study was reviewed and approved by the Institutional Review Board at Pusan National University Hospital (IRB number: E-2013025).
2. Endoscopic submucosal dissection
ESD was performed under conscious sedation, with cardio-respiratory monitoring. For sedation, 5 to 10 mg of midazolam and 25 mg of meperidine were administered intravenously; pro-pofol was administered, as needed, during the procedure. First, marking approximately 2 mm outside the borders of the lesion, identified by chromoendoscopy with iodine staining, was made using argon plasma coagulation (APC) (Fig. 1). After marking, a saline solution (0.9% saline with a small amount of epinephrine and indigo carmine) was injected submucosally around the lesion to elevate it from the muscular layer. A circumferential mucosal incision was made outside the marking dots by using a hook knife (Olympus, Tokyo, Japan). Next, submucosal dissection was performed, with an insulation-tipped knife (ESD-Knife; MTW Endoskopie, Wesel, Germany), to allow complete removal of the lesion. If necessary during the procedure, the submucosal injection was repeated and endoscopic hemostasis was achieved. A high-frequency electrosurgical current generator (Erbotom VIO 300D; ERBE, Tübingen, Germany) was used during marking, mucosal incision, submucosal dissection, and hemostasis.
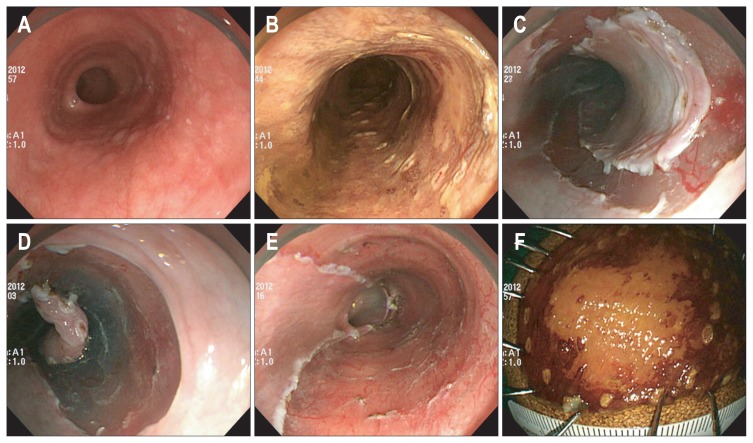
Fig. 1.
Endoscopic submucosal dissection of a superficial esophageal squamous cell carcinoma. (A) A reddish, ambiguous lesion is seen in the lower esophagus. (B) After chromoendoscopy with iodine staining, marking dots are made to demarcate the lesion. (C) A complete circumferential incision is made using a hook knife. (D) Submucosal dissection is made using an insulated-tip knife. (E) The lesion is completely removed. (F) Resected specimen after iodine staining.
3. Histopathological evaluation
Resection specimens were fixed in formalin and serially sectioned at 2 mm intervals to assess the tumor involvement in the lateral and vertical margins. The tumor size, depth of invasion, degree of differentiation, and lymphovascular invasion were evaluated microscopically according to the Japanese Classification of Esophageal Carcinoma.18 A carcinoma that extended up to 200 μm below the lower border of the muscularis mucosae was defined as sm1.19 En bloc resection was defined as resection in a single piece as opposed to piecemeal resection (in multiple segments). Pathologically complete resection was defined when en bloc resection was achieved, with tumor-negative margins and the absence of lymphovascular invasion. Patients whose histopathologic diagnosis involved a cancer invasion depth reaching the muscularis mucosae (m3) with vascular infiltration, or reaching the submucosa with or without vascular infiltration were recommended to undergo additional treatments (e.g., esophagectomy with lymph node dissection or radiation therapy with or without chemotherapy against possible lymph node metastases).
4. Outcomes of ESD
All patients underwent postprocedural chest radiography and a second-look endoscopy on the following day, to detect any perforation and bleeding. After the procedure, intravenous pantoprazole (40 mg daily) for 2 days, followed by oral pantoprazole (40 mg daily) for 4 weeks was administered to relieve pain, prevent procedure-related bleeding, and promote ulcer healing. A sucralfate suspension (4 times a day) was also given to all patients. Patients without serious symptoms or complications were allowed to start food intake on the day after the procedure and were discharged within 3 to 4 days.
The en bloc and pathologically complete resection rates, mean procedure time, complication (perforation and bleeding) rate, and incidence of esophageal stricture after the procedure were evaluated. The procedure time, defined as the time from the marking of the lesion to the completion of lesion resection, was measured. ESD-related bleeding was defined as 1) bleeding proven by routine, second-look endoscopy within 24 hours; 2) clinical evidence of melena or hematemesis; or 3) massive bleeding such as bleeding that required transfusion or bleeding in which the level of hemoglobin fell 2 g/dL after the procedures. Perforation was endoscopically diagnosed during the procedure or by the presence of free air on plain chest radiography after ESD. Esophageal stricture after the procedure was defined as the inability to pass a standard 11-mm-diameter endoscope through the stricture.
5. Follow-up
Follow-up endoscopy with biopsies was conducted 6 months after the ESD and annually thereafter. In cases of noncurative resection or a high possibility of post-ESD stricture, an additional endoscopy was scheduled 1 to 2 months after the ESD. To detect lymph node and distant metastases, chest and abdominal CTs were performed 6 months later after ESD and then annually, and serum tumor markers (carcinoembryonic antigen and carbohydrate antigen 19–9) were also measured. A new lesion detected within 1 year of the initial ESD in a different area from the initial cancer was considered a synchronous cancer, as opposed to a metachronous cancer, which was defined as a lesion that was detected >1 year after treatment.
6. Statistical analysis
Overall and disease-specific survival rates were calculated from the date of ESD. Overall survival included deaths from any cause. Disease-specific survival included deaths from esophageal carcinoma, whereas patients who died of intercurrent disease were counted as “withdrawn alive.” Survival curves were plotted according to the Kaplan-Meier method.
RESULTS
The baseline characteristics of the 28 resected SESCCs in 27 patients (26 men; mean age, 64 years) treated with ESD are described in Table 1. The mean procedure time was 51 minutes (range, 17 to 153 minutes). The mean specimen size and tumor size was 28 mm (range, 11 to 59 mm) and 13 mm (range, 3 to 16 mm), respectively. Of the 28 tumors, 23 (82%) were carcinoma in situ (m1) or intramucosal invasive carcinoma limited to the lamina propria mucosae (m2). Lymphovascular invasion was not seen in any of the tumors included in this study. The post-ESD mucosal defect in 14 lesions (50%) was spread over more than half the circumference of the lumen.
Table 1.
Characteristics of 28 Superficial Esophageal Squamous Cell Carcinomas in 27 Patients Who Underwent Endoscopic Submucosal Dissection
| Characteristic | Value |
|---|---|
| Male:female (n=27) | 26:1 |
| Age, yr | 64 (46–76) |
| Location | |
| Upper esophagus | 4 (14) |
| Mid-esophagus | 7 (25) |
| Lower esophagus | 17 (61) |
| Mucosal defect of the whole circumference after ESD | |
| <1/2 | 14 (50) |
| <3/4 | 6 (21) |
| ≥3/4 | 8 (29) |
| Procedure time, min | 51 (17–153) |
| Resected specimen size, mm | 28 (11–59) |
| Tumor size, mm | 13 (3–26) |
| Histological depth | |
| m1 | 7 (25) |
| m2 | 16 (57) |
| m3 | 3 (11) |
| sm1 | 1 (4) |
| sm2 | 1 (4) |
| Lymphovascular invasion | |
| Absent | 28 (100) |
| Present | 0 |
Data are presented as mean (range) or number (%).
The en bloc and pathologically complete resection rates were 93% (26 of 28) and 86% (24 of 28), respectively (Table 2). In the four lesions with pathologically incomplete resection, three had a positive lateral margin (1 m1, 1 m2, and 1 m3) and the remaining one had a positive vertical margin (sm2).
Table 2.
Outcomes of 28 Superficial Esophageal Squamous Cell Carcinomas in 27 Patients Who Underwent Endoscopic Submucosal Dissection
| No. (%) | |
|---|---|
| En bloc resection | 26 (93) |
| Pathologically complete resection | 24 (86) |
| Causes for pathological incomplete resection | 4 (14) |
| Lateral/vertical involvement | 3/1 |
| Lymphovascular invasion | 0 |
| Procedure-related complications | |
| Bleeding | 0 |
| Perforation | 2 (7) |
| Stricture | 2 (7) |
Of the procedure-related complications, perforation with mediastinal emphysema was observed in two patients (7%). In both patients, perforation was diagnosed endoscopically during ESD and treated successfully with immediate endoscopic closure with hemoclips. These patients were given antibiotics intravenously, and oral intake was discontinued for several days. Minor bleeding during the procedure was frequent but was managed successfully by endoscopic hemostasis with electrocoagulation and hemoclips. None of the patients experienced bleeding that required blood transfusion during or after ESD, nor did they require additional endoscopy for hemostasis.
Among 20 patients with mucosal defects involving less than three-fourths of the esophageal circumference, none experienced post-ESD stricture. In seven patients with mucosal defects involving more than three-fourths of the esophageal circumference, three patients were prescribed oral prednisolone to prevent post-ESD stricture. Oral prednisolone was started at 30 mg daily on the third day post-ESD, the dose was tapered gradually (daily 30, 30, 25, 25, 15, and 10 mg for 7 days each), and then discontinued at 8 weeks. Of them, one patient experienced post-ESD stricture with dysphagia, which was successfully managed with two sessions of balloon dilation. The other four patients with mucosal defects involving more than three-fourths of the esophageal circumference did not take oral prednisolone. Of them, one patient experienced post-ESD stricture, which was successfully managed with balloon dilation and intralesional dexamethasone injection in two sessions.
The follow-up profile of all patients is summarized in Fig. 2. One patient with tumor involvement (m3) in the lateral margin underwent APC in the involved margin. The above-mentioned additional treatments, such as esophagectomy or radiation therapy, were recommended to five patients with m3, sm1, and sm2 cancer; however, all of them refused additional therapy.
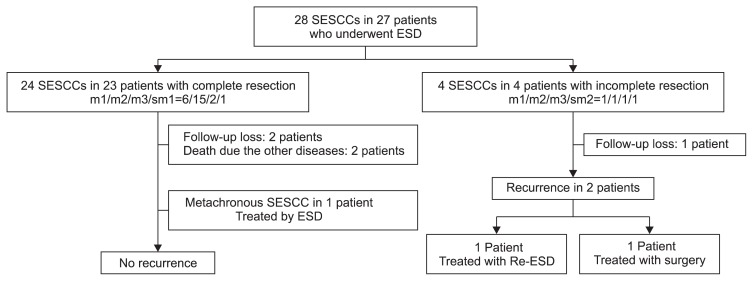
Fig. 2.
Follow-up profile of 28 superficial esophageal squamous cell carcinomas (SESCCs) in 27 patients who underwent endoscopic submucosal dissection (ESD).
Of the 27 patients, three were lost to follow-up (two with m1 cancer and one with sm2 cancer). Finally, 25 lesions in 24 patients were included in the follow-up. The mean follow-up period was 23 months (range, 2 to 78 months). Two patients had a local recurrence after 34 and 38 months, respectively. In one patient, the first lesion was m2 cancer with lateral margin involvement, and the recurred lesion was SESCC. Therefore, the lesion was successfully treated with an additional ESD. In the other patient, the first lesion was m3 cancer with lateral margin involvement; thus, he underwent additional APC therapy in the involved margin. However, the recurred lesion was a submucosal tumor-like elevation, and the cancer was found to invade the muscularis propria.20 Therefore, he underwent surgical esophagectomy with lymph node dissection, and finally the cancer invaded the adventitia without lymph node metastasis. One patient underwent a second ESD session for a metachronous SESCC after 24 months.
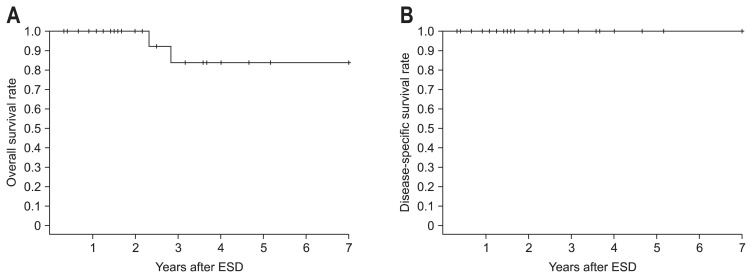
For all patients, including the 3 patients who had no medical records for the most recent year, ending in December 2012, the survival status, cause of death, and date of death were obtained from the Korea Central Cancer Registry. Thus, all 27 patients treated by ESD were eligible for the survival analysis. During a median observation period of 28 months (range, 4 to 84 months), a total of two patients died from causes other than esophageal cancer. The causes of death included tongue cancer and cardiovascular disease. The 5-year overall and disease-specific survival rates were 84% and 100%, respectively (Fig. 3).
Fig. 3.
Survival rates of patients who underwent endoscopic submucosal dissection (ESD) for superficial squamous cell carcinoma. (A) Overall survival rate. (B) Disease-specific survival rate.
DISCUSSION
In this study, the long-term outcomes of ESD for SESCCs demonstrated the excellent curability of m1 or m2 cancers and a low recurrence rate (4%, 1 of 23 lesions). In addition, although two patients with m2 or sm1 cancer died of other causes, the cause-specific survival rate at 5 years was 100% for patients with SESCC. This survival rate is consistent with the findings of previous studies on ESD in SESCCs.8,21,22 Because of the higher comorbidity of esophagectomy and the higher incidence of incomplete resection by EMR,9,23 ESD may be a superior treatment option for SESCCs, regardless of their size or submucosal fibrosis.
Local recurrence is an important issue in curative treatment. Local recurrence occurs in the case of incomplete resection because tumor cells can be left behind in the unresected mucosa. In the present study, all patients with pathologically complete resection did not have local recurrence. Local recurrence occurred in two of four patients in whom the resection was pathologically incomplete. Therefore, if the pathologic reports reveal incomplete resection after ESD, additional treatments, such as re-ESD, esophagectomy, or radiation therapy, should be considered. Furthermore, close follow-up is mandatory.
Because this study was a single-arm, retrospective analysis, we did not evaluate the superiority of ESD over EMR. Although small lesions can be treated by EMR with a single or very few resections, piecemeal resection of large lesions is insufficient for histological assessment of SESCCs. Furthermore, unnecessary nonneoplastic mucosal resection, which is avoidable by ESD, may cause severe postoperative stenosis.8 Considering the risks and benefits, ESD is recommended for m1 or m2 SESCCs >2 cm, and m3 or deeper squamous cell carcinomas of any size, to obtain en bloc resection with sufficient tumor-free margins and to completely remove invasive lesions.14
The major complications associated with ESD include bleeding, perforation and stricture of the esophagus. However, our results indicate that the complications accompanying ESD occurred with the same frequency as in previous studies.8,21 In the present study, esophageal perforation, which is potentially life threatening and may induce severe mediastinitis, occurred in two patients (7%). However, the perforations that occurred during ESD were managed successfully without surgical rescue.
Post-ESD stricture is of great concern in the post-ESD management of these patients, and its incidence is reported to be 5% to 17%.14,21,24 The possibility of esophageal stenosis increases if a circumferential mucosal defect is more than three-fourths of the esophageal circumference after ESD.14,17,24 Moreover, considering the length of longitudinal mucosal defects, longer defects (>30 mm) were a significant risk factor for the development of stricture.25 Post-ESD stricture with dysphagia could be successfully managed with endoscopic balloon dilation in a median of two sessions;6 however, repetitive, periodic endoscopic balloon dilatation is usually needed in controlling and preventing postprocedural stricture after ESD.26 Recently, local injection or systemic administration of steroids has been reported to be effective for preventing post-ESD stricture.25,27 In the present study, we found no post-ESD stricture after ESD when the defect involved less than three-fourths of the esophageal circumference. Besides, post-ESD stricture occurred only in two of seven patients with mucosal defects involving more than three-fourths of the esophageal circumference, and it was successfully managed endoscopically by two sessions of balloon dilation. In our study, three patients took oral prednisolone to prevent post-ESD stricture, and post-ESD stricture occurred in one patient.
This study has several limitations. First, this was a retrospective study that assessed the outcomes of ESD for SESCCs. Therefore, the retrospective nature of the review may have caused a potential bias in the analysis. In addition, exact horizontal and longitudinal mucosal defect size could not be calculated because of the limitation in retrospective design. Only maximal tumor size and the degree of mucosal defect in the circumference of the lumen were evaluated in this study. Second, the number of SESCCs included in this study was relatively small and may be insufficient to adequately evaluate the outcomes of ESD. Third, the mean follow-up period (23 months) was not sufficiently long for the evaluation of the long-term safety of ESD. Fourth, the patients were selected for ESD according to the clinical judgment of physicians at the time of treatment, taking into consideration the patients’ needs.
In conclusion, this study of long-term follow-up data reveals that ESD is a feasible and effective curative treatment for SESCCs. Although further prospective, long-term, multicenter studies are needed to elucidate the appropriate division of treatments for SESCCs, we suggest that ESD plays a central role in the treatment of SESCCs.
ACKNOWLEDGEMENTS
This study was supported by a grant from the National R&D Program for Cancer Control, Ministry for Health, Welfare and Family Affairs, Republic of Korea (0920050).
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Endo M, Takeshita K, Yoshida M. How can we diagnose the early stage of esophageal cancer? Endoscopic diagnosis. Endoscopy. 1986;18(Suppl 3):11–18. doi: 10.1055/s-2007-1018435. [DOI] [PubMed] [Google Scholar]
- 2.Yoshida T, Inoue H, Usui S, Satodate H, Fukami N, Kudo SE. Narrow-band imaging system with magnifying endoscopy for superficial esophageal lesions. Gastrointest Endosc. 2004;59:288–295. doi: 10.1016/S0016-5107(03)02532-X. [DOI] [PubMed] [Google Scholar]
- 3.Kumagai Y, Monma K, Kawada K. Magnifying chromoendoscopy of the esophagus: in-vivo pathological diagnosis using an endocytoscopy system. Endoscopy. 2004;36:590–594. doi: 10.1055/s-2004-814533. [DOI] [PubMed] [Google Scholar]
- 4.Kato H, Tachimori Y, Mizobuchi S, Igaki H, Ochiai A. Cervical, mediastinal, and abdominal lymph node dissection (three-field dissection) for superficial carcinoma of the thoracic esophagus. Cancer. 1993;72:2879–2882. doi: 10.1002/1097-0142(19931115)72:10<2879::AID-CNCR2820721004>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 5.Roth JA, Putnam JB., Jr Surgery for cancer of the esophagus. Semin Oncol. 1994;21:453–461. [PubMed] [Google Scholar]
- 6.Shimizu Y, Tsukagoshi H, Fujita M, Hosokawa M, Kato M, Asaka M. Long-term outcome after endoscopic mucosal resection in patients with esophageal squamous cell carcinoma invading the muscularis mucosae or deeper. Gastrointest Endosc. 2002;56:387–390. doi: 10.1016/S0016-5107(02)70043-6. [DOI] [PubMed] [Google Scholar]
- 7.Ishihara R, Iishi H, Uedo N, et al. Comparison of EMR and endoscopic submucosal dissection for en bloc resection of early esophageal cancers in Japan. Gastrointest Endosc. 2008;68:1066–1072. doi: 10.1016/j.gie.2008.03.1114. [DOI] [PubMed] [Google Scholar]
- 8.Yamashita T, Zeniya A, Ishii H, et al. Endoscopic mucosal resection using a cap-fitted panendoscope and endoscopic submucosal dissection as optimal endoscopic procedures for superficial esophageal carcinoma. Surg Endosc. 2011;25:2541–2546. doi: 10.1007/s00464-011-1584-6. [DOI] [PubMed] [Google Scholar]
- 9.Shimura T, Sasaki M, Kataoka H, et al. Advantages of endoscopic submucosal dissection over conventional endoscopic mucosal resection. J Gastroenterol Hepatol. 2007;22:821–826. doi: 10.1111/j.1440-1746.2006.04505.x. [DOI] [PubMed] [Google Scholar]
- 10.Ishihara R, Iishi H, Takeuchi Y, et al. Local recurrence of large squamous-cell carcinoma of the esophagus after endoscopic resection. Gastrointest Endosc. 2008;67:799–804. doi: 10.1016/j.gie.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 11.Gotoda T. Endoscopic resection of early gastric cancer. Gastric Cancer. 2007;10:1–11. doi: 10.1007/s10120-006-0408-1. [DOI] [PubMed] [Google Scholar]
- 12.Takenaka R, Kawahara Y, Okada H, et al. Risk factors associated with local recurrence of early gastric cancers after endoscopic sub-mucosal dissection. Gastrointest Endosc. 2008;68:887–894. doi: 10.1016/j.gie.2008.03.1089. [DOI] [PubMed] [Google Scholar]
- 13.Park JC, Lee SK, Seo JH, et al. Predictive factors for local recurrence after endoscopic resection for early gastric cancer: long-term clinical outcome in a single-center experience. Surg Endosc. 2010;24:2842–2849. doi: 10.1007/s00464-010-1060-8. [DOI] [PubMed] [Google Scholar]
- 14.Oyama T, Tomori A, Hotta K, et al. Endoscopic submucosal dissection of early esophageal cancer. Clin Gastroenterol Hepatol. 2005;3(7 Suppl 1):S67–S70. doi: 10.1016/S1542-3565(05)00291-0. [DOI] [PubMed] [Google Scholar]
- 15.Fujishiro M, Yahagi N, Kakushima N, et al. Endoscopic submucosal dissection of esophageal squamous cell neoplasms. Clin Gastroenterol Hepatol. 2006;4:688–694. doi: 10.1016/j.cgh.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 16.Ishii N, Itoh T, Horiki N, et al. Endoscopic submucosal dissection with a combination of small-caliber-tip transparent hood and flex knife for large superficial colorectal neoplasias including ileocecal lesions. Surg Endosc. 2010;24:1941–1947. doi: 10.1007/s00464-010-0883-7. [DOI] [PubMed] [Google Scholar]
- 17.Ono S, Fujishiro M, Niimi K, et al. Predictors of postoperative stricture after esophageal endoscopic submucosal dissection for superficial squamous cell neoplasms. Endoscopy. 2009;41:661–665. doi: 10.1055/s-0029-1214867. [DOI] [PubMed] [Google Scholar]
- 18.Japanese Society for Esophageal Diseases. Guidelines for clinical and pathologic studies on carcinoma of the esophagus. Tokyo: Japanese Society for Esophageal Diseases; 2002. [Google Scholar]
- 19.Chiba T, Kawachi H, Kawano T, et al. Independent histological risk factors for lymph node metastasis of superficial esophageal squamous cell carcinoma; implication of claudin-5 immunohistochemistry for expanding the indications of endoscopic resection. Dis Esophagus. 2010;23:398–407. doi: 10.1111/j.1442-2050.2009.01023.x. [DOI] [PubMed] [Google Scholar]
- 20.Choi JC, Kim GH, Park DY, et al. A submucosal tumor-like recurrence of early esophageal cancer after endoscopic submucosal dissection. Clin Endosc. 2013;46:182–185. doi: 10.5946/ce.2013.46.2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ono S, Fujishiro M, Niimi K, et al. Long-term outcomes of endoscopic submucosal dissection for superficial esophageal squamous cell neoplasms. Gastrointest Endosc. 2009;70:860–866. doi: 10.1016/j.gie.2009.04.044. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi H, Arimura Y, Masao H, et al. Endoscopic submucosal dissection is superior to conventional endoscopic resection as a curative treatment for early squamous cell carcinoma of the esophagus (with video) Gastrointest Endosc. 2010;72:255–264. doi: 10.1016/j.gie.2010.02.040. [DOI] [PubMed] [Google Scholar]
- 23.Katada C, Muto M, Manabe T, Ohtsu A, Yoshida S. Local recurrence of squamous-cell carcinoma of the esophagus after EMR. Gastrointest Endosc. 2005;61:219–225. doi: 10.1016/S0016-5107(04)02756-7. [DOI] [PubMed] [Google Scholar]
- 24.Mizuta H, Nishimori I, Kuratani Y, Higashidani Y, Kohsaki T, Onishi S. Predictive factors for esophageal stenosis after endoscopic submucosal dissection for superficial esophageal cancer. Dis Esophagus. 2009;22:626–631. doi: 10.1111/j.1442-2050.2009.00954.x. [DOI] [PubMed] [Google Scholar]
- 25.Ezoe Y, Muto M, Horimatsu T, et al. Efficacy of preventive endoscopic balloon dilation for esophageal stricture after endoscopic resection. J Clin Gastroenterol. 2011;45:222–227. doi: 10.1097/MCG.0b013e3181f39f4e. [DOI] [PubMed] [Google Scholar]
- 26.Isomoto H, Yamaguchi N, Nakayama T, et al. Management of esophageal stricture after complete circular endoscopic submucosal dissection for superficial esophageal squamous cell carcinoma. BMC Gastroenterol. 2011;11:46. doi: 10.1186/1471-230X-11-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamaguchi N, Isomoto H, Shikuwa S, et al. Effect of oral prednisolone on esophageal stricture after complete circular endoscopic submucosal dissection for superficial esophageal squamous cell carcinoma: a case report. Digestion. 2011;83:291–295. doi: 10.1159/000321093. [DOI] [PubMed] [Google Scholar]