Abstract
Background/Aims
Ribonucleotide reductase subunit M2 (RRM2) catalyzes the production of deoxynucleotide triphosphates, which are necessary for DNA synthesis. RRM2 has been reported to play an active role in tumor progression, and elevated RRM2 levels have been correlated with poor prognosis for colorectal cancer patients. This study aimed to elucidate the prognostic significance of RRM2 protein expression in hepatocellular carcinoma after surgery.
Methods
RRM2 protein expression was evaluated using immunohistochemistry in tumor tissues from 259 hepatocellular carcinoma patients who underwent curative hepatectomy.
Results
High RRM2 expression was observed in 210 of 259 patients (81.1%) with hepatocellular carcinomas. High RRM2 expression was significantly associated with viral etiology (p=0.035) and liver cirrhosis (p=0.036). High RRM2 expression was correlated with early recurrence (p=0.004) but not with late recurrence (p=0.144). Logistic regression analysis revealed that high RRM2 expression (p=0.040) and intrahepatic metastasis (p<0.001) were independent predictors of early recurrence. High RRM2 expression unfavorably influenced both shorter recurrence-free survival (p=0.011) and shorter disease-specific survival (p=0.002) and was an independent predictor of shorter disease-specific survival (p=0.008).
Conclusions
High RRM2 protein expression might be a useful marker for predicting early recurrence and may be a marker for poor prognosis of hepatocellular carcinoma after curative hepatectomy.
Keywords: Ribonucleotide reductase subunit M2, Hepatocellular carcinoma, Survival
INTRODUCTION
The prognosis of hepatocellular carcinoma (HCC) remains grave as a result of a high rate of tumor recurrence even after surgical resection.1–3 Predicting clinical outcomes for HCC patients is a challenge for clinicians. Although there are reports on histologic parameters for predicting HCC prognosis, molecular markers for HCC prognosis could provide additional information.4 Cancer classification using prognostic markers can identify patients with a high risk of recurrence or poor prognosis.5,6
Ribonucleotide reductase subunit M2 (RRM2) catalyzes the production of deoxynucleotide triphosphates, which are necessary for DNA synthesis.7 Inhibition of ribonucleotide reductase stops DNA synthesis and cell proliferation, and RRM2 has been considered a promising target for cancer therapy.8 Suppression of RRM2 synthesis inhibited the growth of gastric cancer cell lines in vitro.9 RRM2 depletion significantly reduced antiapoptotic protein Bcl-2 expression and RRM2 suppression led to increased Bcl-2 degradation.10 RRM2 was reported to play an active role in tumor progression11 and elevated RRM2 levels were correlated with poor prognosis for patients with colorectal cancer, pancreas adenocarcinoma, lung adenocarcinoma, and ovarian cancer.12–15 RRM2 was overexpressed in human HCC.16,17 Small-interfering RNA-mediated knockdown of RRM2 inhibited the proliferation of HCC cells and the growth of HCC xenografts transplanted into immunodeficient mice.16 Recent study showed that positive immunoreactivity for RRM2 was found in 84% (16 of 19) of the HCCs.16 However, the prognostic significance of RRM2 protein expression in HCC remains uncertain.
In the present study, we evaluated RRM2 protein expression by immunohistochemistry in order to identify a marker associated with tumor recurrence and to elucidate the prognostic significance of RRM2 in 259 HCC patients with long-term follow-up.
MATERIALS AND METHODS
1. Study subjects
Primary HCC tissues were collected from 259 patients who were treated with curative hepatectomy at the Samsung Medical Center, Seoul, Korea between July 2000 and May 2006. We defined curative resection as the complete resection of all tumor nodules with clear microscopic resection margins and no residual tumors as indicated by a computed tomography scan 1 month after surgery. None of the patients had received preoperative chemotherapy. This study was approved by the Institutional Review Board of the Samsung Medical Center. Tumor differentiation was graded histologically using the Edmondson and Steiner criteria.18 Microvascular invasion was considered present when at least one or more endothelial cells or the tunica media of the vessel surrounded a neoplastic cell group. Intrahepatic metastasis and multicentric occurrence were defined according to the criteria of the Liver Cancer Study Group of Japan.19 Staging of the tumors was performed according to both the American Joint Committee on Cancer (AJCC)20 and the Barcelona Clinic Liver Cancer (BCLC) staging classification.21 Using 2 years as the cutoff, tumor recurrence was classified as either early recurrence or late recurrence.22
We followed up all patients by monitoring serum α-fetoprotein levels and three phase dynamic computed tomography scans every 3 months after surgery. Magnetic resonance imaging was used in order to confirm tumor recurrence in suspected case. The follow-up period for recurrence was at least 26 months and the median follow-up period was 118 months (range, 14.0 to 151.4 months) for survivors. Recurrence-free survival (RFS) was defined from the date of resection until the detection of both intrahepatic and extrahepatic recurrence. We chose disease-specific death (HCC-related death) as the clinical endpoint for survival analysis, defined as 1) tumor occupying more than 80% of the liver, 2) portal venous tumor thrombus proximal to the second bifurcation, 3) obstructive jaundice due to the tumor, 4) distant metastases, or 5) variceal hemorrhage with portal venous tumor thrombus proximal to the first bifurcation.23 Disease-specific survival (DSS) was defined from the date of resection to the date of HCC-related death.
Histologic sections were examined by two pathologists and the representative tumor areas free from necrosis or hemorrhage were premarked in formalin-fixed paraffin-embedded blocks. Two 2.0-mm-diameter tissue cores were obtained from donor blocks and arranged in recipient paraffin blocks. Two cores of normal liver tissue from 12 patients with metastatic colonic carcinoma of the liver were included in each microarray block. Each tissue microarray block contained up to 60 tissue cores.
2. Immunohistochemical analysis
Immunohistochemistry for RRM2 was performed as described elsewhere.24 Epitope retrieval was performed with 0.01 mol/L citrate buffer (pH 6.0) for 30 minutes in a pressure cooker. Sections were incubated with mouse monoclonal antibody to RRM2 (clone 1E1, 1:800; Abcam Corp., Cambridge, MA, USA) for 30 minutes at room temperature. In order to validate the concordance between the tissue microarrays and whole tumor sections, we further detected RRM2 expression in 40 corresponding whole tumor sections randomly chosen from the 259 cases. No immunoreactivity was observed in the tissue sections used as negative control, in which the primary antibody was replaced by isotype-matched irrelevant antibody. The positive control (human normal kidney) showed cytoplasmic RRM2 expression in the epithelial cells of convoluted tubules.
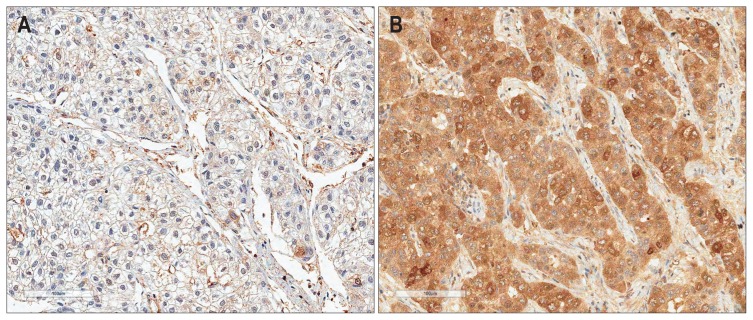
Immunohistochemical staining was assessed by two independent pathologists (C.K.P. and B.L.) who were blinded to clinical details and any discrepancies were resolved by consensus. A nearly homogeneous immunostaining with moderate staining intensity was observed without any predominant expression pattern in whole tumor section of HCC. The percentage of stained tumor cells was scored from 0% to 100% and each sample was rated from 0 to 4 (0, 0%; 1, 1% to 25%; 2, 26% to 50%; 3, 51% to 75%; 4, >75%). Duplicate tissue cores for each tumor showed high levels of homogeneity for proportion of stained cells. In cases of differences between duplicate tissue cores, the higher score was taken. The immunoreactivity of tumor was graded as low expression (0% to 50% stained tumor cells regardless of staining intensity) (Fig. 1A) or high expression (>50% stained tumor cells) (Fig. 1B).
Fig. 1.
Immunostaining of ribonucleotide reductase subunit M2 in hepatocellular carcinomas showing low expression (A) or high expression (B) (horseradish peroxidase stain, ×200).
3. Statistical analysis
All statistical analyses were performed using SPSS software (SPSS Inc., Chicago, IL, USA). The correlation between RRM2 expression and clinicopathologic features was examined by the chi-square and Fisher exact tests. The logistic regression analysis was used for prediction of tumor recurrence. Survival curves were constructed using the Kaplan-Meier method and the differences in survival were evaluated using the log-rank test. The Cox regression hazard model was used to identify factors that were independently associated with survival. A multivariate analysis was performed including all parameters that were significantly associated with survival in a univariate analysis. A p-value of <0.05 was defined as being statistically significant.
RESULTS
1. Clinicopathologic characteristics of patients
The mean patient age was 52.2 years (range, 17 to 76 years), 211 patients were men, and 48 women. One hundred ninety-eight patients (76.4%) were infected with hepatitis B virus and 25 (9.7%) with hepatitis C virus. No viral marker was recognized in 36 patients (13.9%). Tumor recurrence was detected in 185 patients (71.4%), early recurrence in 140 patients (54.1%), late recurrence in 45 patients (17.4%), and 101 patients (39.0%) died of HCC. Nineteen of the 120 deaths in this study were due to non-HCC causes. Twelve of the 19 deaths were due to hepatic failure, five due to nonhepatic causes, and two due to unknown causes.
2. RRM2 protein expression in HCC
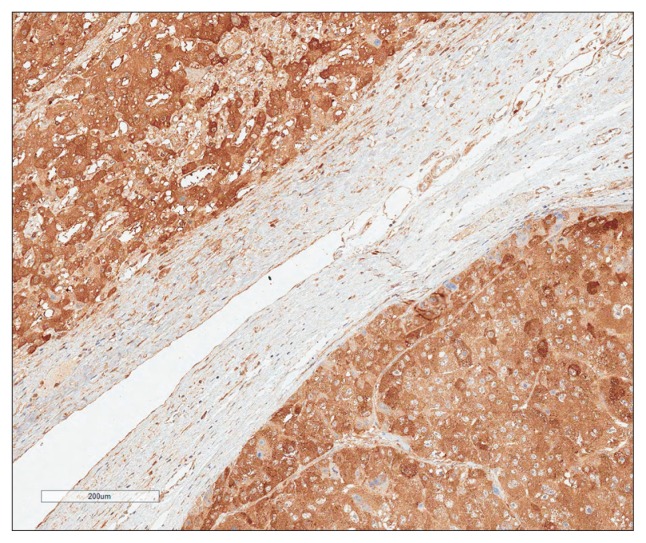
RRM2 expression was observed in the cytoplasm of normal hepatocytes and bile ducts with moderate staining intensity. In HCC, immunoreactivity for RRM2 was similar to that of normal hepatocyte (Fig. 2). In a few cases membranous immunoreactivity was also found. High RRM2 protein expression was observed in 210 of the 259 HCCs (81.1%). High RRM2 expression was significantly associated with viral etiology (p=0.035) and liver cirrhosis (p=0.036). High RRM2 expression was correlated with the early recurrence (p=0.004), but not with the late recurrence (p=0.966) (Table 1).
Fig. 2.
Ribonucleotide reductase subunit M2 immunoreactivity in hepatocellular carcinomas (right lower part) was similar to that in normal hepatocytes (left upper part) (horseradish peroxidase stain, ×100).
Table 1.
Correlations between Ribonucleotide Reductase Subunit M2 Expression and Clinicopathologic Features in 259 Patients with Hepatocellular Carcinomas
| Variable | No. | High RRM2 expression, no. (%) | p-value |
|---|---|---|---|
| Age, yr | |||
| ≤55 | 154 | 123 (79.9) | 0.547 |
| >55 | 105 | 87 (82.9) | |
| Gender | |||
| Female | 48 | 35 (72.9) | 0.110 |
| Male | 211 | 175 (82.9) | |
| Tumor size, cm | |||
| ≤5.0 | 163 | 133 (81.6) | 0.783 |
| >5.0 | 96 | 77 (80.2) | |
| Edmondson grade | |||
| I | 24 | 19 (79.2) | 0.184 |
| II | 178 | 140 (78.7) | |
| III | 57 | 51 (89.5) | |
| Microvascular invasion | |||
| (−) | 114 | 87 (76.3) | 0.083 |
| (+) | 145 | 123 (84.8) | |
| Major portal vein invasion | |||
| (−) | 247 | 200 (81.8) | 1.000 |
| (+) | 12 | 10 (83.3) | |
| Intrahepatic metastasis | |||
| (−) | 195 | 155 (79.5) | 0.253 |
| (+) | 64 | 55 (85.9) | |
| Multicentric occurrence | |||
| (−) | 247 | 202 (81.8) | 0.249 |
| (+) | 12 | 8 (66.7) | |
| AJCC T-stage | |||
| 1 | 110 | 86 (78.2) | 0.550 |
| 2 | 99 | 83 (83.8) | |
| 3 | 44 | 35 (79.5) | |
| 4 | 6 | 6 (100.0) | |
| BCLC stage | |||
| 0–A | 141 | 113 (80.1) | 0.965 |
| B | 104 | 85 (81.7) | |
| C | 14 | 12 (85.7) | |
| Albumin level, g/dL | |||
| >3.5 | 232 | 185 (79.7) | 0.107 |
| ≤3.5 | 27 | 25 (92.6) | |
| AFP level, ng/mL* | |||
| ≤200 | 151 | 127 (84.1) | 0.136 |
| >200 | 98 | 75 (76.5) | |
| Etiology | |||
| Nonviral | 36 | 24 (66.7) | 0.035 |
| HBV | 198 | 167 (84.3) | |
| HCV | 25 | 19 (76.0) | |
| Liver cirrhosis | |||
| (−) | 129 | 98 (76.0) | 0.036 |
| (+) | 130 | 112 (86.2) | |
| Early recurrence (≤2 yr) | |||
| (−) | 119 | 87 (73.1) | 0.004 |
| (+) | 140 | 123 (87.9) | |
| Late recurrence (>2 yr) | |||
| (−)† | 74 | 54 (73.0) | 0.966 |
| (+) | 45 | 33 (73.3) | |
RRM2, ribonucleotide reductase subunit M2; AJCC, American Joint Committee on Cancer; BCLC, Barcelona Clinic Liver Cancer; AFP, α-fetoprotein; HBV, hepatitis B virus; HCV, hepatitis C virus.
Data for 10 patients were unavailable;
No early or late recurrence.
3. Prediction of early recurrence in HCC
Univariate analysis revealed that early recurrence was significantly associated with larger tumor size (p=0.001), Edmondson grade III (p=0.003), microvascular invasion (p<0.001), major portal vein invasion (p=0.028), intrahepatic metastasis (p<0.001), higher AJCC T-stage (p<0.001), higher BCLC stage (p<0.001), viral etiology (p=0.009), and high RRM2 expression (p=0.003). Since AJCC T-stage and BCLC stage were associated with vascular invasion, we did not perform multiple analyses with these variables to avoid potential bias. On multivariate analysis, high RRM2 expression (p=0.040) and intrahepatic metastasis (p<0.001) were independent predictors of early recurrence (Table 2).
Table 2.
Univariate and Multivariate Logistic Regression Models for Predicting Early Tumor Recurrence in 259 Patients with Hepatocellular Carcinomas
| Variable | Univariate model | Multivariate model | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Coefficient | OR (95% CI) | p-value | Coefficient | OR (95% CI) | p-value | |
| Age (≤55 vs >55), yr | 0.210 | 1.23 (0.75–2.03) | 0.410 | - | - | - |
| Gender (female vs male) | 0.097 | 1.10 (0.59–2.07) | 0.762 | - | - | - |
| Tumor size (≤5.0 vs >5.0), cm | 0.900 | 2.46 (1.45–4.17) | 0.001 | 0.198 | 1.22 (0.62–2.41) | 0.568 |
| Edmondson grade (I+II vs III) | 0.981 | 2.67 (1.41–5.06) | 0.003 | 0.504 | 1.66 (0.78–3.52) | 0.190 |
| Microvascular invasion (no vs yes) | 1.343 | 3.83 (2.28–6.43) | <0.001 | 0.430 | 1.54 (0.81–2.94) | 0.192 |
| Major portal vein invasion (no vs yes) | 2.309 | 10.06 (1.28–79.13) | 0.028 | −0.188 | 0.83 (0.081–8.48) | 0.874 |
| Intrahepatic metastasis (no vs yes) | 2.810 | 16.61 (6.38–43.21) | <0.001 | 2.408 | 11.11 (3.52–35.05) | <0.001 |
| Multicentric occurrence (no vs yes) | 0.555 | 1.74 (0.51–5.94) | 0.375 | - | - | - |
| AJCC T-stage (1 vs 2+3+4) | 1.414 | 4.11 (2.44–6.94) | <0.001 | - | - | - |
| BCLC stage (0+A vs B+C) | 1.322 | 3.75 (2.24–6.32) | <0.001 | - | - | - |
| Albumin level (>3.5 vs ≤3.5), g/dL | 0.981 | 2.67 (1.09–6.55) | 0.032 | 0.816 | 2.26 (0.83–6.19) | 0.113 |
| AFP level (≤200 vs >200), ng/mL* | 0.444 | 1.56 (0.93–2.61) | 0.092 | - | - | - |
| Etiology (nonviral vs viral) | 0.991 | 2.70 (1.28–5.66) | 0.009 | 0.847 | 2.33 (0.99–5.53) | 0.054 |
| Liver cirrhosis (no vs yes) | 0.357 | 1.43 (0.88–2.34) | 0.154 | - | - | - |
| RRM2 expression (low vs high) | 0.979 | 2.66 (1.39–5.09) | 0.003 | 0.808 | 2.24 (1.04–4.86) | 0.040 |
OR, odds ratio; CI, confidence interval; AJCC, American Joint Committee on Cancer; BCLC, Barcelona Clinic Liver Cancer; AFP, α-fetoprotein; RRM2, ribonucleotide reductase subunit M2.
Data for 10 patients were unavailable.
4. Correlation between RRM2 expression and clinical outcome
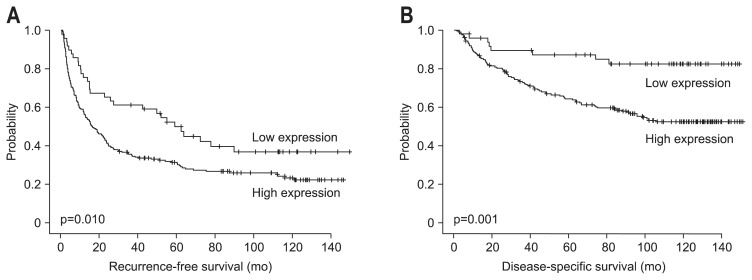
The RFS and DSS rates for 259 HCC patients were 40.9% and 76.4% at 3 years, 35.7% and 69.0% at 5 years, 29.9% and 64.3% at 7 years, and 28.8% and 58.5% at 9 years, respectively. Univariate analysis revealed that larger tumor size, Edmondson grade III, microvascular invasion, major portal vein invasion, intrahepatic metastasis, higher AJCC T-stage, higher BCLC stage, lower albumin level, and higher α-fetoprotein level showed unfavorable influences on both RFS and DSS. Viral etiology and liver cirrhosis showed unfavorable influences on RFS. High RRM2 expression showed an unfavorable influence on both RFS (p=0.011) and DSS (p=0.002) (Table 3). The 5-year RFS rate of the RRM2-high expression group was significantly lower than that of the RRM2-low expression group (31.4% vs 50.0%) (Fig. 3A). The median RFS of the RRM2-high expression group and RRM2-low expression group were 16.4±5.8 and 59.1±16.7 months, respectively. The 5-year DSS rate of the RRM2-high expression group was significantly lower than that of the RRM2-low expression group (64.3% vs 87.3%) (Fig. 3B). The mean DSS of the RRM2-high expression group and RRM2-low expression group were 97.7±8.3 and 129.5±13 months, respectively.
Table 3.
Univariate Analyses of Recurrence-Free Survival and Disease-Specific Survival in 259 Patients with Hepatocellular Carcinomas
| Variable | Recurrence-free survival | Disease-specific survival | ||
|---|---|---|---|---|
|
|
|
|||
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Age (≤55 vs >55), yr | 1.01 (0.76–1.36) | 0.926 | 0.95 (0.64–1.41) | 0.794 |
| Gender (female vs male) | 1.01 (0.70–1.45) | 0.961 | 1.31 (0.77–2.20) | 0.318 |
| Tumor size (≤5.0 vs >5.0), cm | 1.64 (1.22–2.21) | 0.001 | 2.53 (1.71–3.74) | <0.001 |
| Edmondson grade (I+II vs III) | 1.95 (1.40–2.71) | <0.001 | 2.21 (1.45–3.37) | <0.001 |
| Microvascular invasion (no vs yes) | 2.15 (1.59–2.90) | <0.001 | 3.09 (1.99–4.81) | <0.001 |
| Major portal vein invasion (no vs yes) | 3.84 (2.07–7.09) | <0.001 | 6.43 (3.30–12.52) | <0.001 |
| Intrahepatic metastasis (no vs yes) | 4.38 (3.17–6.06) | <0.001 | 5.97 (3.99–8.92) | <0.001 |
| Multicentric occurrence (no vs yes) | 1.59 (0.86–2.92) | 0.138 | 0.88 (0.32–2.39) | 0.799 |
| AJCC T-stage (1 vs 2+3+4) | 2.24 (1.65–3.03) | <0.001 | 3.25 (2.06–5.11) | <0.001 |
| BCLC stage (0+A vs B+C) | 2.05 (1.53–2.74) | <0.001 | 3.45 (2.28–5.20) | <0.001 |
| Albumin level (>3.5 vs ≤3.5), g/dL | 1.89 (1.23–2.90) | 0.004 | 2.47 (1.46–4.17) | 0.001 |
| AFP level (≤200 vs >200), ng/mL* | 1.58 (1.18–2.13) | 0.002 | 1.47 (0.99–2.19) | 0.059 |
| Etiology (nonviral vs viral) | 2.06 (1.25–3.39) | 0.005 | 1.64 (0.85–3.16) | 0.137 |
| Liver cirrhosis (no vs yes) | 1.34 (1.00–1.79) | 0.047 | 1.11 (0.75–1.64) | 0.611 |
| RRM2 expression (low vs high) | 1.68 (1.13–2.49) | 0.011 | 3.22 (1.56–6.63) | 0.002 |
HR, hazard ratio; CI, confidence interval; AJCC, American Joint Committee on Cancer; BCLC, Barcelona Clinic Liver Cancer; AFP, α-fetoprotein; RRM2, ribonucleotide reductase subunit M2.
Data for 10 patients were unavailable.
Fig. 3.
Kaplan-Meier survival curves showing recurrence-free survival (A) and disease-specific survival (B) according to ribonucleotide reductase subunit M2 expression in 259 patients with hepatocellular carcinomas.
Multivariate analysis revealed that intrahepatic metastasis and lower albumin level were independent predictors of both shorter RFS and shorter DSS. High RRM2 expression was an independent predictor of shorter DSS (p=0.008). RRM2-high expression patients were more likely to suffer from disease-specific death than RRM2-low expression patients (hazard ratio, 2.73) (Table 4).
Table 4.
Multivariate Analyses of Recurrence-Free Survival and Disease-Specific Survival in 259 Patients with Hepatocellular Carcinomas
| Variable | Recurrence-free survival | Disease-specific survival | ||
|---|---|---|---|---|
|
|
|
|||
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Tumor size (≤5.0 vs >5.0), cm | 1.16 (0.82–1.66) | 0.407 | 1.38 (0.88–2.16) | 0.163 |
| Edmondson grade (I+II vs III) | 1.16 (0.80–1.69) | 0.427 | 1.09 (0.68–1.74) | 0.721 |
| Microvascular invasion (no vs yes) | 1.35 (0.93–1.97) | 0.114 | 1.47 (0.84–2.57) | 0.173 |
| Major portal vein invasion (no vs yes) | 1.01 (0.50–2.03) | 0.988 | 1.54 (0.75–3.19) | 0.244 |
| Intrahepatic metastasis (no vs yes) | 3.07 (2.04–4.64) | <0.001 | 3.71 (2.21–6.23) | <0.001 |
| Albumin level (>3.5 vs ≤3.5), g/dL | 1.58 (1.01–2.48) | 0.046 | 2.07 (1.20–3.56) | 0.009 |
| Etiology (nonviral vs viral) | 1.59 (0.94–2.68) | 0.082 | - | - |
| Liver cirrhosis (no vs yes) | 1.32 (0.96–1.82) | 0.092 | - | - |
| RRM2 expression (low vs high) | 1.36 (0.90–2.05) | 0.147 | 2.73 (1.30–5.70) | 0.008 |
HR, hazard ratio; CI, confidence interval; RRM2, ribonucleotide reductase subunit M2.
DISCUSSION
Overexpression of RRM2 significantly enhances the invasive potential of human cancer cells.25 Increased invasiveness induced by RRM2 overexpression is associated with nuclear factor-κB activation and matrix metalloproteinase-9 appears to be an important effector of the enhanced invasiveness that RRM2 overexpression induced.26 Levels of RRM2 modulate oncologically important intracellular signaling events that lead to changes in cellular invasiveness. Targeting RRM2 and its downstream signaling intermediaries represents a rational approach for developing novel anticancer therapeutics.26 A molecular biomarker with prognostic value for survival outcomes after surgery can guide further treatment decisions, such as selecting patients at high risk or recurrence.
In the current study, we elucidated the prognostic significance of RRM2 protein expression in a large cohort of HCC patients with long-term follow-up and showed that high RRM2 expression was significantly associated with viral etiology and liver cirrhosis. There were no correlations between high RRM2 expression and tumor aggressiveness, such as tumor size, microvascular invasion, major portal vein invasion, intrahepatic metastasis, AJCC T-stage, or BCLC stage. This is at variance with previous reports using cancer cell lines.9,11,25,26 We suspect that the role of RRM2 might be different in between human cancer tissue and cancer cell line. Using logistic regression analysis, we found that high RRM2 expression and intrahepatic metastasis were independent predictors of early recurrence, suggesting that RRM2 might facilitate the dissemination of tumor cells and clinicians might consider more aggressive treatments for patients with high RRM2 expression in order to prevent recurrence. Moreover, high RRM2 expression showed an unfavorable influence on both RFS and DSS, and was an independent predictor of shorter DSS. Recent studies reported that high RRM2 expression was independent poor prognostic factor of RFS and overall survival in patients with resected pancreas adenocarcinoma or colorectal cancer.12,13 Our results suggest that RRM2 is a potential new marker for predicting the prognosis of HCC after curative hepatectomy and clinicians should possibly have more rigorous follow-up after surgical resection for those patients with poor prognosis identified in this study.
In summary, our data show, for the first time, that high RRM2 protein expression might be a useful marker for predicting early recurrence and a marker for poor prognosis of HCC after curative hepatectomy. Further studies are needed to determine the value of RRM2 as a prognostic predictor in HCCs.
Footnotes
CONFLICTS OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Tung-Ping Poon R, Fan ST, Wong J. Risk factors, prevention, and management of postoperative recurrence after resection of hepatocellular carcinoma. Ann Surg. 2000;232:10–24. doi: 10.1097/00000658-200007000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poon RT. Prevention of recurrence after resection of hepatocellular carcinoma: a daunting challenge. Hepatology. 2011;54:757–759. doi: 10.1002/hep.24569. [DOI] [PubMed] [Google Scholar]
- 3.Llovet JM, Schwartz M, Mazzaferro V. Resection and liver transplantation for hepatocellular carcinoma. Semin Liver Dis. 2005;25:181–200. doi: 10.1055/s-2005-871198. [DOI] [PubMed] [Google Scholar]
- 4.Qin LX, Tang ZY. Recent progress in predictive biomarkers for metastatic recurrence of human hepatocellular carcinoma: a review of the literature. J Cancer Res Clin Oncol. 2004;130:497–513. doi: 10.1007/s00432-004-0572-9. [DOI] [PubMed] [Google Scholar]
- 5.Ho MC, Lin JJ, Chen CN, et al. A gene expression profile for vascular invasion can predict the recurrence after resection of hepatocellular carcinoma: a microarray approach. Ann Surg Oncol. 2006;13:1474–1484. doi: 10.1245/s10434-006-9057-1. [DOI] [PubMed] [Google Scholar]
- 6.Iizuka N, Hamamoto Y, Tsunedomi R, Oka M. Translational microarray systems for outcome prediction of hepatocellular carcinoma. Cancer Sci. 2008;99:659–665. doi: 10.1111/j.1349-7006.2008.00751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D’Angiolella V, Donato V, Forrester FM, et al. Cyclin F-mediated degradation of ribonucleotide reductase M2 controls genome integrity and DNA repair. Cell. 2012;149:1023–1034. doi: 10.1016/j.cell.2012.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shao J, Zhou B, Chu B, Yen Y. Ribonucleotide reductase inhibitors and future drug design. Curr Cancer Drug Targets. 2006;6:409–431. doi: 10.2174/156800906777723949. [DOI] [PubMed] [Google Scholar]
- 9.Morikawa T, Hino R, Uozaki H, et al. Expression of ribonucleotide reductase M2 subunit in gastric cancer and effects of RRM2 inhibition in vitro. Hum Pathol. 2010;41:1742–1748. doi: 10.1016/j.humpath.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 10.Rahman MA, Amin AR, Wang D, et al. RRM2 regulates Bcl-2 in head and neck and lung cancers: a potential target for cancer therapy. Clin Cancer Res. 2013;19:3416–3428. doi: 10.1158/1078-0432.CCR-13-0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furuta E, Okuda H, Kobayashi A, Watabe K. Metabolic genes in cancer: their roles in tumor progression and clinical implications. Biochim Biophys Acta. 2010;1805:141–152. doi: 10.1016/j.bbcan.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu X, Zhang H, Lai L, et al. Ribonucleotide reductase small subunit M2 serves as a prognostic biomarker and predicts poor survival of colorectal cancers. Clin Sci (Lond) 2013;124:567–578. doi: 10.1042/CS20120240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fisher SB, Patel SH, Bagci P, et al. An analysis of human equilibrative nucleoside transporter-1, ribonucleoside reductase subunit M1, ribonucleoside reductase subunit M2, and excision repair cross-complementing gene-1 expression in patients with resected pancreas adenocarcinoma: implications for adjuvant treatment. Cancer. 2013;119:445–453. doi: 10.1002/cncr.27619. [DOI] [PubMed] [Google Scholar]
- 14.Souglakos J, Boukovinas I, Taron M, et al. Ribonucleotide reductase subunits M1 and M2 mRNA expression levels and clinical outcome of lung adenocarcinoma patients treated with docetaxel/gemcitabine. Br J Cancer. 2008;98:1710–1715. doi: 10.1038/sj.bjc.6604344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferrandina G, Mey V, Nannizzi S, et al. Expression of nucleoside transporters, deoxycitidine kinase, ribonucleotide reductase regulatory subunits, and gemcitabine catabolic enzymes in primary ovarian cancer. Cancer Chemother Pharmacol. 2010;65:679–686. doi: 10.1007/s00280-009-1073-y. [DOI] [PubMed] [Google Scholar]
- 16.Satow R, Shitashige M, Kanai Y, et al. Combined functional genome survey of therapeutic targets for hepatocellular carcinoma. Clin Cancer Res. 2010;16:2518–2528. doi: 10.1158/1078-0432.CCR-09-2214. [DOI] [PubMed] [Google Scholar]
- 17.Yu MC, Lee YS, Lin SE, et al. Recurrence and poor prognosis following resection of small hepatitis B-related hepatocellular carcinoma lesions are associated with aberrant tumor expression profiles of glypican 3 and osteopontin. Ann Surg Oncol. 2012;19( Suppl 3):S455–S463. doi: 10.1245/s10434-011-1946-2. [DOI] [PubMed] [Google Scholar]
- 18.Edmondson HA, Steiner PE. Primary carcinoma of the liver: a study of 100 cases among 48,900 necropsies. Cancer. 1954;7:462–503. doi: 10.1002/1097-0142(195405)7:3<462::AID-CNCR2820070308>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 19.Liver Cancer Study Group of Japan. The general rules for the clinical and pathological study of primary liver cancer. 2nd ed. Tokyo: Kanehara; 2003. p. 38. [Google Scholar]
- 20.Edge SB American Joint Committee on Cancer; American Cancer Society; American College of Surgeons. AJCC cancer staging manual. 7th ed. New York: Springer; 2010. pp. 191–199. [Google Scholar]
- 21.Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329–338. doi: 10.1055/s-2007-1007122. [DOI] [PubMed] [Google Scholar]
- 22.Shimada M, Hamatsu T, Yamashita Y, et al. Characteristics of multicentric hepatocellular carcinomas: comparison with intrahepatic metastasis. World J Surg. 2001;25:991–995. doi: 10.1007/s00268-001-0068-6. [DOI] [PubMed] [Google Scholar]
- 23.Hoshida Y, Villanueva A, Kobayashi M, et al. Gene expression in fixed tissues and outcome in hepatocellular carcinoma. N Engl J Med. 2008;359:1995–2004. doi: 10.1056/NEJMoa0804525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahn S, Hyeon J, Park CK. Metadherin is a prognostic predictor of hepatocellular carcinoma after curative hepatectomy. Gut Liver. 2013;7:206–212. doi: 10.5009/gnl.2013.7.2.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou BS, Tsai P, Ker R, et al. Overexpression of transfected human ribonucleotide reductase M2 subunit in human cancer cells enhances their invasive potential. Clin Exp Metastasis. 1998;16:43–49. doi: 10.1023/A:1006559901771. [DOI] [PubMed] [Google Scholar]
- 26.Duxbury MS, Whang EE. RRM2 induces NF-kappaB-dependent MMP-9 activation and enhances cellular invasiveness. Biochem Biophys Res Commun. 2007;354:190–196. doi: 10.1016/j.bbrc.2006.12.177. [DOI] [PubMed] [Google Scholar]