Abstract
Cerebral plasticity includes the adaptation of anatomical and functional connections between parts of the involved brain network. However, little is known about the network dynamics of these connectivity changes. This study investigates the impact of a pure deefferentation, without deafferentation or brain damage, on the functional connectivity of the brain. To investigate this issue, functional MRI was performed on 31 patients in the acute state of Bell's palsy (idiopathic peripheral facial nerve palsy). All of the patients performed a motor paradigm to identify seed regions involved in motor control. The functional connectivity of the resting state within this network of brain regions was compared to a healthy control group. We found decreased connectivity in patients, mainly in areas responsible for sensorimotor integration and supervision (SII, insula, thalamus and cerebellum). However, we did not find decreased connectivity in areas of the primary or secondary motor cortex. The decreased connectivity for the SII and the insula significantly correlated to the severity of the facial palsy. Our results indicate that a pure deefferentation leads the brain to adapt to the current compromised state during rest. The motor system did not make a major attempt to solve the sensorimotor discrepancy by modulating the motor program.
Keywords: fMRI, Resting state, Facial palsy, Functional connectivity, Plasticity
Highlights
-
•
fMRI resting state connectivity analyses after facial palsy
-
•
Decreased connectivity in areas of sensorimotor integration and supervision
-
•
Correlation of connectivity decreases and severity of the facial palsy
-
•
No connectivity changes in motor areas at rest
-
•
Results suggest and adaptation of the brain to a compromised state
1. Introduction
Multiple studies have demonstrated that neural plasticity can change the structure and function of the central nervous system after structural brain damage and rehabilitation (Chen et al., 2010; Hosp and Luft, 2011). An important mechanism is the adaptation of the anatomical and functional connectivities between brain areas. One approach for examining connectivity between brain regions within a network involves quantifying temporal correlation in the blood oxygen level-dependent (BOLD) signal while the brain is in a resting state (Cabral et al., 2014). Multiple studies have demonstrated that resting-state functional connectivity is correlated with behavioral output in multiple domains, particularly in the motor system (Albert et al., 2009). Studies of motor impairments after stroke have demonstrated disrupted connectivity within the motor network (Golestani et al., 2013; Yin et al., 2012). The strength of this disrupted connectivity has been shown to correlate with the clinical outcomes of patients (Yin et al., 2012).
However, little is known about the network dynamics underlying these connectivity changes. The main factors responsible are the effects of structural brain damage itself (Urban et al., 2012), as well as the effects of deafferentation and deefferentation (Werhahn et al., 2002). Because most studies analyzing cortical plasticity after loss of function are conducted in patients suffering from brain damage (e.g., stroke), apportionment can be difficult. Some studies have circumvented this problem by investigating connectivity changes caused by impairment of peripheral nerves or limb amputation (Qiu et al., 2014). However, there are no peripheral pure motor nerves, and therefore, these studies have investigated the combined effect of deafferentation and deefferentation on cortical plasticity. At this time, the effects of a pure deefferentation without structural brain damage are incompletely understood.
Bell's palsy presents an opportunity to investigate a pure deefferentation. The palsy is a transient, unilateral deefferentation of facial muscles, typically of unknown cause (perhaps viral) and resolving within about 6 months. Because efferent activity to facial muscles is carried by the affected facial nerve, whereas somatosensory afference is carried by the trigeminal nerve, the palsy does not affect facial reafference. As we show, Bell's palsy brings into play central nervous system network dynamics pertaining to a pure deefferentation that may illuminate motor systems more generally. We hypothesize that the sensory-motor mismatch alone (without a structural brain lesion) is a sufficient stimulus for adaptation within the facial brain network. Particularly, we investigate whether such a sensory-motor mismatch is a sufficient stimulus for motor adaptation during rest or whether the brain will simply adapt to the current state of the (impaired) sensory-motor information.
We tested this hypothesis in the present study, employing functional magnetic resonance imaging (fMRI) and connectivity analyses in the acute stage of Bell's palsy.
2. Materials and methods
2.1. Subjects
The study population was comprised of 30 patients with Bell's palsy (age 40.9 ± 16.9 years ranging from 21 to 71, 17 male, 14 female, 15 right sided facial palsies), who were recruited from the Neurology and Otorhinolaryngology departments, and 31 age- and gender- matched healthy controls. Right- and left sided facial palsies were investigated in this study together in a balanced design (15 left, 15 right sided palsies) to avoid hemispheric specific effects. Only patients with idiopathic facial nerve palsies without any previous history of neurological disorder were included. The subjects underwent magnetic resonance scans between 2 and 5 days after the onset of their symptoms. Handedness was assessed by the Edinburgh Inventory (Oldfield, 1971), which ranges from −100 for strong left-handedness to +100 for strong right-handedness. Only right-handed (>+79) patients were included. The study was approved by the local ethics committee, and all patients gave their written informed consent according to the declaration of Helsinki.
2.2. Clinical assessment of facial function
Although a variety of scoring systems for the clinical assessment of the severity of peripheral facial nerve palsy are available, the Stennert grading system, which is one of the most widely applied, was selected for use in this study (Stennert et al., 1977). The scale assesses the severity of facial palsy at rest and during voluntary facial movements. This score ranges from 0/0, representing normal facial function, to 4/6, which represents gross facial asymmetry at rest (first value) and complete paralysis (second value).
2.3. MRI experimental design
Subjects were instructed to move the left or right mouth angle up, then relax their facial muscles to regain the starting position. These motor tasks were performed with a frequency of 1 Hz for 30.6 s, followed by a 30.6 s rest. The pace was set visually. The movement effort should be equal on both sides of the face, even if it elicited marginal or no movement on the paretic side. Every subject was trained in this paradigm prior to the MRI scanning. The paradigm during the MRI measurement consisted of 10 blocks (five times the motor task of the left and five times of the right corner of the mouth) in a pseudo-randomized order, which were visually directed.
2.4. MRI recordings
All examinations were performed on the same 3.0 Tesla MR scanner (Trio, Siemens, Erlangen, Germany) to obtain echo-planar T2*-weighted image volumes (EPI) and transaxial T1-weighted structural images. Functional resting state data were acquired in one EPI session of 203 volumes. The patient was instructed to lie down with the eyes closed, to think of nothing in particular, and not fall asleep. The first 3 volumes were subsequently discarded due to equilibration effects. A functional image volume was composed of 44 transaxial slices, including the whole cerebrum and cerebellum (voxel size = 3 × 3 × 3 mm, repetition time = 2.52 s, TE 35 ms). The motor task was performed after the resting state scan, during which 255 images (voxel size = 3 × 3 × 3 mm, repetition time = 2.52 s, TE 35 ms) were acquired. The first 3 volumes were subsequently discarded due to equilibration effects. After functional measurement, high-resolution T1-weighted structural images (voxel size = 1 × 1 × 1 mm) were acquired.
2.5. Preprocessing of functional data (resting state and motor paradigm)
To make patients with right-sided palsy and their age and gender matched healthy control subject comparable to left-sided ones, all their images were flipped along the y-axis prior to analysis.
For each subject, all images were realigned to the first volume using a six-parameter rigid-body transformation that corrected for motion artifacts. The images were co-registered with the subject's corresponding anatomical (T1-weighted) images, re-sliced to correct for acquisition delays, normalized to the Montreal Neurological Institute (MNI) standard brain (Evans et al., 1993) to report MNI coordinates, and smoothed using a 6-mm full-width-at-half-maximum Gaussian kernel.
2.6. fMRI analysis of the motor task
A multiple regression analysis using a general linear model was performed to obtain statistical parametric maps calculated for all three conditions (tongue and right/left sided facial movement). Functional MRI signal time courses were high-pass filtered (128 s) and modeled as an experimental stimulus onset function, convolved by the canonical hemodynamic response function (low-pass filter). Individual results were projected onto the co-registered individual high-resolution T1-weighted 3-D data set. The anatomical localization of activations was analyzed with reference to the standard stereotaxic atlas and by visual inspection of the individual T1-weighted structural data. The resulting statistical maps were thresholded by the family-wise error (FWE; P < 0.05).
2.7. Connectivity analysis of resting state data
In the present study, functional connectivity is examined in the resting state, where temporal correlations of low frequency (<0.1 Hz) blood oxygenation level dependent (BOLD) fMRI signal fluctuations (Biswal et al., 1995; Friston et al., 1993) are presumed to relate to neural activity and reflect information transfer and collaboration between brain areas (Biswal et al., 1995; Greicius et al., 2003). While most studies analyzed the functional connectivity during rest in this frequency range there are also methods to investigate the connectedness between brain areas in other frequency ranges. Particularly, the estimation of the causal influence that one brain area exerts over another (effective connectivity) requires higher frequencies and is usually estimated during a task or the modulation of a task.
Changes in functional connectivity within the facial motor network were investigated in the resting state.
To identify relevant areas of the facial motor network relevant for connectivity analysis, we used the activation maps obtained from the motor task. The point of maximum activation strength, along with its 26 neighbors, was selected from each activated region, and these were further used as regions of interest (ROIs). The resting state data from these identified ROIs were extracted, and cluster-specific time series were estimated by averaging the time series of all voxels within a cluster. Several sources of variance were then removed from the data by linear regression as follows: (1) six parameters obtained by rigid body correction of head motion, (2) the signal from a ventricular ROI and (3) the signal from a region centered in the white matter. All signal intensity time courses were bandpass filtered (0.01 < f < 0.1) to reduce the effect of low-frequency drift and high-frequency noise.
We estimated the functional connectedness using a correlation analysis between different ROIs. The spatial locations of these ROIs were determined using the clusters activated during the motor task in the random effect fMRI analysis. The Pearson's correlation coefficient was computed between all ROIs for each subject. To estimate the global connectivity, we used all combinations of connections in the ROIs (Fig. 1). To estimate the intra-hemispheric connectivity, we used all connections between ROIs within one hemisphere (Fig. 1). These coefficients were transformed to z-scores by Fisher's r- to z-transformation. The z-scores were entered into a paired t-test to determine whether the two groups (healthy vs. facial palsy) showed a significantly different functional connectivity. Findings were considered significant at P < 0.05. In addition to global connectivity values, each chosen ROI was used to determine whether the connections of this ROI to all other areas were different between groups (Fig. 1). Given the resulting number of statistical tests, (16 tests) a correction for multiple tests was necessary, and accordingly, all results were corrected by Bonferroni correction and considered as significant at P < 0.05.
Fig. 1.
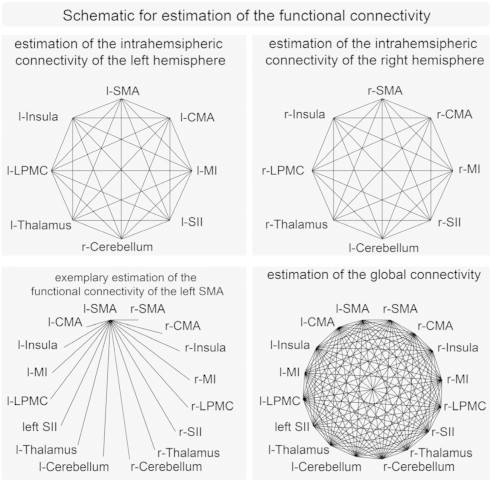
Schematic for estimation of the functional connectivity. The upper left panel shows the connections used to estimate the intrahemispheric connectivity of the left hemisphere, the upper right shows the connections used to estimate the intrahemispheric connectivity of the right hemisphere. The lower left panel shows (exemplary for the left SMA) the connections used to estimate the functional connectivity of one specific area. The lower right panel shows the connections used to estimate the global connectivity (l left, r right).
3. Results
3.1. Clinical assessment of facial function
During the acute state of Bell's palsy, all patients showed a unilateral loss of facial function, with Stennert grades resting index for rest ranging between 0 and 4 (mean 1.5 ± 1.2), and for voluntary facial movements ranging between 1 and 6 (mean 3.7 ± 1.7). All patients reported normal taste and hearing.
3.2. fMRI
The motor tasks of the paretic and non-paretic facial nerves during the acute stage of Bell's palsy evoked highly significant activations (P < 0.01, FDR corrected) in all patients. The same motor task evoked similar activations in the group of healthy subjects. The random effect group analysis of patients and healthy controls revealed significant activations in bilateral MI, bilateral LPMCv, bilateral CMA, bilateral thalamus, bilateral SMA, bilateral secondary somatosensory area (SII), bilateral cerebellum and bilateral insula (Fig. 2). The MNI coordinates with standard deviations, as well as the corresponding t-values for the random effect analysis, are summarized in Table 1. These activated clusters were further used in the following connectivity analysis. Because the aim of the current study is the investigation of connectivity differences between patients with facial palsy and healthy controls, we were mainly interested in the spatial location of activated cortex areas. We compared the spatial location of activity cluster of the eight activity clusters between controls and patients but did not find any significant differences.
Fig. 2.
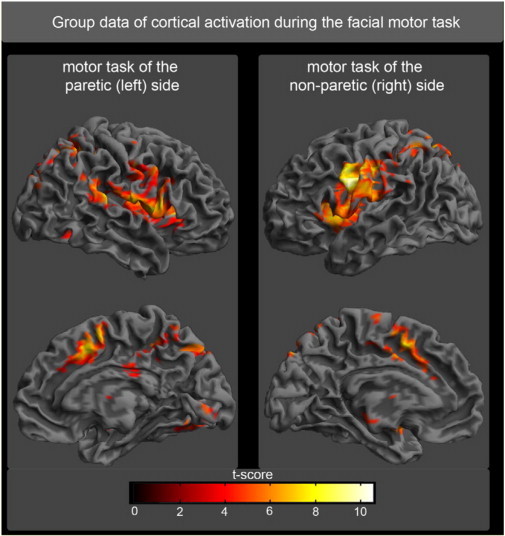
Random effect group analysis of the facial motor task. Activations (P < 0.05, FWE corrected) in response to blocked (30 s) movement of the paretic side (left part of the image) or unaffected side (right part of the image) of the face are shown superimposed on a template cortex.
Table 1.
| Motor task of the paretic side |
Motor task of the non-affected side |
|||||||
|---|---|---|---|---|---|---|---|---|
| x | y | z | t-value | x | y | z | t-value | |
| MI c | 54 ± 4.1 | −10 ± 5.2 | 37 ± 4.6 | 8.87 | −57 ± 3.2 | −10 ± 3.1 | 31 ± 4.1 | 10.3 |
| MI i | −57 ± 4.2 | −16 ± 3.9 | 37 ± 3.7 | 7.37 | 51 ± 3.1 | −10 ± 3.8 | 34 ± 4.3 | 9.73 |
| LPMCv c | 60 ± 6.8 | 2 ± 5.7 | 22 ± 6.9 | 4.42 | −57 ± 4.4 | −1 ± 5.1 | 25 ± 5.1 | 10.19 |
| LPMCv i | −51 ± 5.2 | −1 ± 5.1 | 25 ± 5.9 | 6.97 | 63 ± 4.2 | −1 ± 5.1 | 19 ± 4.4 | 9.62 |
| SMA c | 3 ± 2.9 | 5 ± 3.2 | 55 ± 4.1 | 5.67 | −3 ± 2.1 | 2 ± 4.1 | 55 ± 4.2 | 9.07 |
| SMA i | −3 ± 2.6 | 2 ± 3.4 | 55 ± 3.8 | 6.21 | 3 ± 2.3 | 5 ± 4.4 | 55 ± 4.1 | 8.35 |
| CMA c | 9 ± 4.1 | 8 ± 3.4 | 43 ± 3.3 | 8.50 | −3 ± 4.1 | 5 ± 3.3 | 40 ± 3.5 | 8.94 |
| CMA i | −6 ± 4.5 | 8 ± 4.1 | 43 ± 4.3 | 7.35 | 6 ± 4.8 | 5 ± 4.3 | 37 ± 4.3 | 8.25 |
| Thalamus c | 9 ± 2.3 | −13 ± 2.1 | 10 ± 3.8 | 6.76 | −6 ± 2.1 | −19 ± 2.7 | 7 ± 2.3 | 5.05 |
| Thalamus i | −6 ± 2.2 | −16 ± 2.9 | 7 ± 3.2 | 6.06 | 12 ± 2.7 | −13 ± 2.6 | 7 ± 2.2 | 6.25 |
| SII c | 60 ± 5.1 | −28 ± 3.0 | 19 ± 2.6 | 10.31 | −57 ± 4.6 | −22 ± 4.1 | 16 ± 3.4 | 7.36 |
| SII i | −60 ± 5.1 | −22 ± 4.2 | 16 ± 3.0 | 5.44 | 60 ± 4.4 | −25 ± 4.0 | 19 ± 3.5 | 6.76 |
| Insula c | 36 ± 3.8 | −1 ± 3.5 | 10 ± 3.2 | 8.38 | −39 ± 4.4 | −4 ± 3.9 | 10 ± 3.1 | 10.12 |
| Insula i | −39 ± 3.7 | −4 ± 4.5 | 7 ± 3.1 | 6.10 | 39 ± 4.6 | −1 ± 3.5 | 4 ± 3.4 | 9.92 |
| Cerebellum c | 30 ± 4.3 | −58 ± 3.1 | −32 ± 3.2 | 5.40 | −42 ± 5.5 | −52 ± 4.2 | −29 ± 4.3 | 3.64 |
| Cerebellum i | −15 ± 5.4 | −64 ± 3.4 | −29 ± 3.9 | 3.62 | 33 ± 3.3 | −55 ± 4.3 | −29 ± 4.0 | 3.88 |
MNI coordinates of activation maxima with corresponding t-value and standard deviation for both motor tasks (MI primary motor cortex, LPMCv ventral lateral premotor cortex, SMA supplementary motor area, CMA cingulate motor area, SII secondary somatosensory cortex, c contralateral, i ipsilateral).
3.3. Functional connectivity
We estimated the functional connectivity in the resting state between brain regions that were activated during the motor task. The functional connectivity was estimated for all subjects and compared between both groups (patients with facial palsy and control subjects) by a paired t-test. First, we tested whether there was an overall disturbed connectivity in patients compared with healthy controls. We found a significantly reduced functional connectivity in patients compared to healthy controls (P < 0.01). To determine whether there is a difference in intra-hemispheric connectivity related to the affected side, we averaged all connections between ROIs within one hemisphere. We then tested for differences between groups and between hemispheres. We found a highly significant decreased functional connectivity of the right hemisphere in patients (left facial palsy) compared to the control group (P < 0.01). No group differences were found in the left hemisphere in the same test.
We additionally tested whether this difference in hemispheric connectivity is also significant within the group of patients (testing for differences between both hemispheres within the group of patients). Within the group of patients suffering from facial palsy, the intra-hemispheric connectedness within the right hemisphere was significantly lower compared to the connectedness within the left hemisphere (P < 0.01). The same test for the control group did not reveal any significant differences (P = 0.48).
To investigate which areas contribute to the measured connectivity decrease, we tested for differences between groups for each area (averaged connectivity between one area and all other areas). Because of the number of tests (16 tests), all results were corrected for multiple comparisons using the Bonferroni correction. We found a significantly decreased functional connectivity (healthy vs. patients) in the right thalamus, bilateral SII, right insula and bilateral cerebellum (Fig. 3).
Fig. 3.
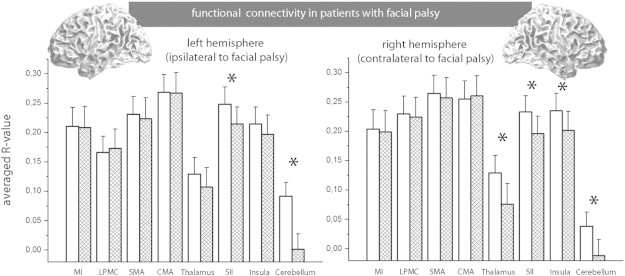
Functional connectivity was measured between one distinct area and all other areas. These values were averaged between subjects. The figure shows the R-values of the Pearson correlation. The white columns represent healthy controls, while the checkered columns represent subjects with facial palsy. A large column indicates a high R-value, corresponding to higher positive connectivity. Significant differences between both groups (P < 0.05, Bonferroni-corrected) are marked by an *.
3.4. Clinical correlation
We estimated the correlation between decrease in connectivity and the clinical severity for all areas that showed a decrease in connectivity. We found a significant correlation between the clinical severity of facial palsy and decreased connectedness for the bilateral SII and the right insula (P < 0.05, Bonferroni corrected −18 tests). The other areas that showed significant decreased connectedness (right thalamus, bilateral cerebellum) failed to show significant correlation with the clinical score (Fig. 4).
Fig. 4.
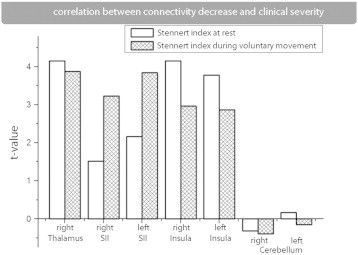
Correlation between connectivity decrease and clinical severity. The difference between the functional connectivity of a patient compared with an age and gender matched control subject was tested for a correlation of clinical severity. We tested all areas that showed significantly decreased connectivity between groups (see also Fig. 3).
4. Discussion
The current study demonstrates decreased functional connectivity in the acute stage of facial palsy in the cerebral hemisphere contralateral to the paralyzed side. We demonstrated that connectedness was most affected in areas of sensorimotor integration, not to primary- or secondary motor areas.At first thought, one might assume that a pure deefferentation should lead to an adaptation of the motor program, and therefore plasticity should primarily affect the motor areas of the brain. However, no altered functional connectivity was found in areas of the primary or secondary motor cortex. Instead, altered functional connectivity was found to the secondary somatosensory cortex, the insula, the thalamus and the cerebellum. The role of each area in the context of the acute deefferentation will be briefly discussed.The insular cortex and the secondary somatosensory areas participate in higher-order processing of somatosensory input and are considered to be crucial nodes for motor-sensory integration detecting the mismatch between perceived and expected movements (Mutschler et al., 2009; Lederman et al., 2001; Simoes and Hari, 1999). In particular, the SII integrates information from both hemispheres, (Disbrow et al., 2001) and perceives asymmetrical facial sensory information at rest and during symmetrical facial movement efforts. Only these areas showed a significant correlation with clinical severity. A further connectivity decrease was found in the dorsolateral thalamus, which is strongly involved in the forwarding and modulation of somatosensory information (Klingner et al., 2013; Klingner et al., 2011).The implications of the decreased functional connectivity to the bilateral cerebellum are harder to interpret due to its complex involvement in the processing of sensory and motor information. However, the cerebellum is known for its contributions to the coordination and quality of movements. For the online control of actions, anticipatory control loops are suggested which predict the sensory feedback (forward modeling) due to a chosen motor program (Desmurget and Grafton, 2000; Shadmehr et al., 2010). We suggest that the decreased connectivity to the cerebellum is due to the substantial prediction error caused by the motor-sensory mismatch. Our knowledge about the role of the involved brain areas could be extended in further studies by analyzing the effective connectivity by using fMRI at higher temporal resolution or by using EEG and MEG. These methods would not only allow to estimate the causal relationship between brain areas but might also shed light on the importance of the sensory feedback as possible driving force for the adaptation during the course of Bell's palsy.Our results demonstrated connectivity changes in areas responsible for sensory processing and motor-sensory integration while no alterations were found in the primary or secondary motor areas.This pattern might be due to the fact that the brain has no information on the cause of the sensory-motor mismatch and particularly no information about where a possible damage might be. Compared to the motor program, the brain detects an unexpectedly low sensory feedback. Because of the brain's inability to detect whether the efferent or afferent information transfer is impaired, we interpret the current results as the principal mechanism that the brain is using in the case of a detected sensory motor discrepancy of unknown origin. Similarly reduced sensory feedback might also arise from a peripheral deafferentation. Accordingly, studies investigating cortical plasticity after long-term sensory deprivation found representational map changes in the somatosensory cortex (Merzenich et al., 1983). However, a deafferentation (in contrary to a deefferentation) reduces not only information about performed movements but also information about external stimuli. This condition is often associated with dysesthesia, which limits the comparability to a deefferentation.In clinical practice, most patients suffering from an acute Bell's palsy receive an exercise physiotherapy although there is no clear evidence for its effectiveness (de Almeida et al., 2014) and also no compelling theory of how such an exercise therapy might influence the outcome after facial nerve palsy. The results of this study indicate that the brain tries to adapt to the actual impaired state of sensorimotor mismatch without any major attempt to solve this discrepancy by modulating motor programs. The question remains whether increased training in the acute state is helpful by activating motor programs and increasing the total amount of the remaining sensory information or whether training solidified the adaptation to this pathologic state. In a previous study of Bell's palsy, we found reduced functional connectivity during motor activity in the motor network (Klingner et al., 2011). These results indicate that motor training is necessary for adaptation of the motor system, while the pure existence of a sensorimotor mismatch is not a sufficient stimulus for adaptation. The training-associated adaptation of the motor system together with our current results (no adaptation of the motor network at rest) further indicates that the adaptation of brain networks is highly context sensitive. It can therefore be hypothesized that motor training is helpful in the acute state of this disease. This result is in line with existing studies suggesting that facial exercise therapy is effective for facial palsy for the functional outcome (Pereira et al., 2011). If we assume that the increased amount of sensory information is important in avoiding adaptation to the impaired state of sensorimotor information, then the question becomes whether additional sensory information from other modalities is also beneficial via impeding the adaptation to the current pathologic state. For example, one might hypothesize that mirror feedback training avoids the adaptation to the pathologic state by additional visual information. Another interesting approach to overcome the sensorimotor mismatch could be a repetitive manual stimulation of the face (e.g., with the hands of the patient). With this maneuver, the brain could be supported in realizing, that no afferent but a pure efferent deficit causes the sensorimotor discrepancy.
5. Conclusion
In conclusion, we demonstrated that connections in Bell's palsy areas of sensory-motor integration were most affected, and primary- or secondary motor areas were not. This pattern indicates that a pure deefferentation leads the brain to adapt to its current state without any major attempts to modulate the motor program. These results suggest an important role of sensory feedback in plasticity after an acute deefferentation, but its exact role and impact have to be investigated in further studies.
References
- Albert N.B., Robertson E.M., Miall R.C. The resting human brain and motor learning. Current Biology: CB. 2009;19:1023–1027. doi: 10.1016/j.cub.2009.04.028. 19427210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B., Yetkin F.Z., Haughton V.M., Hyde J.S. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magnetic Resonance in Medicine: Official Journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 1995;34:537–541. doi: 10.1002/mrm.1910340409. 8524021 [DOI] [PubMed] [Google Scholar]
- Cabral J., Kringelbach M.L., Deco G. Exploring the network dynamics underlying brain activity during rest. Progress in Neurobiology. 2014;114:102–131. doi: 10.1016/j.pneurobio.2013.12.005. 24389385 [DOI] [PubMed] [Google Scholar]
- Chen H., Epstein J., Stern E. Neural plasticity after acquired brain injury: evidence from functional neuroimaging. PM & R: the Journal of Injury, Function, and Rehabilitation. 2010;2:S306–S312. doi: 10.1016/j.pmrj.2010.10.006. 21172692 [DOI] [PubMed] [Google Scholar]
- de Almeida J.R., Guyatt G.H., Sud S., Dorion J., Hill M.D., Kolber M.R., Lea J., Loong S., Somogyi B.K., Westerberg B.D., White C., Chen J.M. Management of Bell Palsy: Clinical Practice Guideline. CMAJ; 2014. 24934895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmurget M., Grafton S. Forward modeling allows feedback control for fast reaching movements. Trends in Cognitive Sciences. 2000;4:423–431. doi: 10.1016/s1364-6613(00)01537-0. 11058820 [DOI] [PubMed] [Google Scholar]
- Disbrow E., Roberts T., Poeppel D., Krubitzer L. Evidence for interhemispheric processing of inputs from the hands in human S2 and PV. Journal of Neurophysiology. 2001;85:2236–2244. doi: 10.1152/jn.2001.85.5.2236. 11353038 [DOI] [PubMed] [Google Scholar]
- Evans A.C., Collins D.L., Mills S.R., Brown E.D., Kelly R.L., Peters T.M. 3D statistical neuroanatomical models from 305 MRI volumes. IEEE. Nuclear Science Symposium and Medical Imaging Conference. 1993:1813–1817. [Google Scholar]
- Friston K.J., Frith C.D., Liddle P.F., Frackowiak R.S. Functional connectivity: the principal-component analysis of large (PET) data sets. Journal of Cerebral Blood Flow and Metabolism: Official Journal of the International Society of Cerebral Blood Flow and Metabolism. 1993;13:5–14. doi: 10.1038/jcbfm.1993.4. 8417010 [DOI] [PubMed] [Google Scholar]
- Hosp J.A., Luft A.R. Cortical plasticity during motor learning and recovery after ischemic stroke. Neural Plasticity. 2011;2011:871296. doi: 10.1155/2011/871296. 22135758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golestani A.M., Tymchuk S., Demchuk A., Goodyear B.G., VISION-2 Study Group Longitudinal evaluation of resting-state fMRI after acute stroke with hemiparesis. Neurorehabilitation and Neural Repair. 2013;27:153–163. doi: 10.1177/1545968312457827. 22995440 [DOI] [PubMed] [Google Scholar]
- Greicius M.D., Krasnow B., Reiss A.L., Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:253–258. doi: 10.1073/pnas.0135058100. 12506194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingner C.M., Hasler C., Brodoehl S., Axer H., Witte O.W. Perceptual plasticity is mediated by connectivity changes of the medial thalamic nucleus. Human Brain Mapping. 2013;34:2343–2352. doi: 10.1002/hbm.22074. 22451353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingner C.M., Nenadic I., Hasler C., Brodoehl S., Witte O.W. Habituation within the somatosensory processing hierarchy. Behavioural Brain Research. 2011;225:432–436. doi: 10.1016/j.bbr.2011.07.053. 21840344 [DOI] [PubMed] [Google Scholar]
- Merzenich M.M., Kaas J.H., Wall J., Nelson R.J., Sur M., Felleman D. Topographic reorganization of somatosensory cortical areas 3b and 1 in adult monkeys following restricted deafferentation. Neuroscience. 1983;8:33–55. doi: 10.1016/0306-4522(83)90024-6. 6835522 [DOI] [PubMed] [Google Scholar]
- Mutschler I., Wieckhorst B., Kowalevski S., Derix J., Wentlandt J., Schulze-Bonhage A., Ball T. Functional organization of the human anterior insular cortex. Neuroscience Letters. 2009;457:66–70. doi: 10.1016/j.neulet.2009.03.101. 19429164 [DOI] [PubMed] [Google Scholar]
- Oldfield R.C. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. 5146491 [DOI] [PubMed] [Google Scholar]
- Pereira L.M., Obara K., Dias J.M., Menacho M.O., Lavado E.L., Cardoso J.R. Facial exercise therapy for facial palsy: systematic review and meta-analysis. Clinical Rehabilitation. 2011;25:649–658. doi: 10.1177/0269215510395634. 21382865 [DOI] [PubMed] [Google Scholar]
- Qiu T.M., Chen L., Mao Y., Wu J.S., Tang W.J., Hu S.N., Zhou L.F., Gu Y.D. Sensorimotor cortical changes assessed with resting-state fMRI following total brachial plexus root avulsion. Journal of Neurology, Neurosurgery, and Psychiatry. 2014;85:99–105. doi: 10.1136/jnnp-2013-304956. 23761913 [DOI] [PubMed] [Google Scholar]
- Lederman S., Gati J., Servos P., Wilson D. fMRI-derived cortical maps for haptic shape, texture, and hardness. Cognitive Brain Research. 2001;12:307–313. doi: 10.1016/s0926-6410(01)00041-6. [DOI] [PubMed] [Google Scholar]
- Shadmehr R., Smith M.A., Krakauer J.W. Error correction, sensory prediction, and adaptation in motor control. Annual Review of Neuroscience. 2010;33:89–108. doi: 10.1146/annurev-neuro-060909-153135. [DOI] [PubMed] [Google Scholar]
- Simões C., Hari R. Relationship between responses to contra- and ipsilateral stimuli in the human second somatosensory cortex SII. Neuroimage. 1999;10:408–416. doi: 10.1006/nimg.1999.0476. 10493899 [DOI] [PubMed] [Google Scholar]
- Stennert E., Limberg C.H., Frentrup K.P. An index for paresis and defective healing — an easily applied method for objectively determining therapeutic results in facial paresis (author's transl) HNO. 1977;25:238–245. 893151 [PubMed] [Google Scholar]
- Urban E.T.R., 3rd, Bury S.D., Barbay H.S., Guggenmos D.J., Dong Y., Nudo R.J. Gene expression changes of interconnected spared cortical neurons 7 days after ischemic infarct of the primary motor cortex in the rat. Molecular and Cellular Biochemistry. 2012;369:267–286. doi: 10.1007/s11010-012-1390-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werhahn K.J., Mortensen J., Kaelin-Lang A., Boroojerdi B., Cohen L.G. Cortical excitability changes induced by deafferentation of the contralateral hemisphere. Brain: A Journal of Neurology. 2002;125:1402–1413. doi: 10.1093/brain/awf140. 12023328 [DOI] [PubMed] [Google Scholar]
- Yin D., Song F., Xu D., Peterson B.S., Sun L., Men W., Yan X., Fan M. Patterns in cortical connectivity for determining outcomes in hand function after subcortical stroke. PloS One. 2012;7:e52727. doi: 10.1371/journal.pone.0052727. 23285171 [DOI] [PMC free article] [PubMed] [Google Scholar]


