Abstract
Epidemiological and clinical studies have reported that olive oil reduces the incidence of cardiovascular disease. However, the mechanisms involved in this beneficial effect have not been delineated. The endothelium plays an important role in blood pressure regulation through the release of potent vasodilator and vasoconstrictor agents such as nitric oxide (NO) and endothelin-1 (ET-1), respectively, events that are disrupted in type 2 diabetes. Extra virgin olive oil contains polyphenols, compounds that exert a biological action on endothelial function. This study analyzes the effects of olive oil polyphenols on endothelial dysfunction using an in vitro model that simulates the conditions of type 2 diabetes. Our findings show that high glucose and linoleic and oleic acids decrease endothelial NO synthase phosphorylation, and consequently intracellular NO levels, and increase ET-1 synthesis by ECV304 cells. These effects may be related to the stimulation of reactive oxygen species production in these experimental conditions. Hydroxytyrosol and the polyphenol extract from extra virgin olive oil partially reversed the above events. Moreover, we observed that high glucose and free fatty acids reduced NO and increased ET-1 levels induced by acetylcholine through the modulation of intracellular calcium concentrations and endothelial NO synthase phosphorylation, events also reverted by hydroxytyrosol and polyphenol extract. Thus, our results suggest a protective effect of olive oil polyphenols on endothelial dysfunction induced by hyperglycemia and free fatty acids.
Keywords: Type 2 diabetes, Hyperglycemia, Oleic acid, Linoleic acid, ECV304 cells, Reactive oxygen species, Cell oxidative stress
Abbreviations: EVOO, extra virgin olive oil; NO, nitric oxide; ET-1, endothelin-1; FFAs, free fatty acids; ROS, reactive oxygen species; Ach, acetylcholine; DAF-DA, 4,5-diaminofluorescein diacetate; CM-H2DCF-DA, 5,6-chloromethyl 2′,7′-dichlorodihydrofluorescein diacetate; eNOS, endothelial nitric oxide synthase; MAPK, mitogen-activated protein kinase; PEEVOO, polyphenol extract from extra virgin olive oil; PI3K, phosphoinositide 3-kinase; PREDIMED, The PREvention con DIeta MEDiterranea
Graphical abstract
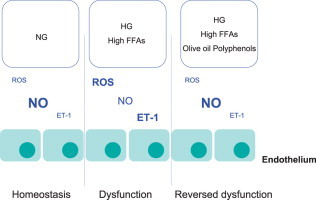
Highlights
-
•
Insulin resistance increases plasma levels of free fatty acids and glucose.
-
•
Hyperglycemia and free fatty acids increase reactive oxygen species and decrease nitric oxide.
-
•
High glucose and free fatty acids augment endothelin-1 release by ECV304 cells.
-
•
Olive oil polyphenols revert endothelial dysfunction induced by high glucose/free fatty acids.
-
•
Extra virgin olive oil is more beneficial than high oleic seed oils on endothelial dysfunction.
Introduction
Epidemiological studies of the dietary patterns of Mediterranean countries have demonstrated that olive oil consumption lowers the incidence of cardiovascular disease [1,2] and that olive oil is associated with lower blood pressure [3]. The PREvencion con DIeta MEDiterranea (PREDIMED) study is a large-scale, multicenter, randomized trial that observed that the traditional Mediterranean diet enriched with extra virgin olive oil (EVOO) reduced the incidence of cardiovascular events in high-risk patients [4]. The results of the 3-month intervention on the first 772 patients entering the study showed that, compared with a low-fat diet, the Mediterranean diet rich in EVOO reduced blood pressure in a hypertensive population [5]. This finding was also observed in a 4-year follow-up study [6]. However, the mechanisms involved in this beneficial effect on the blood pressure have not been delineated. We must consider that minor compounds, such as polyphenols, which constitute the unsaponifiable fraction of EVOO prevent autooxidation of the oil, contribute to its characteristic flavor and taste, and are responsible, at least in part, for their beneficial effects.
Endothelium plays an important role in the regulation of vascular tone via the synthesis and release of vascular modulators such as nitric oxide (NO), a potent vasodilator [7], and endothelin-1 (ET-1), a vasoconstrictor agent [8] that acts as the natural counterpart to endothelium-derived NO [9]. An imbalance between these vasoactive molecules results in endothelial dysfunction.
Endothelium-dependent vascular relaxation is impaired in diabetes [10]. Insulin resistance is known to be associated with increased plasma levels of free fatty acids (FFAs), a consequence of hyperglycemia [11]. An increase in FFAs is likely to contribute to functional and structural vascular changes through vascular NO/ET-1 levels [12]. Moreover, hyperglycemia and FFAs cause an excessive formation of reactive oxygen species (ROS), including superoxide anion which reacts with NO to generate peroxynitrite. Very little research on the mechanisms underlying the modulation of endothelial dysfunction induced by FFAs in type 2 diabetes has been conducted. This study was designed to elucidate the effects of EVOO polyphenols on NO/ET-1 synthesized by endothelial cells in in vitro experimental conditions that simulated type 2 diabetes (high glucose and elevated FFAs) in the culture medium to understand the effects of polyphenols on cell signaling as well as the mechanisms involved in endothelial homeostasis.
Materials and methods
Materials
Cell culture medium, heat-inactivated fetal bovine serum, penicillin G, streptomycin and trypsin/EDTA were supplied by Bio Whittaker Europe (Verviers, Belgium). Aprotinin, phenylmethylsulfonyl fluoride, leupeptin, dimethyldithiocarbamic acid, β-sitosterol from soya beans, oleic and linoleic acids, acetylcholine (Ach) chloride, 4,5-diaminofluorescein diacetate (DAF-DA), PD 98059, SB 202190, wortmannin, Fura-2AM, superoxide dismutase (SOD) and catalase from human erythrocytes were obtained from Sigma Chem. Co. (St. Louis, MO, USA). 5,6 Chloromethyl 2′,7′-dichlorodihydrofluorescein diacetate (CM-H2DCF-DA) was obtained from Invitrogen SA (Barcelona, Spain). Hydroxytyrosol and bovine recombinant endothelial NO synthase (eNOS) were supplied by Cayman Chem. Co. (Ann Arbor, MI, USA). Apocynin came from Calbiochem (Darmstadt, Germany). All other chemicals were of the highest quality commercially available. Oleic and linoleic acids were dissolved in 0.1 M NaOH at 70 °C and then complexed with 10% BSA at 55 °C for 10 min. These stock solutions of 5 mM FFAs were prepared the day before the experiment, placed in a sealed tube in nitrogen atmosphere and stored overnight at −20 °C, then added to the cell culture medium. A solution with 10% BSA was added to control cultures.
Polyphenols present in EVOO were extracted three times with 80% methanol (1/1, vol/vol). The methanolic extract was evaporated under vacuum in a nitrogen flow, and the total polyphenol concentration in the aqueous extract was determined [13]. Total polyphenols were expressed as mg gallic acid equivalents. Our samples had a total polyphenol content of 76±3 mg gallic acid equivalents/kg olive oil. Polyphenol extract from EVOO (PEEVOO) was prepared to 0.5 mM gallic acid equivalents.
Cells and cell culture
ECV304 cells have been considered as endothelial like cells. They were obtained from the European Collection of Cell Cultures (Salisbury, UK) and grown in medium 199 with Earl’s salts, 25 mM HEPES supplemented with 2 mM l-glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin, and 10% FBS. Cells were split at confluence 1–3 or at higher passages 1–2 every 5–7 days and plated on gelatin-coated flasks up to passage 20 [14]. ECV304 cells were washed and incubated in normal glucose (5 mM glucose and 25 mM mannitol as an osmotic control of high glucose) or co-incubated with high glucose (30 mM) in the presence or absence of 0–120 µM FFAs (oleic or linoleic acid) and 10 µM hydroxytyrosol or 10 µM gallic acid equivalents of PEEVOO for 48 h.
Intracellular reactive oxygen species generation
Cells were rinsed twice with PBS supplemented with 5 mM glucose and 5 mM HEPES. Intracellular ROS were detected once 1 µM CM-H2DCF-DA was oxidized to a fluorescent DCF product within the cell after incubation at 37 °C for 20 min. We performed the experiments in the presence of SOD or catalase to confirm that CM-H2DCF-DA probe measure ROS in our experimental conditions [15]. The fluorescence intensity was analyzed using a FLUOstar OPTIMA fluorometer (BMG Labtech, Ortenburg, Germany), with excitation at 495 nm and emission at 515 nm.
Intracellular nitric oxide generation
Cells were rinsed with PBS and incubated with 10 µM DAF-DA for 10 min. at 37 °C. Intracellular basal NO production and NO production in response to 10 µM Ach were measured by the level of change in fluorescence intensity (DAF-2T), which was detected under the FLUOstar OPTIMA fluorometer (BMG Labtech, Ortenburg, Germany), with excitation at 495 nm and emission at 515 nm. The real-time changes in intracellular fluorescence intensity were measured for 15 min. There are some pitfalls and limitation for using DAF-DA to measure NO concentrations. The intermediate probe DAF-2 is autofluorescence [16]. For this reason we always perform the experiments in the dark and we express the results by the fluorescence relative intensity (F1/F0) before and after the addition of Ach.
To investigate the role of phosphoinositide 3-kinase (PI3K/Akt) and mitogen-activated protein kinase (MAPK) (ERK and p38) signaling cascades on the effects of olive oil polyphenols on NO synthesized by ECV304 cells, we used Akt, ERK or p38 specific inhibitors such as wortmannin [17], PD98059 [18] and SB202190 [19], respectively.
Intracellular calcium concentration
ECV304 cells grown in clusters were loaded with 25 µM Fura-2AM (a selective fluorescent Ca2+ indicator) in DMEM for 1 h at 37 °C as we previously described [20]. Cells were stimulated with 10 µM Ach and [Ca2+]i was calculated in accordance with Grynkiewicz et al. [21].
Western blot analysis of endothelial nitric oxide synthase
ECV304 cells were washed twice in ice-cold PBS, scraped off into PBS containing 2 mM EDTA and pelleted. Cell pellets were sonicated in PBS containing 2 mM EDTA, 2 µg/ml phenylmethylsulfonyl fluoride, 20 µg/ml aprotinin, 20 µg/ml leupeptin and 200 µg/ml dimethyldithiocarbamic acid, separated by SDS-PAGE and blotted onto a nitrocellulose membrane using a Mini-Protean II system (Bio-Rad, Hercules, CA, USA). Finally, the membranes were blocked and eNOS or phospho-eNOS (Ser 1107) were immunodetected using rabbit polyclonal antiserums (Cayman Chem. Co., Ann Arbor, MI, USA and Cell Signaling, Beverly, MA, USA, respectively) in a 1/2000 dilution for 1 h. All blots were developed using an enhanced chemiluminescence kit (Supersignal West Dura Extended Duration Substrate) from Pierce (Rockford, IL, USA).
Detection of endothelin-1 production
ET-1 concentrations in supernatant cell cultures were analyzed by enzyme immunoassay (R&D Systems, Minneapolis, MN, USA). The minimum detectable concentration of ET-1 was 0.02–0.03 pg/ml. The inter-assay and intra-assay coefficient of variation were 4.5% and 2.6%, respectively.
Statistical analysis
Results were expressed as mean±SEM. Statistical evaluation of the data was performed by Wilcoxon and Mann–Whitney test. Values were considered to be statistically different at p<0.05.
Results
Incubation of ECV304 cells with growing concentrations of oleic and linoleic acids lowered intracellular NO levels (Fig. 1A) and increased ROS (Fig. 1B). Interestingly, these changes were less marked in the presence of oleic acid. These effects were concentration-dependent and reached significant changes up to 40 µM FFAs. The impairment of NO concentrations induced by linoleic and oleic acids may be related to a reduction in eNOS phosphorylation as shown in Fig. 1C. High glucose concentration also significantly decreased intracellular NO concentration (22%) in the ECV304 cell culture (Fig. 2A), whereas it markedly enhanced ROS formation (110%) (Fig. 2B) and ET-1 concentration (95%) (Fig. 2C). The change in NO bioavailability by high glucose may be related to a reduction in eNOS phosphorylation (Fig. 3). Moreover, the impairment of NO levels and the enhancement of ROS and ET-1 in ECV304 cells induced by high glucose levels were heightened by the presence of 60 µM FFAs, and the effect of linoleic acid was higher than that of oleic acid (Fig. 2A–C). Our findings also show that the NADPH oxidase inhibitor apocynin [22] and antioxidant enzymes such as SOD and catalase reversed the impairment of NO levels induced by high glucose/oleic acid (Table 1).
Fig. 1.
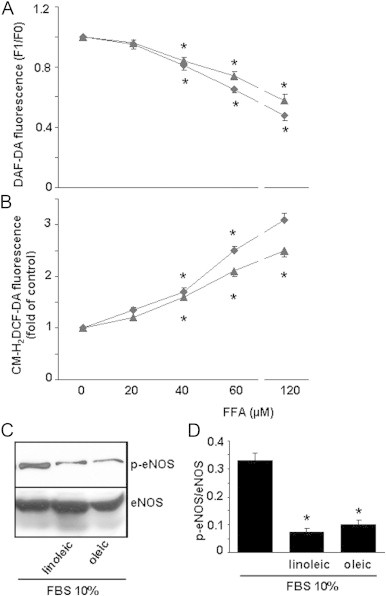
Effect of oleic and linoleic acids on nitric oxide (NO) and reactive oxygen species (ROS) produced by ECV304 cells. Cells were cultured in 199 medium and 10% FBS for 48 h with oleic (triangle) or linoleic (romb) acid (0–120 µM). NO (A) and ROS (B) levels were measured using DAF-DA or CM-H2DCF-DA, respectively. Results are means±SEM of at least three experiments performed in triplicate. *p<0.05 vs control cells. The changes in endothelial NO synthase (eNOS) and phosphorylated eNOS (p-eNOS) in the presence of oleic or linoleic acid (60 µM) were measured by Western blot. A representative blot (C) and the means of three blots (D) are shown.
Fig. 2.
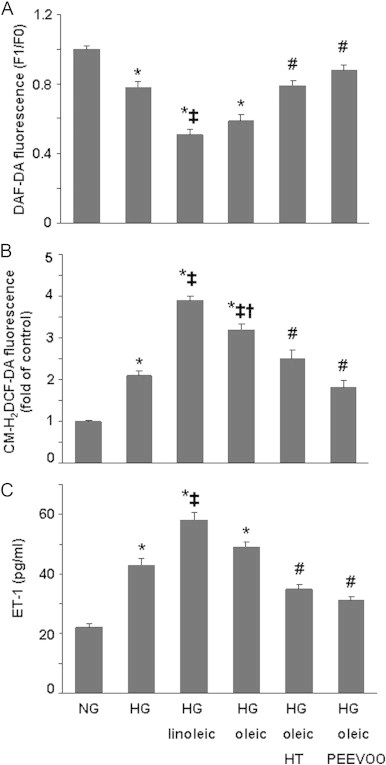
Effect of hydroxytyrosol (HT) and polyphenol extract from extra virgin olive oil (PEEVOO) on the changes in nitric oxide (NO), reactive oxygen species (ROS) and endothelin-1 (ET-1) induced by the presence of high glucose (HG) and/or linoleic and oleic acids in ECV304 cells. Cells were cultured in 199 medium and 10% FBS for 48 h in normal glucose (NG, 5 mM), HG (30 mM) or HG together with linoleic or oleic acid (60 µM) in the presence or absence of HT (10 µM) or PEEVOO (10 µM gallic acid equivalents). NO (A), ROS (B) and ET-1 (C) concentrations in the culture supernatants were determined. Results are means±SEM of at least three experiments performed in triplicate. *p<0.05 vs control cells; ‡p<0.05 vs HG condition; †p<0.05 vs HG plus linoleic acid; #p<0.05 vs HG plus oleic acid.
Fig. 3.
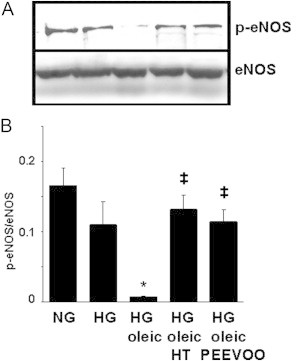
Effect of hydroxytyrosol (HT) and polyphenol extract from extra virgin olive oil (PEEVOO) on endothelial nitric oxide synthase (eNOS) phosphorylation induced by high glucose (HG) and oleic acid. ECV304 cells were cultured in 199 medium and 10% FBS for 48 h in normal glucose (NG, 5 mM), HG (30 mM) or HG together with oleic acid (60 µM) in the presence or absence of HT (10 µM) or PEEVOO (10 µM gallic acid equivalents). Cells were scraped off and eNOS and p-eNOS were determined by Western blot. A representative blot (A) and means from three westerns blots (B) are shown. Bars are means±SEM. *p<0.05 vs control cells; ‡p<0.05 vs HG plus oleic acid.
Table 1.
Effect of NADPH, Akt, ERK and p38 inhibitors on nitric oxide concentrations in the experimental conditions of the present study.
| DAF-DA fluorescence (F1/F0) | |
|---|---|
| NG | 1.05±0.03 |
| HG | 0.73±0.05* |
| HG+oleic acid | 0.58±0.04* |
| HG+oleic acid+apocynin (100 µM) | 0.69±0.03# |
| HG+oleic acid+SOD (50 units/ml) | 0.74±0.03# |
| HG+oleic acid+catalase (100 units/ml) | 0.71±0.02# |
| HG+oleic acid+HT | 0.75±0.05# |
| HG+oleic acid+HT+wortmannin (5 µM) | 0.65±0.02† |
| HG+oleic acid+HT+PD 98059 (10 µM) | 0.72±0.04 |
| HG+oleic acid+HT+SB 202190 (1 µM) | 0.71±0.03 |
| HG+oleic acid+HT+PD 98059+SB 202190 | 0.62±0.02† |
| HG+oleic acid+PEEVOO | 0.82±0.03# |
| HG+oleic acid+PEEVOO+wortmannin (5 µM) | 0.67±0.02† |
| HG+oleic acid+PEEVOO+PD 98059 (10 µM) | 0.78±0.03 |
| HG+oleic acid+PEEVOO+SB202190 (1 µM) | 0.77±0.04 |
| HG+oleic acid+PEEVOO+PD 98059+SB 202190 | 0.60±0.04† |
Data are expressed as means±SEM from three independent experiments performed in duplicate.
p<0.05 vs normal glucose (NG).
p<0.05 vs high glucose (HG) plus oleic acid.
p<0.05 vs HG plus oleic acid plus hydroxytyrosol (HT) or polyphenol extract from extra virgin olive oil (PEEVOO).
Ach induced an increase in intracellular NO production in endothelial ECV304 cells, as well as [Ca2+]i (Fig. 4). Our results also demonstrate that 30 mM glucose lessened the enhancement of NO production and [Ca2+]i induced by Ach (Fig. 4).
Fig. 4.
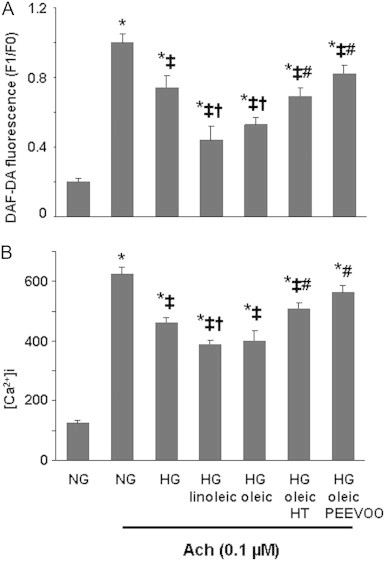
Effect of hydroxytyrosol (HT) and polyphenol extract from extra virgin olive oil (PEEVOO) on the changes in nitric oxide (NO) and intracellular calcium concentration ([Ca2+]i) induced by acetylcholine (Ach) in the presence of high glucose (HG) and/or oleic and linoleic acids in ECV304 cells. Cells were cultured in 199 medium and 10% FBS for 48 h in normal glucose (NG, 5 mM), HG (30 mM) or HG together with linoleic or oleic acid (60 µM) in the presence or absence of HT (10 µM) or PEEVOO (10 µM gallic acid equivalents). Ach (0.1 µM) was added and NO concentration (A) and [Ca2+]i (B) were determined. Results are means±SEM of at least three experiments performed in triplicate. *p<0.05 vs control cells; ‡p<0.05 vs HG condition; †p<0.05 vs HG/linoleic acid; #p<0.05 vs HG/oleic acid.
Hydroxytyrosol and PEEVOO prevented the reduction in NO (Fig. 2A), the increase in ROS (Fig. 2B) and ET-1 (Fig. 2C) induced by high glucose and elevated FFA concentrations. Moreover, we observed that the reversal of the NO production impairment induced by hydroxytyrosol and PEEVOO (Fig. 2B) was related to the phosphorylation of eNOS in these experimental conditions (Fig. 3) and that both treatments blocked the reduction induced by high glucose/FFAs on NO and [Ca2+]i and production stimulated by Ach (Fig. 4). As reported in Table 1, the inhibition of PI3K/Akt, ERK and p38 by 5 µM wortmannin, 10 µM PD98059 and 1 µM SB202190, respectively, blocked the beneficial action of hydroxytyrosol or PEEVOO on the change in NO levels induced by high glucose/oleic acid.
Discussion
There is now compelling evidence that there are subtle alterations in the endothelium in the early stages of atherogenesis. This is frequently described as endothelial dysfunction, which involves a decline in the bioavailability of NO as a consequence of reduced biosynthesis and/or increased degradation. Moreover, longitudinal studies have shown that impaired NO dependent vasodilation can predict arterial hypertension risk, future cardiac events [23] and the development of coronary artery disease [24].
Several authors have reported that FFAs such as oleic and linoleic acids reduce the levels of intracellular NO synthesized by endothelial cells [25,26]. Our data confirm that linoleic and oleic acids at concentrations reached in human plasma [27] markedly reduce NO levels, and the effect of linoleic acid was higher than that of oleic acid. This effect of the FFAs studied may be related to the enhancement of ROS also observed by Du et al. [28] and attributed to FFAs-induced overproduction of superoxide by the mitochondrial electron transport chain. However, our findings also suggest that NA(D)PH oxidases are involved as it was previously observed in mice with type 2 diabetes [29], a disorder in which ROS derived from these oxidases play a key role in vascular endothelial dysfunction [30]. We demonstrate that the NAD(P)H inhibitor apocynin, SOD and catalase effectively reversed the intracellular NO impairment induced by high glucose/oleic acid, which would indicate the involvement of ROS produced by NAD(P)H oxidases. However, we must consider that Heumüller et al. [31] reported that apocynin predominantly acts as an antioxidant in endothelial cells but not as NADPH oxidase inhibitor. The present results are also consistent with reports on the overexpression of NAD(P)H elements in high glucose-stimulated ROS generation in endothelial cells [30,32] and in diabetic rat arteries [33].
Stress is known to elevate the expression of certain genes in several cell types including endothelial and smooth muscle cells [34]. Furthermore, hyperglycemia increases ROS production, PKC activity, and advanced glycation end-product formation which are known to inhibit the PI3K/Akt/eNOS pathway [35], decrease NO bioavailability [36] and increase ET-1 plasma concentrations [37]. Considering all together, we examined the simultaneous effects of oleic or linoleic acid and high glucose concentration on NO production and ET-1 release by ECV304 cell cultures. Our findings show that these experimental conditions, which simulate those of type 2 diabetes, induce endothelial dysfunction through the impairment of NO caused by the reduction in eNOS phosphorylation and the enhancement of ET-1, related to oxidative stress.
The PREDIMED study recently showed that EVOO consumption led to a reduction in the blood pressure of elderly Mediterranean participants with high risk for cardiovascular disease [6,38]. However, there is little information concerning the EVOO components in its beneficial effects and the mechanisms involved. In Mediterranean countries, the main dietary source of polyphenols is coffee and fruits, and the most important differentiating factor with respect to other countries is the consumption of polyphenols from olives and olive oil [39]. Nicholson et al. [40] reported that physiological concentrations of dietary polyphenols increased basal eNOS expression and lowered ROS-induced ET-1 mRNA expression. For the first time, we demonstrated that olive oil polyphenol concentration reached in plasma following olive oil consumption [41] may prevent, at least in part, the effects of high glucose/FFAs on basal NO/ET-1 levels in endothelial cell cultures.
eNOS is a constitutively expressed enzyme, controlled at transcriptional and post-transcriptional level [42]. The post-transcriptional regulation of eNOS is dependent on the phosphorylation state, substrate and cofactor availability, endogenous inhibitors (caveolins) as well as calcium. eNOS can be phosphorylated on serine, threonine, and tyrosine residues but the most functional changes are a consequence of Ser 1177 phosphorylation [43]. The notion of disrupted insulin signaling via impaired PI3K/Akt/eNOS phosphorylation, as the major determinant of decreased NO and endothelial dysfunction, has been challenged [11]. In this regard, polyphenol-induced NO formation is due to the redox-sensitive activation of the PI3K/Akt pathway, which leads to eNOS activation subsequent to its phosphorylation on Ser 1177 [44] as observed with polyphenolic-rich fraction of black tea [45] through the estrogen receptor dependent pathway [46]. The present study demonstrates for the first time that olive oil polyphenols such as hydroxytyrosol stimulate eNOS activity, primarily through non-genomic activation in endothelial cells. New findings have shown the importance of crosstalk between PI3K/Akt and MAPK pathways. The regulatory interaction between components of Akt and MAPK cascades and NO synthesis by endothelial cells has been well documented [47]. Our results show that Akt, ERK and p38 may be involved in eNOS phosphorylation induced by olive oil polyphenols.
We observed that high glucose and FFAs decrease NO bioavailability induced by Ach and that olive oil polyphenols can significantly impede this action. Thus, a polyphenol extract from red wine significantly increased [Ca2+]i which led to the endothelial formation of NO [48]. We observed a similar effect induced by olive oil polyphenols on [Ca2+]i and NO synthesis stimulated by Ach. It is likely that olive oil polyphenols modulate NO production through the control of Ser 1177 eNOS phosphorylation and changes in [Ca2+]i.
In conclusion, the present study provides new evidences that hydroxytyrosol and PEEVOO reduce the oxidative stress and modulate NO and ET-1 in experimental conditions that simulated hyperglycemia and high FFA levels observed in diabetes. These findings suggest that EVOO is potentially more effective than high oleic seed oils, which do not contain polyphenols, in the dietary treatment of physiopathological events involved in endothelial dysfunction. Although, numerous studies including morphological, cytochemical and genetic analyzes validated ECV304 as an endothelial cell model, several differences between ECV304 and human endothelial cells are reported [49]. It should be necessary to confirm our results using umbilical vein endothelial cells.
Acknowledgments
This study was partly supported by the Spanish Ministry of Health (Instituto de Salud Carlos III) (RD06/0045), Fondo Europeo de Desarrollo Regional (FEDER), the Spanish Ministry of Science (BFU2007-61727/BFI) and the Generalitat de Catalunya (2009SGR00438).
References
- 1.Keys A., Menotti A., Karvonen M.J., Aravanis C., Blackburn H., Buzina R., Djordjevic B.S., Dontas A.S., Fidanza F., Keys M.H. The diet and 15-year death rate in the Seven Countries Study. American Journal of Epidemiology. 1986;124:903–915. doi: 10.1093/oxfordjournals.aje.a114480. 3776973 [DOI] [PubMed] [Google Scholar]
- 2.Kris-Etherton P.M., Hecker K.D., Bonanome A., Coval S.M., Binkoski A.E., Hilpert K.F., Griel A.E., Etherton T.D. Bioactive compounds in foods: their role in the prevention of cardiovascular disease and cancer. American Journal of Medicine. 2002;113(Suppl. 9B):71S–88S. doi: 10.1016/s0002-9343(01)00995-0. 12566142 [DOI] [PubMed] [Google Scholar]
- 3.Strazzullo P., Ferro-Luzzi A., Siani A., Scaccini C., Sette S., Catasta G., Mancini M. Changing the Mediterranean diet: effects on blood pressure. Journal of Hypertension. 1986;4:407–412. doi: 10.1097/00004872-198608000-00003. 3534087 [DOI] [PubMed] [Google Scholar]
- 4.Estruch R., Ros E., Salas-Salvadó J., Covas M.I., Corella D., Arós F., Gómez-Gracia E., Ruiz-Gutiérrez V., Fiol M., Lapetra J., Lamuela-Raventos R.M., Serra-Majem L., Pintó X., Basora J., Muñoz M.A., Sorlí J.V., Martínez J.A., Martínez-González M.A. Primary prevention of cardiovascular disease with a Mediterranean diet. New England Journal of Medicine. 2013;368:1279–1290. doi: 10.1056/NEJMc1806491. 23432189 [DOI] [PubMed] [Google Scholar]
- 5.Estruch R., Martínez-González M.A., Corella D., Salas-Salvadó J., Ruiz-Gutiérrez V., Covas M.I., Fiol M., Gómez-Gracia E., López-Sabater M.C., Vinyoles E., Arós F., Conde M., Lahoz C., Lapetra J., Sáez G., Ros E. Effects of a Mediterranean-style diet on cardiovascular risk factors: a randomized trial. Annals of Internal Medicine. 2006;145:1–11. doi: 10.7326/0003-4819-145-1-200607040-00004. 16818923 [DOI] [PubMed] [Google Scholar]
- 6.Toledo E., Hu F.B., Estruch R., Buil-Cosiales P., Corella D., Salas-Salvadó J., Covas M.I., Arós F., Gómez-Gracia E., Fiol M., Lapetra J., Serra-Majem L., Pinto X., Lamuela-Raventós R.M., Saez G., Bulló M., Ruiz-Gutiérrez V., Ros E., Sorli J.V., Martinez-Gonzalez M.A. Effect of the Mediterranean diet on blood pressure in the PREDIMED trial: results from a randomized controlled trial. BMC Medicine. 2013;11:207. doi: 10.1186/1741-7015-11-207. 24050803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ignarro L.J., Buga G.M., Wood K.S., Byrns R.E., Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proceedings of the National Academy of Sciences of the United States of America. 1987;84:9265–9269. doi: 10.1073/pnas.84.24.9265. 2827174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yanagisawa M., Kurihara H., Kimura S., Tomobe Y., Kobayashi M., Mitsui Y., Yazaki Y., Goto K., Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332:411–415. doi: 10.1038/332411a0. 2451132 [DOI] [PubMed] [Google Scholar]
- 9.Lüscher T.F., Yang Z., Tschudi M., von Segesser L., Stulz P., Boulanger C., Siebenmann R., Turina M., Bühler F.R. Interaction between endothelin-1 and endothelium-derived relaxing factor in human arteries and veins. Circulation Research. 1990;66:1088–1094. doi: 10.1161/01.res.66.4.1088. 2180587 [DOI] [PubMed] [Google Scholar]
- 10.Ding H., Triggle C.R. Endothelial dysfunction in diabetes: multiple targets for treatment. Pflügers Archiv: European Journal of Physiology. 2010;459:977–994. doi: 10.1007/s00424-010-0807-3. 20238124 [DOI] [PubMed] [Google Scholar]
- 11.Imrie H., Abbas A., Kearney M. Insulin resistance, lipotoxicity and endothelial dysfunction. Biochimica et Biophysica Acta. 2010;1801:320–326. doi: 10.1016/j.bbalip.2009.09.025. 19818873 [DOI] [PubMed] [Google Scholar]
- 12.Monti L.D., Landoni C., Setola E., Galluccio E., Lucotti P., Sandoli E.P., Origgi A., Lucignani G., Piatti P., Fazio F. Myocardial insulin resistance associated with chronic hypertriglyceridemia and increased FFA levels in Type 2 diabetic patients. American Journal of Physiology: Heart and Circulatory Physiology. 2004;287:H1225–H1231. doi: 10.1152/ajpheart.00629.2003. 15130883 [DOI] [PubMed] [Google Scholar]
- 13.Medina-Remón A., Barrionuevo-González A., Zamora-Ros R., Andres-Lacueva C., Estruch R., Martínez-González M.A., Diez-Espino J., Lamuela-Raventos R.M. Rapid folin-ciocalteu method using microtiter 96-well plate cartridges for solid phase extraction to assess urinary total phenolic compounds, as a biomarker of total polyphenols intake. Analytica Chimica Acta. 2009;634:54–60. doi: 10.1016/j.aca.2008.12.012. 19154810 [DOI] [PubMed] [Google Scholar]
- 14.Moreno J.J. Antiflammin-2 prevents HL-60 adhesion to endothelial cells and prostanoid production induced by lipopolysaccharides. Journal of Pharmacology and Experimental Therapeutics. 2001;296:884–889. 11181920 [PubMed] [Google Scholar]
- 15.Kalyanaraman B., Darley-Usmar V., Davies K.J., Dennery P.A., Forman H.J., Grisham M.B., Mann G.E., Moore K., Roberts L.J., Ischiropoulos H. Measuring reactive oxygen and nitrogen species with fluorescent probes: challenges and limitations. Free Radical Biology & Medicine. 2012;52:1–6. doi: 10.1016/j.freeradbiomed.2011.09.030. 22027063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wardman P. Fluorescent and luminescent probes for measurement of oxidative and nitrosative species in cells and tissues: progress, pitfalls, and prospects. Free Radical Biology & Medicine. 2007;43:995–1022. doi: 10.1016/j.freeradbiomed.2007.06.026. 17761297 [DOI] [PubMed] [Google Scholar]
- 17.Wymann M.P., Bulgarelli-Leva G., Zvelebil M.J., Pirola L., Vanhaesebroeck B., Waterfield M.D., Panayotou G. Wortmannin inactivates phosphoinositide 3-kinase by covalent modification of lys-802, a residue involved in the phosphate transfer reaction. Molecular and Cellular Biology. 1996;16:1722–1733. doi: 10.1128/mcb.16.4.1722. 8657148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dudley D.T., Pang L., Decker S.J., Bridges A.J., Saltiel A.R. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proceedings of the National Academy of Sciences of the United States of America. 1995;92:7686–7689. doi: 10.1073/pnas.92.17.7686. 7644477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frantz B., Klatt T., Pang M., Parsons J., Rolando A., Williams H., Tocci M.J., O’Keefe S.J., O’Neill E.A. The activation state of p38 mitogen-activated protein kinase determines the efficiency of ATP competition for pyridinylimidazole inhibitor binding. Biochemistry. 1998;37:13846–13853. doi: 10.1021/bi980832y. 9753474 [DOI] [PubMed] [Google Scholar]
- 20.Rodríguez-Lagunas M.J., Martín-Venegas R., Moreno J.J., Ferrer R. PGE2 promotes Ca2+-mediated epithelial barrier disruption through EP1 and EP4 receptors in Caco-2 cell monolayers. American Journal of Physiology. Cell Physiology. 2010;299:C324–C334. doi: 10.1152/ajpcell.00397.2009. 20484658 [DOI] [PubMed] [Google Scholar]
- 21.Grynkiewicz G., Poenie M., Tsien R.Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. Journal of Biological Chemistry. 1985;260:3440–3450. 3838314 [PubMed] [Google Scholar]
- 22.Abid M.R., Kachra Z., Spokes K.C., Aird W.C. NADPH oxidase activity is required for endothelial cell proliferation and migration. FEBS Letters. 2000;486:252–256. doi: 10.1016/s0014-5793(00)02305-x. 11119713 [DOI] [PubMed] [Google Scholar]
- 23.Schächinger V., Britten M.B., Zeiher A.M. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation. 2000;101:1899–1906. doi: 10.1161/01.cir.101.16.1899. 10779454 [DOI] [PubMed] [Google Scholar]
- 24.Bugiardini R., Manfrini O., Pizzi C., Fontana F., Morgagni G. Endothelial function predicts future development of coronary artery disease: a study of women with chest pain and normal coronary angiograms. Circulation. 2004;109:2518–2523. doi: 10.1161/01.CIR.0000128208.22378.E3. 15136498 [DOI] [PubMed] [Google Scholar]
- 25.Kuroda R., Hirata K., Kawashima S., Yokoyama M. Unsaturated free fatty acids inhibit Ca2+ mobilization and NO release in endothelial cells. Kobe Journal of Medical Sciences. 2001;47:211–219. 11781499 [PubMed] [Google Scholar]
- 26.Kim F., Tysseling K.A., Rice J., Pham M., Haji L., Gallis B.M., Baas A.S., Paramsothy P., Giachelli C.M., Corson M.A., Raines E.W. Free fatty acid impairment of nitric oxide production in endothelial cells is mediated by IKKβ. Arteriosclerosis, Thrombosis, and Vascular Biology. 2005;25:989–994. doi: 10.1161/01.ATV.0000160549.60980.a8. 15731493 [DOI] [PubMed] [Google Scholar]
- 27.Kopf T., Schmitz G. Analysis of non-esterified fatty acids in human samples by solid-phase-extraction and gas chromatography/mass spectrometry. Journal of Chromatography. 2013;938:22–26. doi: 10.1016/j.jchromb.2013.08.016. 24036177 [DOI] [PubMed] [Google Scholar]
- 28.Du X.L., Edelstein D., Obici S., Higham N., Zou M.H., Brownlee M. Insulin resistance reduces arterial prostacyclin synthase and eNOS activities by increasing endothelial fatty acid oxidation. Journal of Clinical Investigation. 2006;116:1071–1080. doi: 10.1172/JCI23354. 16528409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wong W.T., Tian X.Y., Xu A., Ng C.F., Lee H.K., Chen Z.Y., Au C.L., Yao X., Huang Y. Angiotensin II type 1 receptor-dependent oxidative stress mediates endothelial dysfunction in type 2 diabetic mice. Antioxidants & Redox Signaling. 2010;13:757–768. doi: 10.1089/ars.2009.2831. 20136508 [DOI] [PubMed] [Google Scholar]
- 30.Liu S., Ma X., Gong M., Shi L., Lincoln T., Wang S. Glucose down-regulation of cGMP-dependent protein kinase I expression in vascular smooth muscle cells involves NAD(P)H oxidase-derived reactive oxygen species. Free Radical Biology & Medicine. 2007;42:852–863. doi: 10.1016/j.freeradbiomed.2006.12.025. 17320767 [DOI] [PubMed] [Google Scholar]
- 31.Heumüller S., Wind S., Barbosa-Sicard E., Schmidt H.H., Busse R., Schröder K. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension. 2008;51:211–217. doi: 10.1161/HYPERTENSIONAHA.107.100214. 18086956 [DOI] [PubMed] [Google Scholar]
- 32.Patel H., Chen J., Das K.C., Kavdia M. Hyperglycemia induces differential change in oxidative stress at gene expression and functional levels in HUVEC and HMVEC. Cardiovascular Diabetology. 2013;12:142. doi: 10.1186/1475-2840-12-142. 24093550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Serizawa K., Yogo K., Aizawa K., Tashiro Y., Ishizuka N. Nicorandil prevents endothelial dysfunction due to antioxidative effects via normalisation of NADPH oxidase and nitric oxide synthase in streptozotocin diabetic rats. Cardiovascular Diabetology. 2011;10:105. doi: 10.1186/1475-2840-10-105. 22107602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu J.C., Chen J.J., Chan P., Cheng C.F., Cheng T.H. Inhibition of cyclic strain-induced endothelin-1 gene expression by resveratrol. Hypertension. 2003;42:1198–1205. doi: 10.1161/01.HYP.0000103162.76220.51. 14623829 [DOI] [PubMed] [Google Scholar]
- 35.Du X.L., Edelstein D., Dimmeler S., Ju Q., Sui C., Brownlee M. Hyperglycemia inhibits endothelial nitric oxide synthase activity by posttranslational modification at the Akt site. Journal of Clinical Investigation. 2001;108:1341–1348. doi: 10.1172/JCI11235. 11696579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wallis M.G., Smith M.E., Kolka C.M., Zhang L., Richards S.M., Rattigan S., Clark M.G. Acute glucosamine-induced insulin resistance in muscle in vivo is associated with impaired capillary recruitment. Diabetologia. 2005;48:2131–2139. doi: 10.1007/s00125-005-1887-z. 16059714 [DOI] [PubMed] [Google Scholar]
- 37.Cardillo C., Campia U., Bryant M.B., Panza J.A. Increased activity of endogenous endothelin in patients with type II diabetes mellitus. Circulation. 2002;106:1783–1787. doi: 10.1161/01.cir.0000032260.01569.64. 12356630 [DOI] [PubMed] [Google Scholar]
- 38.Medina-Remón A., Zamora-Ros R., Rotchés-Ribalta M., Andres-Lacueva C., Martínez-González M.A., Covas M.I., Corella D., Salas-Salvadó J., Gómez-Gracia E., Ruiz-Gutiérrez V., García de la Corte F.J., Fiol M., Pena M.A., Saez G.T., Ros E., Serra-Majem L., Pinto X., Warnberg J., Estruch R., Lamuela-Raventos R.M., PREDIMED Study Investigators Total polyphenol excretion and blood pressure in subjects at high cardiovascular risk. Nutrition, Metabolism and Cardiovascular Diseases. 2011;21:323–331. doi: 10.1016/j.numecd.2009.10.019. 20167460 [DOI] [PubMed] [Google Scholar]
- 39.Tresserra-Rimbau A., Medina-Remón A., Pérez-Jiménez J., Martínez-González M.A., Covas M.I., Corella D., Salas-Salvadó J., Gómez-Gracia E., Lapetra J., Arós F., Fiol M., Ros E., Serra-Majem L., Pintó X., Muñoz M.A., Saez G.T., Ruiz-Gutiérrez V., Warnberg J., Estruch R., Lamuela-Raventós R.M. Dietary intake and major food sources of polyphenols in a Spanish population at high cardiovascular risk: the PREDIMED study. Nutrition, Metabolism and Cardiovascular Diseases. 2013;23:953–959. doi: 10.1016/j.numecd.2012.10.008. 23332727 [DOI] [PubMed] [Google Scholar]
- 40.Nicholson S.K., Tucker G.A., Brameld J.M. Physiological concentrations of dietary polyphenols regulate vascular endothelial cell expression of genes important in cardiovascular health. British Journal of Nutrition. 2010;103:1398–1403. doi: 10.1017/S0007114509993485. 20021702 [DOI] [PubMed] [Google Scholar]
- 41.Weinbrenner T., Fitó M., Farré Albaladejo M., Saez G.T., Rijken P., Tormos C., Coolen S., De la Torre R., Covas M.I. Bioavailability of phenolic compounds from olive oil and oxidative/antioxidant status at postprandial state in healthy humans. Drugs Under Experimental and Clinical Research. 2004;30:207–212. 15700748 [PubMed] [Google Scholar]
- 42.Atochin D.N., Wang A., Liu V.W., Critchlow J.D., Dantas A.P., Looft-Wilson R., Murata J., Salomone S., Shin H.K., Avata C., Moskowitz H.A. The phosphorylation state of eNOS modulates vascular reactivity and outcome of cerebral ischemia in vivo. Journal of Clinical Investigation. 2007;117:1961–1967. doi: 10.1172/JCI29877. 17557122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fleming I. Molecular mechanisms underlying the activation of eNOS. Pflügers Archiv: European Journal of Physiology. 2010;459:793–806. doi: 10.1007/s00424-009-0767-7. 20012875 [DOI] [PubMed] [Google Scholar]
- 44.Dimmeler S., Fleming I., Fisslthaler B., Hermann C., Busse R., Zeiher A.M. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. 10376603 [DOI] [PubMed] [Google Scholar]
- 45.Anter E., Thomas S.R., Schulz E., Shapira O.M., Vita J.A., Keaney J.F., Jr. Activation of endothelial nitric-oxide synthase by the p38 MAPK in response to black tea polyphenols. Journal of Biological Chemistry. 2004;279:46637–46643. doi: 10.1074/jbc.M405547200. 15333638 [DOI] [PubMed] [Google Scholar]
- 46.Anter E., Chen K., Shapira O.M., Karas R.H., Keaney J.F., Jr. p38 mitogen-activated protein kinase activates eNOS in endothelial cells by an estrogen receptor α-dependent pathway in response to black tea polyphenols. Circulation Research. 2005;96:1072–1078. doi: 10.1161/01.RES.0000168807.63013.56. 15879307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vouyouka A.G., Jiang Y., Rastogi R., Basson M.D. Ambient pressure upregulates nitric oxide synthase in a phosphorylated-extracellular regulated kinase- and protein kinase C-dependent manner. Journal of Vascular Surgery. 2006;44:1076–1084. doi: 10.1016/j.jvs.2006.06.033. 17098545 [DOI] [PubMed] [Google Scholar]
- 48.Martin S., Andriambeloson E., Takeda K., Andriantsitohaina R. Red wine polyphenols increase calcium in bovine aortic endothelial cells: a basis to elucidate signalling pathways leading to nitric oxide production. British Journal of Pharmacology. 2002;135:1579–1587. doi: 10.1038/sj.bjp.0704603. 11906973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brown J., Reading S.J., Jones S., Fitchett C.J., Howl J., Martin A., Longland C.L., Michelangeli F., Dubrova Y.E., Brown C.A. Critical evaluation of ECV304 as a human endothelial cell model defined by genetic analysis and functional responses: a comparison with the human bladder cancer derived epithelial cell line T24/83. Laboratory Investigation. 2000;80:37–45. doi: 10.1038/labinvest.3780006. 10653001 [DOI] [PubMed] [Google Scholar]


