Abstract
LPFC dysfunction is a well-established neural impairment in schizophrenia and is associated with worse symptoms. However, how LPFC activation influences symptoms is unclear. Previous findings in healthy individuals demonstrate that lateral prefrontal cortex (LPFC) activation during cognitive control of emotional information predicts mood and behavior in response to interpersonal conflict, thus impairments in these processes may contribute to symptom exacerbation in schizophrenia. We investigated whether schizophrenia participants show LPFC deficits during cognitive control of emotional information, and whether these LPFC deficits prospectively predict changes in mood and symptoms following real-world interpersonal conflict. During fMRI, 23 individuals with schizophrenia or schizoaffective disorder and 24 healthy controls completed the Multi-Source Interference Task superimposed on neutral and negative pictures. Afterwards, schizophrenia participants completed a 21-day online daily-diary in which they rated the extent to which they experienced mood and schizophrenia-spectrum symptoms, as well as the occurrence and response to interpersonal conflict. Schizophrenia participants had lower dorsal LPFC activity (BA9) during cognitive control of task-irrelevant negative emotional information. Within schizophrenia participants, DLPFC activity during cognitive control of emotional information predicted changes in positive and negative mood on days following highly distressing interpersonal conflicts. Results have implications for understanding the specific role of LPFC in response to social stress in schizophrenia, and suggest that treatments targeting LPFC-mediated cognitive control of emotion could promote adaptive response to social stress in schizophrenia.
Keywords: Emotion processing, Experience sampling, fMRI, Inhibitory control, Social stress, DLPFC, Emotion interference, MSIT
1. Introduction
Interpersonal conflicts are emotionally difficult and require regulation of negative affect and behavior for successful resolution (Arriaga and Rusbult, 1998; Lopes et al., 2011). These self-regulatory mechanisms are reliant on cognitive control processes mediated by the lateral prefrontal cortex (LPFC; Heatherton and Wagner, 2011; Ochsner et al., 2012). LPFC dysfunction in cognitive control is a well-established neural impairment in schizophrenia (Barch, 2005; Manoach, 2003; Minzenberg et al., 2009) that is associated with worse symptoms (Goghari et al., 2010; MacDonald et al., 2005; Menon et al., 2001; Nishimura et al., 2011; Perlstein et al., 2001; van Veelen et al., 2010) and global functioning deficits (Sanz et al., 2009; Yoon et al., 2008). However, there is a paucity of research examining how LPFC dysfunction contributes to illness severity. Consistent with the diathesis–stress model, one proposal is that LPFC dysfunction is a biological vulnerability that, in the presence of an interpersonal stressor, contributes to symptom exacerbation via impaired cognitive control of emotion (Hooker et al., 2010; Krabbendam et al., 2014; Kring and Werner, 2004).
Cognitive control of emotion comprises the dual processes of explicit and effortful control of the experience and expression of internal emotional states (emotion regulation) and the implicit and more automatic control of how external emotional information influences behavior (Gyurak et al., 2011; Gross and Thompson, 2007, Ochsner et al., 2005; Quirk et al., 2006). The LPFC, comprising both dorsolateral (DLPFC) and ventrolateral (VLPFC) regions, is consistently implicated in laboratory assessed cognitive control of emotion (Ochsner and Gross, 2005; Ochsner et al., 2012; Pessoa, 2008), including paradigms assessing explicit emotion regulation (e.g. reappraisal paradigms) as well as paradigms assessing implicit cognitive control of external and/or irrelevant emotional information (e.g. emotional Stroop, emotional flanker tasks) (Gyurak et al., 2011).
Evidence suggests that response to interpersonal stressors may be mediated by LPFC function. Lower VLPFC activity during social exclusion predicts higher self-reported distress (Eisenberger et al., 2003). Similarly, lower VLPFC activity when viewing negative facial expressions predicts increased negative mood and maladaptive behavior following interpersonal conflicts (Hooker et al., 2010). In schizophrenia, interpersonal conflicts, especially conflicts characterized by criticism, predict symptom exacerbation and higher relapse rates (Hooley, 2007), and symptom exacerbation in response to interpersonal criticism is related to poor working memory and cognitive control (Rosenfarb et al., 2000) — neurocognitive processes known to be mediated by the LPFC (Aron et al., 2004; Curtis and D'Esposito, 2003) and to predict functional outcome (Milev et al., 2005). Collectively, these data suggest that compromised LPFC function is a vulnerability for symptom exacerbation and functional difficulties in response to interpersonal conflict.
However, research attempting to connect LPFC activity, interpersonal conflict, and schizophrenia symptomatology is sparse. To date, studies have primarily focused on the direct relationship between the LPFC and symptoms (Goghari et al., 2010; MacDonald et al., 2005; Menon et al., 2001; Nishimura et al., 2011; Perlstein et al., 2001; van Veelen et al., 2010). To our knowledge, only one study has examined the interaction between LPFC activation, symptoms, and real-world social interactions. In a sample of healthy individuals characterized along the schizophrenia-risk dimension social anhedonia, individuals at high-risk for schizophrenia (i.e. those with high social anhedonia) with low LPFC activation had worse symptoms of paranoia on days with distressing interpersonal conflicts compared to days without distressing interpersonal conflicts (Hooker et al., 2014). To date, no study has examined this relationship in individuals with schizophrenia. The scarcity of studies directly linking LPFC function to social interactions and subsequent symptoms in schizophrenia may result from limitations inherent in the currently available and commonly used methods.
First, the tasks traditionally used to assess LPFC function, such as response inhibition or working memory tasks (Barch, 2005), although robust activators, may not be the most sensitive measures for assessing how LPFC activity relates to real-world social functioning because they do not directly capture cognitive control of emotional information. Given the inherently affective nature of social interactions and the accompanying need for self-regulation (Arriaga and Rusbult, 1998), tasks assessing the interaction between LPFC mediated cognitive control and emotional information may provide a more accurate reflection of the inhibitory demands of real-world social contexts. By using “cold cognitive” tasks and not assessing LPFC mediated cognitive control in relation to emotional information, previous studies may have lacked the sensitivity necessary to identify the role of the LPFC in individuals' response to social conflict.
Second, prior research has primarily assessed social interactions using laboratory-based one-time retrospective measures of functioning. Although these provide an overview of an individual's general level of functioning, they are not well suited for capturing the multidimensional nature of social interactions in daily life, and rarely provide the context in which they occur (Trull and Ebner-Priemer, 2009). Social interactions do not occur in a vacuum; day-to-day changes in social behavior may be prompted at a specific time, in a specific setting, or in the context of a particular interpersonal relationship. One-time retrospective evaluations of social functioning miss these nuances, calling into question their ecological validity (Yager and Ehmann, 2006). Experience sampling methods (ESM), a technique in which assessments are collected in the person's natural environment and repeated over time, have revealed a nuanced relationship between changes in the social environment, particularly social stressors, and symptoms (Myin-Germeys et al., 2009; Oorschot et al., 2009), Thus, using ESM in conjunction with neuroimaging techniques may provide a more sensitive and ecologically valid approach to understanding the contribution of LPFC dysfunction to social deficits in schizophrenia.
The present study addressed these prior limitations by combining fMRI and ESM to test whether people with schizophrenia have LPFC deficits in cognitive control of negative emotional information, and, if so, whether these LPFC deficits are related to changes in mood and symptoms following interpersonal conflict. Individuals with schizophrenia and demographically-matched healthy controls completed an adapted version of the Multi-Source Interference Task (MSIT), a cognitive control task specifically designed to activate the cingulo–frontal–parietal cognitive control network (Bush and Shin, 2006). In our adapted version, the MSIT-Emotion, MSIT stimuli are superimposed on a negative emotional scene that is irrelevant to the central task demand. Thus, rather than requiring explicit manipulation of emotional material and/or the explicit regulation of internal emotional states (i.e. emotion regulation through reappraisal/suppression), the task requires participants to engage cognitive control mechanisms to inhibit the effect of irrelevant emotional information in the external environment on task performance. This process is thought to more accurately reflect the interaction of emotion and cognitive control in real-world social contexts (Silbersweig et al., 2007), and considered to be a form of implicit cognitive control of emotion (Gyurak et al., 2011). Our measure of cognitive control of emotional information was LPFC activity when inhibiting the effect of irrelevant emotional information during high interference trials (when cognitive control skills are most challenged). Stimuli were also superimposed on neutral pictures, included to test the specificity of schizophrenia participants' LPFC deficits for controlling emotional information. Following the scan, schizophrenia participants completed an online, structured daily-diary questionnaire of mood and symptoms every evening for 3 weeks. End-of-the-day reports provide data on daily events and day-to-day symptom fluctuations whilst minimizing interference with participants' daily experience. Participants rated the extent to which they experienced mood and schizophrenia-spectrum symptoms, as well as the occurrence of interpersonal conflict and associated distress. We hypothesized that: 1) schizophrenia participants would show reduced LPFC activity during cognitive control of emotional information compared to healthy participants; and 2) among schizophrenia participants, LPFC activity during cognitive control of emotional information will predict changes in mood and symptoms following interpersonal conflict. Specifically, we expect that schizophrenia participants with low LPFC activity will have an increase in negative mood and psychotic symptoms the day after highly distressing interpersonal conflict.
2. Material and methods
2.1. Participants
23 individuals with schizophrenia or schizoaffective disorder and 24 healthy controls were recruited from the Greater Boston area. Groups were matched for gender, age, education, and IQ (Table 1). Inclusion criteria for all participants were: age 18–65, primary English speaker, no neurological or major medical illness, no head trauma history, no substance abuse within 6 months, and no current/past substance dependence. Inclusion criteria for schizophrenia participants were: diagnosis of schizophrenia or schizoaffective disorder, no comorbid axis I disorders, and no history of electroconvulsive therapy. Inclusion criteria for healthy participants were: no current/past axis I disorders, no first-degree relative with a psychotic disorder, scores within 1.5 standard deviations of the population mean on five measures of schizotypal personality (perceptual aberration scale (Chapman et al., 1978), magical ideation scale (Eckblad & Chapman, 1983), referential thinking scale (Lenzenweger et al., 1997), physical anhedonia scale (Chapman et al., 1976), and revised social anhedonia scale (Eckblad et al., 1982)). Psychopathology was assessed with the Structured Clinical Interview for DSM-IV Axis I Disorders (First et al., 2002); symptoms were assessed using the Positive and Negative Syndrome Scale (PANSS; Kay et al., 1987); social functioning was assessed with the Social Adjustment Scale – Self-Report (SAS-SR; Weissman et al., 1978) and the Global Functioning: Social scale (GF:S; Auther et al., 2006); positive and negative mood were assessed using the Positive And Negative Affect Schedule (PANAS; Watson et al., 1988). Clinical assessments were conducted by trained PhD-level clinical psychologists (LMT, SHL) and supervised by a licensed clinical psychologist (CIH).
Table 1.
Participant characteristics, social functioning, and MSIT-Emotion performance.
| SZ group | Control group | Differences between groups | |
|---|---|---|---|
| N | 23 | 24 | |
| Gender: (M/F) | 14/9 | 15/9 | χ2(1) = 0.013, p = 0.91 |
| Age | 39.3 (9.60) [21–58] | 34.54 (12.23) [19–55] | t(45) = 1.481, p = 0.15 |
| Education | 14.78 (2.19) [10–18] | 14.62 (2.84) [11–21] | t(45) = 0.212, p = 0.83 |
| IQa | 108.35 (14.14) [82–133] | 111.29 (11.44) [88–130] | t(45) = 0.786, p = 0.44 |
| Diagnosis: N (%)b | |||
| Schizophrenia | 17 (74%) | ||
| Schizoaffective | 6 (26%) | ||
| Age of onset | 21.64 (4.55) [13–30] | ||
| Antipsychotic medication: N (%)c | |||
| Atypical | 16 (70%) | ||
| Typical | 3 (13%) | ||
| None | 3 (13%) | ||
| CPZ equivalent | 394.20 (395.80) [0–1600] | ||
| PANSS symptoms | |||
| Positive symptoms | 16.26 (5.69) [7–30] | ||
| Negative symptoms | 13.35 (5.69) [7–27] | ||
| Disorganized symptoms | 8.13 (4.39) [5–18] | ||
| Social functioninge | 68.87 (17.22) [43–97] | 52.00 (9.37) [36–76] | t(45) = 4.163, p < 0.001, d = 1.24d |
| MSIT-Emotion behavioral dataf | |||
| RT mean (msec), (SD) | |||
| Neutral control | 843.45 (134.25) | 783.89 (111.75) | t(43) = 1.62, p = 0.11 |
| Neutral interference | 1046.39 (128.50) | 1012.14 (117.33) | t(43) = 0.93. p = 0.36 |
| Within group Interference effect | Int > Con; t(20) = 12.24, p < 0.001 | Int > Con; t(23) = 18.98, p < 0.001 | |
| Negative control | 872.93 (125.99) | 819.97 (121.35) | t(43) = 1.44, p = 0.16 |
| Negative interference | 1059.84 (116.09) | 1050.97 (135.04) | t(43) = 0.24, p = 0.82 |
| Within group Interference effect | Int > Con; t(20) = 13.72, p < 0.001 | Int > Con; t(23) = 22.85, p < 0.001 | |
| Within group condition × emotion interaction | F(1,22) = 4.48, p = 0.047 | F(1,23) = 0.58, p = 0.8 | |
| Accuracy (%), (SD) | |||
| Neutral control | 98.51 (2.10) | 98.26 (4.46) | t(43) = 0.23, p = 0.82 |
| Neutral interference | 96.43 (5.67) | 93.58 (7.03) | t(43) = 1.48, p = 0.15 |
| Within group Interference effect | Con > Int; t(20) = 1.81, p = 0.09 | Con > Int; t(23) = 4.23, p < 0.001 | |
| Negative control | 97.82 (2.67) | 97.40 (3.95) | t(43) = 0.41, p = 0.68 |
| Negative interference | 93.15 (8.59) | 93.32 (9.28) | t(43) = 0.60, p = 0.95 |
| Within group Interference effect | Con > Int; t(20) = 2.61, p = 0.02 | Con > Int; t(23) = 2.55, p = 0.02 | |
| Within group condition × emotion interaction | F(1,22) = 4.88, p = 0.039 | F(1,23) = 25, p = 0.62 |
All data are presented as: mean (SD), [range], unless otherwise noted.
Full scale IQ scores were estimated using the vocabulary and matrix reasoning subtests of the Weschler Abbreviated Scale of Intelligence (WASI).
Subtypes of the 17 participants with schizophrenia were: 13 paranoid, 3 residual, and 1 undifferentiated. Subtypes of the 6 participants with schizoaffective disorder were: 3 bipolar, and 3 depressive.
One patient did not report medication.
Cohen's d effect size.
Overall T score from Social Adjustment Scale — Self Report.
Due to technical problems, behavioral data for the MSIT-Emotion was not collected for two SZ participants.
Harvard University Institutional Review Board approved the study. Participants gave written informed consent and were paid for their participation. Participants completed behavioral assessments, returned a separate day for the scan, and were subsequently oriented to the daily-diary.
2.2. fMRI task: MSIT-Emotion
Cognitive control of emotional information was assessed with the MSIT-Emotion (Fig. 1), an adaptation of the MSIT, a standard cognitive control paradigm that combines the Flanker (Eriksen and Eriksen, 1974) and Simon (Simon and Berbaum, 1990) effects to robustly activate the cingulo–frontal–parietal cognitive control network within individual subjects (Bush and Shin, 2006). In the MSIT, subjects see sets of three numbers (1, 2, or 3) and report the identity of the number that differs from the other two. During control trials, position of the target number is always congruent with its position on the button box and the other two ‘distracter’ numbers are zeros; thus there is no spatial or semantic interference. During interference trials, position of the target number is incongruent with its position on the button box and the distracters are other numbers. This creates two sources of interference: spatial incongruence between the target and response (Simon effect) and semantic incongruence between the target and distracter numbers (Flanker effect). In the MSIT-Emotion, task stimuli (i.e. the numbers for interference and control trials) are shown on a background of either a neutral or negative picture, resulting in four conditions: neutral control (NeuCon), neutral interference (NeuInt), negative control (NegCon), and negative interference (NegInt). 48 neutral pictures and 48 negative pictures were selected from the International Affective Picture System (IAPS; Lang et al., 2005; see supplemental methods). Trials were presented in an event-related design so that correct and incorrect trials could be analyzed separately. Each trial was presented for 1.75 s followed by a variable inter-trial interval (central fixation-cross) for 4–10 s. Pictures were randomly assigned to conditions; trial types were presented in a fixed, pseudo-random order.
Fig. 1.
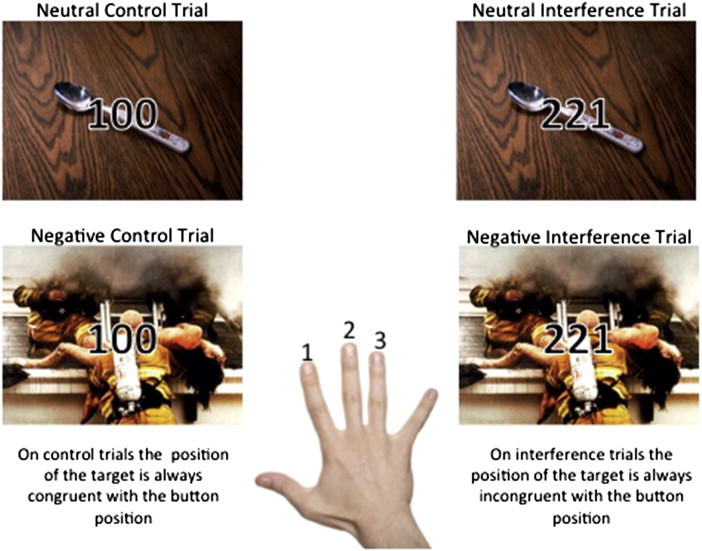
The MSIT-Emotion.The MSIT-Emotion is presented in an event-related design format. Control and interference trials are presented on a background of either a neutral or negative picture for 1.75 s followed by a variable inter-trial interval (central fixation cross) of 4–10 s. Participants see sets of three numbers and are required to report the identity of the number that is different from the other two. During control trials the position of the target number is always congruent with its position on the button box, and distracter numbers are always zeros. During interference trials the position of the target number is always incongruent with its position on the button box and distracter numbers are other numbers (either 1, 2, or 3). In all examples shown, the correct answer would be to press the button “1” with the index finger.
Before entering the scanner, participants completed a practice version of the task (10 trials for each of the 4 conditions). Participants were required to achieve 80% accuracy or higher before entering the scanner to ensure task competency; thus any group differences would not be due to task difficulty/performance differences. All participants reached 80% accuracy on their first practice. After the scan, participants rated the valence of each neutral and negative picture (see supplemental methods).
2.3. fMRI single-subject analysis
Images were acquired on a Siemens 3T TimTrio scanner and analyzed using SPM8 within the general linear model (GLM) framework. For single-subject GLMs, vectors of onset times with 0 s duration were defined for each event in each condition (NeuCon, NeuInt, NegCon, NegInt) and convolved with the canonical HRF. The data were high-pass filtered (cut-off period: 128 s). Artifact detection and movement correction were conducted using the Artifact detection tools software package (ART; Whitfield-Gabrieli, 2009). Regressors were created to exclude volumes with gross motion (>3 mm relative to previous time frame) or spiking artifacts (global mean image intensity greater than 3SD from the mean of the entire time series within a scan) from analysis. There were no group differences in number of outliers identified (SZ max = 30; HC max = 28). Contrasts were calculated for each of the four conditions relative to fixation periods, and for interference versus control for neutral and negative pictures (i.e. NeuInt > NeuCon; NegInt > NegCon). See supplemental methods for further details of image acquisition, processing, and analysis.
2.4. fMRI group analysis
Hypothesis 1
Schizophrenia participants have LPFC deficits in cognitive control of emotional information.
We tested our central hypothesis that, compared to healthy controls (HC), schizophrenia (SZ) participants would show reduced LPFC activity during cognitive control of emotional information using a 2 × 2 × 2 full factorial ANOVA with group (HC/SZ), emotion (negative/neutral) and condition (interference/control) as factors. The predicted group × emotion × condition interaction was that HC would have greater cognitive control related activity (interference–control) than SZ when inhibiting irrelevant negative emotional information on interference trials versus control trials compared to inhibiting irrelevant neutral information on interference versus control trials. Clusters showing the predicted group × emotion × condition interaction within the LPFC were identified and contrast values for each condition for each subject were extracted from the peak voxel. The difference between cognitive control activation on negative trials and cognitive control activation on neutral trials (i.e. (NegInt − Neg Con) − (NeuInt − NeuCon)) was calculated and used in the analysis with the daily-diary data.
Group maps were thresholded at t = 3.18, p < 0.001 (uncorrected) with 10 voxels/270 mm cluster size. We used WFU pickatlas (Maldjian et al., 2004; Maldjian et al., 2003) to create our LPFC region of interest mask, comprising Brodmann's Areas (BA), 9 and 46 (dorsal LPFC; middle frontal gyri extending into portions of the superior frontal gyri) and BA 44, 45, and 47 (ventral LPFC; inferior frontal gyri). Activation clusters surviving small volume corrections within this mask (FWE, p < 0.05) are reported.
2.5. The daily-diary
The daily-diary consisted of a structured questionnaire completed online at the end of each day (i.e. ‘right before bed’) for 21 consecutive days. Table 2 lists diary variables, questions, and descriptive statistics. Participants rated the extent to which they experienced mood and psychotic symptoms, including positive and negative mood, paranoia, hallucinations/odd experiences, negative symptoms, and disorganized symptoms. Questions were rated on a 1-to-5 scale (1 = not at all; 5 = extremely). Participants also reported whether they had an interpersonal conflict (yes/no), and if so, the extent to which the conflict caused distress and was resolved. Mood items were selected from the Profile of Mood States-Short Form (POMS-SF; Curran et al., 1995). Diary questions for psychotic symptoms were adapted from previous literature using ESM in schizophrenia (Myin-Germeys et al., 2002; Myin-Germeys and van Os, 2007; Thewissen et al., 2008) as well as commonly used diagnostic interviews and questionnaires (e.g. Schizotypal Personality Questionnaire; Psychotic-Like Experiences Scale). Consistent with prior ESM literature on stress, we used the subjective rating of conflict distress as the measure of social stress. If a conflict occurred, participants rated distress after each conflict (1-to-5). If more than one conflict occurred, distress ratings were summed. If no conflict occurred that day, distress was coded as 0.
Table 2.
Descriptive information regarding daily-diary questions and average response across the 21 diary days. Data shown is mean (SD) [range].
| Daily-diary variable |
Diary questions. Rating scale: 1 = not at all; 5 = extremely | SZ participants Mean (SD) [range] |
|---|---|---|
| Daily symptom | ||
| Negative mood (α = .91) |
Today I felt: anxious, on edge, uneasy, sad, hopeless, discouraged, depressed, angry, resentful, annoyed, lonely | 1.5 (0.6) [1.0–4.0] |
| Positive mood (α = .85) |
Today I felt: cheerful, lively, happy, accepted, supported, trusting, friendly | 2.8 (0.7) [1.0–4.7] |
| Paranoia (α = .77) |
I had a sense that people were looking at me oddly because of my appearance or something I did. I felt that others dislike me. I felt that others may hurt me. I felt that I had to be ‘on guard’ with other people, even with my friends. I felt like other people were watching me or taking notice of what I was saying or doing. I felt like people were giving me a hard time or were out to get me. |
1.5 (0.6) [1.0–3.4] |
| Positive symptoms (hallucinations & odd experiences) (α = .71) |
I heard voices or whispers (that did not seem to be coming from anywhere identifiable). I felt like my mind was playing tricks on me. I had the experience of thinking I heard a sound and then realizing there was nothing there. I noticed unusual bodily sensations today, like tingling, pin pricks, burning, numbness, or pain that I do not usually have. I had the experience of seeing people, animals, or things, and then I realized they were not really there. |
1.4 (0.7) [1.0–5.0] |
| Negative symptoms (α = .58) |
I felt emotionally dull or blunted I did not care about my appearance today (e.g. clothes, cleanliness, etc.) I felt like I did not care about anything I felt unmotivated and could not get things done |
1.5 (0.6) [1.0–3.7] |
| Disorganized symptoms (α = .80) |
I had a hard time communicating thoughts and ideas to others. I felt like my thoughts were jumbled. I had a hard time collecting my thoughts I found myself going off track or rambling a lot when I talked today |
1.4 (0.6) [1.0–3.7] |
| Interpersonal conflict | ||
| Conflict occurrencea | Did you have a disagreement, irritation, annoyance or other negative encounter with another person today? | |
| • Total number of conflicts across 21 day diary periodb | 11.6 (14.3) [0–46] | |
| • Percent (%) of diary days in which at least one conflict occurred | 28.8 (29.4) [0–90.5] | |
| • Number of diary days in which at least one conflict occurred | 5.8 (6.2) [0–19.0] | |
| Conflict distressc | If yes, how distressing was this encounter? (Distress rated: 1–5; no conflict coded as 0) |
1.5 (3.1) [0–19] |
This question was the section heading and was followed by examples of specific types of conflicts — e.g. I felt someone was hostile toward me (yes/no).
Total number of conflicts is the sum of all conflicts across the 21 day period (i.e. if multiple conflicts occurred, each was counted individually).
Conflict distress was the sum of distress ratings each day (i.e. if more than one conflict occurred, the distress ratings for each conflict were added together).
Internet-enabled laptop computers were provided for participants without computer and/or Internet access. Responses were time-stamped and could not be modified after completion. Research staff monitored diary entries daily and sent a reminder email if a day was missed. Participants could not miss more than 6 days.
Hypothesis 2
Among schizophrenia participants, LPFC activity during cognitive control of emotional information will predict changes in mood and symptoms following interpersonal conflict.
Analysis of daily-diary responses and LPFC activity used a hierarchical linear modeling (HLM) approach with the mixed procedure in SAS (SAS Institute, Cary, NC). The daily diary has a hierarchical structure in which the 21 daily assessments are nested within participant. We expected that the relationship between conflict distress and symptom severity within each participant would vary as a function of the between-subject variable, LPFC activity. Thus, we conducted two-level models with the mixed procedure in SAS, which allows for the simultaneous analysis of within and between subject variables. The repeated assessments over 21 days allowed us to examine the longitudinal effect of conflict distress on the change in mood and symptoms the following day.
The main analyses tested whether daily symptom-severity is predicted by the interaction of LPFC activity and the previous day's conflict distress. All models controlled for previous day's symptom-level. Thus, by accounting for previous day symptom-severity, we are able to test the effect of LPFC activity on the change in symptom-severity the day after conflict.
We expected that the interaction of LPFC activity and previous day conflict distress would significantly predict symptom-severity, such that SZ participants with low LPFC activity would have an increase in symptoms the day after high conflict distress. Separate HLM regression models were conducted for each symptom. All variables were grand-mean centered. If the interaction between yesterday's conflict distress and LPFC activity was significant, the effects of the interaction were further examined using simple slope analysis as described by Aiken and West (1991). Simple slope analyses for high and low groups were tested at 1SD above and below each centered mean.
For completeness, we also report the relationship between symptom severity and: 1) LPFC activity; 2) same day conflict distress; and 3) the interaction of same day conflict distress and LPFC activity.
Test–retest reliability (i.e. stability of the diary ratings) was examined by correlating average diary ratings for the first and second halves of the diary period. Internal consistency of diary items in each symptom construct was measured with standardized alpha coefficients. Construct validity was examined by correlating diary variables with corresponding mood and symptom constructs assessed with standard laboratory-based instruments.
3. Results
3.1. Preliminary analyses and data quality
3.1.1. fMRI task validation
One sample t-tests of interference versus control conditions (i.e. NeuInt > NeuCon and NegInt > NegCon) confirmed that the MSIT-Emotion task activates the expected cognitive control network. Both HC and SZ participants demonstrated greater activity for interference versus control conditions in cognitive control regions, including inferior, middle and superior frontal gyri (LPFC), and anterior cingulate cortex (ACC) (Fig. 2; Supplementary Tables 1 and 2).
Fig. 2.
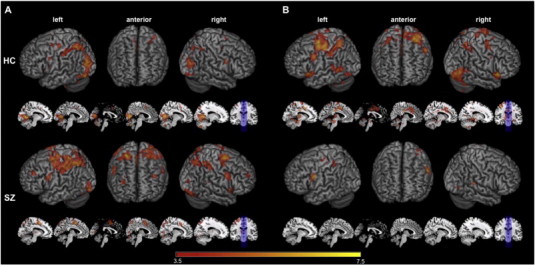
MSIT-Emotion task validation.Within-Group Interference Effect Related fMRI BOLD responses: A, one sample t-tests in neutral picture conditions (NeuInt–NeuCon) in both HC and SZ groups. B, One sample t-tests in negative picture conditions (NegInt–NegCon) in both HC and SZ groups. HC group results are displayed on the top row; SZ group results are displayed on the bottom row. 3D renderings are displayed in left, right, and anterior views alongside sagittal slices of an averaged MNI structural volume. Slices were chosen to focus more directly on midline and subcortical structures; slice numbers from left to right are: −10, −6, 0, 4, 10, 14. Coronal view of slice location is provided for reference. Neural activity clusters are based on one-sample t-tests within each group with a significance threshold of p < 0.001 uncorrected and cluster threshold of 10 voxels/270 mm. See Supplemental Tables 1 and 2 for complete list of neuroanatomical labels, MNI coordinates, and t-values for cluster peaks.
3.1.2. Behavioral results from fMRI scan
The MSIT-Emotion elicited expected interference effects (Table 1). All participants responded slower and were less accurate on interference trials compared to control trials; participants also responded slower on negative picture trials compared to neutral picture trials. There were no group differences in reaction time or accuracy. A significant condition × emotion interaction in the SZ group indicated that individuals with schizophrenia were slower and less accurate on negative interference trials relative to neutral interference trials and negative and neutral control trials. There was no condition × emotion interaction in the HC group. These data indicate that the negative interference trials, posited to be the most taxing on cognitive control resources, are particularly challenging for SZ participants.
3.1.3. Daily diary assessments — verification of data quality
Twenty schizophrenia participants completed the daily-diary. Preliminary analyses of diary responses indicate that data quality is adequate for planned analyses. Compliance was high; average number of diary days completed was 20.0 (SD 1.8). On average, participants had 11.6 conflicts over the diary period and conflicts occurred on 28.8% of diary days. The consistent daily responses and number of conflicts indicate sufficient data to analyze the relationship between conflict and symptoms. Test–retest reliability shows that diary estimates are stable (i.e. correlations between the first 10 days and last 11 days had rs > .75 for all variables). The items included in each mood and symptom construct were chosen according to existing literature and a priori face validity. After the data were collected, standardized alpha of each construct was examined to verify internal consistency, and items that were not correlated with the construct were removed. This analysis revealed that reverse-coded items were not effective for assessing symptoms and were removed (i.e. two items were dropped from the negative symptom construct and one item from the disorganized symptom construct). Alpha is reported for final constructs (Table 2). Alpha was low for the negative symptom construct (α = .58) but acceptable for all other mood and symptom constructs (α > .70). Analysis of construct validity showed that 21-day average daily-diary ratings for negative mood significantly correlated with laboratory measures of trait negative affect (PANAS Negative Affect, r = .55, p < .05). Daily-diary ratings of positive mood were not correlated with PANAS Positive Affect (r = .01); however, there is no overlap between the PANAS and POMS positive mood items, so a different lab-based assessment of positive mood may be necessary to test construct validity. Daily diary measures of positive, negative, and disorganized symptoms were not correlated with Positive and Negative Symptom Scale (PANSS) measures of positive, negative, and disorganized symptoms. This does not necessarily indicate that the diary measures are inaccurate or invalid, but it does suggest that different factors may be influencing symptom assessments from the diary and the PANSS. Research comparing multiple methods of assessment may provide more information regarding the validity of different methods.
In addition, there were significant correlations between daily-diary measures of interpersonal conflict and lab-based measure social functioning. For example, participants with worse social impairment as measured by the Social Adjustment Scale had more interpersonal conflicts over the 21 day diary period (r = .50, p = .03) and worse conflict distress (r = .50, p = .03). A similar pattern was observed for the Global Functioning: Social scale but the correlations were not significant. PANSS measures of interpersonal functioning were also related to daily-diary measures of interpersonal conflict. Higher ratings on PANSS Hostility were associated with more interpersonal conflicts (r = .58, p = .008) and more conflict distress (r = .66, p = .002). A higher degree of PANSS Uncooperativeness was also associated with more interpersonal conflicts (r = .50, p = .02) and more conflict distress (r = .69, p = .001). Together these data indicate that the daily-diary assessments of mood and interpersonal conflict are measuring the intended construct.
Since prior studies have found a relationship between social stress and symptoms, we conducted simple regression analysis to look at the relationship between conflict distress and symptom severity. Consistent with prior findings, daily ratings of high conflict distress were associated with worse symptom severity, including worse negative mood, paranoia, negative symptoms and disorganized symptoms (Table 3).
Table 3.
Results from hierarchical linear regression models predicting daily-diary ratings of symptom severity. Significant findings are shown with p values in bold type.
| LPFC activity |
Conflict distress |
LPFC activity × same day conflict distress |
LPFC activity × previous day conflict distress |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| b (S.E.) | F | p | b (S.E.) | F | p | b (S.E.) | F | p | b (S.E.) | F | p | |
| Negative mood | 0.02 (0.03) | 0.29 | 0.6 | 0.04 (0.01) | 39.26 | < 0.0001 | 0.0004 (0.01) | 0.01 | 0.94 | −0.01 (0.003) | 4.49 | 0.047 |
| Positive mood | 0.01 (0.04) | 0.12 | 0.73 | −0.02 (0.01) | 3.30 | 0.08 | 0.001 (0.01) | 0.11 | 0.75 | 0.02 (0.01) | 8.03 | 0.01 |
| Paranoia | −0.001 (0.04) | 0.00 | 0.98 | 0.02 (0.01) | 11.62 | 0.003 | 0.001 (0.01) | 0.04 | 0.84 | −0.01 (0.01) | 2.16 | 0.16 |
| Hallucinations Odd experience |
−0.03 (0.05) | 0.41 | 0.53 | 0.01 (0.01) | 1.06 | 0.32 | 0.01 (0.01) | 2.04 | 0.17 | −0.01 (0.01) | 1.68 | 0.21 |
| Negative symptoms | 0.01 (0.03) | 0.25 | 0.63 | 0.03 (0.01) | 11.44 | 0.003 | 0.003 (0.01) | 0.20 | 0.66 | −0.01 (0.01) | 0.89 | 0.36 |
| Disorganized Symptoms |
0.01 (0.04) | 0.02 | 0.89 | 0.02 (0.01) | 5.33 | 0.03 | 0.01 (0.01) | 1.30 | 0.27 | 0.001 (0.004) | 0.07 | 0.79 |
We also conducted simple regression analysis to examine the relationship between LPFC activity and symptoms regardless of interpersonal conflict: LPFC activity did not relate to any of the symptom measures.
We used HLM to investigate the interaction of right DLPFC activity with conflict distress on the same day of the conflict (i.e. “same day conflict distress”). No significant interactions were found; that is, on the same day as an interpersonal conflict, individuals' DLPFC activation during cognitive control of emotion did not interact with conflict distress in predicting symptom severity that day (Table 3).
3.2. Hypothesis testing
Hypothesis 1
Schizophrenia participants have LPFC deficits in the cognitive control of emotional information.
Analysis revealed a single cluster of activation in our LPFC region of interest mask, in the right superior frontal gyrus (BA9; DLPFC), with a significant group × emotion × condition interaction(HC > SZ) that survived small volume correction (FWE p < 0.05; see Fig. 3). Fig. 3 barplots illustrate that HC participants consistently demonstrate the expected pattern of activation in the right DLPFC: increased activation in NegInt compared to NegCon conditions; SZ participants show the opposite pattern, deactivating in NegInt compared to NegCon conditions.
Fig. 3.
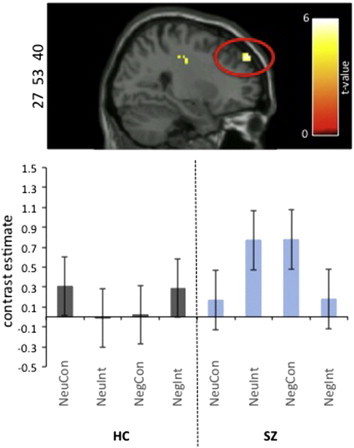
fMRI BOLD responses during cognitive control of negative emotional information in HC vs. SZ groups.A single cluster of activation in our LPFC region of interest mask, specifically, the right superior frontal gyrus (BA9; DLPFC), showed a significant group × emotion × condition interaction(HC > SZ) that survived small volume correction (FWE p < 0.05). See Supplemental Table 3. Cluster size: 50 voxels/1350 mm. MNI coordinates: x = 27, y = 53, z = 40. t-value = 4.25. FWE p = 0.009. Results are based on a 2 × 2 × 2 full factorial ANOVA implemented in SPM8. The NegInt contrast was compared with the NegCon, NeuInt, and NeuCon contrast for HC and SZ participants. Contrast estimates were extracted from the peak voxel of the cluster and plotted for each group and each condition. Cluster is displayed with a significance threshold of p < 0.001 uncorrected and a cluster threshold of 10 voxels/270 mm.
Activation clusters with significant group × emotion × condition interaction(HC > SZ) were also present in the right lateral orbital frontal gyrus extending into the insula (LOFG; BA11) and the left dorsal anterior cingulate (dACC; BA32), but these did not survive small volume correction in either the LPFC region of interest mask (i.e. the LOFG cluster) or an anterior cingulate mask comprising BA24 and BA32 (see Supplemental Table 3).
No regions showed an interaction in the opposite direction (i.e. SZ > HC).
The cluster in the right superior frontal gyrus (DLPFC; BA 9, peak: x = 27, y = 53, z = 40) was the only cluster that survived multiple comparison correction within our LPFC region of interest mask. All subsequent analyses investigating LPFC activity and diary data were conducted using the difference score (NegInt − NegCon) − (NeuInt − NeuCon), i.e. our measure of cognitive control of emotional information, calculated from the contrast values in each condition extracted from the peak of this cluster in the right DLPFC.
Hypothesis 2
Among schizophrenia participants, LPFC activity during cognitive control of emotional information will predict changes in mood and symptoms following interpersonal conflict.
We used HLM to examine the interaction of DLPFC activity with conflict distress from the previous day (i.e. “previous day conflict distress”) in predicting symptom severity on days following interpersonal conflicts. DLPFC activity significantly interacted with previous day conflict distress in predicting change in positive mood [F = 8.03, b = 0.02, p = 0.01] and negative mood [F = 4.49, b = −0.01, p = 0.047] the following day. Results for positive and negative mood are plotted in Fig. 4. The interaction of previous day conflict distress and DLPFC activity did not significantly predict any other symptom (see Table 3).
Fig. 4.
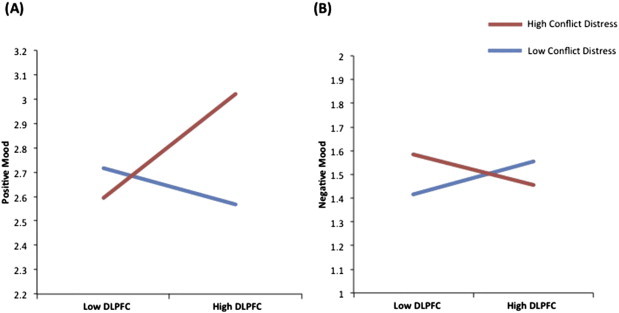
Daily diary simple slope analysis.Daily diary ratings of positive and negative mood are plotted as a function of DLPFC activity and conflict distress. Mood is plotted on the y-axis. DLPFC activity is plotted on the x-axis. High conflict distress ratings from the preceding day are shown in red. Low conflict distress ratings from the preceding day are shown in blue. (A) When previous day conflict distress was high, schizophrenia participants with high DLPFC activity had better positive mood. No relationship to positive mood was found among individuals with low DLPFC activity, regardless of level of previous day conflict distress. (B) When previous day conflict distress was high, schizophrenia participants with low DLPFC activity had worse negative mood. No relationship to negative mood was found among individuals with high DLPFC activity, regardless of level of previous day conflict distress.
Simple slope analyses were conducted to better understand the interactions predicting positive and negative mood. Analysis of effects influencing positive mood showed the following: The effect of DLPFC activity on positive mood was examined separately for days when previous day conflict distress was low and days when previous day conflict distress was high. On days when previous day conflict distress was low, DLPFC activity did not predict positive mood [b = −0.02(S.E. 0.02), t(19) = 1.51, p = 0.30] (blue line plotted in Fig. 4A). However, when previous day conflict distress was high, DLPFC activity significantly predicted positive mood the following day [b = 0.07(S.E. 0.02), t(19) = 3.1, p = 0.006], such that schizophrenia participants with higher DLPFC activity had higher positive mood compared to participants with lower DLPFC activity (red line plotted in Fig. 4A).
Next we examined the effect of conflict distress on positive mood for schizophrenia participants with high DLPFC activity and low DLPFC activity. For schizophrenia participants with low DLPFC activity, previous day conflict distress was not related to positive mood [b = −0.02(S.E. 0.02), t(19) = 1.0, p = 0.35]. However, for schizophrenia participants with high DLPFC activity, previous day conflict distress had a significant effect on positive mood the next day [b = 0.07(S.E. 0.02), t(19) = 3.6, p = 0.002], such that positive mood was higher on days following high conflict distress. These results indicate that, among schizophrenia participants with high DLPFC activity, positive mood is significantly higher on days preceded by high conflict distress as compared to days preceded by low conflict distress. No relationship to positive mood was found among individuals with low DLPFC activity, regardless of level of previous day conflict distress.
Analysis of effects influencing negative mood showed the following: On days when previous day conflict distress was low, DLPFC activity had no relationship to negative mood [b = 0.02(S.E. 0.02), t(19) = 1.54, p = 0.14] (blue line plotted in Fig. 4B). Similarly, on days when previous day conflict distress was high, DLPFC activity had no significant influence on negative mood [b = −0.02(S.E. 0.01), t(19) = 1.52, p = 0.15] (red line plotted in Fig. 4B).
We then examined the effect of previous day conflict distress for schizophrenia participants with high and low DLPFC activities. For schizophrenia participants with low DLPFC activity, previous day conflict distress was a trend-level significant predictor of the change in negative mood the next day [b = 0.03(S.E. 0.01), t(19) = 2.0, p = 0.06], such that when previous day conflict distress was high, participants with low DLPFC activity had worse negative mood the next day. However, for schizophrenia participants with high DLPFC activity, previous day conflict distress was not related to the change in negative mood the next day [b = −0.02(S.E. 0.01), t(19) = 1.2, p = 0.26]. These results indicate that, among schizophrenia participants with low DLPFC activity, negative mood is significantly higher on days preceded by high conflict distress as compared to days preceded by low conflict distress. No relationship to negative mood was found among individuals with high DLPFC activity, regardless of level of previous day conflict distress.
4. Discussion
This study combines fMRI and experience sampling methods to investigate whether people with schizophrenia have LPFC deficits in the cognitive control of emotional information, and, if so, whether these LPFC deficits prospectively predict the influence of real-world interpersonal conflict on changes in mood and psychotic symptoms. Two key findings emerged.
First, analysis of the MSIT-Emotion task revealed a group × emotion × condition interaction in the right DLPFC (superior frontal gyrus, BA9). Inspection of activity in this region indicates that, compared to healthy participants, individuals with schizophrenia had lower DLPFC activity during cognitive control of task-irrelevant negative emotional information. This is consistent with prior literature demonstrating that schizophrenia is associated with LPFC deficits in cognitive control of emotion (Ursu et al., 2011; Vercammen et al., 2012). Here, schizophrenia participants showed deficient neural activity in the right DLPFC when controlling emotional information on trials with the greatest cognitive demand (i.e. negative interference trials) suggesting that schizophrenia participants' ability to control the influence of irrelevant negative emotional information on task performance is particularly impacted during high cognitive load tasks. This is consistent with research demonstrating a relationship between DLPFC dysfunction and cognitive load in non-emotional cognitive control tasks (Callicott et al., 2003), and further indicates that tasks probing cognition–emotion interactions may be a more sensitive tool for understanding the role of emotion processing in the pathophysiology of schizophrenia.
Second, among schizophrenia participants, the interaction of right DLPFC activity during cognitive control of emotional information and the previous day's conflict distress predicted changes in positive and negative mood the following day. Specifically, analysis of positive mood shows that schizophrenia participants with higher DLPFC activity had better positive mood the day after high conflict distress. However, DLPFC activity had no influence on days following low conflict distress, and for schizophrenia participants with low DLPFC activity, positive mood was no different on days following high or low conflict distress. Thus, higher DLPFC activity appears to convey a boost to positive mood following an interpersonal conflict, but only when that conflict is experienced as highly distressing.
Conversely, analysis of negative mood showed that schizophrenia participants with lower DLPFC activity had worse negative mood when the previous day's conflict distress was high. However, on days when previous day conflict distress was low, DLPFC activation had no relationship to negative mood. Thus, lower DLPFC activity appears to contribute to an exacerbation of negative mood following an interpersonal conflict, but only when that conflict is experienced as highly distressing.
These data provide the first evidence tying LPFC dysfunction in schizophrenia to daily ratings of mood following real-world social interactions, and have substantive implications for understanding the specific role of LPFC in response to social stress. First, our finding that low DLPFC activity during cognitive control of negative emotional information predicts increased negative mood on days following highly distressing interpersonal conflicts is consistent with our previous finding in healthy individuals (Hooker et al., 2010), and prior literature demonstrating reduced LPFC engagement in response to social stress predicts higher self-reported social distress (Eisenberger et al., 2003; Eisenberger et al., 2007; Hooker et al., 2010). Together, these data support the well-documented proposal that LPFC control-related mechanisms act to down-regulate negative affect/mood (Kohn et al., 2014; Ochsner and Gross, 2005), and suggest that adaptive response to social stressors requires LPFC control-related functions to down-regulate negative emotional information.
Second, our finding that individuals with high DLPFC activity show an increase in their positive mood the day after highly distressing interpersonal conflicts is particularly interesting, especially given that the paradigm we used to probe LPFC-mediated cognitive control focused on control of negative emotional information. This finding supports the proposal that LPFC-mediated control mechanisms are also important in the up-regulation of positive mood (Kim and Hamann, 2007; Mak et al., 2009) — even in the context of incidental/automatic cognitive control of emotional information, and replicates parallel findings in our previous study (Hooker et al., 2010). In the context of explicit emotion regulation, one explanation of this finding is that people with high LPFC control-related activation use more effective cognitive strategies to reevaluate distressing interpersonal conflicts and reframe them in a positive light, possibly by thinking about what they have learned from the interpersonal conflict and how it makes them stronger/better (i.e. positive reappraisal). In line with this interpretation, the use of positive reappraisal is related to increased experience and expression of positive emotions (Gross and John, 2003), and has consistently been shown to predict well-being (Helgeson et al., 2006) and promote an “upward-spiral” of adaptive responding to negative events (Fredrickson, 2004). Interestingly, several studies examining explicit emotion regulation in mood psychopathology (e.g. depression, bipolar disorder) have not found strong group differences (e.g. Dillon and Pizzagalli, 2013; Ehring et al., 2010; Gruber et al., 2014). This suggests that, when given clear instructions, individuals with emotion regulation difficulties can effectively engage emotion control mechanisms, but that in the absence of such clear instructions – as is typically the case in daily life – they struggle to regulate emotional responses. Thus, using the MSIT-Emotion to probe incidental/automatic LPFC-mediated emotion control processes may have enhanced the probability of finding group differences and detecting a relationship between LPFC activity and daily social interactions.
Finally, the results are in line with the diathesis–stress model of neural mechanisms of social stress (e.g. Dillon and Pizzagalli, 2013; Ehring et al., 2010; Gruber et al., 2014) and have implications for the development of treatment interventions. Low or compromised LPFC function may be a vulnerability that contributes to the increased negative impact of highly distressing interpersonal conflicts via impaired cognitive control of emotion. Conversely, high or intact LPFC function may be protective against the negative impact of interpersonal conflicts, and appear to convey a benefit in the form of increased positive mood. Given that greater DLPFC engagement during our laboratory-based measure of control of emotional information predicted increased positive mood the day after a distressing interpersonal conflict, interventions that aim to improve LPFC function and/or cognitive control of emotional information may also improve real-world forms of emotion control, and consequently, adaptive response to social conflict. Consistent with this proposal, normalization/improvement of LPFC function, either in response to medication (Garnefski and Kraaij, 2006; Zlomke and Hahn, 2010), cognitive therapy (Heller et al., 2013), or computerized cognitive training (Edwards et al., 2010), is associated with improved symptoms in depression, psychosis, and global functioning respectively.
This study is also consistent with mounting consensus that ecological measures of daily life can be combined with neuroimaging data to meaningfully connect neural indicators of psychological processes to real-world behavior (Subramaniam et al., 2014). In healthy individuals, LPFC activity predicts daily levels of social support (Berkman and Lieberman, 2011), successful smoking cessation (Eisenberger et al., 2007), and maladaptive behavior following a conflict with a partner (Berkman et al., 2011). Our findings extend this “brain-as-predictor” (Hooker et al., 2010) approach to understanding the consequences of well-established LPFC deficits in schizophrenia in the context of social stress (i.e. interpersonal conflict). Using a task that specifically assessed the interaction between cognitive control and emotion processing likely increased sensitivity to this brain–behavior relationship, and provides further support for the proposal that the consequences of cognitive and emotion processing deficits in schizophrenia may be better understood in the context of cognition–emotion interactions (Berkman et al., 2011). By using these methods together, this study demonstrates the value of the “brain-as-predictor” approach to research attempting to delineate the mechanisms that contribute to the development and persistence of problematic mood and symptoms in schizophrenia.
Limitations must be acknowledged. First, mood and symptom measures on the daily-diary were self-report and thus subject to bias. Future research could include more objective measures of interpersonal functioning and associated symptomatic responses. Second, we designed the MSIT-Emotion with the assumption that in order to meet task demands participants must engage cognitive control mechanisms to inhibit the influence of the negative picture on performance. It is possible, however, that participants could use an alternative, non-control related strategy to counteract the influence of the negative picture (e.g. squint their eyes to blur the picture and focus on the numerical stimuli). Future investigations should use a paradigm that precludes the use of such alternative strategies by incorporating the emotional stimuli into the response pattern of the task, for example, the face-emotion Stroop (Kring and Elis, 2013; Pessoa, 2008). Third, based on our previous findings regarding predictive value of LPFC activity (Etkin et al., 2006), we chose to focus specifically on the role of the LPFC in social behavior. Next steps should investigate the contribution of other regions involved in cognitive control of emotional information (e.g. ACC, amygdala) and cognitive control network connectivity (e.g. cingulo–fronto–parietal connectivity) to mood changes in response to social stress.
In sum, this study integrates fMRI and experience sampling methods to demonstrate a direct link between LPFC activation during cognitive control of emotional information and response to real-world social conflict in schizophrenia. Results indicate that LPFC activity during a laboratory-based measure of cognitive control of emotional information predicts changes in positive and negative mood on days following highly distressing interpersonal conflicts. These findings suggest that treatment interventions targeting LPFC mechanisms of cognitive control of emotion could promote adaptive responding to real-world social conflict in schizophrenia.
Funding
This work was supported by Harvard University research funds to Christine I. Hooker and a Sackler Scholar Fellowship from the Sackler Scholar Program in Psychobiology to Laura M. Tully.
Financial disclosures
The authors have no financial disclosures of conflicts of interest.
Acknowledgments
We are grateful to Beverly Pozuelos, Chinmayi Tengshe, Nadia Liyanage-Don, Todd Wright, Emily Carol, and Caitlin Carey for their assistance with data collection and processing, and to Dr. Dost Ongur and Danielle Pfaff for their assistance with participant recruitment. We would also like to thank the participants for their involvement and dedication to this research. This study was supported by Harvard University research funds to Christine I. Hooker and a Sackler Scholar Fellowship from the Sackler Scholar Program in Psychobiology to Laura M. Tully.
Appendix A. Supplementary data
Supplementary methods and tables.
References
- Aiken L.S., West S.G. Multiple Regression: Testing and Interpreting Interactions. Sage; Thousand Oaks, CA: 1991. [Google Scholar]
- Aron A.R., Robbins T.W., Poldrack R.A. Inhibition and the right inferior frontal cortex. Trends in Cognitive Sciences. 2004;8(4):170–177. doi: 10.1016/j.tics.2004.02.010. 15050513 [DOI] [PubMed] [Google Scholar]
- Arriaga X.B., Rusbult C.E. Standing in my partner's shoes: partner perspective taking and reactions to accommodative dilemmas. Personality and Social Psychology Bulletin. 1998;24(9):927–948. [Google Scholar]
- Auther A., Smith C., Cornblatt B. Global Functioning: Social Scale (GF: Social) Zucker Hillside Hospital; Glen Oaks, N.Y.: 2006. [Google Scholar]
- Barch D.M. The cognitive neuroscience of schizophrenia. Annual Review of Clinical Psychology. 2005;1(1):321–353. doi: 10.1146/annurev.clinpsy.1.102803.143959. [DOI] [PubMed] [Google Scholar]
- Berkman E.T., Falk E.B., Lieberman M.D. In the trenches of real-world self-control: neural correlates of breaking the link between craving and smoking. Psychological Science. 2011;22(4):498–506. doi: 10.1177/0956797611400918. 21378368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkman E.T., Lieberman M.D. What's outside the black box?: the status of behavioral outcomes in neuroscience research. Psychological Inquiry. 2011;22(2):100–107. doi: 10.1080/1047840X.2011.550182. 21984865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G., Shin L.M. The multi-source interference task: an fMRI task that reliably activates the cingulo–frontal–parietal cognitive/attention network. Nature Protocols. 2006;1(1):308–313. doi: 10.1038/nprot.2006.48. 17406250 [DOI] [PubMed] [Google Scholar]
- Callicott J.H., Mattay V.S., Verchinski B.A., Marenco S., Egan M.F., Weinberger D.R. Complexity of prefrontal cortical dysfunction in schizophrenia: more than up or down. American Journal of Psychiatry. 2003;160(12):2209–2215. doi: 10.1176/appi.ajp.160.12.2209. 14638592 [DOI] [PubMed] [Google Scholar]
- Chapman L.J., Chapman J.P., Raulin M.L. Scales for physical and social anhedonia. Journal of Abnormal Psychology. 1976;85(4):374–382. doi: 10.1037//0021-843x.85.4.374. 956504 [DOI] [PubMed] [Google Scholar]
- Chapman L.J., Chapman J.P., Raulin M.L. Body-image aberration in schizophrenia. Journal of Abnormal Psychology. 1978;87(4):399–407. doi: 10.1037//0021-843x.87.4.399. 681612 [DOI] [PubMed] [Google Scholar]
- Curran S.L., Andrykowski M.A., Studts J.L. Short form of the profile of mood states (POMS-SF): psychometric information. Psychological Assessment. 1995;7(1):80–83. [Google Scholar]
- Curtis C.E., D'Esposito M. Persistent activity in the prefrontal cortex during working memory. Trends in Cognitive Sciences. 2003;7(9):415–423. doi: 10.1016/s1364-6613(03)00197-9. 12963473 [DOI] [PubMed] [Google Scholar]
- Dillon D.G., Pizzagalli D.A. Evidence of successful modulation of brain activation and subjective experience during reappraisal of negative emotion in unmedicated depression. Psychiatry Research. 2013;212(2):99–107. doi: 10.1016/j.pscychresns.2013.01.001. 23570916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckblad M., Chapman L.J. Magical ideation as an indicator of schizotypy. Journal of Consulting and Clinical Psychology. 1983;51(2):215–225. doi: 10.1037//0022-006x.51.2.215. 6841765 [DOI] [PubMed] [Google Scholar]
- Eckblad, M., Chapman, L. J., Chapman, J. P., & Mishlove, M. The revised social anhedonia scale (1982). Unpublished Test
- Edwards B.G., Barch D.M., Braver T.S. Improving prefrontal cortex function in schizophrenia through focused training of cognitive control. Frontiers in Human Neuroscience. 2010;4:32. doi: 10.3389/fnhum.2010.00032. 20461148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehring T., Tuschen-Caffier B., Schnülle J., Fischer S., Gross J.J. Emotion regulation and vulnerability to depression: spontaneous versus instructed use of emotion suppression and reappraisal. Emotion (Washington, D.C.) 2010;10(4):563–572. doi: 10.1037/a0019010. 20677873 [DOI] [PubMed] [Google Scholar]
- Eisenberger N.I., Lieberman M.D., Williams K.D. Does rejection hurt? An fMRI study of social exclusion. Science (New York, N.Y.) 2003;302(5643):290–292. doi: 10.1126/science.1089134. 14551436 [DOI] [PubMed] [Google Scholar]
- Eisenberger N.I., Taylor S.E., Gable S.L., Hilmert C.J., Lieberman M.D. Neural pathways link social support to attenuated neuroendocrine stress responses. Neuroimage. 2007;35(4):1601–1612. doi: 10.1016/j.neuroimage.2007.01.038. 17395493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksen B.A., Eriksen C.W. Effects of noise letters upon the identification of a target letter in a nonsearch task. Perception & Psychophysics. 1974;16(1):143–149. [Google Scholar]
- Etkin A., Egner T., Peraza D.M., Kandel E.R., Hirsch J. Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron. 2006;51(6):871–882. doi: 10.1016/j.neuron.2006.07.029. 16982430 [DOI] [PubMed] [Google Scholar]
- First M.B., Spitzer R.L., Gibbon M., Williams J.B.W. Structured Clinical Interview for DSM-IV-TR Axis I Disorders. research version, patient edition (SCID-I/P) Biometrics Research, New York State Psychiatric Institute; New York: 2002. [Google Scholar]
- Fredrickson B.L. The broaden-and-build theory of positive emotions. Philosophical Transactions of the Royal Society of London Series B. Biological Sciences. 2004:1367–1378. doi: 10.1098/rstb.2004.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garnefski N., Kraaij V. Relationships between cognitive emotion regulation strategies and depressive symptoms: a comparative study of five specific samples. Personality and Individual Differences. 2006;40(8):1659–1669. [Google Scholar]
- Goghari V.M., Sponheim S.R., MacDonald A.W. The functional neuroanatomy of symptom dimensions in schizophrenia: a qualitative and quantitative review of a persistent question. Neuroscience and Biobehavioral Reviews. 2010;34(3):468–486. doi: 10.1016/j.neubiorev.2009.09.004. 19772872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J.J., John O.P. Individual differences in two emotion regulation processes: implications for affect, relationships, and well-being. Journal of Personality and Social Psychology. 2003;85(2):348–362. doi: 10.1037/0022-3514.85.2.348. 12916575 [DOI] [PubMed] [Google Scholar]
- Gross J.J., Thompson R.A. Emotion regulation: Conceptual foundations. Handbook of emotion regulation. 2007;3:24. [Google Scholar]
- Gruber J., Hay A.C., Gross J.J. Rethinking emotion: cognitive reappraisal is an effective positive and negative emotion regulation strategy in bipolar disorder. Emotion (Washington, D.C.) 2014;14(2):388–396. doi: 10.1037/a0035249. 24364852 [DOI] [PubMed] [Google Scholar]
- Gyurak A., Gross J.J., Etkin A. Explicit and implicit emotion regulation: a dual-process framework. Cognition & Emotion. 2011;25(3):400–412. doi: 10.1080/02699931.2010.544160. 21432682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton T.F., Wagner D.D. Cognitive neuroscience of self-regulation failure. Trends in Cognitive Sciences. 2011;15(3):132–139. doi: 10.1016/j.tics.2010.12.005. 21273114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helgeson V.S., Reynolds K.A., Tomich P.L. A meta-analytic review of benefit finding and growth. Journal of Consulting and Clinical Psychology. 2006;74(5):797–816. doi: 10.1037/0022-006X.74.5.797. 17032085 [DOI] [PubMed] [Google Scholar]
- Heller A.S., Johnstone T., Peterson M.J., Kolden G.G., Kalin N.H., Davidson R.J. Increased prefrontal cortex activity during negative emotion regulation as a predictor of depression symptom severity trajectory over 6 months. JAMA Psychiatry. 2013;70(11):1181–1189. doi: 10.1001/jamapsychiatry.2013.2430. 24173657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooker C.I., Benson T.L., Gyurak A., Yin H., Tully L.M., Lincoln S.H. Neural activity to positive expressions predicts daily experience of schizophrenia-spectrum symptoms in adults with high social anhedonia. Journal of Abnormal Psychology. 2014;123(1):190–204. doi: 10.1037/a0035223. 24661170 [DOI] [PubMed] [Google Scholar]
- Hooker C.I., Gyurak A., Verosky S.C., Miyakawa A., Ayduk Ö. Neural activity to a partner's facial expression predicts self-regulation after conflict. Biological Psychiatry. 2010;67(5):406–413. doi: 10.1016/j.biopsych.2009.10.014. 20004365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooley J.M. Expressed emotion and relapse of psychopathology. Annual Review of Clinical Psychology. 2007;3(1):329–352. doi: 10.1146/annurev.clinpsy.2.022305.095236. 17716059 [DOI] [PubMed] [Google Scholar]
- Kay S.R., Opler L.A., Fiszbein A. The positive and negative syndrome rating scale (PANSS) for schizophrenia. Schizophrenia Bulletin. 1987;45:20–31. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kim S.H., Hamann S. Neural correlates of positive and negative emotion regulation. Journal of Cognitive Neuroscience. 2007;19(5):776–798. doi: 10.1162/jocn.2007.19.5.776. 17488204 [DOI] [PubMed] [Google Scholar]
- Kohn N., Eickhoff S.B., Scheller M., Laird A.R., Fox P.T., Habel U. Neural network of cognitive emotion regulation — an ALE meta-analysis and MACM analysis. Neuroimage. 2014;87(0):345–355. doi: 10.1016/j.neuroimage.2013.11.001. 24220041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krabbendam L., Hooker C.I., Aleman A. Neural effects of the social environment. Schizophrenia Bulletin. 2014;40(2):248–251. doi: 10.1093/schbul/sbt233. 24442853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kring A.M., Elis O. Emotion deficits in people with schizophreniaAnnual Review of Clinical Psychology. 2013;9(1):409–433. doi: 10.1146/annurev-clinpsy-050212-185538. 23245340 [DOI] [PubMed] [Google Scholar]
- Kring A.M., Werner K.H. Emotion Regulation and Psychopathology. In: Feldman P.P.R.S., editor. The Regulation of Emotion. Lawrence Erlbaum Associates; Mahwah, NJ, US: 2004. pp. 359–385. [Google Scholar]
- Lang, P.J., Bradley, M.M., Cuthbert, B.N. (2005), International Affective Picture System (IAPS): affective ratings of pictures and instruction manual. Technical Report, p. A-6. Gainesville, FL: University of Florida
- Lenzenweger M.F., Bennett M.E., Lilenfeld L.R. The referential thinking scale as a measure of schizotypy: scale development and initial construct validation. Psychological Assessment. 1997;9(4):452–463. [Google Scholar]
- Lopes P.N., Nezlek J.B., Extremera N., Hertel J., Fernández-Berrocal P., Schütz A., Salovey P. Emotion regulation and the quality of social interaction: does the ability to evaluate emotional situations and identify effective responses matter? Journal of Personality. 2011;79(2):429–467. doi: 10.1111/j.1467-6494.2010.00689.x. 21395594 [DOI] [PubMed] [Google Scholar]
- MacDonald A.W., Carter C.S., Kerns J.G., Ursu S., Barch D.M., Holmes A.J., Stenger V.A. Specificity of prefrontal dysfunction and context processing deficits to schizophrenia in never-medicated patients with first-episode psychosis. American Journal of Psychiatry. 2005;162(3):475–484. doi: 10.1176/appi.ajp.162.3.475. 15741464 [DOI] [PubMed] [Google Scholar]
- Mak A.K., Hu Z.G., Zhang J.X., Xiao Z.W., Lee T.M. Neural correlates of regulation of positive and negative emotions: an fMRI study. Neuroscience Letters. 2009;457(2):101–106. doi: 10.1016/j.neulet.2009.03.094. 19429172 [DOI] [PubMed] [Google Scholar]
- Maldjian J.A., Laurienti P.J., Burdette J.H. Precentral gyrus discrepancy in electronic versions of the Talairach atlas. Neuroimage. 2004;21(1):450–455. doi: 10.1016/j.neuroimage.2003.09.032. 14741682 [DOI] [PubMed] [Google Scholar]
- Maldjian J.A., Laurienti P.J., Kraft R.A., Burdette J.H. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19(3):1233–1239. doi: 10.1016/s1053-8119(03)00169-1. 12880848 [DOI] [PubMed] [Google Scholar]
- Manoach D.S. Prefrontal cortex dysfunction during working memory performance in schizophrenia: reconciling discrepant findings. Schizophrenia Research. 2003;60(2–3):285–298. doi: 10.1016/s0920-9964(02)00294-3. 12591590 [DOI] [PubMed] [Google Scholar]
- Menon V., Anagnoson R.T., Mathalon D.H., Glover G.H., Pfefferbaum A. Functional neuroanatomy of auditory working memory in schizophrenia: relation to positive and negative symptoms. Neuroimage. 2001;13(3):433–446. doi: 10.1006/nimg.2000.0699. 11170809 [DOI] [PubMed] [Google Scholar]
- Milev P., Ho B.-C., Arndt S., Andreasen N.C. Predictive values of neurocognition and negative symptoms on functional outcome in schizophrenia: a longitudinal first-episode study with 7-year follow-up. American Journal of Psychiatry. 2005;162(3):495–506. doi: 10.1176/appi.ajp.162.3.495. 15741466 [DOI] [PubMed] [Google Scholar]
- Minzenberg M.J., Laird A.R., Thelen S., Carter C.S., Glahn D.C. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Archives of General Psychiatry. 2009;66(8):811–822. doi: 10.1001/archgenpsychiatry.2009.91. 19652121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myin-Germeys I., Krabbendam L., Jolles J., Delespaul P.A., van Os J. Are cognitive impairments associated with sensitivity to stress in schizophrenia? An experience sampling study. American Journal of Psychiatry. 2002;159(3):443–449. doi: 10.1176/appi.ajp.159.3.443. 11870009 [DOI] [PubMed] [Google Scholar]
- Myin-Germeys I., Oorschot M., Collip D., Lataster J., Delespaul P., van Os J. Experience sampling research in psychopathology: opening the black box of daily life. Psychological Medicine. 2009;39(9):1533–1547. doi: 10.1017/S0033291708004947. 19215626 [DOI] [PubMed] [Google Scholar]
- Myin-Germeys I., van Os J. Stress-reactivity in psychosis: evidence for an affective pathway to psychosis. Clinical Psychology Review. 2007;27(4):409–424. doi: 10.1016/j.cpr.2006.09.005. 17222489 [DOI] [PubMed] [Google Scholar]
- Nishimura Y., Takizawa R., Muroi M., Marumo K., Kinou M., Kasai K. Prefrontal cortex activity during response inhibition associated with excitement symptoms in schizophrenia. Brain Research. 2011;1370(0):194–203. doi: 10.1016/j.brainres.2010.11.003. 21059348 [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Gross J.J. The cognitive control of emotion. Trends in Cognitive Sciences. 2005;9(5):242–249. doi: 10.1016/j.tics.2005.03.010. 15866151 [DOI] [PubMed] [Google Scholar]
- Ochsner K.N., Silvers J.A., Buhle J.T. Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Annals of the New York Academy of Sciences. 2012;1251(1):E1–E24. doi: 10.1111/j.1749-6632.2012.06751.x. 23025352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oorschot M., Kwapil T., Delespaul P., Myin-Germeys I. Momentary assessment research in psychosis. Psychological Assessment. 2009;21(4):498–505. doi: 10.1037/a0017077. 19947784 [DOI] [PubMed] [Google Scholar]
- Perlstein W.M., Carter C.S., Noll D.C., Cohen J.D. Relation of prefrontal cortex dysfunction to working memory and symptoms in schizophrenia. American Journal of Psychiatry. 2001;158(7):1105–1113. doi: 10.1176/appi.ajp.158.7.1105. 11431233 [DOI] [PubMed] [Google Scholar]
- Pessoa L. On the relationship between emotion and cognition. Nature Reviews. Neuroscience. 2008;9(2):148–158. doi: 10.1038/nrn2317. 18209732 [DOI] [PubMed] [Google Scholar]
- Quirk G.J., Beer J.S. Prefrontal involvement in the regulation of emotion: Convergence of Rat and Human Studies. Current Opinion in Neurobiology. 2006;16(6):723–727. doi: 10.1016/j.conb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Rosenfarb I.S., Nuechterlein K.H., Goldstein M.J., Subotnik K.L. Neurocognitive vulnerability, interpersonal criticism, and the emergence of unusual thinking by schizophrenic patients during family transactions. Archives of General Psychiatry. 2000;57(12):1174–1179. doi: 10.1001/archpsyc.57.12.1174. 11115332 [DOI] [PubMed] [Google Scholar]
- Sanz J.H., Karlsgodt K.H., Bearden C.E., van Erp T.G.M., Nandy R.R., Ventura J., Cannon T.D. Symptomatic and functional correlates of regional brain physiology during working memory processing in patients with recent onset schizophrenia. Psychiatry Research. 2009;173(3):177–182. doi: 10.1016/j.pscychresns.2009.02.008. 19692211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbersweig M.D.D., Clarkin P.D.J., Goldstein M.D.M., Kernberg M.D.O., Tuescher M.D.P.D.O., Levy P.D.K., Brendel G. Failure of frontolimbic inhibitory function in the context of negative emotion in borderline personality disorder. American Journal of Psychiatry. 2007;164(12):1832–1841. doi: 10.1176/appi.ajp.2007.06010126. 18056238 [DOI] [PubMed] [Google Scholar]
- Simon J.R., Berbaum K. Effect of conflicting cues on information processing: the ‘Stroop effect’ vs. the ‘Simon effect’. Acta Psychologica. 1990;73(2):159–170. doi: 10.1016/0001-6918(90)90077-s. 2343770 [DOI] [PubMed] [Google Scholar]
- Subramaniam K., Luks T.L., Garrett C., Chung C., Fisher M., Nagarajan S., Vinogradov S. Intensive cognitive training in schizophrenia enhances working memory and associated prefrontal cortical efficiency in a manner that drives long-term functional gains. Neuroimage. 2014;99:281–292. doi: 10.1016/j.neuroimage.2014.05.057. 24867353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thewissen V., Bentall R.P., Lecomte T., van Os J., Myin-Germeys I. Fluctuations in self-esteem and paranoia in the context of daily life. Journal of Abnormal Psychology. 2008;117(1):143–153. doi: 10.1037/0021-843X.117.1.143. 18266492 [DOI] [PubMed] [Google Scholar]
- Trull T.J., Ebner-Priemer U.W. Using experience sampling methods/ecological momentary assessment (ESM/EMA) in clinical assessment and clinical research: introduction to the special section. Psychological Assessment. 2009;21(4):457–462. doi: 10.1037/a0017653. 19947780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ursu S., Kring A.M., Gard M.G., Minzenberg M.J., Yoon J.H., Ragland J.D., Carter C.S. Prefrontal cortical deficits and impaired cognition-emotion interactions in schizophrenia. American Journal of Psychiatry. 2011;168(3):276–285. doi: 10.1176/appi.ajp.2010.09081215. 21205806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Veelen N.M.J., Vink M., Ramsey N.F., Kahn R.S. Left dorsolateral prefrontal cortex dysfunction in medication-naive schizophrenia. Schizophrenia Research. 2010;123(1):22–29. doi: 10.1016/j.schres.2010.07.004. 20724113 [DOI] [PubMed] [Google Scholar]
- Vercammen A., Morris R., Green M.J., Lenroot R., Kulkarni J., Carr V.J., Weickert T.W. Reduced neural activity of the prefrontal cognitive control circuitry during response inhibition to negative words in people with schizophrenia. Journal of Psychiatry & Neuroscience: JPN. 2012;37(6):379–388. doi: 10.1503/jpn.110088. 22617625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D., Clark L.A., Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. Journal of Personality and Social Psychology. 1988;54(6):1063–1070. doi: 10.1037//0022-3514.54.6.1063. 3397865 [DOI] [PubMed] [Google Scholar]
- Weissman M.M., Prusoff B.A., Thompson W.D. Social adjustment by self-report in a community sample and in psychiatric outpatients. Journal of Nervous and Mental Disease. 1978;166(5):317–326. doi: 10.1097/00005053-197805000-00002. 650195 [DOI] [PubMed] [Google Scholar]
- Whitfield-Gabrieli S. 2009. Artifact Detection Tools (ART) software package. [Google Scholar]
- Yager J.A., Ehmann T.S. Untangling social function and social cognition: a review of concepts and measurement. Psychiatry. 2006;69(1):47–68. doi: 10.1521/psyc.2006.69.1.47. 16704332 [DOI] [PubMed] [Google Scholar]
- Yoon J.H., Minzenberg M.J., Ursu S., Ryan Walter B.S., Walters R., Wendelken C., Ragland J.D. Association of dorsolateral prefrontal cortex dysfunction with disrupted coordinated brain activity in schizophrenia: relationship with impaired cognition, behavioral disorganization, and global function. American Journal of Psychiatry. 2008;165(8):1006–1014. doi: 10.1176/appi.ajp.2008.07060945. 18519527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlomke K.R., Hahn K.S. Cognitive emotion regulation strategies: gender differences and associations to worry. Personality and Individual Differences. 2010;48(4):408–413. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary methods and tables.


