Abstract
The neurobiological underpinnings of effort-related monetary reward processing of gambling disorder have not been previously studied. To date neuroimaging studies lack in large sample sizes and as a consequence less attention has been given to brain reward processing that could potentially be attributed to comorbid conditions such as depressive mood state. We assessed monetary reward processing using an effort-dependent task during 3 tesla functional magnetic resonance imaging. We investigated a large sample of male, right-handed, slot-machine-playing disordered gamblers (DGs; N = 80) as well as age- and smoking-matched male healthy controls (HCs; N = 89). Depressive symptoms were assessed using the Beck Depression Inventory (BDI). DGs and HCs were divided into subgroups (“high” and “low”) based on their BDI scores. Effort-related monetary reward processing did not differ between the complete groups of HCs and DGs. Brain activation during receipt of monetary reward though revealed a significant Group × BDI interaction: DGs with higher BDI scores compared to DGs with lower BDI scores showed greater brain activity in the right insula cortex and dorsal striatum while no differences were observed for HCs with higher versus lower BDI scores. Our results suggest that effort-related aspects of monetary motivation, i.e. when monetary output is tied to performance, are not altered in DG. Additionally, our findings strengthen the need for subgroup comparisons in future investigations of the disorder as part of a personalized medicine approach.
Keywords: Gambling disorder, Depressive mood, Monetary reward, Dorsal striatum, Insula, fMRI
Highlights
-
•
We studied effort-related monetary reward processing in disordered gamblers (DGs).
-
•
Anticipatory and feedback-related activity did not differ between DGs and controls.
-
•
Impact of depressive symptoms on feedback-related brain activity in DGs.
-
•
DGs with higher BDI scores showed increased insula and dorsal striatum activity.
-
•
Results highlight the need of subgroup comparisons in gambling disorder.
1. Introduction
Gambling disorder (GD) is a common psychiatric disorder, with lifetime prevalence estimates of almost 1.0% for Germany (Erbas et al., 2012) and between 0.2% and 3.5% worldwide (Kessler et al., 2008; Stucki et al., 2007). GD is characterized by persistent and recurrent maladaptive gambling behaviors. These include loss of control over gambling as well as preoccupation with gambling or with obtaining money with which to gamble (continuously or periodically). In addition, disordered gamblers (DGs) exhibit irrational thinking and failure to change their behavior despite adverse consequences (American Psychiatric Association, 2003). GD (referred to as pathological gambling in the DSM-IV) has been recently reclassified in the 5th DSM edition under the category of “Addictions and Related Disorders” because of similarities to substance use disorder (SUD) (i.e. genetic predisposition, treatment response, clinical characteristics, cognitive deficits and underlying neurobiological mechanisms; e.g. Petry et al., 2006; Potenza et al., 2006; Shaffer et al. 2004; Goudriaan et al., 2004).
Neuroimaging studies evaluating monetary-reward processing in DGs have found altered brain responses in DGs during processing of gambling-associated stimuli (cue-reactivity) (e.g. Crockford et al., 2005; Potenza et al., 2003b; van Holst et al., 2012a; Goudriaan et al., 2010); risky decision making (e.g. Tanabe et al., 2007); inhibitory control (Potenza et al., 2003a); presentation of non-monetary reward, such as personally relevant stimuli (e.g. de Greck et al., 2010); probability and delay discounting of monetary reward (e.g. Miedl et al., 2012) as well as processing of monetary gains and losses (Miedl et al., 2012; Sescousse et al., 2013; Miedl et al., 2010; Reuter et al., 2005). These studies evaluated probability- or delay-modulated effects on monetary-reward processing or used gambling-related tasks involving risk or uncertainty elements. Monetary reward processing has been shown to be altered not only by delay and risk costs in healthy individuals (e.g. Prevost et al., 2010; Burke et al., 2013) but also by physical effort required to obtain the reward (i.e. motivation) (e.g. Bühler et al., 2010). Outside a gambling context, financially motivated behaviors are often characterized by a high degree of behavioral effort and work output. To date it is not known whether DGs show a deficit in such effort-related aspects of monetary motivation.
GD is often accompanied by other mental disorders, with mood disorders and substance use disorders (SUD) being the most frequently occurring comorbid psychiatric Axis I disorders (Kessler et al., 2008; Petry et al., 2005). The majority of studies have found 12-month comorbid mood disorders (mainly depression) to be around 50% (Kessler et al., 2008; Petry et al., 2005; Kim et al., 2006). Several lines of evidence suggest that DGs suffering from depressive mood state might not have two discrete and unrelated conditions, but should instead be considered as an important subgroup of DGs. There are several points supporting this reasoning. First, depressive symptoms have been successfully used to predict both the urge to gamble and the duration of gambling in DGs (Romer-Thomsen et al., 2009), indicating DGs with more severe depressive mood state to have more severe gambling symptoms. Second, GD and major depression have been found to have a shared genetic provenance in men, suggesting common etiological mechanisms (Potenza et al., 2005). Third, a single conceptual model that applies universally to all DGs can hardly account for the heterogeneity observed in GD (Milosevic et al., 2010; Blaszczynski and Nower, 2002).
To our knowledge neuroimaging studies to date apart from lacking in large sample sizes (usually N < 20; e.g. Balodis et al., 2011, 2012; Goudriaan et al., 2010; Crockford et al., 2005; Potenza et al., 2003a; Miedl et al., 2012; vanHolst et al., 2012a; de Ruiter et al., 2012; Choi et al., 2012; Habib et al., 2010; de Ruiter et al., 2009; Reuter et al., 2005) they also do not account for brain reward processing alterations that could potentially be attributed to comorbid conditions such as depressive mood state. Neuroimaging studies on monetary reward processing in depression alone show differences in depressed versus non-depressed participants especially for receipt for monetary reward (feedback phase) (Knutson et al., 2008; Pizzagalli et al. (2008)). For example, findings indicate blunted activation in depression at receipt for monetary reward in the left putamen but not for the anticipation phase (Pizzagalli et al. (2008)). In a similar fashion, another investigation found decreased brain activation including parts of the frontal cortex as well as the putamen and insula in the patient group (Knutson et al., 2008). It remains unknown how and whether comorbid depressive symptoms in DGs impact reward processing.
The aim of the current study was to assess effort-dependent monetary reward processing in a large group of DGs and HCs using an instrumental-motivation fMRI task without any gambling-like features (the task involved no chance or uncertainty). Assessing whole brain activation we hypothesized that gamblers will show altered reward processing brain activity compared to healthy controls. Additionally, we examined the impact of depressive mood state in a statistically-powered way, acknowledging that way the heterogeneity of DG (e.g. Milosevic et al., 2010; Blaszczynski and Nower, 2002). Since DGs with comorbid depressive symptoms represent a distinct subgroup of GD characterized by elevated gambling severity, we anticipated finding neurobiological brain responses that would be specific to elevated depressive mood in particular for feedback of monetary reward as shown by studies on depression.
2. Material and methods
2.1. Subjects
The assessment took place within the scope of the Baden-Württemberg study on GD, which was financially supported by the Ministry for Work and Social Affairs (Ministerium für Arbeit und Sozialordnung, Familien und Senioren), Baden-Württemberg, Germany. DGs were either recruited from three different centers in Germany (Central Institute of Mental Health in Mannheim (CIMH) (inpatient treatment/day clinic), Münzesheim and Münchwies (inpatient treatment)). HCs were recruited using advertisements in local newspapers as well as from a departmental pool of volunteers. The assessment took place at the CIMH. Data (neuroimaging, socio-demographic and psychometric) from 80 DGs and 89 HCs were included in the statistical analysis. All participants were male and right handed according to the Edinburgh Handedness Inventory (Edinburgh Handedness Inventory; Oldfield et al., 1971). Participants needed to fulfill the standard requirements for fMRI assessments (no metal implants, pacemaker, etc.). All participants' vision was either normal or corrected to normal. Inclusion criteria for DGs were a diagnosis of pathological gambling according to the DSM-IV criteria (≥5 affirmative answers) and a minimum score of 5 on the South Oaks Gambling Screen [(SOGS (Lesieur and Blume, 1987)]. In the manuscript we will make use of the new DSM-V terminology DGs as the label “pathological” is a pejorative term that only reinforces the social stigma of being a problem gambler. Subjects were only assigned to the HC group if they scored 2 or below on the SOGS. Exclusion criteria that applied to both groups included positive urine drug screen on the day of scanning, current use of psychotropic medications, any major physical disorders, acute psychosis as well as inadequate German-language communication skills. HCs were excluded if they were diagnosed with any Axis I psychiatric disorder, according to DSM-IV (within the past 12 months, except nicotine dependence and specific phobias), using a psychiatric interview (SCID-I). Detailed face-to-face interviews were conducted to acquire demographic information and gambling-related behavioral characteristics. Psychiatric disorders including SUD were diagnosed based on the DSM-IV criteria as part of detailed clinical assessments using SCID-I. A new variable was created for the purposes of our analysis including yes/no answers. We administered the South Oaks Gambling Screen (SOGS; Lesieur and Blume, 1987) and the Yale–Brown Obsessive Compulsive Scale adapted for Pathological Gambling (PG-YBOCS) (Pallanti et al., 2005) to assess gambling severity. Depressive mood state was assessed using the Beck Depression Inventory (BDI IA; Beck et al., 1961). Anxiety was measured using the State Trait Anxiety Inventory (STAI; Spielberger et al., 1993). In addition, the Fagerström Test for Nicotine Dependence (Heatherton et al., 1991) was used to investigate smoking severity. A summary of participants' scores on demographic and clinical measures is provided in Table 1. The study was approved by the ethics committee of the Mannheim Medical Faculty of Heidelberg University (Ref: 2009-207N-MA), and all participants provided written informed consent prior to participating.
Table 1.
Group comparisons of demographic and clinical characteristics.
| HCs (N = 89) |
DGs (N = 80) |
DGshigh BDI (N = 43) | DGslow BDI (N = 37) |
Sign.a | |
|---|---|---|---|---|---|
| Age (years) | 36.2 (9.4) |
37.4 (9.1) |
t = –.84 p = .403 |
||
| 37.2 (9.5) |
37.6 (8.7) |
t = .18 p = .86 |
|||
| Depts (€) | 787 (742) |
28.828 (47.227) |
t = –5.52* p < .0005 |
||
| 33.962 (50.063) |
23.544 (44.236) |
t = –.92 p = .36 |
|||
| SOGS | .2 (.5) |
11.1 (3) |
t = –33.48* p < .00025 |
||
| 11.7 (2.7) |
10.4 (3.3) |
t = –1.73* p = .044 |
|||
| FTND | .9 (2.1) |
4.5 (2.8) |
t = –9.19* p < .0005 |
||
| 4.8 (2.6) |
4.2 (2.9) |
t = –.9 p = .38 |
|||
| Mean # of hours played per day | 0 | 4.5 (2.4) |
t = –17.78* p < .00025 |
||
| 5.2 (2.4) |
3.8 (2.2) |
t = –2.49* p = .0075 |
|||
| Max # of hours played per day | 0 | 11 (5.8) |
t = –17.97* p < .00025 |
||
| 13.1 (6.3) |
8.8 (4.2) |
t = –3.30* p = .001 |
|||
| PG-YBOCS: sum | 2.1 (3.3) |
18.7 (8.8) |
t = –16.46* p < .00025 |
||
| 22.5 (7.1) |
14.6 (8.6) |
t = –4.17* p < .00025 |
|||
| PG-YBOCS: thought | 0.9 (1.7) |
9.1 (4.3) |
t = –16.31* p < .00025 |
||
| 10.8 (3.9) |
7.2 (3.9) |
t = –3.81* p < .00025 |
|||
| PG-YBOCS: impulse | 1.2 (1.8) |
9.7 (4.8) |
t = –15.48* p < .00025 |
||
| 11.6 (3.8) |
7.7 (4.9) |
t = –3.74* p < .00025 |
|||
| Anxiety (STAI) | 34.3 (7.9) |
45.1 (12) |
t = –6.95* p < .0005 |
||
| 51.5 (10.8) |
37.5 (8.6) |
t = –6.34* p < .0005 |
|||
| Depression (BDI) | 5.2 (4.9) |
14.5 (9.1) |
t = –8.36* p < .0005 |
||
| 20.9 (7.6) |
7.1 (3.2) |
t = –10.23* p < .0005 |
DGs: Disordered gamblers; HCs: Healthy controls; SOGS: South Oaks Gambling Screen; PG-YBOCS: Yale–Brown Obsessive Compulsive Scale adapted for Pathological Gambling; STAI: State Trait Anxiety Inventory; BDI: the Beck Depression Inventory; FTND: Fagerström Test for Nicotine Dependence (Heatherton et al., 1991);
Sign.: Significance: two-sample t-test (one-sided for variables with a-priori hypotheses, including SOGS, YBOCS, mean/max # of hours played per day); standard deviation is shown in parenthesis.
p < 0.05.
2.2. Study design/subgroup definition
We defined subgroups of DGs and HCs using a median split of the overall BDI score. Considering that the HCs' BDI scores showed no clinical relevance to depression, a median split was selected as an appropriate approach compared to using clinical/non-clinical BDI cut-off scores. The median BDI score for the patient group was 12. DGs with a BDI < 12 were assigned to the “BDI low” group (N = 37; 7.1 ± 3.2) and DGs with a BDI ≥ 12 were assigned to the “BDI high” group (N = 43; 20.9 ± 7.6). The median BDI score for the control group was 4. HCs with a BDI < 4 were assigned to the “BDI low” group (N = 41; 1.8 ± .8) and HCs with a BDI ≥ 4 to the “BDI high” group (N = 48; 8.1 ± 7). The distribution of the BDI scores in HCs and DGs is provided in the supplementary material (see Fig. S1). Please note that the average score for the BDI high group is in a lower range than the usual clinical values.
2.3. Psychometric data analysis
Psychometric and behavioral data were analyzed using SPSS (version 20.0, IBM Corp., Somers, NY, USA). We used t-tests to reveal any differences in demographic and clinical indicators between HCs and DGs, as well as DGs with “high” vs. “low” BDI scores (Table 1). The relationships between BDI scores and SOGS scores, YBOCS measures (sum, thought, impulses) as well as gambling duration were assessed using Pearson's correlation analysis. The level of significance was again set to p < 0.05 uncorrected. The choice of an uncorrected threshold was justified by the exploratory nature of this analysis.
2.4. Instrumental-motivation task
During the fMRI session, participants completed an effort-dependent event-related instrumental-motivation task (Bühler et al., 2010; Fig. 1). The task is designed to measure brain activity to stimuli predicting increasing levels of monetary reward, together with behavioral assessment of subsequent instrumental responding to obtain the respective reward on a trial-by-trial basis. The motivational task used in the current investigation has been adopted from the Bühler et al. (2010) study. The use of the particular task allows for brain activity measurement for stimuli predicting monetary reward. There is also behavioral assessment of successive instrumental responding so as to obtain the reward on a trial-by-trial basis. Physical effort is thus used as a measure of motivation. The current version of the task consisted of 48 trials with 4 reward levels presented 12 times each, displayed in a pseudorandom order with total task duration of 15:59 min. Participants, prior to entering the scanner, had to complete a practice session consisting of eight trials to learn how to perform the task. The scanning session would then begin with a test run of eight trials to assess the individual maximum response speed under scanning conditions. This was defined as the maximum of achieved number of button presses in a trial during the test run. We then used this information to standardize the cumulative gain to about €30 in the subsequent main run irrespective of inter-individual performance differences.
Fig. 1.

Graphical representation of the effort-dependent instrumental-motivation task employed in this study and fMRI task effects in healthy controls (HCs) and disordered gamblers (DGs).
At the beginning of each trial, subjects were shown the reward magnitude/level (0, 1, 10, and 100) which was represented by a block with a red line placed inside (anticipation phase). The higher the position of the red bar, the higher the reward level. Participants apart from visually attending each cue, only needed to stay still and do nothing during this phase. The anticipation phase lasted for 3 s. A 2 s fixation period followed during which period subjects had to inhibit any button press responses. If they failed to do so then the particular trials were characterized as invalid due to premature motor response (button press). Thereafter, the motor response phase began during which subjects viewed an image with an exclamation mark in the middle. This 3 s interval was when participants had to respond by pressing a button; amount won in the end solely depended on the number of button presses accomplished in this phase. Feedback phase then followed (3 s) which indicated not only the end of the trial but also the amount participants had managed to win in the particular trial as well as the cumulative amount up to that trial. Participants won higher amounts of money the more button presses they committed to. The reward per trial was defined by multiplying the number of button presses in the trial by the reward level. This means that the amount won at the end increased with higher effort and reward level. An individual reward unit was created. The result then was multiplied by the individual reward element using the following equation: ru = Rc/bmax • ΣLi consisting of the standard cumulative gain of €30 (Rc), the individual number of maximum button presses in the test run (bmax), times the reward level (Li). In trials where the reward level was 0, no matter how many button presses participants committed to, the monetary gain never exceeded 0.
2.5. fMRI instrumental-response data analysis
We averaged the number of button presses for each trial type. We subjected these means to repeated-measures analysis of variance (ANOVA) 2 × 2 × 4 with group (HCs and DGs) and BDI (“high” and “low”) as the between-subject factors and reward level (0, 1, 10 and 100) as the within-subject factor.
2.6. fMRI data acquisition
Scanning was performed using a 3 T whole-body Siemens scanner (MAGNETOM Trio, TIM-technology; Siemens, Erlangen, Germany). We used a tilted plane of acquisition (30° to the anterior commissure–posterior commissure line, rostral > caudal) to reduce signal dropout in orbitofrontal regions. Forty-two slices were acquired in a descending order (2 mm and an interslice gap of 1 mm) using a gradient-echo T2*-weighted sequence (EPI) with an in-plane resolution of 64 × 64 pixels (FOV 192 × 192 mm) and with the following parameters: TR = 2.41 s; TE = 0.025 s; α = 80°. Three hundred ninety-six volumes were acquired for each subject, covering the entire brain. Visual stimuli were presented via magnetic resonance imaging audio/video system goggles (Resonance Technology, Inc., Los Angeles, California). In order to present the tasks and record participants' behavioral responses, we used Presentation® software (Version 9.9, Neurobehavioral Systems, Inc, Albany, CA, USA).
2.7. fMRI data analysis
Preprocessing and statistical analysis of the neuroimaging data were carried out using Statistical Parametric Mapping 8 (SPM; Wellcome Trust Centre for Neuroimaging, London). The first five images of each data set were discarded in order to reduce T1 saturation effects. Slice time correction was performed in order to minimize temporal differences in slice acquisition. All individual data were spatially realigned to correct for head movement. The first functional T2* image was normalized to a standard EPI template (MNI brain) using a 12-parameter affine transformation with additional non-linear components. The same non-linear transformation was subsequently applied to all functional T2* data and voxels were re-sampled at a resolution of 2 × 2 × 2 mm. The functional data were smoothed using an isotropic Gaussian kernel for group analysis (8 mm full width at half maximum). Subjects with transformation parameters >3 mm and rotation >3° were removed from the analysis. In addition, we inspected the mask image of each subject and excluded those participants whose mask image did not have sufficient brain coverage.
First level statistics of the pre-processed fMRI data were conducted by modeling the four monetary reward levels as explanatory variables for the reward anticipation, motor response and feedback phase within the context of the general linear model on a voxel-by-voxel basis. The hemodynamic responses for the anticipation and feedback phase were modeled as single events (delta functions) convolved with a synthetic hemodynamic response function. The motor-response phase was modeled as a short box-car function with a 3 s duration convolved with a synthetic hemodynamic-response function. We chose this modeling technique because we expected sustained activity for the motor-response phase. This resulted in 12 regressors (4 conditions × 3 phases). Realignment parameters (6 in total) were also added to the model. Individual contrast images modeling a parametric linear increase in reward level during reward anticipation, motor responding and feedback (contrast: −1.5, −0.5, 0.5, 1.5) were generated and subsequently included in one-sample t-tests. A linear model was chosen as it has been previously shown to be a good model fit (Bühler et al., 2010).
Individual brain activation contrast-maps for each task phase (reward anticipation, motor responding and feedback) were entered to a full factorial model in SPM8 with factors group (HCs and DGs) and BDI (“low” and “high”). Main effect of group (DGs vs. HCs), interaction effects (Group × BDI) as well as differences between BDI “high” and “low” DGs as well as between BDI “high” and “low” HCs were assessed for reward anticipation, motor responding and feedback. For main effect of group and interaction effects we applied a threshold of p < .001 whole brain voxel-wise uncorrected (minimum cluster size of 20 adjacent voxels). For planned contrasts between subgroups (DGs with “high” vs. “low” BDI, HCs with “high” vs. “low” BDI) we used a p < .05 family-wise error (FWE) whole brain corrected threshold (minimum cluster size of 20 adjacent voxels). To identify the anatomical brain regions, we used the Automated Anatomical Labelling (AAL) atlas (Tzourio-Mazoyer et al., 2002).
3. Results
3.1. Behavioral-response data
The instrumental-response rates in the motivation task (i.e. the number of button presses in the motor-response phase) increased as reward levels increased (main effect of reward level;F(3, 495) = 415.14, p < .001) indicating the motivation task to be working as expected. We found no significant differences between DGs and HCs (main effect Group; [F(1, 165) = 2.85, p = .093]) and individuals with a “high” vs. “low” BDI [main effect BDI; (F(1, 165) = .09, p = .767)] nor a significant interaction between the two factors [F(1, 165) = .04, p = .842]. For controls and gamblers the mean and standard deviations for button presses for the different reward levels are presented in Table 2.
Table 2.
Number of button presses in the motor-response phase for HCs and DGs with low and high BDIs.
| BDI low mean ± SD | BDI high mean ± SD | Total mean ± SD | |
|---|---|---|---|
| 0 reward | |||
| HCs | 6.4 ± 6.9 | 4.6 ± 5.8 | 5.4 ± 6.3 |
| DGs | 5.2 ± 5.1 | 5.4 ± 5.3 | 5.3 ± 5.2 |
| 1 reward | |||
| HCs | 14.9 ± 5.5 | 15.1 ± 6 | 15.0 ± 5.7 |
| DGs | 13.6 ± 3.9 | 12.7 ± 6.7 | 13.1 ± 5.6 |
| 10 reward | |||
| HCs | 17.7 ± 2.4 | 18.3 ± 2.8 | 18 ± 2.6 |
| DGs | 17.3 ± 2.2 | 17.1 ± 3.8 | 17.2 ± 3.1 |
| 100 reward | |||
| HCs | 18.2 ± 2.4 | 19 ± 2.4 | 18.6 ± 2.4 |
| DGs | 18.3 ± 2.5 | 18.1 ± 3.7 | 18.2 ± 3.2 |
3.2. Psychometric data
A detailed description of demographic and clinical characteristics of the groups assessed is provided in Table 1. We found no differences in SUD (alcohol, nicotine, other) between the BDI “low” and BDI “high” DGs [for smoking, χ2 (1, N = 80) = .87, p = .351; for alcohol, χ2 (1, N = 80) = 0.61, p = .434; for other substances, χ2 (1, N = 80) = 1.06, p = .304].
In DGs, BDI scores correlated significantly with the mean number of hours played per day (r = .41, p < .001), maximum number of hours played per day (r = .37, p = .001), SOGS score (r = .21, p = .044), YBOCS sum score (r = .48, p < .001), YBOCS thought score (r = .47, p < .001), and YBOCS impulse score (r = .42, p < .001).
3.3. Functional imaging data
3.3.1. Task-related brain activity
To check that the task was working as expected, we assessed the parametric influence of reward magnitude on anticipation, response and feedback for both HCs and DGs. Task-related results were in line with a previous finding of our research group using this task (Bühler et al., 2010) (Fig. 1). Please also see supplementary material (see Figs. S2 and S3a–c).
3.4. Group differences
To assess group differences in the full factorial model, we compared the parametric influence of reward magnitude on anticipation, response and feedback between HCs and DGs using a p = .001 uncorrected threshold. We found no differences between the groups for reward anticipation (no main effect of group or BDI and no interaction).
For the motor response phase we found a significant interaction that revealed clusters in the left olfactory gyrus and inferior frontal gyrus [whole brain p = .001 uncorrected, k = 20; left olfactory (x = −2, y = 22, z = –2) F = 16.63, k = 60; left inferior frontal (x = −26, y = 40, z = –8) F = 13.81, k = 49]. No difference was found when comparing “high” vs. “low” DGs for the motor response phase in post-hoc comparisons using FWE correction. We also found no differences in task-related brain-activity during motor responding for HCs with a “high” and “low” BDI.
Brain activation during feedback phase revealed a significant Group × BDI interaction and a BDI main effect. The BDI main effect revealed three clusters including the left insula [(x = −32, y = 6, z = 16) F = 17.10, k = 33], right insula [(x = 34, y = 2, z = 16) F = 15.73, k = 62] and right putamen [(x = 32, y = −12, z = 12) F = 14.08, k = 23] whereas the interaction showed greater brain BOLD response revealing three clusters including the right insula and putamen as well as the inferior occipital gyrus [whole brain p = .001 uncorrected, k = 20] (Fig. 2).
Fig. 2.

Group × BDI interaction for brain activation during monetary feedback in the instrumental-motivation task.
Pairwise comparisons were applied thereafter to determine which group activations differed. We found that the interaction was driven by the following pattern. BDI “high” DGs had significantly higher brain activation at feedback than the BDI “low” DGs in the insular cortex (x = 34, y = 2, z = 16, k = 24, t = 5.75, p = .010, FWE) and dorsal striatum (x = 26, y = −6, z = –8, k = 14, t = 5.55, p = .016, FWE) (Table 3, Fig. 3). No brain area was significantly more activated for BDI “low” DGs than for BDI “high” DGs.
Table 3.
Feedback-related brain activity in response to monetary reward during the instrumental-motivation task: DGs with “high” BDI versus DGs with “low” BDI.
| Local maximum |
||||||
|---|---|---|---|---|---|---|
| Brain area | Side | Cluster size | MNI |
t-Value | ||
| x | y | z | ||||
| Insula | R | 25 | 34 | 2 | 16 | 5.75 |
| Dorsal striatum (putamen) |
R | 14 | 26 | −6 | −8 | 5.51 |
p < .05 (FWE) whole brain; MNI: Montreal Neurological Institute; R: right.
Fig. 3.
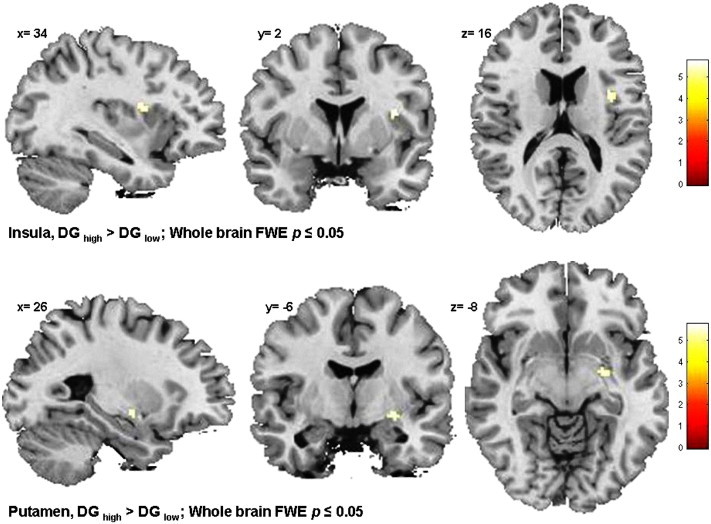
Feedback-related brain activity in DGs with “high” BDI versus DGs with “low” BDI.
In a second model, gambling severity (SOGS) was included as covariate in the group comparison of BDI “high” versus BDI “low” DG groups to assess which of the group differences in brain activity survive controlling for differences in severity of gambling addiction (SOGS). The same pattern of brain activation was found with two significant clusters (whole brain, p < .05, FWE) on the right insula cortex (x = 36, y = 0, z = 18, k = 27, t = 5.94, p = .008) and right dorsal striatum (x = 26, y = −6, z = –8, k = 11, t = 5.48, p = .019). Analysis was also repeated using FTND as covariate for the same group comparison (DG BDI “high” vs. BDI “low” groups). Results confirmed the same finding for whole brain (FWE p < .05; insula x = 36, y = 0, z = 18, t = 5.71, p = .015, k = 15 and DS x = 26, y = −6, z = –8, t = 5.62, p = .016, k = 14).
4. Discussion
We examined differences in effort-dependent monetary reward processing in a large group of disordered gamblers (DGs) and healthy controls (HCs) using an instrumental-motivation task without any gambling-like features (i.e. the task involved no chance or uncertainty). We found no brain activation differences for the anticipation, motor response and feedback phases between DGs and HCs. Response rates in the motor response phase of the task did not differ significantly between groups either.
Using primarily gambling-related tasks or tasks involving some sort of uncertainty about monetary outcome previous fMRI studies found significantly diminished DGs' fronto-striatal activation compared to HCs' for both unpredictable monetary gains and losses (e.g. Balodis et al., 2012; de Ruiter et al., 2009). Ventromedial activation has also been found to be reduced when participants were given positive reinforcement for their correct responses (monetary gain) and punished for giving incorrect answers (monetary loss) on a probabilistic reversal task (e.g. de Ruiter et al., 2009). By contrast, fMRI studies that varied the amount of risk involved (e.g. Miedl et al., 2010) or used tasks with different probabilities of winning or losing varying amounts of money (e.g. van Holst et al., 2012b) have found increased activity in the mesocorticolimbic brain regions. To explain these seemingly contradictory findings it has been suggested that under most conditions problem gamblers are characterized by a hypo-responsive reward circuitry. However, highly salient cues or reward anticipation could lead to enhanced attention that can enable normal to even levels of striatal activation (e.g. van Holst et al., 2012a).
Our results in a large group of patients and controls extend these previous findings by showing that although monetary reward processing with uncertainty or risk elements (e.g. delay, outcome probabilities) is altered in DG, no differences in effort-related monetary reward processing become obvious in the patient group compared to HCs. Outside a gambling context, effort-related aspects of monetary motivation seem not to be altered in DG. Given that we found no differences in cue-induced brain activity our results suggest that incentive salience of monetary cues is not increased in DGs. Additionally, DGs show no differences to HCs on the brain and on the behavioral level (button presses) in the motor response phase. The evaluation of monetary feedback was also comparable between groups. In summary all phases of monetary reward processing were comparable when receipt of reward purely depended on effort with no chance or gambling-like elements present.
Taking into account that DGs are a heterogeneous group who vary in their presentation of comorbid psychopathology (Milosevic et al., 2010; Blaszczynski and Nower, 2002), we also assessed the association between monetary reward processing in DGs and depressive mood state. We compared “low” vs. “high” scores on the BDI in DGs and HCs after performing a median split. Results indicated a difference specific to the right insula and dorsal striatum regions. Patients with higher BDI scores compared to those with “low” BDI scores exhibited greater right insula and dorsal striatum activity during feedback of monetary reward. Severity of gambling addiction and nicotine dependence did not affect these group differences. No difference was found for HCs with a “high” vs. “low” BDI.
As part of the mesolimbic reward system the dorsal striatum is relevant for decision-making by selecting and initiating actions through integration of motivational/emotional, cognitive and sensorimotor information (Koob et al., 2010). A growing body of evidence from animal as well as human studies suggests the dorsal part of the striatum to play a role in habitual responding and in initiating automatic stimulus-response tendencies (Everitt and Robbins, 2005; Vollstädt-Klein et al., 2010). Increased dorsal striatal activity in the BDI “high” compared to the BDI “low” DG groups might represent a tendency to continue with gambling following monetary reward (Everitt and Robbins, 2013).
The anterior insula cortex is known to be associated with interception of bodily states including conscious emotional feelings and drug urges (Critchley et al., 2004; Damasio et al., 2000). Neuroimaging studies in the addiction field have provided evidence of the insula's association with craving and drug urges (Naqvi et al., 2009). The insula is one of the brain areas activated when exposed to different drug related cues (i.e. alcohol, nicotine, opiates, or cocaine) (Garavan et al., 2000) and this structure also plays a role in relapse behavior (Paulus et al., 2005). Moreover, smokers with damage in this particular region due to stroke reported less subjective nicotine craving and were more likely to quit smoking (Naqvi et al., 2007). In a recent and well-designed study (Clark et al., 2014), the role of the insula in gambling has been highlighted in a way that distorted cognitive processing of near-miss outcomes seems to involve the insula. Increased activation in the anterior part of the insular cortex in the present study could highlight the importance of this particular region for comorbid psychiatric conditions such as a depressed mood state. When it comes to depression, research suggests volumetric alterations in this region in depressed individuals (Takahashi et al., 2010). In line with this we speculate that the observed insula activation in the DG BDI “high” group might also reflect a morphological insula alteration that forms the basis of the functional activation we see. Interestingly recent findings even go as far as to suggest that the insula metabolic rate in depressed individuals distinguishes those patients who will benefit from medication treatment and those who will only benefit from psychotherapy for the same condition (McGarth et al., 2013). Future studies have to explore whether increased activity in the insula cortex for gamblers with higher BDI score might be an indication that those individuals could potentially benefit more from pharmacological treatment for their condition.
Finally, our results revealed that DGs with more pronounced depressive symptoms are more severely dependent, spend more time gambling and experience more gambling-related craving. This is in line with previous findings, indicating that depressive symptoms in DGs predict both gambling urges and duration of gambling (Romer-Thomsen et al., 2009). Whether excessive gambling is applied by these patients as a kind of self-medication to relieve depressive symptoms or whether depressive symptoms are a consequence of excessive gambling and associated problems remains an open question. Available retrospective data suggest that depressive symptoms precede the onset of GD (Kessler et al., 2008). Excessive gambling might therefore be a strategy used to overcome a preexisting anhedonic state.
One limitation of the present study is the restriction of its sample to male participants. No conclusions can thus be drawn from our data concerning female gamblers. Moreover, although the median split was helpful in investigating the differences in gamblers with “high” and “low” depressive symptoms, future investigations should extend those findings and the design of this study by including an additional control sample with elevated depressive symptoms (i.e. high BDI scores). Furthermore we would like to mention that for the motor response FWE correction in the post-hoc test did not lead to significant differences. Lowering the statistical threshold to .001 uncorrected we found that DG with higher BDI scores hypo-activated in the inferior frontal gyrus and the right superior temporal gyrus. With regard to the inferior frontal gyrus we could argue that DGs with “high” BDI compared to DGs with “low” BDI are characterized by reduced inhibition. For this reason they might be less risk averse and more prone to engage in risky situations (Christopoulos et al., 2009). We think though that any interpretation of that finding would be too speculative considering that the threshold applied for the post-hoc comparison is low.
4.1. Conclusion
No differences in monetary reward processing between HCs and DGs were found using an instrumental-motivation task with no chance (gambling-like) elements during fMRI. Our result suggests that effort-related aspects of monetary motivation are not altered in DG, when monetary output is tied to performance.
Notably, DGs with higher compared to those with lower BDI scores exhibited greater brain activity in the insula and dorsal striatum, regions often associated with craving and addiction in general, while no differences were observed for HCs with higher versus lower BDI. Given our finding of a relationship between BDI scores and insula and dorsal striatum activity during effort-related monetary reward processing, future neuroimaging studies need to take into account the potential impact of comorbid depressive symptoms in GD.
Our results highlight the need for subgroup comparisons in future investigations of the disorder as well as the development and efficacy testing of subgroup specific treatment approaches as part of a personalized medicine approach.
Conflicts of interest
None of the authors report financial relationships with commercial interests or any other potential conflicts of interest. The study was supported financially by the Ministry for Work and Social Affairs (Ministerium für Arbeit und Sozialordnung, Familien und Senioren), Baden-Württemberg, Germany (reference number: 53-5072-7.1).
Acknowledgments
We would like to thank Helmut Nakovics for his advice on statistical aspects of this manuscript. We would also like to thank Dr. med. Monika Vogelgesang and colleagues from AHG Klinik Münchwies, Dr. med. Martin Beutel and colleagues from Therapiezentrum Münzesheim and Nina Kämmerer from CIMH for their assistance with patient recruitment and identification.
Contributor Information
Mira Fauth-Bühler, Email: mira.fauth-buehler@zi-mannheim.de.
Evangelos Zois, Email: evangelos.zois@zi-mannheim.de.
Sabine Vollstädt-Klein, Email: S.Vollstaedt-Klein@zi-mannheim.de.
Tagrid Lemenager, Email: tagrid.lemenager@zi-mannheim.de.
Martin Beutel, Email: martin.beutel@kraichtal-kliniken.de.
Karl Mann, Email: karl.mann@zi-mannheim.de.
Appendix A. Supplementary data
Supplementary material.
References
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. fourth edition. American Psychiatric Publishing; Washington, DC: 2003. [Google Scholar]
- Balodis I.M., Kober H., Worhunsky P.D., Stevens M.C., Pearlson G.D., Potenza M.N. Diminished frontostriatal activity during processing of monetary rewards and losses in pathological gambling. Biological Psychiatry. 2012;71:749–757. doi: 10.1016/j.biopsych.2012.01.006. 22336565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A.T., Ward C.H., Mendelson M., Mock J., Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. 13688369 [DOI] [PubMed] [Google Scholar]
- Blaszczynski A., Nower L. A pathways model of problem and pathological gambling. Addiction (Abingdon, England) 2002;97:487–499. doi: 10.1046/j.1360-0443.2002.00015.x. 12033650 [DOI] [PubMed] [Google Scholar]
- Bühler M., Vollstädt-Klein S., Kobiella A., Budde H., Reed L.J., Braus D.F. Nicotine dependence is characterized by disordered reward processing in a network driving motivation. Biological Psychiatry. 2010;67:745–752. doi: 10.1016/j.biopsych.2009.10.029. 20044075 [DOI] [PubMed] [Google Scholar]
- Burke C.J., Brünger C., Kahnt T., Park S.Q., Tobler P.N. Neural integration of risk and effort costs by the frontal pole: only upon request. Journal of Neuroscience: the Official Journal of the Society for Neuroscience. 2013;33:1706–1713. doi: 10.1523/JNEUROSCI.3662-12.2013. 23345243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark L., Studer B., Bruss J., Tranel D., Bechara A. Damage to insula abolishes cognitive distortions during simulated gambling. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:6098–6103. doi: 10.1073/pnas.1322295111. 24711387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J.S., Shin Y.C., Jung W.H., Jang J.H., Kang D.H., Choi C.H., Choi S.W., Lee J.Y., Hwang J.Y., Kwon J.S. Altered brain activity during reward anticipation in pathological gambling and obsessive-compulsive disorder. PLoS One. 2012;7(9):e45938. doi: 10.1371/journal.pone.0045938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopoulos G.I., Tobler P.N., Bossaerts P., Dolan R.J., Schultz W. Neural correlates of value, risk, and risk aversion contributing to decision making under risk. Journal of Neuroscience: the Official Journal of the Society for Neuroscience. 2009;29(40) doi: 10.1523/JNEUROSCI.2614-09.2009. 19812332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley H.D., Wiens S., Rotshtein P., Ohman A., Dolan R.J. Neural systems supporting interoceptive awareness. Nature Neuroscience. 2004;7(2):189–195. doi: 10.1038/nn1176. 14730305 [DOI] [PubMed] [Google Scholar]
- Crockford D.N., Goodyear B., Edwards J., Quickfall J., el-Guebaly N. Cue-induced brain activity in pathological gamblers. Biological Psychiatry. 2005;58:787–795. doi: 10.1016/j.biopsych.2005.04.037. 15993856 [DOI] [PubMed] [Google Scholar]
- Damasio A.R., Grabowski T.J., Bechara A., Damasio H., Ponto L.L., Parvizi J., Hichwa R.D. Subcortical and cortical brain activity during the feeling of self-generated emotions. Nature Neuroscience. 2000;3:1049–1056. doi: 10.1038/79871. 11017179 [DOI] [PubMed] [Google Scholar]
- de Greck M., Enzi B., Prösch U., Gantman A., Tempelmann C., Northoff G. Decreased neuronal activity in reward circuitry of pathological gamblers during processing of personal relevant stimuli. Human Brain Mapping. 2010;31:1802–1812. doi: 10.1002/hbm.20981. 20162606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Ruiter M.B., Veltman D.J., Goudriaan A.E., Oosterlaan J., Sjoerds Z., van den Brink W. Response perseveration and ventral prefrontal sensitivity to reward and punishment in male problem gamblers and smokers. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2009;34(4):1027–1038. doi: 10.1038/npp.2008.175. 18830241 [DOI] [PubMed] [Google Scholar]
- de Ruiter M.B., Oosterlaan J., Veltman D.J., van den Brink W., Goudriaan A.E. Similar hyporesponsiveness of the dorsomedial prefrontal cortex in problem gamblers and heavy smokers during an inhibitory control task. Drug Alcohol Depend. 2012;121(1–2):81–89. doi: 10.1016/j.drugalcdep.2011.08.010. [DOI] [PubMed] [Google Scholar]
- Erbas B., Buchner U.G. Pathological gambling: prevalence, diagnosis, comorbidity, and intervention in Germany. Deutsches Ärzteblatt International. 2012;109(10):173–179. doi: 10.3238/arztebl.2012.0173. 22470406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt B.J., Robbins T.W. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nature Neuroscience. 2005;8:1481–1489. doi: 10.1038/nn1579. 16251991 [DOI] [PubMed] [Google Scholar]
- Everitt B.J., Robbins T.W. From the ventral to the dorsal striatum: devolving views of their roles in drug addiction. Neuroscience and Biobehavioral Reviews. 2013;37:1946–1954. doi: 10.1016/j.neubiorev.2013.02.010. [DOI] [PubMed] [Google Scholar]
- Garavan H., Pankiewicz J., Bloom A., Cho J.K., Sperry L., Ross T.J. Cue-induced cocaine craving: neuroanatomical specificity for drug users and drug stimuli. American Journal of Psychiatry. 2000;157:1789–1798. doi: 10.1176/appi.ajp.157.11.1789. 11058476 [DOI] [PubMed] [Google Scholar]
- Goudriaan A.E., de Ruiter M.B., van den Brink W., Oosterlaan J., Veltman D.J. Brain activation patterns associated with cue reactivity and craving in abstinent problem gamblers, heavy smokers and healthy controls: an fMRI study. Addiction Biology. 2010;15:491–503. doi: 10.1111/j.1369-1600.2010.00242.x. 20840335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudriaan A.E., Oosterlaan J., de Beurs E., van den Brink W. Pathological gambling: a comprehensive review of biobehavioral findings. Neuroscience and Biobehavioral Reviews. 2004;28:124–141. doi: 10.1016/j.neubiorev.2004.03.001. 15172761 [DOI] [PubMed] [Google Scholar]
- Habib R., Dixon M.R. Neurobehavioral evidence for the "Near-Miss" effect in pathological gamblers. J Exp Anal Behav. 2010;93(3):313–328. doi: 10.1901/jeab.2010.93-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton T.F., Kozlowski L.T., Frecker R.C., Fagerström K.O. The Fagerström Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. 1932883 [DOI] [PubMed] [Google Scholar]
- Kessler R.C., Hwang I., LaBrie R., Petukhova M., Sampson N.A., Winters K.C., Shaffer H.J. DSM-IV pathological gambling in the National Comorbidity Survey Replication. Psychological Medicine. 2008;38:1351–1360. doi: 10.1017/S0033291708002900. 18257941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.W., Grant J.E., Eckert E.D., Faris P.L., Hartman B.K. Pathological gambling and mood disorders: clinical associations and treatment implications. Journal of Affective Disorders. 2006;92:109–116. doi: 10.1016/j.jad.2005.12.040. 16443282 [DOI] [PubMed] [Google Scholar]
- Knutson B., Bhanji J.P., Cooney R.E., Atlas L.Y., Gotlib I.C.H. Neural responses to monetary incentives in major depression. Biological Psychiatry. 2008;63(7):686–692. doi: 10.1016/j.biopsych.2007.07.023. 17916330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob G.F., Volkow N.D. Neurocircuitry of addiction. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. 19710631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesieur H.R., Blume S.B. The South Oaks gambling screen (SOGS): a new instrument for the identification of pathological gamblers. American Journal of Psychiatry. 1987;144:1184–1188. doi: 10.1176/ajp.144.9.1184. 3631315 [DOI] [PubMed] [Google Scholar]
- McGrath C.L., Kelley M.E., Holtzheimer P.E., Dunlop D.B.W., III, Craighead W.E., Franco A.R., Craddock R.C., Mayberg H.S. Toward a neuroimaging treatment selection biomarker for major depressive disorder. JAMA Psychiatry. 2013;70(8):821–829. doi: 10.1001/jamapsychiatry.2013.143. 23760393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miedl S.F., Fehr T., Meyer G., Herrmann M. Neurobiological correlates of problem gambling in a quasi-realistic blackjack scenario as revealed by fMRI. Psychiatry Research. 2010;181:165–173. doi: 10.1016/j.pscychresns.2009.11.008. 20138482 [DOI] [PubMed] [Google Scholar]
- Miedl S.F., Peters J., Büchel C. Altered neural reward representations in pathological gamblers revealed by delay and probability discounting. Archives of General Psychiatry. 2012;69:177–186. doi: 10.1001/archgenpsychiatry.2011.1552. 22310505 [DOI] [PubMed] [Google Scholar]
- Milosevic A., Ledgerwood D.M. The subtyping of pathological gambling: a comprehensive review. Clinical Psychology Review. 2010;30:988–998. doi: 10.1016/j.cpr.2010.06.013. 20655134 [DOI] [PubMed] [Google Scholar]
- Naqvi N.H., Bechara A. The hidden island of addiction: the insula. Trends in Neurosciences. 2009;32:56–67. doi: 10.1016/j.tins.2008.09.009. 18986715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi N.H., Rudrauf D., Damasio H., Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science (New York, N.Y.) 2007;315:531–534. doi: 10.1126/science.1135926. 17255515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield R.C. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. 5146491 [DOI] [PubMed] [Google Scholar]
- Pallanti S., DeCaria C.M., Grant J.E., Urpe M., Hollander E. Reliability and validity of the pathological gambling adaptation of the Yale–Brown Obsessive–Compulsive Scale (PG-YBOCS) Journal of Gambling Studies / Co-Sponsored by the National Council on Problem Gambling and Institute for the Study of Gambling and Commercial Gaming. 2005;21:431–443. doi: 10.1007/s10899-005-5557-3. 16311876 [DOI] [PubMed] [Google Scholar]
- Paulus M.P., Tapert S.F., Schuckit M.A. Neural activation patterns of methamphetamine-dependent subjects during decision making predict relapse. Archives of General Psychiatry. 2005;62:761–768. doi: 10.1001/archpsyc.62.7.761. 15997017 [DOI] [PubMed] [Google Scholar]
- Petry N.M. Should the scope of addictive behaviors be broadened to include pathological gambling? Addiction (Abingdon, England) 2006;101(Suppl. 1):152–160. doi: 10.1111/j.1360-0443.2006.01593.x. 16930172 [DOI] [PubMed] [Google Scholar]
- Petry N.M., Stinson F.S., Grant B.F. Comorbidity of DSM-IV pathological gambling and other psychiatric disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Journal of Clinical Psychiatry. 2005;66:564–574. doi: 10.4088/jcp.v66n0504. 15889941 [DOI] [PubMed] [Google Scholar]
- Pizzagalli D.A., Iosifescu D., Hallett L.A., Ratner K.G., Fava M. Reduced hedonic capacity in major depressive disorder: evidence from a probabilistic reward task. Journal of Psychiatric Research. 2008;43(1):76–87. doi: 10.1016/j.jpsychires.2008.03.001. 18433774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potenza M.N. Should addictive disorders include non-substance related conditions? Addiction (Abingdon, England) 2006;101(Suppl. 1):142–151. doi: 10.1111/j.1360-0443.2006.01591.x. 16930171 [DOI] [PubMed] [Google Scholar]
- Potenza M.N., Leung H.C., Blumberg H.P., Peterson B.S., Fulbright R.K., Lacadie et al An FMRI Stroop task study of ventromedial prefrontal cortical function in pathological gamblers. American Journal of Psychiatry. 2003;160:1990–1994. doi: 10.1176/appi.ajp.160.11.1990. 14594746 [DOI] [PubMed] [Google Scholar]
- Potenza M.N., Steinberg M.A., Skudlarski P., Fulbright R.K., Lacadie C.M., Wilber M.K. Gambling urges in pathological gambling: a functional magnetic resonance imaging study. Archives of General Psychiatry. 2003;60:828–836. doi: 10.1001/archpsyc.60.8.828. 12912766 [DOI] [PubMed] [Google Scholar]
- Potenza M.N., Xian H., Shah K., Scherrer J.F., Eisen S.A. Shared genetic contributions to pathological gambling and major depression in men. Archives of General Psychiatry. 2005;62:1015–1021. doi: 10.1001/archpsyc.62.9.1015. 16143733 [DOI] [PubMed] [Google Scholar]
- Prévost C., Pessiglione M., Météreau E., Cléry-Melin M.L., Dreher J.C. Separate valuation subsystems for delay and effort decision costs. Journal of Neuroscience: the Official Journal of the Society for Neuroscience. 2010;30:14080–14090. doi: 10.1523/JNEUROSCI.2752-10.2010. 20962229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter J., Raedler T., Rose M., Hand I., Gläscher J., Büchel C. Pathological gambling is linked to reduced activation of the mesolimbic reward system. Nature Neuroscience. 2005;8:147–148. doi: 10.1038/nn1378. 15643429 [DOI] [PubMed] [Google Scholar]
- Rømer Thomsen K., Callesen M.B., Linnet J., Kringelbach M.L., Møller A. Severity of gambling is associated with severity of depressive symptoms in pathological gamblers. Behavioural Pharmacology. 2009;20:527–536. doi: 10.1097/FBP.0b013e3283305e7a. 19654506 [DOI] [PubMed] [Google Scholar]
- Sescousse G., Barbalat G., Domenech P., Dreher J.C. Imbalance in the sensitivity to different types of rewards in pathological gambling. Brain: A Journal of Neurology. 2013;136:2527–2538. doi: 10.1093/brain/awt126. 23757765 [DOI] [PubMed] [Google Scholar]
- Shaffer H.J., LaPlante D.A., LaBrie R.A., Kidman R.C., Donato A.N., Stanton M.V. Toward a syndrome model of addiction: multiple expressions, common etiology. Harvard Review of Psychiatry. 2004;12:367–374. doi: 10.1080/10673220490905705. 15764471 [DOI] [PubMed] [Google Scholar]
- Spielberger C.D., Gorsuch R.L., Luschene R.E., Vagg P.R., Jacobs G.A. Mind Garden; Palo Alto, CA: 1993. State-Trait Anxiety Inventory for Adults. [Google Scholar]
- Stucki S., Rihs-Middel M. Prevalence of adult problem and pathological gambling between 2000 and 2005: an update. Journal of Gambling Studies / Co-Sponsored by the National Council on Problem Gambling and Institute for the Study of Gambling and Commercial Gaming. 2007;23:245–257. doi: 10.1007/s10899-006-9031-7. 17216582 [DOI] [PubMed] [Google Scholar]
- Takahashi T., Yücel M., Lorenzetti V. Volumetric MRI study of the insular cortex in individuals with current and past major depression. Journal of Affective Disorders. 2010;121(3):231–238. doi: 10.1016/j.jad.2009.06.003. 19540599 [DOI] [PubMed] [Google Scholar]
- Tanabe J., Thompson L., Claus E., Dalwani M., Hutchison K., Banich M.T. Prefrontal cortex activity is reduced in gambling and nongambling substance users during decision-making. Human Brain Mapping. 2007;28:1276–1286. doi: 10.1002/hbm.20344. 17274020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Delcroix N., Mazoyer B. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. 11771995 [DOI] [PubMed] [Google Scholar]
- van Holst R.J., van Holstein M., van den Brink W., Veltman D.J., Goudriaan A.E. Response inhibition during cue reactivity in problem gamblers: an fMRI study. PloS One. 2012;7:e30909. doi: 10.1371/journal.pone.0030909. 22479305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Holst R.J., Veltman D.J., Büchel C., van den Brink W., Goudriaan A.E. Distorted expectancy coding in problem gambling: is the addictive in the anticipation? Biological Psychiatry. 2012;71:741–748. doi: 10.1016/j.biopsych.2011.12.030. 22342105 [DOI] [PubMed] [Google Scholar]
- Vollstädt-Klein S., Wichert S., Rabinstein J., Bühler M., Klein O., Ende G. Initial, habitual and compulsive alcohol use is characterized by a shift of cue processing from ventral to dorsal striatum. Addiction (Abingdon, England) 2010;105:1741–1749. doi: 10.1111/j.1360-0443.2010.03022.x. 20670348 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.


