Abstract
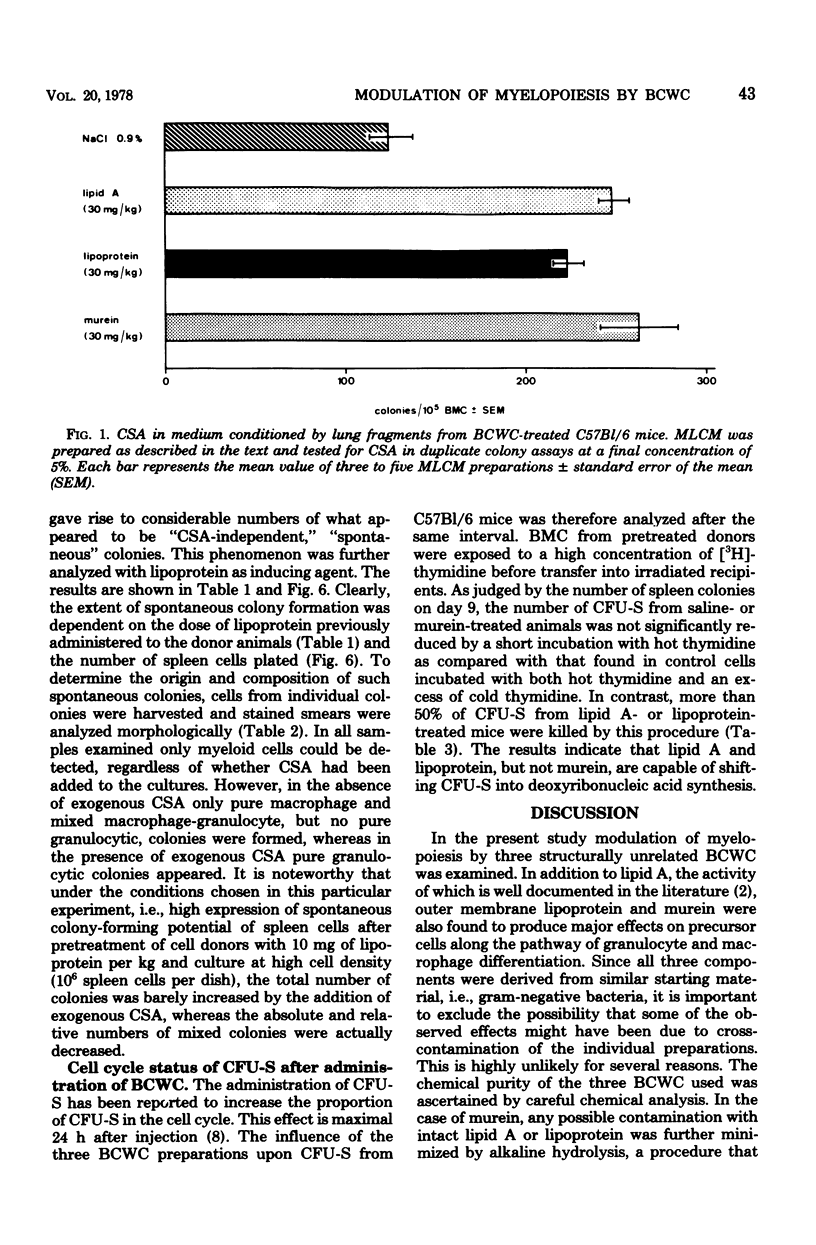
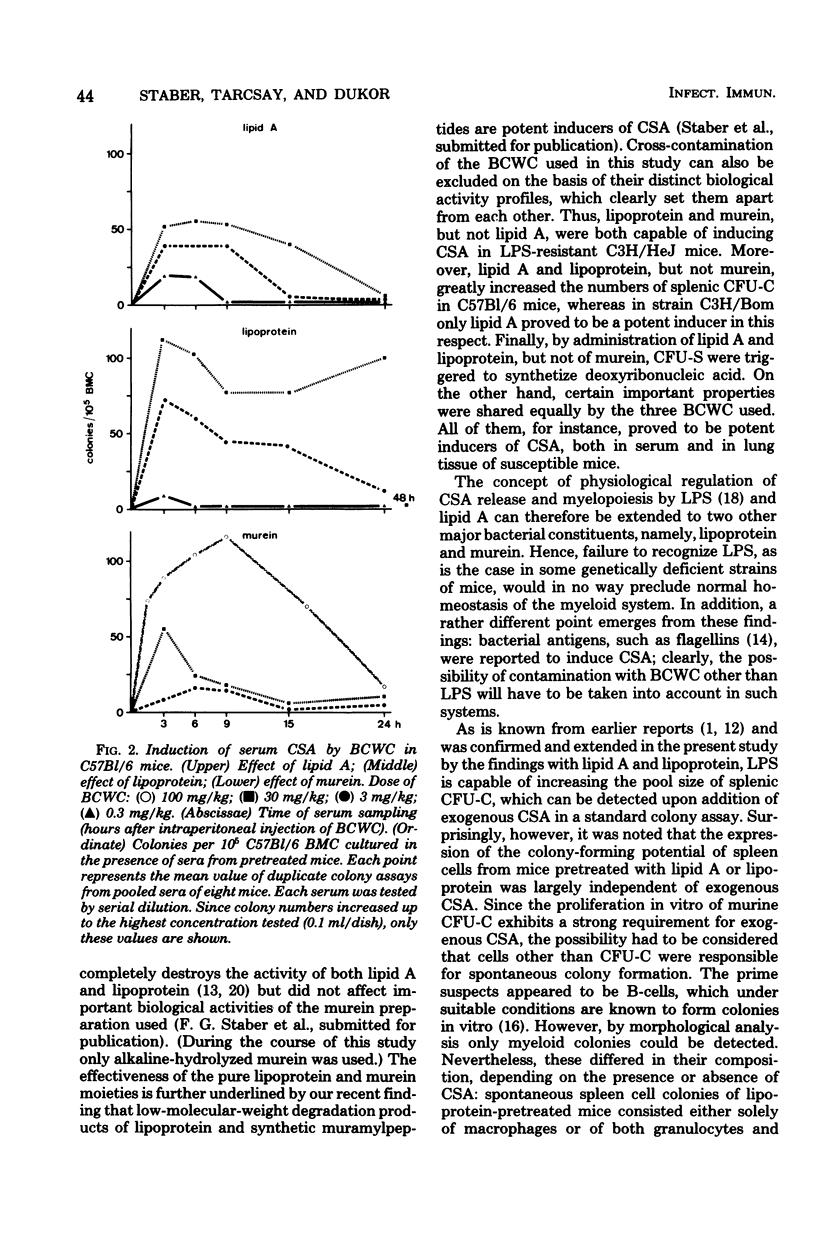
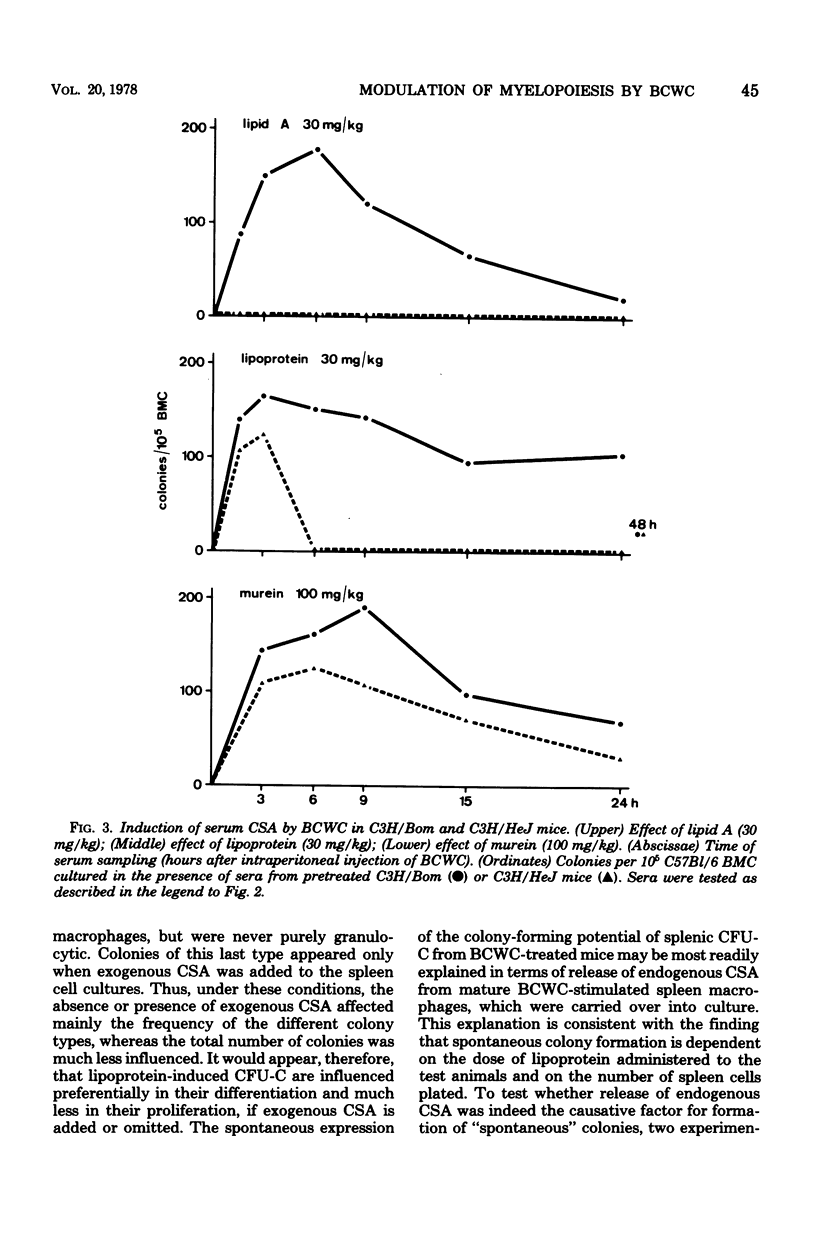
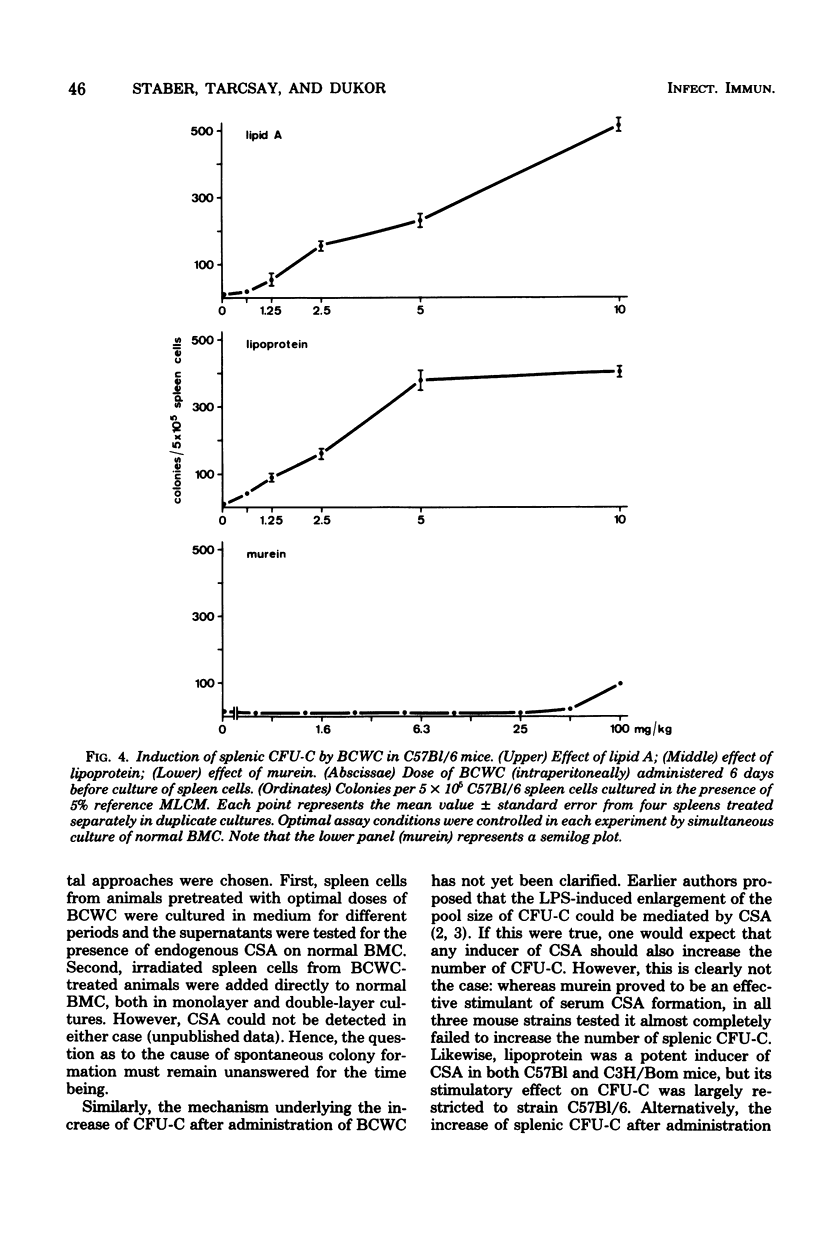
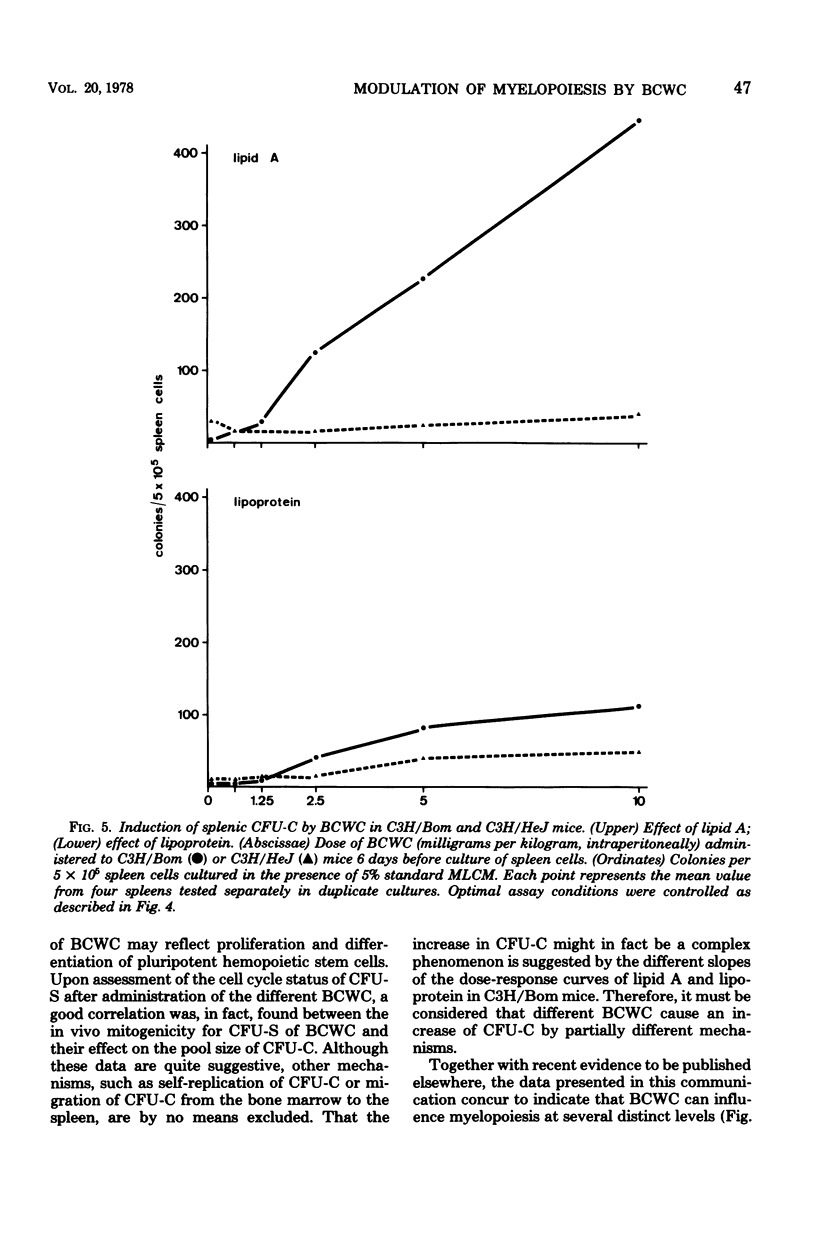
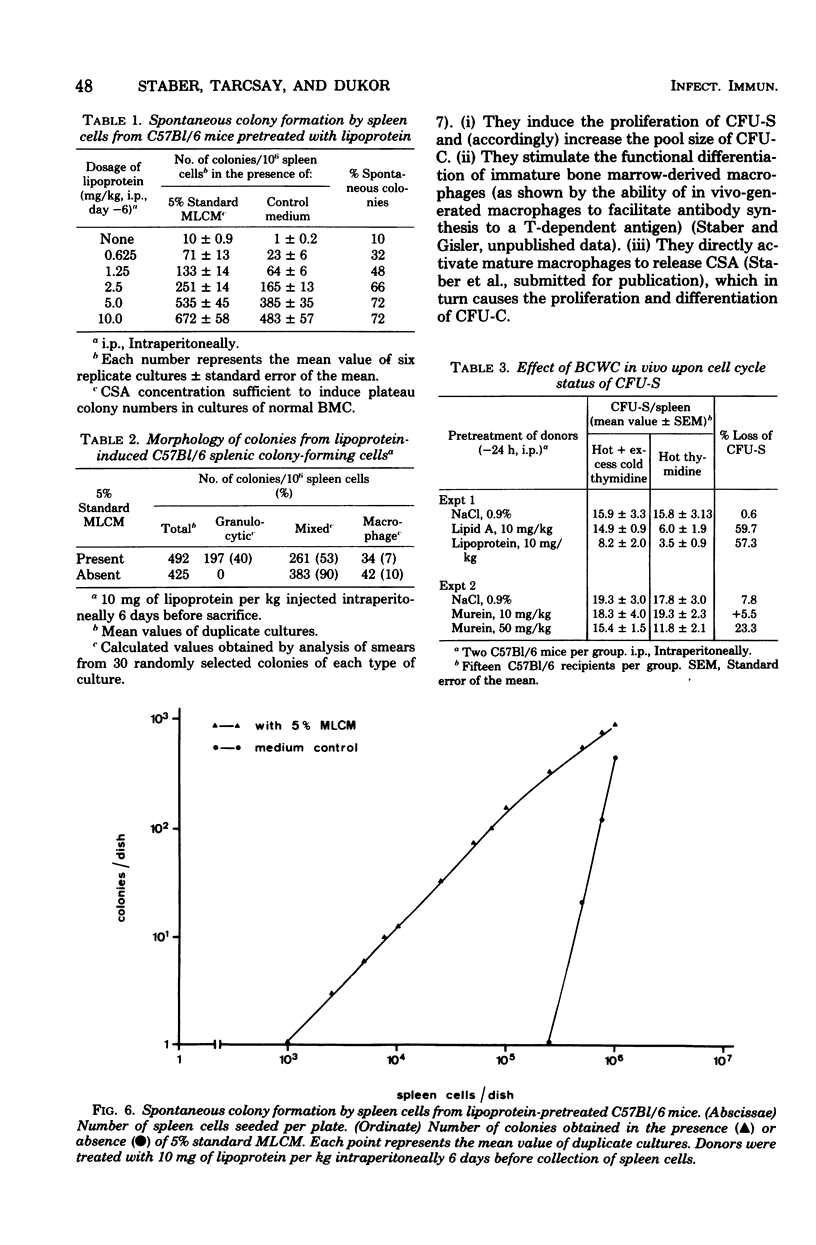
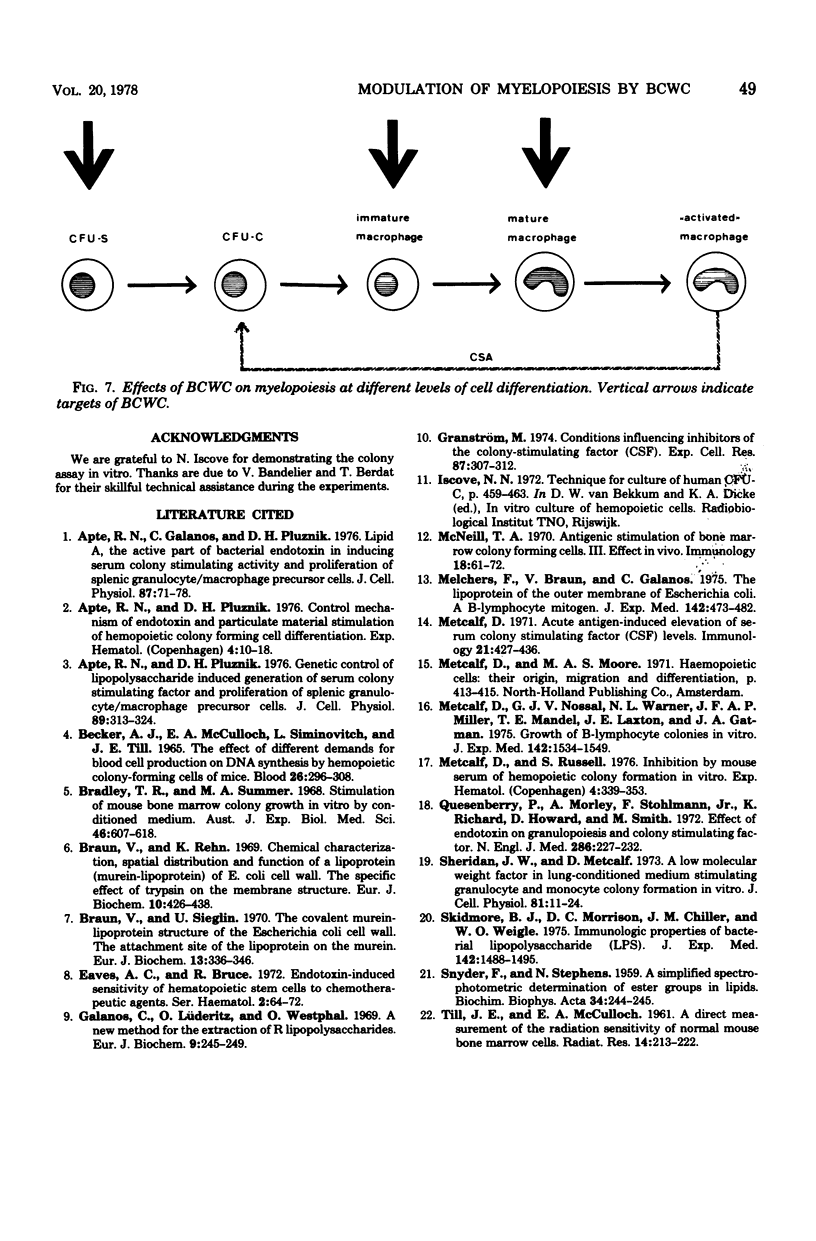
Modulation of myelopoiesis by chemically pure preparations of different cell wall components from gram-negative bacteria was investigated in vivo. The effects of lipid A, outer membrane lipoprotein, and murein were evaluated at several distinct stages: induction of colony-stimulating activity (CSA) in the serum, increase in the number of committed splenic precursor cells (CFU-C) forming granulocyte-macrophage colonies in vitro, and triggering into the cell cycle of noncommitted hemopoietic stem cells (CFU-S) from bone marrow. The results reveal different patterns of activity of the bacterial cell wall components (BCWC) tested. (i) In C57Bl/6 mice and C3H/Bom mice, all three preparations were potent inducers of CSA. In C3H/HeJ mice, CSA was only induced by lipoprotein and murein and not by lipid A. After injection of lipid A or lipoprotein, but not murein, the number of CFU-C in spleens of C57Bl/6 mice was increased up to 100-fold. In C3H/Bom and C3H/HeJ mice, not only murein but also lipoprotein were much less potent in this respect. (iii) In C57Bl/6 mice, both lipid A and lipoprotein, but not murein, were capable of inducing the proliferation of CFU-S, as demonstrated by a hot thymidine cytocide technique. Thus, induction of CSA and changes in the pool size of splenic CFU-C after administration of BCWC may be unrelated events. On the other hand, the increase of CFU-C might reflect the mitogenicity of BCWC for CFU-S.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apte R. N., Galanos C., Pluznik D. H. Lipid A, the active part of bacterial endotoxins in inducing serum colony stimulating activity and proliferation of splenic granulocyte/macrophage progenitor cells. J Cell Physiol. 1976 Jan;87(1):71–78. doi: 10.1002/jcp.1040870110. [DOI] [PubMed] [Google Scholar]
- Apte R. N., Pluznik D. H. Control mechanisms of endotoxin and particulate material stimulation of hemopoietic colony forming cell differentiation. Exp Hematol. 1976 Jan;4(1):10–18. [PubMed] [Google Scholar]
- Apte R. N., Pluznik D. H. Genetic control of lipopolysaccharide induced generation of serum colony stimulating factor and proliferation of splenic granulocyte/macrophage precursor cells. J Cell Physiol. 1976 Oct;89(2):313–323. doi: 10.1002/jcp.1040890214. [DOI] [PubMed] [Google Scholar]
- BECKER A. J., MCCULLOCH E. A., SIMINOVITCH L., TILL J. E. THE EFFECT OF DIFFERING DEMANDS FOR BLOOD CELL PRODUCTION ON DNA SYNTHESIS BY HEMOPOIETIC COLONY-FORMING CELLS OF MICE. Blood. 1965 Sep;26:296–308. [PubMed] [Google Scholar]
- Bradley T. R., Sumner M. A. Stimulation of mouse bone marrow colony growth in vitro by conditioned medium. Aust J Exp Biol Med Sci. 1968 Oct;46(5):607–618. doi: 10.1038/icb.1968.167. [DOI] [PubMed] [Google Scholar]
- Braun V., Rehn K. Chemical characterization, spatial distribution and function of a lipoprotein (murein-lipoprotein) of the E. coli cell wall. The specific effect of trypsin on the membrane structure. Eur J Biochem. 1969 Oct;10(3):426–438. doi: 10.1111/j.1432-1033.1969.tb00707.x. [DOI] [PubMed] [Google Scholar]
- Braun V., Sieglin U. The covalent murein-lipoprotein structure of the Escherichia coli cell wall. The attachment site of the lipoprotein on the murein. Eur J Biochem. 1970 Apr;13(2):336–346. doi: 10.1111/j.1432-1033.1970.tb00936.x. [DOI] [PubMed] [Google Scholar]
- Eaves A. C., Bruce W. R. Endotoxin-induced sensitivity of hematopoietic stem cells to chemotherapeutic agents. Ser Haematol. 1972;5(2):64–72. [PubMed] [Google Scholar]
- Galanos C., Lüderitz O., Westphal O. A new method for the extraction of R lipopolysaccharides. Eur J Biochem. 1969 Jun;9(2):245–249. doi: 10.1111/j.1432-1033.1969.tb00601.x. [DOI] [PubMed] [Google Scholar]
- Granström M. Conditions influencing inhibitors of the colony-stimulating factor (CSF). Exp Cell Res. 1974 Aug;87(2):307–312. doi: 10.1016/0014-4827(74)90486-8. [DOI] [PubMed] [Google Scholar]
- McNeill T. A. Antigenic stimulation of bone marrow colony forming cells. 3. Effect in vivo. Immunology. 1970 Jan;18(1):61–72. [PMC free article] [PubMed] [Google Scholar]
- Melchers F., Braun V., Galanos C. The lipoprotein of the outer membrane of Escherichia coli: a B-lymphocyte mitogen. J Exp Med. 1975 Aug 1;142(2):473–482. doi: 10.1084/jem.142.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf D. Acute antigen-induced elevation of serum colony stimulating factor (CFS) levels. Immunology. 1971 Sep;21(3):427–436. [PMC free article] [PubMed] [Google Scholar]
- Metcalf D., Nossal G. J., Warner N. L., Miller J. F., Mandel T. E., Layton J. E., Gutman G. A. Growth of B-lymphocyte colonies in vitro. J Exp Med. 1975 Dec 1;142(6):1534–1549. doi: 10.1084/jem.142.6.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf D., Russell S. Inhibition by mouse serum of hemopoietic colony formation in vitro. Exp Hematol. 1976 Nov;4(6):339–353. [PubMed] [Google Scholar]
- Quesenberry P., Morley A., Stohlman F., Jr, Rickard K., Howard D., Smith M. Effect of endotoxin on granulopoiesis and colony-stimulating factor. N Engl J Med. 1972 Feb 3;286(5):227–232. doi: 10.1056/NEJM197202032860502. [DOI] [PubMed] [Google Scholar]
- SNYDER F., STEPHENS N. A simplified spectrophotometric determination of ester groups in lipids. Biochim Biophys Acta. 1959 Jul;34:244–245. doi: 10.1016/0006-3002(59)90255-0. [DOI] [PubMed] [Google Scholar]
- Sheridan J. W., Metcalf D. A low molecular weight factor in lung-conditioned medium stimulating granulocyte and monocyte colony formation in vitro. J Cell Physiol. 1973 Feb;81(1):11–23. doi: 10.1002/jcp.1040810103. [DOI] [PubMed] [Google Scholar]
- Skidmore B. J., Morrison D. C., Chiller J. M., Weigle W. O. Immunologic properties of bacterial lipopolysaccharide (LPS). II. The unresponsiveness of C3H/HeJ Mouse spleen cells to LPS-induced mitogenesis is dependent on the method used to extract LPS. J Exp Med. 1975 Dec 1;142(6):1488–1508. doi: 10.1084/jem.142.6.1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TILL J. E., McCULLOCH E. A. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res. 1961 Feb;14:213–222. [PubMed] [Google Scholar]


