Abstract
Cancer cells, relative to normal cells, demonstrate significant alterations in metabolism that are proposed to result in increased steady-state levels of mitochondrial-derived reactive oxygen species (ROS) such as O2•−and H2O2. It has also been proposed that cancer cells increase glucose and hydroperoxide metabolism to compensate for increased levels of ROS. Given this theoretical construct, it is reasonable to propose that forcing cancer cells to use mitochondrial oxidative metabolism by feeding ketogenic diets that are high in fats and low in glucose and other carbohydrates, would selectively cause metabolic oxidative stress in cancer versus normal cells. Increased metabolic oxidative stress in cancer cells would in turn be predicted to selectively sensitize cancer cells to conventional radiation and chemotherapies. This review summarizes the evidence supporting the hypothesis that ketogenic diets may be safely used as an adjuvant therapy to conventional radiation and chemotherapies and discusses the proposed mechanisms by which ketogenic diets may enhance cancer cell therapeutic responses.
Keywords: Ketogenic diet, Oxidative stress, Cancer therapy
Introduction
Numerous dietary components and supplements have been evaluated as possible cancer prevention agents; however, until recently few studies have investigated diet as a possible adjuvant to cancer treatment. One of the most prominent and universal metabolic alterations seen in cancer cells is an increase in the rate of glycolytic metabolism even in the presence of oxygen [1]. Although increased glucose uptake by tumor cells was thought to support increased cancer cell proliferation and energy demands, recent studies suggest that increased tumor cell glycolytic metabolism may represent an adaptive response to escape metabolic oxidative stress caused by altered mitochondrial oxygen metabolism [2–4]. These data support the hypothesis that cancer cells are reliant on increased glucose consumption to maintain redox homeostasis due to increased one electron reductions of O2 to form O2•− and H2O2 in mitochondria. This divergence from normal cell metabolism has sparked a growing interest in targeting mitochondrial oxygen metabolism as a means of selectively sensitizing cancer cells to therapy [5–17]. In this regard, dietary modifications, such as high-fat, low-carbohydrate ketogenic diets that enhance mitochondrial oxidative metabolism while limiting glucose consumption could represent a safe, inexpensive, easily implementable, and effective approach to selectively enhance metabolic stress in cancer cells versus normal cells.
What is a ketogenic diet?
A ketogenic diet consists of high fat, with moderate to low protein content, and very low carbohydrates, which forces the body to burn fat instead of glucose for adenosine triphosphate (ATP) synthesis. Generally, the ratio by weight is 3:1 or 4:1 fat to carbohydrate+protein, yielding a diet that has an energy distribution of about 8% protein, 2% carbohydrate, and 90% fat. Fig. 1 shows the composition of the clinically available 4:1 KetoCal© ketogenic diet in comparison to other related diets.
Fig. 1.
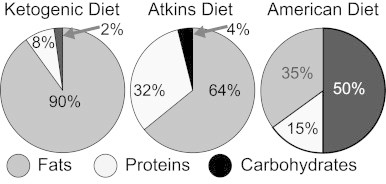
Comparison of the caloric composition of the ketogenic diet, Atkins diet, and American diet. On any given day Americans consume an average of 265 g of carbohydrates (50% of total calories), 78.3 g of total fat (35% of total calories), and 78.1 g of protein (15% of total calories). Using percentage of total calories, these values are consistent with current 2010 United States Department of Agriculture recommendations that call for 45–65% of total calories from carbohydrate, 20–35% of total calories from fat, and 10–15% of total calories from protein (80).
When an individual ingests a ketogenic diet, fat metabolism occurs via the oxidation of fatty acids by the liver, producing the ketone bodies including acetoacetate, β-hydroxybutyrate, and acetone. Ketones are transported in the blood to tissues where they are converted to acetyl-CoA, a substrate in the first step of the citric acid cycle. The low carbohydrate content of the ketogenic diet may cause a modest reduction of blood glucose and overall greater glycemic control resulting in lower hemoglobin A1C levels [18]. This treatment can also stimulate gluconeogenesis in humans to compensate for the drop in blood glucose levels [19]. The adherence and effectiveness of ketogenic diets can be monitored by measuring serum and urine β-hydroxybutyrate [20].
The discovery of ketogenic diets as a disease therapy
Since Hippocrates, prolonged periods of fasting have been recorded as a therapeutic tool for epilepsy [21]. Early 20th century medical literature has multiple case reports suggesting patients with various illnesses, including epilepsy, benefited from short, 2–3 week fasts and these studies attributed the success of fasting to dehydration, ketosis, or acidosis [21]. In 1921, Dr. R.M. Wilder at the Mayo Clinic proposed a diet in which the major portion of calories were derived from fat, mimicking the biochemical changes of fasting for the treatment of epilepsy. He coined the term ketogenic diet for this dietary composition [21]. With the development of safe and effective anticonvulsant drugs such as phenytoin and sodium valproate in the 1950s, interest in the ketogenic diet waned but the therapy was still utilized in cases where the symptoms of disease were refractory to other drug therapies.
Based on clinical experiences, ketogenic diets began to re-emerge in the mid-1990s as a frontline and acceptable alternative in childhood epilepsy patients who were unresponsive to other anticonvulsant drug therapies. A recent randomized controlled study from University College London showed a clear benefit of the ketogenic diet in controlling childhood seizures. In the final analysis of the 54 patients in the diet group, 61% experienced significant reductions in seizures compared to 8% of patients in the control group [22]. In addition, after consuming the diet for approximately 6 months, there was no evidence of significant adverse effects on childhood cognition or social adaptation [23].
Clinical applications of ketogenic diet
Increased recognition of the safety and efficacy of using ketogenic diets in the treatment of epilepsy has resulted in successful application of this dietary intervention to other disorders. The most notable and well-studied use of a ketogenic diet is for the treatment of obesity popularized by Dr. Robert Atkins (see Fig. 1) (Dr. Atkins Diet Revolution 1972). Ketogenic diets have also been shown to be beneficial in the treatment of patients with glucose transporter defects and other inborn metabolic disorders [24]. The diet is reported to show promise in slowing the progression of amyotrophic lateral sclerosis [25], and there is a growing body of evidence suggesting ketogenic diets may be beneficial in other neurodegenerative diseases including Alzheimer’s disease and Parkinson’s disease [26]. In addition, there are case reports and small case studies indicating improvement in patients with autism [27] depression [28], polycystic ovary syndrome [29], and type 2 diabetes mellitus [18].
Ketogenic diets in cancer therapy
Recently, ketogenic diets have been studied as an adjuvant to cancer therapy in both animal models and human case reports. As early as 1987, Tisdale et al. saw decreased tumor weight and improved cachexia in mice with colon adenocarcinoma xenografts eating a ketogenic diet [30]. Additional studies have shown that ketogenic diets reduce tumor growth and improve survival in animal models of malignant glioma [31–33], colon cancer [34], gastric cancer [35], and prostate cancer [36–38]. Furthermore, ketogenic diets have been hypothesized, with some supporting evidence, to potentiate the effects of radiation in malignant glioma models [39] as well as in non-small cell lung cancer models [5]. Fasting, which also induces a state of ketosis, has been shown to enhance responsiveness to chemotherapy in pre-clinical cancer therapy models as well as possibly ameliorating some of the normal tissue side effects seen with chemotherapy [40]. Fasting cycles are also reported to retard the growth of tumors and sensitize a range of cancer cell types to chemotherapy [40,41].
Some of the clinical results include a case report of two female pediatric patients, with advanced stage malignant astrocytoma who demonstrated a 21.8% decrease in tumor SUV when these patients were fed a ketogenic diet, as determined by uptake of 2-deoxy-2[18F]fluoro-d-glucose (FDG) using positron emission tomography (PET) [42]. A more recent case report showed improvement in a 65 year old female patient with glioblastoma multiforme treated with calorie-restricted ketogenic diet together with standard treatment [43]. Importantly, a quality of life study in patients with advanced cancer found that a ketogenic diet had no severe adverse effects, improved emotional functioning, and reduced insomnia [44].
Proposed mechanism of action of ketogenic diet in cancer
Mitochondrial metabolism and cancer
Most cancer therapies are designed to take advantage of the metabolic and physiological differences that exist between cancer cells and normal cells. Compared to normal cells, cancer cells exhibit increased glucose metabolism as well as alterations in mitochondrial oxidative metabolism that are believed to be the result of chronic metabolic oxidative stress [3,4,45] (Fig. 2). Mitochondria are involved in the regulation of cellular energy production through the process of oxidative phosphorylation where electron transport chain (ETC) activity is used in the generation of cellular ATP [46]. In the mitochondrial ETC, electrons are shuttled down complexes I–IV, resulting in the generation of transmembrane proton gradient that is coupled to ATP production through ATP synthase (Complex V). Studies have shown increased prevalence of mitochondrial DNA mutations as well as alterations in the expression of nuclear encoded mitochondrial proteins in many human cancers [47–49] including head and neck [50], prostate [51], ovary [52], and liver cancers [53]. Previous data suggests that the susceptibility of mitochondrial DNA to mutations is largely due to the increase in ROS levels in this organelle [6,49,54–57]. Furthermore, recent studies have shown that breast and colon cancer cells demonstrate significantly increased steady-state levels of ROS relative to normal colon and breast cells [3]. These differences were even more pronounced in the presence of mitochondrial ETC blockers, suggesting dysfunctional mitochondrial ETCs as the major source of elevated ROS production in cancer cells [3]. Overall, there is substantial literature indicating that there is a significant increase in intracellular O2•− and H2O2 in cancer cell mitochondria relative to normal cells and that this could represent a target for enhancing cancer therapy [5,7,9–14,16,17].
Fig. 2.
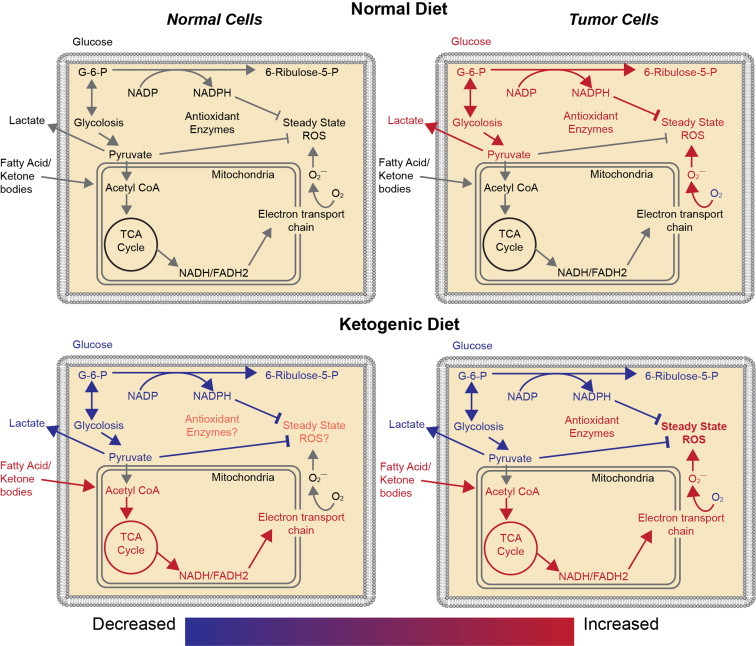
Comparison of normal cell and tumor cell metabolism on an American diet and a ketogenic diet. Relative to normal cells, tumor cells have been hypothesized to have increased mitochondrial DNA mutations as well as alterations in the expression of nuclear encoded mitochondrial proteins, resulting in increased production of reactive oxygen species (ROS) during mitochondrial respiration. Increased tumor cell ROS increases tumor cell dependence upon glucose metabolism, resulting in generation of NADPH and pyruvate via the pentose phosphate shunt and pyruvate from glycolysis. NADPH and pyruvate reduce hydroperoxides. Ketogenic diets decrease the capability of tumor cells to produce NADPH because, in most tissues, fat metabolism is unable to undergo gluconeogenesis to form glucose-6-phosphate (G-6-P) necessary to enter the pentose phosphate shunt. Thus, ketogenic diets should further increase the oxidative stress in tumor cells relative to normal cells by limiting NADPH regeneration.
Glucose dependence of cancer cells
Glycolysis mediates the enzymatic breakdown of glucose to pyruvate, which in the presence of oxygen is converted to acetyl-CoA and enters the Citric Acid Cycle in the mitochondria. In the absence of oxygen, pyruvate is alternatively converted into lactate. Normal cells link pyruvate production to mitochondrial respiration to efficiently generate ATP via oxidative phosphorylation and usually demonstrate low levels of glycolysis as well as lactate production. In contrast to normal cells, cancer cells demonstrate increased glucose consumption, even in the presence of oxygen [1] which was suggested to occur because of defective mitochondrial respiration requiring increased glycolysis as a compensatory response.
Numerous animal studies over the past 60 years have not only confirmed the observation of increased glucose consumption in cancer cells but also demonstrate the importance of glucose for tumor survival and metastasis. The flux of energy-yielding substrates across colon carcinomas in patients has demonstrated that net glucose uptake and lactate release by malignant tumors exceeds the peripheral non-malignant exchange rates by 30 and 43 fold, respectively, while no significant differences existed between tumor and peripheral tissue in fatty acid or ketone balance. FDG PET conclusively demonstrates that most human carcinomas have an increased glucose demand when compared to the surrounding normal tissue [58].
In addition to abnormal aerobic glycolysis, cancer cells have increased pentose phosphate pathway activity [3,59]. The pentose phosphate pathway oxidizes glucose to produce two molecules of the reducing equivalent nicotinamide adenine dinucleotide phosphate (NADPH) and ribose-5-phosphate. NADPH acts as a cofactor for the glutathione/glutathione peroxidase system as well as the thioredoxin/thioredoxin peroxidase system [60]. These thiol systems are responsible for detoxifying H2O2 and organic peroxides, thereby maintaining the redox balance by preventing and repairing oxidative damage.
Glucose metabolism is known to play a major role in the detoxification of peroxides both through the formation of pyruvate (which scavenges peroxides directly through a deacetylation reaction) and the regeneration of the redox cofactor NADPH. Previous studies have shown glucose deprivation selectively causes oxidative stress and toxicity in human cancer cells relative to normal cells that is reversed upon addition of superoxide and peroxide scavengers [2,3]. Furthermore, many in vitro and in vivo studies have successfully investigated the use of glycolytic inhibitors to cause selective cancer cell toxicity via a mechanism involving metabolic oxidative stress [3,7,61–65].
Ketogenic diets increase cancer cell oxidative stress
Ketogenic diets may act as an adjuvant cancer therapy by two different mechanisms that both increase the oxidative stress inside cancer cells. Lipid metabolism limits the availability of glucose for glycolysis restricting the formation of pyruvate and glucose-6 phosphate which can enter the pentose phosphate pathway forming NADPH necessary for reducing hydroperoxides (Fig. 2). Additionally, lipid metabolism forces cells to derive their energy from mitochondrial metabolism. Because cancer cells are believed to have dysfunctional mitochondrial ETCs resulting in increased one electron reductions of O2 leading to ROS production, cancer cells will be predicted to selectively experience oxidative stress, relative to normal cells, when glucose metabolism is restricted in the case of feeding ketogenic diets (Fig. 2). Similar to fat metabolism, protein derived energy production, such as in glutaminolysis, forces cells to derive their energy from mitochondrial metabolism and would be expected to increase cancer cell oxidative stress. However, many amino acids enter the Citric Acid Cycle through alpha-keto-glutarate which may undergo gluconeogenesis allowing for the production of NADPH. Thus, protein metabolism may not lead to the same levels of increased tumor cell oxidative stress as fat metabolism.
Evidence of ketogenic diets increasing cancer cell oxidative stress is present both clinically and in animal models. Hyperketotic diabetic humans have a higher level of lipid peroxidation in red blood cells and a significant decrease in cellular glutathione relative to normal ketonic diabetic controls [66]. Jain et al. also found elevated indices of lipid peroxidation in cultured human endothelial cells treated with acetoacetate [66]. Acetoacetate was also found to deplete cellular glutathione and increase intracellular peroxides in primary rat hepatocytes [67]. Chronic exposure to β-hydroxybutyrate was shown to increase ROS production in cardiomyocytes [68]. Combining a ketogenic diet with hyperbaric oxygen therapy decreased tumor growth rate, increased mean survival time, and increased β-hydroxybutyrate compared to controls in a metastatic mouse cancer model [69]. Thus combining a ketogenic diet with hyperbaric oxygen may further increase the oxidative stress inside of tumor cells. Furthermore, animals bearing lung cancer xenografts fed a ketogenic diet and treated with chemotherapy and radiation had increased 4-hydroxy-2-nonenal (4HNE)-modified protein relative to tumors treated with chemotherapy and radiation therapy alone [5]. 4HNE is a product of lipid peroxidation that damages proteins by forming adducts and is therefore both a marker of lipid and protein damage during oxidative stress.
Potential risks of ketogenic diets
Ketogenic diets haves been recognized to be effective at controlling seizures and inducing weight loss but have been suggested to cause some potential side effects. The acute side effects of high fat intake are typically lethargy, nausea, and vomiting due to intolerance of the diet, especially in children [70] (Fig. 3). Children can be prone to hypoglycemia due to low glucose intake and nausea [70]. In contrast, gastrointestinal discomfort is a common side effect in adults due to the high fat content of the diet [71]. A prospective pilot study on ketogenic diets reported a substantial and progressive increase in the cholesterol levels in patients after 1 year [72]. Past studies have also reported some deficiencies in trace minerals like selenium, copper, and zinc in the serum levels of patients on ketogenic diets, suggesting that appropriate supplementation of trace minerals is needed while on the diet [73].
Fig. 3.
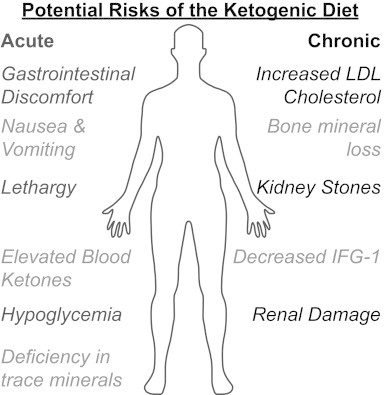
Possible acute and chronic side effects associated with the ketogenic diet.
Although no severe adverse changes have been reported with long term consumption of a ketogenic diet, renal damage due to excretion of nitrogenous waste products is also a possible side effect [74]. While no studies report absolute renal damage associated with ketogenic diet use, 6% of cases involving children with intractable epilepsy have reported the presence of kidney stones following eating the ketogenic diet for 1–5 years [75,76]. Most studies that examine the adverse effects of ketogenic diets have been done in children with epilepsy that had prolonged consumption of the diet over a period of 1–6 years. Most adverse effects reported in children only occur in patients who are on the ketogenic diet for greater than 1 year and include hypertriglyceridemia, decreased growth (decreased levels of insulin-like growth factor–1) and progressive bone mineral content loss. In addition, the most serious adverse effects of ketogenic diets can be prevented or corrected with appropriate measures such as vitamin supplements, assessment of bone function, and use of oral potassium citrate to decrease the risk of kidney stones [76,77].
In contrast, studies of ketogenic diets in adults show fewer and more minor adverse effects. In a 6-month study of adults on low-carbohydrate ketogenic diets, the only adverse effects noted were an increase in low-density lipoprotein (LDL) cholesterol levels, shakiness, and uneasiness [78]. In another trial, only 3 out of 72 adult patients on ketogenic diets for 1 year had adverse effects, with two showing elevated LDL cholesterol and one developing a kidney stone [79]. Another expected change associated with ketogenic diets is elevated blood ketones. This raises some concern in diabetic patients who are at an increased risk of developing ketoacidosis, a potentially life-threatening condition. However, the level of blood ketones as a result of ketogenic diet use in most adult patients is modest and is not accompanied with high blood glucose and therefore presents a low risk for ketoacidosis.
Clinical trials using the ketogenic diet for cancer control
There are currently 62 trials assessing low carbohydrate diets as a potential therapy for a variety of diseases of which 11 trials are assessing ketogenic diets as an adjuvant cancer therapy. In the University of Würzburg, Germany, patients having failed traditional cancer therapy and with no other salvage options have been enrolled in trials involving the ketogenic diet. Preliminary reports indicate that patients who were able to continue the ketogenic diet therapy for over 3 months showed improvement with a stable physical condition, tumor shrinkage, or slowed growth [44].
At the University Hospital in Tübingen, Germany a Phase 1 ERGO study designed to determine whether a mild ketogenic diet can influence quality of life and survival of patients with glutamino recurrent glioblastoma was conducted by Dr. Johannes Rieger and Dr. J. Steinbachand. No severe adverse events were reported (http://ClinicalTrials.gov/show/NCT00575146).
At the University of Iowa, three phase I trials assessing the tolerability of a ketogenic diet in combination with chemotherapy and radiation therapy are on-going in locally advanced pancreas, lung cancer as well as head and neck cancer (http://ClinicalTrials.gov). The typical schema is shown in Fig. 4a. While receiving standard of care radiation and chemotherapy, patients are consuming a ketogenic diet for 5 weeks; serum glucose and ketone levels are assessed daily in combination with weekly oxidative stress markers. A sample ketogenic diet is demonstrated in Fig. 4b.
Fig. 4.
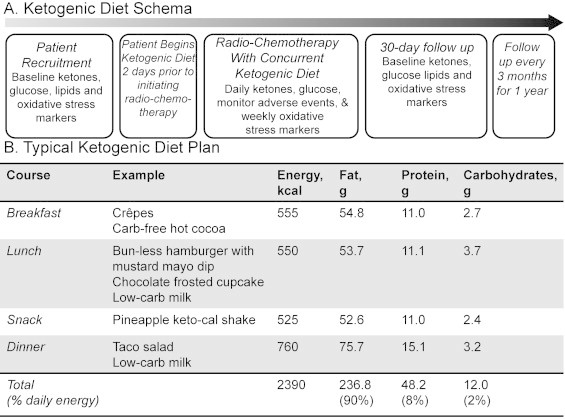
(A) Ketogenic diet phase I clinical trial schema and (B) sample ketogenic diet meal with a similar 4:1 ratio of fat to carbohydrate+protein as provided in the nutritionally complete KetoCal©. Ketosis is confirmed by laboratory measurement prior to beginning radiation therapy.
Conclusions
Despite recent advances in chemo-radiation, the prognosis for many cancer patients remains poor, and most current treatments are limited by severe adverse events. Therefore, there is a great need for complimentary approaches that have limited patient toxicity while selectively enhancing therapy responses in cancer versus normal tissues. Ketogenic diets could represent a potential dietary manipulation that could be rapidly implemented for the purpose of exploiting inherent oxidative metabolic differences between cancer cells and normal cells to improve standard therapeutic outcomes by selectively enhancing metabolic oxidative stress in cancer cells.
Although the mechanism by which ketogenic diets demonstrate anticancer effects when combined with standard radio-chemo-therapies has not been fully elucidated, preclinical results have demonstrated the safety and potential efficacy of using ketogenic diets in combination with radio-chemo-therapy to improve responses in murine cancer models. These preclinical studies have provided the impetus for extending the use of ketogenic diets into phase I clinical trials that are currently ongoing.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgements
The authors would like to thank the manufacturers of KetoCal for providing their 4:1 diet formulation for ongoing pre-clinical and clinical trials. This work was supported in part by the Carver Research Program of Excellence in Redox Biology and Medicine (R01CA133114, R21CA161182, R01182804-01, R21CA139182, P30CA086862, and UL1TR000442), RSNA Research and Educational Foundation Grant RR1020, as well as a generous gift from Mrs. Nellie K. Spitz, Mrs. Marie Foster, and the IBM Corporation.
References
- 1.Warburg O. On the origin of cancer cells. Science. 1956;123(3191):309–314. doi: 10.1126/science.123.3191.309. 13298683 [DOI] [PubMed] [Google Scholar]
- 2.Ahmad I.M., Aykin-Burns N., Sim J.E., Walsh S.A., Higashikubo R., Buettner G.R., Venkataraman S., Mackey M.A., Flanagan S.W., Oberley L.W., Spitz D.R. Mitochondrial O2•− and H2O2 mediate glucose deprivation-induced stress in human cancer cells. Journal of Biological Chemistry. 2005;280(6):4254–4263. doi: 10.1074/jbc.M411662200. 15561720 [DOI] [PubMed] [Google Scholar]
- 3.Aykin-Burns N., Ahmad I.M., Zhu Y., Oberley L.W., Spitz D.R. Increased levels of superoxide and H2O2 mediate the differential susceptibility of cancer cells versus normal cells to glucose deprivation. Biochemical Journal. 2009;418(1):29–37. doi: 10.1042/BJ20081258. 18937644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spitz D.R., Sim J.E., Ridnour L.A., Galoforo S.S., Lee Y.J. Glucose deprivation-induced oxidative stress in human tumor cells. A fundamental defect in metabolism? Annals of the New York Academy of Sciences. 2000;899:349–362. doi: 10.1111/j.1749-6632.2000.tb06199.x. 10863552 [DOI] [PubMed] [Google Scholar]
- 5.Allen B.G., Bhatia S.K., Buatti J.M., Brandt K.E., Lindholm K.E., Button A.M., Szweda L.I., Smith B.J., Spitz D.R., Fath M.A. Ketogenic diets enhance oxidative stress and radio-chemo-therapy responses in lung cancer xenografts. Clinical Cancer Research: 2013;19(14):3905–3913. doi: 10.1158/1078-0432.CCR-12-0287. 23743570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bize I.B., Oberley L.W., Morris H.P. Superoxide dismutase and superoxide radical in Morris hepatomas. Cancer Research. 1980;40(10):3686–3693. 6254638 [PubMed] [Google Scholar]
- 7.Coleman M.C., Asbury C.R., Daniels D., Du J., Aykin-Burns N., Smith B.J., Li L., Spitz D.R., Cullen J.J. 2-deoxy-d-glucose causes cytotoxicity, oxidative stress, and radiosensitization in pancreatic cancer. Free Radical Biology & Medicine. 2008;44(3):322–331. doi: 10.1016/j.freeradbiomed.2007.08.032. 18215740 [DOI] [PubMed] [Google Scholar]
- 8.Dixon S.J., Stockwell B.R. The role of iron and reactive oxygen species in cell death. Nature Chemical Biology. 2013;10(1):9–17. doi: 10.1038/nchembio.1416. [DOI] [PubMed] [Google Scholar]
- 9.Fath M.A., Ahmad I.M., Smith C.J., Spence J., Spitz D.R. Enhancement of carboplatin-mediated lung cancer cell killing by simultaneous disruption of glutathione and thioredoxin metabolism. Clinical Cancer Research: 2011;17(19):6206–6217. doi: 10.1158/1078-0432.CCR-11-0736. 21844013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fath M.A., Diers A.R., Aykin-Burns N., Simons A.L., Hua L., Spitz D.R. Mitochondrial electron transport chain blockers enhance 2-deoxy-d-glucose induced oxidative stress and cell killing in human colon carcinoma cells. Cancer Biology & Therapy. 2009;8(13):1228–1236. doi: 10.4161/cbt.8.13.8631. 19411865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hadzic T., Aykin-Burns N., Zhu Y., Coleman M.C., Leick K., Jacobson G.M., Spitz D.R. Paclitaxel combined with inhibitors of glucose and hydroperoxide metabolism enhances breast cancer cell killing via H2O2-mediated oxidative stress. Free Radical Biology & Medicine. 2010;48(8):1024–1033. doi: 10.1016/j.freeradbiomed.2010.01.018. 20083194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishikawa K., Takenaga K., Akimoto M., Koshikawa N., Yamaguchi A., Imanishi H., Nakada K., Honma Y., Hayashi J. ROS-generating mitochondrial DNA mutations can regulate tumor cell metastasis. Science. 2008;320(5876):661–664. doi: 10.1126/science.1156906. 18388260 [DOI] [PubMed] [Google Scholar]
- 13.Oberley L.W. Anticancer therapy by overexpression of superoxide dismutase. Antioxidants & Redox Signaling. 2001;3(3):461–472. doi: 10.1089/15230860152409095. 11491657 [DOI] [PubMed] [Google Scholar]
- 14.Oberley L.W. Mechanism of the tumor suppressive effect of MnSOD overexpression. Biomedicine & Pharmacotherapy. 2005;59(4):143–148. doi: 10.1016/j.biopha.2005.03.006. 15862707 [DOI] [PubMed] [Google Scholar]
- 15.Springer E.L. Comparative study of the cytoplasmic organelles of epithelial cell lines derived from human carcinomas and nonmalignant tissues. Cancer Research. 1980;40(3):803–817. 7193514 [PubMed] [Google Scholar]
- 16.Wen S., Zhu D., Huang P. Targeting cancer cell mitochondria as a therapeutic approach. Future Medicinal Chemistry. 2013;5(1):53–67. doi: 10.4155/fmc.12.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wondrak G.T. Redox-directed cancer therapeutics: molecular mechanisms and opportunities. Antioxidants & Redox Signaling. 2009;11(12):3013–3069. doi: 10.1089/ars.2009.2541. 19496700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Westman E.C., Yancy W.S., Jr., Mavropoulos J.C., Marquart M., McDuffie J.R. The effect of a low-carbohydrate, ketogenic diet versus a low-glycemic index diet on glycemic control in type 2 diabetes mellitus. Nutrition & Metabolism. 2008;5:36. doi: 10.1186/1743-7075-5-36. 19099589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Valayannopoulos V., Bajolle F., Arnoux J.B., Dubois S., Sannier N., Baussan C., Petit F., Labrune P., Rabier D., Ottolenghi C., Vassault A., Broissand C., Bonnet D., de Lonlay P. Successful treatment of severe cardiomyopathy in glycogen storage disease type III with D,l-3-hydroxybutyrate, ketogenic and high-protein diet. Pediatric Research. 2011;70(6):638–641. doi: 10.1203/PDR.0b013e318232154f. 21857385 [DOI] [PubMed] [Google Scholar]
- 20.Gilbert D.L., Pyzik P.L., Freeman J.M. The ketogenic diet: seizure control correlates better with serum beta-hydroxybutyrate than with urine ketones. Journal of Child Neurology. 2000;15(12):787–790. doi: 10.1177/088307380001501203. 11198492 [DOI] [PubMed] [Google Scholar]
- 21.Wheless J.W. History and origin of the ketogenic diet. In: Stafstrom C.E., Rho J.M., editors. Epilepsy and the Ketogenic Diet. Humana Press; Totowa, NJ: 2004. pp. 31–50. [Google Scholar]
- 22.Neal E.G., Chaffe H., Schwartz R.H., Lawson M.S., Edwards N., Fitzsimmons G., Whitney A., Cross J.H. The ketogenic diet for the treatment of childhood epilepsy: a randomised controlled trial. Lancet Neurology. 2008;7(6):500–506. doi: 10.1016/S1474-4422(08)70092-9. 18456557 [DOI] [PubMed] [Google Scholar]
- 23.Lambrechts D.A., Bovens M.J., de la Parra N.M., Hendriksen J.G., Aldenkamp A.P., Majoie M.J. Ketogenic diet effects on cognition, mood, and psychosocial adjustment in children. Acta Neurologica Scandinavica. 2013;127(2):103–108. doi: 10.1111/j.1600-0404.2012.01686.x. 22690843 [DOI] [PubMed] [Google Scholar]
- 24.Klepper J., Diefenbach S., Kohlschütter A., Voit T. Effects of the ketogenic diet in the glucose transporter 1 deficiency syndrome. Prostaglandins, Leukotrienes, and Essential Fatty Acids. 2004;70(3):321–327. doi: 10.1016/j.plefa.2003.07.004. 14769490 [DOI] [PubMed] [Google Scholar]
- 25.Zhao Z., Lange D.J., Voustianiouk A., MacGrogan D., Ho L., Suh J., Humala N., Thiyagarajan M., Wang J., Pasinetti G.M. A ketogenic diet as a potential novel therapeutic intervention in amyotrophic lateral sclerosis. BMC Neuroscience. 2006;7:29. doi: 10.1186/1471-2202-7-29. 16584562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barañano K.W., Hartman A.L. The ketogenic diet: uses in epilepsy and other neurologic illnesses. Current Treatment Options in Neurology. 2008;10(6):410–419. doi: 10.1007/s11940-008-0043-8. 18990309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Evangeliou A., Vlachonikolis I., Mihailidou H., Spilioti M., Skarpalezou A., Makaronas N., Prokopiou A., Christodoulou P., Liapi-Adamidou G., Helidonis E., Sbyrakis S., Smeitink J. Application of a ketogenic diet in children with autistic behavior: pilot study. Journal of Child Neurology. 2003;18(2):113–118. doi: 10.1177/08830738030180020501. 12693778 [DOI] [PubMed] [Google Scholar]
- 28.Murphy P., Likhodii S., Nylen K., Burnham W.M. The antidepressant properties of the ketogenic diet. Biological Psychiatry. 2004;56(12):981–983. doi: 10.1016/j.biopsych.2004.09.019. 15601609 [DOI] [PubMed] [Google Scholar]
- 29.Mavropoulos J.C., Yancy W.S., Hepburn J., Westman E.C. The effects of a low-carbohydrate, ketogenic diet on the polycystic ovary syndrome: a pilot study. Nutrition & Metabolism. 2005;2:35. doi: 10.1186/1743-7075-2-35. 16359551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tisdale M.J., Brennan R.A., Fearon K.C. Reduction of weight loss and tumour size in a cachexia model by a high fat diet. British Journal of Cancer. 1987;56(1):39–43. doi: 10.1038/bjc.1987.149. 3620317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maurer G.D., Brucker D.P., Bähr O., Harter P.N., Hattingen E., Walenta S., Mueller-Klieser W., Steinbach J.P., Rieger J. Differential utilization of ketone bodies by neurons and glioma cell lines: a rationale for ketogenic diet as experimental glioma therapy. BMC Cancer. 2011;11:315. doi: 10.1186/1471-2407-11-315. 21791085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seyfried T.N., Sanderson T.M., El-Abbadi M.M., McGowan R., Mukherjee P. Role of glucose and ketone bodies in the metabolic control of experimental brain cancer. British Journal of Cancer. 2003;89(7):1375–1382. doi: 10.1038/sj.bjc.6601269. 14520474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stafford P., Abdelwahab M.G., Kim Y., Preul M.C., Rho J.M., Scheck A.C. The ketogenic diet reverses gene expression patterns and reduces reactive oxygen species levels when used as an adjuvant therapy for glioma. Nutrition & Metabolism. 2010;7:74. doi: 10.1186/1743-7075-7-74. 20831808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beck S.A., Tisdale M.J. Nitrogen excretion in cancer cachexia and its modification by a high fat diet in mice. Cancer Research. 1989;49(14):3800–3804. 2736521 [PubMed] [Google Scholar]
- 35.Otto C., Kaemmerer U., Illert B., Muehling B., Pfetzer N., Wittig R., Voelker H.U., Thiede A., Coy J.F. Growth of human gastric cancer cells in nude mice is delayed by a ketogenic diet supplemented with omega-3 fatty acids and medium-chain triglycerides. BMC Cancer. 2008;8:122. doi: 10.1186/1471-2407-8-122. 18447912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Freedland S.J., Mavropoulos J., Wang A., Darshan M., Demark-Wahnefried W., Aronson W.J., Cohen P., Hwang D., Peterson B., Fields T., Pizzo S.V., Isaacs W.B. Carbohydrate restriction, prostate cancer growth, and the insulin-like growth factor axis. Prostate. 2008;68(1):11–19. doi: 10.1002/pros.20683. 17999389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Masko E.M., Thomas J.A., 2nd, Antonelli J.A., Lloyd J.C., Phillips T.E., Poulton S.H., Dewhirst M.W., Pizzo S.V., Freedland S.J. Low-carbohydrate diets and prostate cancer: How low is “low enough”? Cancer Prevention Research. 2010;3(9):1124–1131. doi: 10.1158/1940-6207.CAPR-10-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mavropoulos J.C., Buschemeyer W.C., 3rd, Tewari A.K., Rokhfeld D., Pollak M., Zhao Y., Febbo P.G., Cohen P., Hwang D., Devi G., Demark-Wahnefried W., Westman E.C., Peterson B.L., Pizzo S.V., Freedland S.J. The effects of varying dietary carbohydrate and fat content on survival in a murine LNCaP prostate cancer xenograft model. Cancer Prevention Research. 2009;2(6):557–565. doi: 10.1158/1940-6207.CAPR-08-0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abdelwahab M.G., Fenton K.E., Preul M.C., Rho J.M., Lynch A., Stafford P., Scheck A.C. The ketogenic diet is an effective adjuvant to radiation therapy for the treatment of malignant glioma. PloS One. 2012;7(5):e36197. doi: 10.1371/journal.pone.0036197. 22563484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee C., Raffaghello L., Brandhorst S., Safdie F.M., Bianchi G., Martin-Montalvo A., Pistoia V., Wei M., Hwang S., Merlino A., Emionite L., de Cabo R., Longo V.D. Fasting cycles retard growth of tumors and sensitize a range of cancer cell types to chemotherapy. Science Translational Medicine. 2012;4(124) doi: 10.1126/scitranslmed.3003293. 124ra127 22323820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Safdie F.M., Dorff T., Quinn D., Fontana L., Wei M., Lee C., Cohen P., Longo V.D. Fasting and cancer treatment in humans: a case series report. Aging. 2009;1(12):988–1007. doi: 10.18632/aging.100114. 20157582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nebeling L.C., Miraldi F., Shurin S.B., Lerner E. Effects of a ketogenic diet on tumor metabolism and nutritional status in pediatric oncology patients: two case reports. Journal of the American College of Nutrition. 1995;14(2):202–208. doi: 10.1080/07315724.1995.10718495. 7790697 [DOI] [PubMed] [Google Scholar]
- 43.Zuccoli G., Marcello N., Pisanello A., Servadei F., Vaccaro S., Mukherjee P., Seyfried T.N. Metabolic management of glioblastoma multiforme using standard therapy together with a restricted ketogenic diet: case report. Nutrition & Metabolism. 2010;7:33. doi: 10.1186/1743-7075-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schmidt M., Pfetzer N., Schwab M., Strauss I., Kämmerer U. Effects of a ketogenic diet on the quality of life in 16 patients with advanced cancer: a pilot trial. Nutrition & Metabolism. 2011;8(1):54. doi: 10.1186/1743-7075-8-54. 21794124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kroemer G., Pouyssegur J. Tumor cell metabolism: cancer’s Achilles’ heel. Cancer Cell. 2008;13(6):472–482. doi: 10.1016/j.ccr.2008.05.005. 18538731 [DOI] [PubMed] [Google Scholar]
- 46.Attardi G., Schatz G. Biogenesis of mitochondria. Annual Review of Cell. Biology. 1988;4:289–333. doi: 10.1146/annurev.cb.04.110188.001445. [DOI] [PubMed] [Google Scholar]
- 47.Carew J.S., Huang P. Mitochondrial defects in cancer. Molecular Cancer. 2002;1:9. doi: 10.1186/1476-4598-1-9. 12513701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fliss M.S., Usadel H., Caballero O.L., Wu L., Buta M.R., Eleff S.M., Jen J., Sidransky D. Facile detection of mitochondrial DNA mutations in tumors and bodily fluids. Science. 2000;287(5460):2017–2019. doi: 10.1126/science.287.5460.2017. 10720328 [DOI] [PubMed] [Google Scholar]
- 49.Polyak K., Li Y., Zhu H., Lengauer C., Willson J.K., Markowitz S.D., Trush M.A., Kinzler K.W., Vogelstein B. Somatic mutations of the mitochondrial genome in human colorectal tumours. Nature Genetics. 1998;20(3):291–293. doi: 10.1038/3108. 9806551 [DOI] [PubMed] [Google Scholar]
- 50.Zhou S., Kachhap S., Sun W., Wu G., Chuang A., Poeta L., Grumbine L., Mithani S.K., Chatterjee A., Koch W., Westra W.H., Maitra A., Glazer C., Carducci M., Sidransky D., McFate T., Verma A., Califano J.A. Frequency and phenotypic implications of mitochondrial DNA mutations in human squamous cell cancers of the head and neck. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(18):7540–7545. doi: 10.1073/pnas.0610818104. 17456604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Petros J.A., Baumann A.K., Ruiz-Pesini E., Amin M.B., Sun C.Q., Hall J., Lim S., Issa M.M., Flanders W.D., Hosseini S.H., Marshall F.F., Wallace D.C. mtDNA mutations increase tumorigenicity in prostate cancer. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(3):719–724. doi: 10.1073/pnas.0408894102. 15647368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu V.W., Shi H.H., Cheung A.N., Chiu P.M., Leung T.W., Nagley P., Wong L.C., Ngan H.Y. High incidence of somatic mitochondrial DNA mutations in human ovarian carcinomas. Cancer Research. 2001;61(16):5998–6001. 11507041 [PubMed] [Google Scholar]
- 53.Nishikawa M., Nishiguchi S., Shiomi S., Tamori A., Koh N., Takeda T., Kubo S., Hirohashi K., Kinoshita H., Sato E., Inoue M. Somatic mutation of mitochondrial DNA in cancerous and noncancerous liver tissue in individuals with hepatocellular carcinoma. Cancer Research. 2001;61(5):1843–1845. 11280735 [PubMed] [Google Scholar]
- 54.Esposito L.A., Melov S., Panov A., Cottrell B.A., Wallace D.C. Mitochondrial disease in mouse results in increased oxidative stress. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(9):4820–4825. doi: 10.1073/pnas.96.9.4820. 10220377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Szatrowski T.P., Nathan C.F. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Research. 1991;51(3):794–798. 1846317 [PubMed] [Google Scholar]
- 56.Brandon M., Baldi P., Wallace D.C. Mitochondrial mutations in cancer. Oncogene. 2006;25(34):4647–4662. doi: 10.1038/sj.onc.1209607. 16892079 [DOI] [PubMed] [Google Scholar]
- 57.Wallace D.C., Fan W. The pathophysiology of mitochondrial disease as modeled in the mouse. Genes & Development. 2009;23(15):1714–1736. doi: 10.1101/gad.1784909. 19651984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rigo P., Paulus P., Kaschten B.J., Hustinx R., Bury T., Jerusalem G., Benoit T., Foidart-Willems J. Oncological applications of positron emission tomography with fluorine-18 fluorodeoxyglucose. European Journal of Nuclear Medicine. 1996;23(12):1641–1674. doi: 10.1007/BF01249629. 8929320 [DOI] [PubMed] [Google Scholar]
- 59.Boros L.G., Lee P.W., Brandes J.L., Cascante M., Muscarella P., Schirmer W.J., Melvin W.S., Ellison E.C. Nonoxidative pentose phosphate pathways and their direct role in ribose synthesis in tumors: Is cancer a disease of cellular glucose metabolism? Medical Hypotheses. 1998;50(1):55–59. doi: 10.1016/s0306-9877(98)90178-5. 9488183 [DOI] [PubMed] [Google Scholar]
- 60.Buettner G.R. Superoxide dismutase in redox biology: the roles of superoxide and hydrogen peroxide. Anti-Cancer Agents in Medicinal Chemistry. 2011;11(4):341–346. doi: 10.2174/187152011795677544. 21453242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Geschwind J.F., Ko Y.H., Torbenson M.S., Magee C., Pedersen P.L. Novel therapy for liver cancer: direct intraarterial injection of a potent inhibitor of ATP production. Cancer Research. 2002;62(14):3909–3913. 12124317 [PubMed] [Google Scholar]
- 62.Le A., Cooper C.R., Gouw A.M., Dinavahi R., Maitra A., Deck L.M., Royer R.E., Vander Jagt D.L., Semenza G.L., Dang C.V. Inhibition of lactate dehydrogenase A induces oxidative stress and inhibits tumor progression. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(5):2037–2042. doi: 10.1073/pnas.0914433107. 20133848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lin X., Zhang F., Bradbury C.M., Kaushal A., Li L., Spitz D.R., Aft R.L., Gius D. 2-Deoxy-d-glucose-induced cytotoxicity and radiosensitization in tumor cells is mediated via disruptions in thiol metabolism. Cancer Research. 2003;63(12):3413–3417. 12810678 [PubMed] [Google Scholar]
- 64.Simons A.L., Ahmad I.M., Mattson D.M., Dornfeld K.J., Spitz D.R. 2-deoxy-d-glucose combined with cisplatin enhances cytotoxicity via metabolic oxidative stress in human head and neck cancer cells. Cancer Research. 2007;67(7):3364–3370. doi: 10.1158/0008-5472.CAN-06-3717. 17409446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Trachootham D., Alexandre J., Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nature Reviews. Drug Discovery. 2009;8(7):579–591. doi: 10.1038/nrd2803. 19478820 [DOI] [PubMed] [Google Scholar]
- 66.Jain S.K., Kannan K., Lim G. Ketosis (acetoacetate) can generate oxygen radicals and cause increased lipid peroxidation and growth inhibition in human endothelial cells. Free Radical Biology & Medicine. 1998;25(9):1083–1088. doi: 10.1016/s0891-5849(98)00140-3. 9870562 [DOI] [PubMed] [Google Scholar]
- 67.Abdelmegeed M.A., Kim S.K., Woodcroft K.J., Novak R.F. Acetoacetate activation of extracellular signal-regulated kinase 1/2 and p38 mitogen-activated protein kinase in primary cultured rat hepatocytes: role of oxidative stress. Journal of Pharmacology and Experimental Therapeutics. 2004;310(2):728–736. doi: 10.1124/jpet.104.066522. 15051799 [DOI] [PubMed] [Google Scholar]
- 68.Pelletier A., Coderre L. Ketone bodies alter dinitrophenol-induced glucose uptake through AMPK inhibition and oxidative stress generation in adult cardiomyocytes. American Journal of Physiology: Endocrinology and Metabolism. 2007;292(5):E1325–E1332. doi: 10.1152/ajpendo.00186.2006. 17227964 [DOI] [PubMed] [Google Scholar]
- 69.Poff A.M., Ari C., Seyfried T.N., D’Agostino D.P. The ketogenic diet and hyperbaric oxygen therapy prolong survival in mice with systemic metastatic cancer. PloS One. 2013;8(6):e65522. doi: 10.1371/journal.pone.0065522. 23755243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dhamija R., Eckert S., Wirrell E. Ketogenic diet. Canadian Journal of Neurological Sciences. 2013;40(2):158–167. doi: 10.1017/s0317167100013676. 23419562 [DOI] [PubMed] [Google Scholar]
- 71.Nangia S., Caraballo R.H., Kang H.C., Nordli D.R., Scheffer I.E. Is the ketogenic diet effective in specific epilepsy syndromes? Epilepsy Research. 2012;100(3):252–257. doi: 10.1016/j.eplepsyres.2012.01.015. 22424762 [DOI] [PubMed] [Google Scholar]
- 72.Mosek A., Natour H., Neufeld M.Y., Shiff Y., Vaisman N. Ketogenic diet treatment in adults with refractory epilepsy: a prospective pilot study. Seizure: The Journal of the British Epilepsy Association. 2009;18(1):30–33. doi: 10.1016/j.seizure.2008.06.001. 18675556 [DOI] [PubMed] [Google Scholar]
- 73.Hayashi A., Kumada T., Nozaki F., Hiejima I., Miyajima T., Fujii T. Changes in serum levels of selenium, zinc and copper in patients on a ketogenic diet using Keton formula. No to Hattatsu. Brain and Development. 2013;45(4):288–293. 23951940 [PubMed] [Google Scholar]
- 74.Westerterp-Plantenga M.S., Nieuwenhuizen A., Tomé D., Soenen S., Westerterp K.R. Dietary protein, weight loss, and weight maintenance. Annual Review of Nutrition. 2009;29:21–41. doi: 10.1146/annurev-nutr-080508-141056. 19400750 [DOI] [PubMed] [Google Scholar]
- 75.McNally M.A., Pyzik P.L., Rubenstein J.E., Hamdy R.F., Kossoff E.H. Empiric use of potassium citrate reduces kidney-stone incidence with the ketogenic diet. Pediatrics. 2009;124(2):e300–e304. doi: 10.1542/peds.2009-0217. 19596731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sampath A., Kossoff E.H., Furth S.L., Pyzik P.L., Vining E.P. Kidney stones and the ketogenic diet: risk factors and prevention. Journal of Child Neurology. 2007;22(4):375–378. doi: 10.1177/0883073807301926. 17621514 [DOI] [PubMed] [Google Scholar]
- 77.Bergqvist A.G., Schall J.I., Stallings V.A., Zemel B.S. Progressive bone mineral content loss in children with intractable epilepsy treated with the ketogenic diet. American Journal of Clinical Nutrition. 2008;88(6):1678–1684. doi: 10.3945/ajcn.2008.26099. 19064531 [DOI] [PubMed] [Google Scholar]
- 78.Yancy W.S., Jr., Olsen M.K., Guyton J.R., Bakst R.P., Westman E.C. A low-carbohydrate, ketogenic diet versus a low-fat diet to treat obesity and hyperlipidemia: a randomized, controlled trial. Annals of Internal Medicine. 2004;140(10):769–777. doi: 10.7326/0003-4819-140-10-200405180-00006. 15148063 [DOI] [PubMed] [Google Scholar]
- 79.Yancy W.S., Jr., Westman E.C., McDuffie J.R., Grambow S.C., Jeffreys A.S., Bolton J., Chalecki A., Oddone E.Z. A randomized trial of a low-carbohydrate diet vs orlistat plus a low-fat diet for weight loss. Archives of Internal Medicine. 2010;170(2):136–145. doi: 10.1001/archinternmed.2009.492. 20101008 [DOI] [PubMed] [Google Scholar]


