Abstract
Objective
Children with epilepsy often have reorganization of language networks and abnormal brain anatomy, making determination of language lateralization difficult. We characterized the proportion and distribution of language task activation in the cerebellum to determine the relationship to cerebral language lateralization.
Methods
Forty-six pediatric epilepsy surgery candidates (aged 7–19 years) completed an fMRI auditory semantic decision language task. Distribution of activated voxels and language laterality indices were computed using: (a) Broca's and Wernicke's areas and their right cerebral homologues; and (b) left and right cerebellar hemispheres. Language task activation was anatomically localized in the cerebellum.
Results
Lateralized language task activation in either cerebral hemisphere was highly correlated with lateralized language task activation in the contralateral cerebellar hemisphere (Broca vs. cerebellar: ρ = −0.54, p < 0.01). Cerebellar language activation was located within Crus I/II, areas previously implicated in non-motor functional networks.
Conclusions
Cerebellar language activation occurs in homologous regions of Crus I/II contralateral to cerebral language activation in patients with both right and left cerebral language dominance. Cerebellar language laterality could contribute to comprehensive pre-operative evaluation of language lateralization in pediatric epilepsy surgery patients. Our data suggest that patients with atypical cerebellar language activation are at risk for having atypical cerebral language organization.
Keywords: Functional MRI, Child, Aphasia, Functional Connectivity, Broca's area, Wernicke's area, Laterality index, Cerebellum
Highlights
-
•
We examine fMRI cerebellar language activation in pediatric epilepsy surgery patients.
-
•
A semantic decision task is employed to lateralize cerebral and cerebellar language.
-
•
Cerebral and contralateral cerebellar language activations are highly correlated.
-
•
Cerebellar language activation is located in right or left Crus I/II.
-
•
Cerebellar language laterality may aid pre-operative cerebral language localization.
1. Introduction
Epilepsy, a common childhood condition, is medically refractory in one third of patients (Kwan, and Brodie, 2000). Epilepsy surgery improves seizure control and developmental outcomes in a significant proportion of these children (Engel, 1996; Freitag, and Tuxhorn, 2005). Children with epilepsy are also more likely to have reorganization of language networks outside of classical language areas (Broca's and Wernicke's areas) in the typically dominant left hemisphere (Gaillard, 2007; Yuan, 2006). Although clinical or neuropsychological characteristics may provide clues to localization of language (Berl, 2014a), these features are not sufficiently reliable for prediction of language laterality in individual patients (Anderson, 2006; Baxendale, and Thompson, 2010). Therefore, methods to accurately localize language networks are an integral part of pre-operative pediatric epilepsy surgery work-up in order to predict and minimize post-operative language deficits (Bookheimer, 2007).
Pediatric functional MRI (fMRI) language methodology has been developed to non-invasively localize language in children (Gaillard, 2004; Wilke, 2006; Wood et al., 2004). fMRI protocols can target “expressive” (for instance, using verbal fluency (Gaillard, 2004) or letter tasks (Wilke, 2006)), and “receptive” (for instance, using auditory or reading comprehension tasks (Gaillard, 2004; Wilke, 2005)) aspects of language. Here we use the semantic decision task, which has been investigated in both adult and pediatric epilepsy populations (Szaflarski, 2008; You, 2009) and demonstrates good agreement with previously standard, invasive methodologies, such as intracarotid amobarbital injection (Gaillard, 2004; Binder, 1996; Desmond, 1995). Furthermore, this task generates consistent, strongly left cerebral hemisphere-lateralized activation that is highly specific for linguistic processing in healthy adults (Binder, 2008). Using this task, patients have been defined as having atypical language if one or both classical language areas demonstrate right hemisphere-lateralized or non-lateralized activation patterns (Gaillard, 2004; You, 2009; Fernández, 2001; Gaillard, 2002).
However, multiple different approaches have been developed to classify patients' cerebral language laterality (Wang et al., 2014; Wilke, and Lidzba, 2007; You, 2013; Abbott, et al., 2010; Knecht, 2003), and there is little consensus as to which methods are most reliable for clinical decision-making in individual patients. Most methods are particularly challenged by abnormal cerebral anatomy, which is a relatively common finding in patients with refractory epilepsy (Wellmer, 2009). Cerebellar activation has been given less attention than cerebral activation in presurgical language evaluation (for exception see: Brázdil, 2005; You, 2011). Interestingly, crossed cerebro-cerebellar activation has been noted during fMRI language tasks (Jansen, 2005). If a strong correlation between cerebral and cerebellar language activation patterns exists in epilepsy patients, analysis of cerebellar language laterality could provide complementary information about cerebral organization of language networks.
In support of potentially relevant lateralized cerebellar language activation, the cerebellum has strong neuroanatomical connectivity with the contralateral frontal lobe, including Broca's area (Schmahmann, 1996). Patients with cerebellar lesions often demonstrate semantic and syntactical processing deficits in addition to abnormal motor patterns of speech (Silveri, and Misciagna, 2000). Functional neuroimaging studies also converge upon a role for the cerebellum in various aspects of language function (Petersen, 1988) (for review, see Murdoch, 2010) and identify functional cerebellar topography for performance of different tasks (Stoodley, 2012). Intrinsic functional connectivity analyses have provided further support for the existence of segregated fronto-cerebellar circuits potentially capable of playing a role in cognitive processes, such as language (Krienen, and Buckner, 2009).
We hypothesized that cerebellar language activation would reflect cerebral language laterality in epilepsy patients, possibly representing an under-recognized indicator of language reorganization in this population. To address this hypothesis, we examined the relationship between cerebral and cerebellar language activation during a semantic decision task in pediatric epilepsy surgery patients.
2. Material and methods
2.1. Enrollment of subjects
Between 2006 and 2011, pediatric epilepsy surgery candidates were enrolled (n = 60; aged 4–19 years) from the Pediatric Epilepsy Surgery Program at British Columbia Children's Hospital (BCCH, Vancouver, Canada) into an ongoing multi-center collaborative study of pediatric pre-surgical language fMRI methodology (You, 2011). Written, informed consent was obtained from each subject and/or legal guardian upon enrollment. The study was approved by the Research Review Committee at BCCH, and by the Clinical Research Ethics Board at the University of British Columbia (UBC).
Sixty patients met initial criteria for inclusion in the study. Of these, 14 subjects were excluded because: (a) subjects did not (n = 5) or could not (n = 3) successfully perform the semantic decision task; (b) fMRI image quality was excessively degraded by subject head motion (n = 2); (c) fMRI data acquisition was unsatisfactory due to equipment malfunction (unable to hear stimuli, n = 1); (d) valid laterality analysis could not be completed due to extensive cerebral pathology (n = 2); or (e) an insufficient volume of cerebellum was imaged (<50%; n = 1).
2.2. Clinical data
Clinical records were reviewed and neurological, neurophysiological and neuroimaging data were tabulated (see Table 1). Neuropsychological test data were also collected but not reported in detail here. Seizure onset zone was established by clinical seizure semiology and EEG characteristics, as verified by a pediatric epileptologist. Neuroimaging data were reviewed by a pediatric neuroradiologist to identify and characterize brain lesions. Resected lesions were further characterized by histopathological examination.
Table 1.
Characteristics of pediatric epilepsy surgery candidates completing semantic decision fMRI task.
| Epilepsy surgery candidates | |
|---|---|
| Number of patients | 46 |
| Males | 28 |
| Right-handed | 39 |
| Age at fMRI (years; mean ± sd) | 14.2 ± 2.8 |
| Age at seizure onset (years; mean ± sd) | 7.2 ± 4.7 |
| Subsequent cortical resection | 19 |
| Lesion location | |
| Right hemisphere | 5 |
| Left hemisphere | 20 |
| Bilateral | 4 |
| Nonlesional | 17 |
| Seizure focus | |
| Right hemisphere | 9 |
| Left hemisphere | 34 |
| Bilateral | 3 |
| Lesion type | |
| Cortical dysplasia | 7 |
| Tumor | 5 |
| Cortical dysplasia + tumor | 1 |
| Inflammatory | 1 |
| Infarct | 3 |
| Vascular malformation | 2 |
| Hippocampal sclerosis | 3 |
| Unknown | 24 |
2.3. MRI data
Neuroimaging data were acquired at BCCH, on a 1.5 T Siemens Avanto (Siemens Canada Ltd., Mississauga, ON, Canada) MRI system, and at the UBC, High Field MRI Center, on a 3.0 T Philips Achieva (Philips Medical Systems, Best, Netherlands) MRI system. Some subjects were scanned at both sites. Each site uses a phased array 8-element receive headcoil. For anatomical co-registration with functional MRI datasets, high-resolution sagittal 3D T1-weighted datasets were obtained as follows: (a) for 1.5 T scans, data were collected using a FLASH sequence with the integrated parallel acquisition technique (iPAT) (TR = 18 ms, TE = 9.2 ms, flip angle = 30°, matrix size = 256 × 256, FOV = 256 × 256); (b) for 3.0 T scans, data were obtained using an MPRAGE sequence with sensitivity encoding (SENSE) (TR = 8.3 ms, TE = 3.9 s, flip angle = 8°, matrix size = 256 × 256, FOV = 284 × 284). Functional MRI data were acquired axially, with slices aligned parallel to each subject's own AC–PC plane, using BOLD echo-planar imaging sequences, with coverage of the entire cerebrum and most or all of the cerebellum (see Fig. 1): (a) for 1.5 T data (TR = 3000 ms, TE = 48 ms, flip angle = 90°, matrix size = 64 × 64, FOV = 220 × 220, 36 × 3.5 mm slices); (b) for 3.0 T data (TR = 2000 ms, TE = 30 ms, flip angle = 90°, matrix size = 80 × 80, FOV = 240 × 240, 36 × 3 mm slices, 1 mm gap).
Fig. 1.
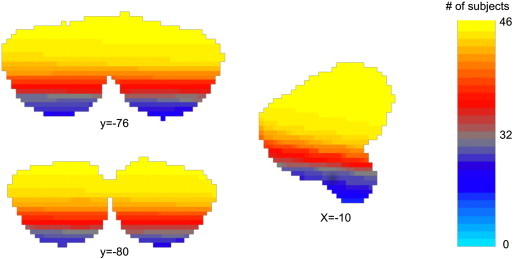
Summated cerebellar coverage. Sampling of data from the most inferior voxels of the cerebellum was incomplete. The figure demonstrates cerebellar coverage summated across all subjects after linearly registering (FLIRT, 12-dof) binarized functional image to the MNI space. Voxels sampled in >75% of subjects are depicted in yellow. Voxels sampled in <25% of subjects are depicted in gray or blue.
Audiovisual stimuli were presented at BCCH using MRI-compatible goggles and headphones (Resonance Technology Inc., CA, USA) and at UBC using a projector, projecting stimuli onto a screen attached to the MRI bore and viewed using a rear-facing mirror. Behavioral responses were recorded using a fiber-optic push button system (Photon Control, Burnaby, BC, Canada), monitored in the control room, and recorded by a Dell workstation running customized Matlab (Mathworks, Natick, MA, USA) software. Behavioral data were recorded during fMRI tasks, to monitor task performance. Anatomical and functional MRI datasets were acquired and reconstructed on the scanner console, and transferred to independent workstations for all subsequent data analysis.
2.4. fMRI paradigm
The experimental paradigm was an auditory decision (semantic) task previously described by Gaillard (2007). Given the wide age range of potential subjects, the paradigm was developed with several levels of difficulty available, varying in vocabulary, sentence structure, and semantic content provided. Each subject was individually trained on a grade-level appropriate version of the task prior to scanning. If the subject was unable to successfully complete training on one version of the task, a less difficult version was provided. This training ensured successful subsequent task performance. The 5 minute paradigm alternated between 30 s blocks of (a) an auditory semantic decision task: listen to a series of statements, e.g., “Something monkeys eat, is a banana”, and press a button each time a statement is “true”, and (b) an auditory tone detection task: listen for a tone embedded into a recording of reversed speech, and press a button each time a tone is heard. The number of targets was balanced across the two conditions. Subjects were instructed to maintain eye fixation on a cross, presented at the center of the screen.
2.5. Structural data
Structural data were processed following the methodology introduced by Klein et al. (2009). MNI152-1 mm brain and the MNI structural atlas package, available through the FMRIB Software Library (FSL, version 4.1.9) (Jenkinson, 2012; Smith, 2004; Woolrich, 2009), were used as template cerebellum ROIs (Collins et al., 1995; Mazziotta, 2001). Colin27 brain and the cytoarchitectonic probabilistic atlas were used as template Broca's and Wernicke's area ROIs (Holmes, 1998; Eickhoff, 2005). This methodology delineates the anatomical boundaries of Broca's and Wernicke's areas based on a probabilistic atlas, thereby avoiding derivation of ROIs from fMRI activation clusters and preventing “circular analysis” (Kriegeskorte, 2009). A cutoff value of 1/10 was used for the cytoarchitectonic templates so that each cytoarchitectonic region was at its largest size and included the effect of all the available post-mortem cytoarchitectonic data when masking the fMRI activations. FSL's Brain Extraction Tool (BET) (Smith, 2002) and FMRIB's Linear Image Registration Tool (FLIRT) (Jenkinson, 2002) were used for aligning each subject to template spaces, then the Automatic Registration Toolbox (Ardekani, 2005) was used for nonlinear registration of templates to aligned subject space. Population-based cytoarchitectural variability is not constrained by macroscopic sulcal and gyral boundaries; hence ROIs derived from the cytoarchitectonic atlas can span across macroscopic anatomical regions defined by surface anatomy (Rademacher, 1993), leading to overlap between the ROIs defining Broca's and Wernicke's areas and their homologues. To resolve this during laterality index computation, voxels simultaneously assigned to overlapping probabilistic cytoarchitectonic areas were equally divided between the two overlapping regions, so that no voxel would be counted twice. This rule was only applied to a small number of voxels, with negligible effect on results.
2.6. Functional MRI data analysis
FMRI data analysis was performed using FSL. fMRI pre-processing was performed using FSL's FMRI Expert Analysis Tool (FEAT) version 5.98 (Smith, 2004; Woolrich, 2009). Images underwent motion correction using MCFLIRT (Jenkinson, 2002), non-brain removal using BET, spatial smoothing using a Gaussian kernel of FWHM 4.0 mm, grand-mean intensity normalization of the entire 4D datasets by a single multiplicative factor, and highpass temporal filtering (Gaussian-weighted least-squares straight line fitting, with sigma = 50.0 s). Functional images were then registered to the high-resolution anatomical images using FLIRT. Time-series statistical analysis was carried out using FMRIB's Improved Linear Model (FILM) with local autocorrelation correction, and Z (Gaussianised T/F) statistic images were thresholded using clusters determined by Z > 2.3 and a corrected cluster significance threshold of p = 0.05 (from the Gaussian random field theory) (Worsley, 2001). Z-statistic images were masked with the regional masks created previously and the number of significant voxels was extracted.
2.7. Language laterality index (LI) computation
After extracting the number of significant voxels, laterality indices (LIs) were independently calculated for each pair of anatomically homologous regions (i.e., for Broca's area, Wernicke's area, combined cerebral (Broca's area + Wernicke's area), and hemi-cerebellum), using the following formula (Desmond, 1995):
We tabulated language lateralization of each of the above regions based on the following categories: right-lateralized (LI ≤ 0.2), left-lateralized (LI ≥ 0.2), non-lateralized (−0.2 < LI < 0.2) (Gaillard, 2007). Using these cutoffs, we defined atypical language as any combination of LIs that does not consist of left lateralization of Broca's and Wernicke's areas paired with right lateralization of the cerebellum. Patients with atypical language were identified for further qualitative analysis. Classification of patients for further quantitative group analysis was based on lateralization in Broca's area. Thus, patients were placed into one of three groups: Broca Right, Broca Left, or Broca Non-lateralized. Because only one subject was classified as Broca Non-lateralized, this group could not be included in statistical group analysis.
2.8. Statistics
Group analysis was performed on cerebral and cerebellar activation data, using Broca's area LI as described above. Cerebral group analysis was done using mixed effect modeling (Beckmann, Jenkinson, and Smith, 2003). Because alignment of subject data to a common cerebral space leads to suboptimal alignment of cerebellar data, we utilized the spatially unbiased infratentorial template (SUIT) toolbox, version 2.5.2 (Diedrichsen, 2009; Diedrichsen, 2006) running on SPM8 (Friston, et al., 2007), to bring functional statistics maps of each subject into a common cerebellar space. Then, cerebellar group analysis consisted of voxel-wise, one-sample t-tests of SUIT-aligned Z-statistical maps, obtained previously from FEAT analysis. Given that right cerebral language lateralization is uncommon, we expected that the Broca Right subjects would be under-represented in group-wise analysis, with reduced statistical power compared with the larger Broca Left group. To compensate for this lower statistical power and to better demonstrate comparable activation distribution between the groups, we constructed proportional “summary maps” (Eickhoff, 2005)” of the cerebellum, for the two groups.
Correlations between LIs were calculated using Spearman's rank correlation coefficient (Broca vs. cerebellar, Wernicke vs. cerebellar, combined cerebral vs. cerebellar). Comparison of means was accomplished using Student's t-test. Data are reported as mean ± standard error or mean ± standard deviation as appropriate.
2.9. Relationship to functional connectivity atlas
To relate data from our subjects to a general cerebro-cerebellar connectivity network model, the 7-Network functional connectivity atlases for the cerebellum (Buckner, 2011; Yeo, 2011) were coregistered to the Broca Left and Broca Right group averaged left and right cerebral and cerebellar hemispheres, using methods described previously. Then, we overlaid our cerebral and cerebellar language-dependent group activation statistical maps on the coregistered 7-Network atlas. This assigned each activation voxel to a membership in one of the seven networks.
3. Results
3.1. Subject demographics and clinical characteristics
Results are reported on 46 subjects, aged 7–19 years (Table 1). Average language function as assessed by scaled scores obtained on the Vocabulary, Similarities, and Comprehension subtests of the WISC or WAIS was 8.3 ± 2.9 (data available for 37/46 patients). Within our cohort, the majority of patients' zone of ictal onset and location of cerebral lesions (when present) were localized to the left cerebral hemisphere. A wide variety of cerebral lesions were identified, but cerebral lesion pathology was unverifiable in cases where neuroimaging findings were nonspecific and surgery was not performed. Neuroimaging did not reveal any congenital or acquired abnormalities of the cerebellum. Cortical resection, when performed, occurred after fMRI scanning.
3.2. Cerebral and cerebellar language task activation and laterality
Task-correlated cerebral activation was demonstrated in patterns expected for the semantic decision task (Binder, 2008) (Fig. 2). Three cerebral LIs were calculated based on: (1) Broca's area + right cerebral homologue; (2) Wernicke's area + right cerebral homologue; and (3) combined Broca's and Wernicke's areas + right cerebral homologues (Table 2). One cerebellar LI was calculated based on right and left cerebellar hemispheres.
Fig. 2.
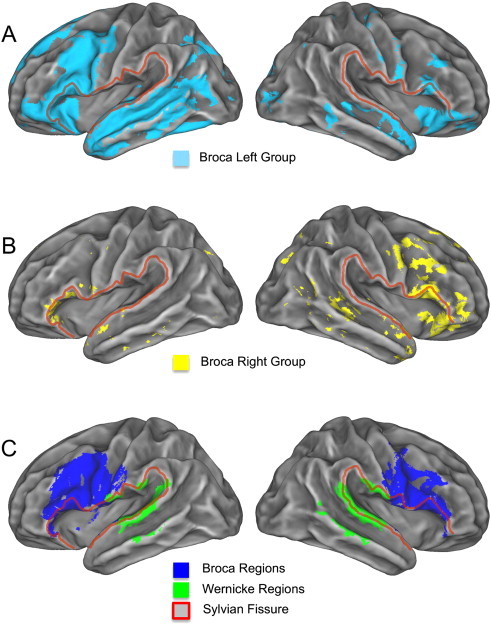
Group average activation and ROI masks. (A) Group average activations (Z > 2.3, p = 0.01) for the Broca Left group and (B) Broca Right group displayed on FreeSurfer average subject surface. (C) The cytoarchitectonic masks for the Broca and Wernicke areas are also overlaid on the surface. The Sylvian fissure is outlined in red.
Table 2.
Distribution of subjects based on cerebral and cerebellar laterality indices (LI). Each subject has been categorized as left lateralized (left), right lateralized (right) or non-lateralized (non) by several LIs: (1) Broca's area, (2) Wernicke's area, (3) combined cerebral (Broca's + Wernicke's areas), and (4) cerebellum. Crossed-cerebrocerebellar lateralization of language is evident.
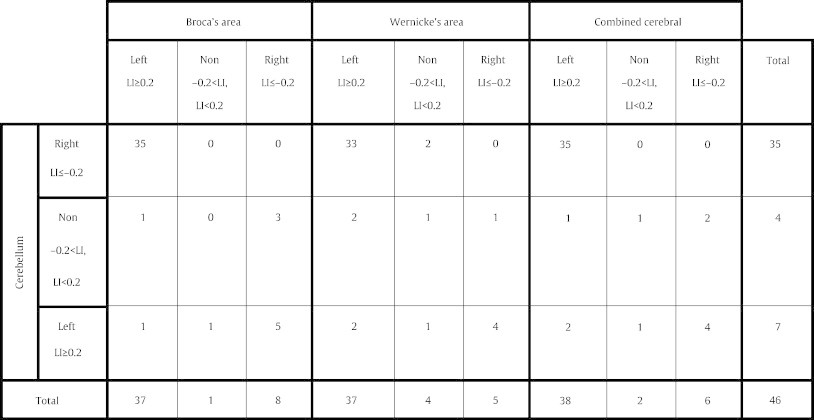
The combined cerebral LI was highly correlated with both Broca's area LI (ρ = 0.94) and Wernicke's area LI (ρ = 0.72), but did not accurately reflect patients with dissociated language laterality (i.e., contralateral language dominance in Wernicke's area relative to Broca's area). In addition, neuroanatomical connectivity between classical language areas and the cerebellum is more firmly established for Broca's area, and activation in Broca's area homologue is the least variable component of language activation in subjects with right hemisphere-dominant language (You, 2011). For this reason, we used the language lateralization in Broca's area to divide our patients into three groups: (1) Broca Left (n = 37), (2) Broca Right (n = 8), and (3) Broca Non-lateralized (n = 1).
In our pediatric epilepsy subjects, we found that lateralized language task activation in either cerebral hemisphere was highly correlated with lateralized language task activation in the contralateral cerebellar hemisphere. This relationship was readily apparent in single subject datasets and in pooled group analysis, regardless of cerebral lateralization (Fig. 3).
Fig. 3.
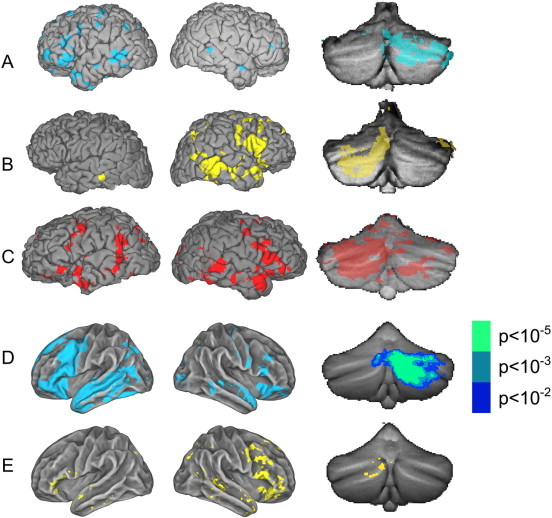
Typical and group averaged activation of the cerebrum and cerebellum.(A) Single subject activation data from a representative Broca Left subject, overlaid on left and right cerebral surfaces rendered in the subject's native space (FreeSurfer); at far right, the same single subject's cerebellar activation rendered, after alignment with the spatially unbiased infratentorial template (SUIT) (Z > 2.3, p = 0.01). (B) Single subject activation data from a typical Broca Right subject, with cerebral and cerebellar rendering as in (A). (C) Single Broca Non-lateralized subject data, with cerebral and cerebellar rendering as in (A). (D) Group averaged activation data from all Broca Left subjects overlaid on FreeSurfer average subject surfaces, with cerebellar data rendered on SUIT. (E) Group averaged activation data from all Broca Right subjects. For group cerebellum activations, the Broca Left group is shown with 3 levels of threshold: p = 0.01, p = 0.001, and p = 0.00001. For the Broca Right group, activation is shown only at threshold p = 0.01.
3.3. Relationship of cerebral and cerebellar LIs to clinical features
The majority of patients had cerebral language network activation that was left-lateralized, both in the inferior frontal gyrus (Broca's area) and in the superior temporal gyrus (Wernicke's area), whereas it was right-lateralized in the cerebellum. This is the typical pattern. Subjects with language organization that differs from this pattern (i.e., those with an atypical pattern) are uncommon, and of special interest. Therefore, we identified patients from each group with atypical patterns for further qualitative analysis (Table 3).
Table 3.
Detailed clinical characteristics of patients with atypical language lateralizationa.
| Patient number | Broca laterality index | Wernicke laterality index | Cerebellar laterality index | Handedness L = left R = right |
Age at fMRI (years) |
Seizure onset (years) | Seizure focus | Lesion location | Lesion type | Language (scaled scores)b |
|---|---|---|---|---|---|---|---|---|---|---|
| Broca Right patients | ||||||||||
| 6 | –0.36 | 0.05 | 0.03 | R | 14 | 0.25 | Left parietal | Left parietal occipital | Cortical dysplasia | 6 |
| 8 | –1.00 | –1.00 | 0.82 | R | 12 | 8 | Left parietal | Normal | Unknown | Unknown |
| 13 | –0.48 | –1.00 | –0.01 | R | 15 | 0.003 | Left parietal | Left frontal parietal; right frontal | Perinatal stroke | Unknown |
| 25 | –0.90 | –1.00 | 1.00 | L | 10 | 8 | Left frontal | Left frontal temporal | Perinatal stroke | 7 |
| 37 | –1.00 | 0.26 | n/ac | R | 12 | 7 | Left frontal | Normal | Cortical dysplasia | 10 |
| 38 | –0.28 | –0.74 | 0.27 | L | 16 | 1 | Left frontal | Left frontal parietal occipital | Pachygyria, polymicrogyria | 5 |
| 49 | –0.50 | 1.00 | 1.00 | R | 16 | 7 | Left temporal | Left temporal | Tumor | 9 |
| 60 | –0.25 | –0.98 | 0.60 | R | 11 | 3 | Left temporal | Left temporal | Polymicrogyria | 4 |
| Additional atypical patients | ||||||||||
| 5 | 0.25 | 0.16 | –0.48 | R | 14 | 7 | Left temporal | Left temporal | Unknown | 8 |
| 40 | –0.10 | 0.17 | 0.42 | R | 10 | 0.003 | Left temporal | Left temporal | Tumor + cortical dysplasia | 7 |
| 43 | 0.84 | 0.13 | –0.38 | R | 10 | 9 | Left temporal | Left & right temporal | Cortical dysplasia | Unknown |
| 51 | 0.22 | 0.89 | 0.98 | R | 14 | 7 | Left frontal | Normal | Unknown | 9 |
| 55 | 0.46 | 0.21 | 0.19 | R | 16 | 15 | Left & right temporal | Left frontal | Vascular malformation | 10 |
Atypical language defined as any patient who does not have left lateralization of Broca's and Wernicke's areas paired with right lateralization of the cerebellum.
Language function of patients with right or mixed cerebral language dominance was assessed using an average of the scaled scores obtained on the Vocabulary, Similarities, and Comprehension subtests of the Weschler Intelligence Scale for Children (WISC) or Weschler Adult Intelligence Scale (WAIS) depending on age of the child being tested.
Not applicable (n/a) as patient did not have above-threshold cerebellar language task activation.
All Broca Left subjects had right-lateralized cerebellar LIs except for two patients. Cerebellar LIs in these patients were left-lateralized (n = 1) or non-lateralized (n = 1). One of these patients had bilateral seizure foci in the context of a vascular malformation, and the other had a left sided seizure focus with a normal structural MRI.
Each Broca Right subject had a left cerebral seizure focus, and all but one had left cerebral pathology confirmed by neuroimaging and/or histopathology. The type and extent of lesion were variable, however, ranging from a relatively small area of microdysgenesis to large perinatal infarction. Similarly, only three patients had associated right-sided incoordination or hemiparesis. Among these subjects, cerebellar activation was either left-lateralized (n = 5), non-lateralized (n = 2), or subthreshold (n = 1), but was never right-lateralized (n = 0); see Fig. 4 for Broca Right subjects' individual cerebellar activation data. The only patient with subthreshold cerebellar activation exhibited crossed cerebral language laterality (right-lateralized in Broca's area and left-lateralized in Wernicke's area) and a left frontal seizure focus despite normal structural neuroimaging (patient 37).
Fig. 4.
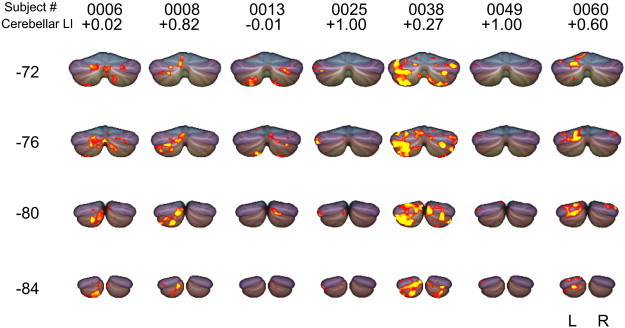
Individual cerebellar activation of the Broca Right subjects. Functional Z-statistics map (Z > 2.3) of the Broca Right subjects. Coronal sections of y = –72, –76, –80, –84 in MNI standard space shown.
The single Broca Non-lateralized patient had left-lateralized cerebellar activation (patient 40). This patient had neonatal seizure onset associated with left temporal lobe pathology (mixed tumor and cortical dysplasia).
3.4. Cerebellar language task activation and laterality
Next, we quantified the relationship between cerebral and cerebellar language laterality in our patient cohort. There was a negative linear correlation between LIs derived from Broca's and Wernicke's areas, and those from the cerebellar hemispheres in our patient cohort (Fig. 5A: Broca vs. cerebellar, ρ = −0.54, p < 0.01 (two-tailed); Fig. 5B: Wernicke vs. cerebellar, ρ = −0.45, p < 0.01 (two-tailed)). This relationship was evident throughout a wide range of cerebral LIs. The combined cerebral LI similarly demonstrated a negative correlation with cerebellar LIs (ρ = −0.58).
Fig. 5.
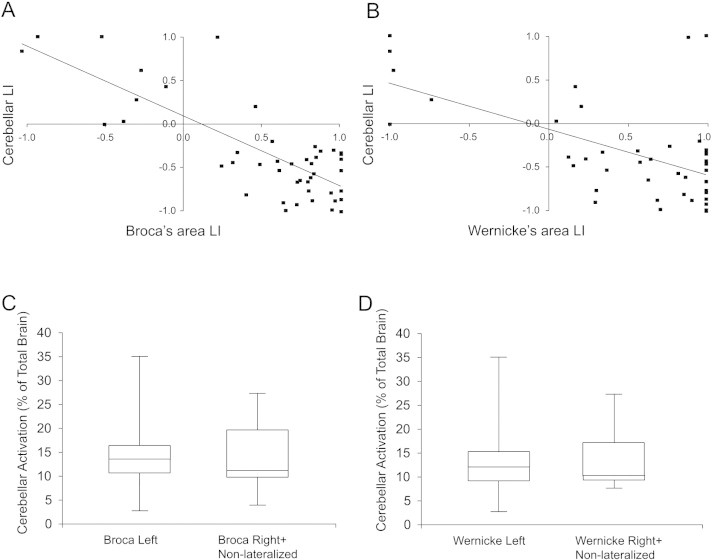
Relationship of cerebral and cerebellar laterality indices.(A) Plot of Broca's area laterality indices (LIs) vs. cerebellar LIs for all subjects. Left lateralization is represented by positive LIs and right lateralization by negative LIs. The contralateral relationship between Broca's area and cerebellar LIs can be seen. (B) Plot of Wernicke's area LIs vs. cerebellar LIs for all subjects. (C) Percentage of cerebellar-activated voxels to whole brain activated voxel counts in patients with left and right lateralized language in Broca's area. (D) Percentage of cerebellar-activated voxel to whole brain activated voxel counts in patients with left and right lateralized language in Wernicke's area.
To determine the contribution of cerebellar language-task activation to overall brain activation during semantic decision task, we calculated the proportion of significantly active voxels in the whole brain that were located in the cerebellum. Activation located in the cerebellum represented 12 ± 1% (mean ± standard error) of total brain language-task activation in our patient cohort. This percentage was similar in patients with left compared to right cerebral lateralization based on Broca's area LI (Fig. 5C) or Wernicke's area LI (Fig. 5D).
3.5. Localization of cerebellar activation
To investigate the cerebellar language task activation in more detail, we examined localization of cerebellar activation in our groups defined by Broca's area LI. Group analysis of Broca Left subjects (n = 37) demonstrated activation in the right cerebellar hemisphere, predominantly localized above and below the horizontal fissure, in Crus I and Crus II of Lobule VII (Fig. 6 lower panel, column A; cluster centroid at SUIT Atlas coordinates 22, –76, –35). In contrast, group analysis of Broca Right subjects with above-threshold cerebellar activation (n = 7) demonstrated homologous activation in left cerebellar Crus I and II, though with smaller cluster size and less activation (Fig. 6 lower panel, column B; cluster centroid SUIT atlas coordinates –7, –81, –33).
Fig. 6.
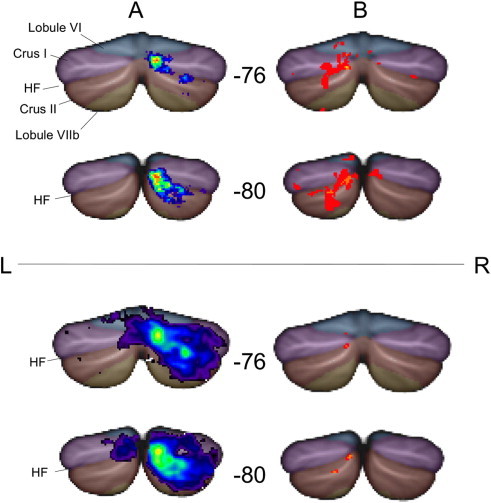
Localization of language task activation in the cerebellum. Cerebellar activation data from Broca Left (column A) and Broca Right (column B) subjects are displayed using the spatially unbiased infratentorial template (SUIT) in MNI standard space, with SUIT anatomical parcellation overlaid (horizontal fissure is denoted HF). Coronal slices at y = −76, and y = −80 are shown. Data were generated by two methods. The upper panel displays functional activation summary maps, thresholded to show only those voxels that are commonly activated by at least 2 of 7 subjects for the Broca Right group, and by a similar fraction (27%, i.e., at least 10/37) of the Broca Left group. The lower panel displays the 2nd level group analysis data, using one-sample t-tests in SPM8 (p = 0.01, uncorrected). Regardless of the method of analysis, lateralized cerebellar activation (contralateral to cerebral activation) is evident in Crus I & Crus II.
Proportional summary maps of cerebellar activation for Broca Left subjects (Fig. 6 upper panel, column A) and Broca Right subjects (Fig. 6 upper panel, column B) exemplify that despite the smaller number of Broca Right subjects represented in the cohort, activation occurs in strikingly homologous left cerebellar regions.
3.6. Relationship to functional connectivity data
To shed light on the possible functional implications of the observed localized cerebellar language activation, we related data from our subjects to a general cerebro-cerebellar connectivity network model (You, 2011; Buckner, 2011). Among Broca Left subjects, the default network and the frontoparietal network accounted for 40.5% and 15.7% of left cerebral activation, and for 58.2% and 30.9% of right cerebellar activation, respectively. Among Broca Right subjects, the default network and the frontoparietal network accounted for 31.1% and 42.8% of right cerebral activation, and for 31.2% and 31.1% of left cerebellar activation, respectively. For both groups, much smaller proportions were assigned to the other five networks in each of the cerebral and cerebellar hemispheres.
4. Discussion
We established that significant language task activation occurs in the cerebellum in pediatric epilepsy surgery patients engaged in a semantic decision task. This activation is consistently located in Crus I/II of the cerebellar hemisphere contralateral to cerebral language activation, in subjects with left or right lateralized cerebral language.
Our data provide support for the hypothesis that cerebellar activation during a language task importantly reflects cerebral language laterality, and that crossed cerebro-cerebellar language activation may be a key conserved feature of language networks despite reorganization due to cerebral lesions and/or epilepsy.
Above-threshold cerebellar activation during language tasks has not been consistently demonstrated across all language tasks in children. One study, using verb generation and orthographic lexical retrieval tasks, found that 100% of normal adult subjects exhibited language task-dependent cerebellar activation, compared with only 67% of normal children (Wood, 2004). In contrast, our results show that all patients except one (45/46; 98%) had above-threshold cerebellar activation during the semantic decision task. Moreover, cerebellar activation represents on average 12% of whole brain language-activated voxels in our patients, with the majority of patients ranging between 5 and 15%. There was no significant difference in the proportion of cerebellar activation between patients with left or right lateralized cerebral language. Language task cerebellar activation therefore appears to be a conserved and substantial finding in our population. It is unclear whether the higher rate of cerebellar activation in our single subject pediatric data is due to enhanced cerebellar network participation in semantic tasks in this age group, differences in level of general task difficulty, or both. In support of the importance of semantic processing in recruiting cerebellar networks, studies in normal adults show that cerebellar activation increases in response to progressively more difficult semantic tasks (Xiang, 2003). Cerebellar activation during semantic decision tasks has also been noted, though not quantified, in epilepsy surgery candidates (Binder, 1996) and children (Wilke, 2006).
Current evidence suggests that language processing is lateralized in the cerebellum (Marien, 2001; Stoodley, Valera, and Schmahmann, 2010). In healthy right-handed subjects, cerebellar language task activation occurs in the right cerebellar hemisphere (Frings, 2006). Note has been made of right cerebellar activation in adult and pediatric epilepsy surgery patients with left cerebral activation during semantic language tasks (You, 2011; Szaflarski, 2002). Here, we extend this finding by showing a highly significant linear correlation between Broca's area and contralateral cerebellar activation across a wide range of cerebral LIs in pediatric epilepsy patients. In normally developing children, stronger language task activation in the right cerebellum is correlated with better language skills (Berl, 2014b), implying possible functional ramifications of cerebellar language lateralization. Furthermore, we found that the lateralized activation generated by semantic decision task in our pediatric patients predominantly engaged regions that demonstrate functional connectivity in the resting state. Similar results were found in the left cerebral and right cerebellar hemispheres of normal adult subjects (Wang, Buckner, and Liu, 2013). Taken together, these results suggest that co-activation of the cerebral and contralateral cerebellar regions associated with common networks may represent a key component of language task engagement.
Crossed cerebro-cerebellar language activation in subjects with right lateralized cerebral language has been observed, but the fidelity of this relationship has not been fully established due to small numbers of subjects. For instance, contralateral right cerebral–left cerebellar language lateralization has been demonstrated in small numbers of normal adults (7 subjects in Jansen, 2005; 1 subject in Hubrich-Ungureanu, 2002; 1 subject in Chee, 1999 and in patients with congenital left cerebral hemisphere lesions (5 subjects; (Lidzba, 2008)). Left handedness, right cerebral and left cerebellar language lateralization has also been demonstrated in a monozygotic twin with equivalent language function to her right handed, left cerebral and right cerebellar language lateralized sibling (Lux, 2008). Our data extend these observations by showing that pediatric epilepsy patients with right lateralized cerebral language display linearly correlated left cerebellar activation. The majority of these patients did not have large congenital left cerebral hemisphere lesions, right hemiparesis, or left-handedness, suggesting that left cerebellar language dominance is not necessarily a consequence of extensive left cerebral pathology.
A small subset of our cohort demonstrated non-lateralized or subthreshold cerebellar language activation. This finding was associated with bilateral cortical pathology, bilateral electrographic seizure onset, dissociated cerebral language (right-lateralized in Broca's area, and left-lateralized in Wernicke's area), and neonatal seizure onset. Although the number of patients in this subset is small, there is a suggestion that non-lateralized or subthreshold cerebellar language activation is indicative of bilateral cerebral pathologic processes that may induce substantial inter- and intra-hemispheric language reorganization.
We found that cerebellar language activation in patients with left cerebral language dominance was located primarily in right Crus I/II, in keeping with previously identified functional topography of the cerebellum (Stoodley, and Schmahmann, 2009). Although statistically underpowered, our analysis of patients with right cerebral language dominance demonstrated cerebellar activation in an approximately homologous region of left Crus I/II. Healthy adults with right cerebral language dominance demonstrated similarly localized left cerebellar language activation (Jansen, 2005). Taken together, these data imply that homologous regions of the cerebellum support language function, akin to right cerebral hemisphere language area homologues.
This functional relationship of contralateral cerebellum to the cerebral cortex was more evident in Broca’s area than Wernicke’s area (or homologues) in our patients. Correspondingly, neuroanatomical studies demonstrate a closed loop network between frontal non-motor areas (including Broca's area) and lateral cerebellar regions, providing a neuroanatomical substrate for these interactions (Buckner, 2011; Leiner et al., 1986; Strick, Dum, and Fiez, 2009). This crossed cerebro-cerebellar connectivity appears to be a key component of language network organization, as demonstrated by response to various brain insults. Right cerebellar hemisphere damage in previously normal adults can generate a variety of language deficits, some of which are associated with frontoparietal SPECT hypoperfusion in the absence of any other observable cortical pathology (Marien, 2001; Mariën, 1996; Fiez, 1992). Right cerebral and left cerebellar language task activation also increases during recovery after left hemisphere stroke in patients with previous left cerebral hemisphere language dominance (Connor, 2006). Our data similarly demonstrate that crossed cerebro-cerebellar language activation was maintained in across our patient population despite refractory seizure activity that might disrupt normal network development. These findings are in keeping with the significant evidence for the role of the cerebellum in non-motor aspects of language (Murdoch, 2010).
One limitation of our dataset was that not all our patients had complete imaging coverage of the inferior cerebellum (see Fig. 1 for cerebellar coverage). However, our results should provide valid lateralization of cerebellar language activation, which involves the upper and middle cerebellum in normal subjects (Stoodley, and Schmahmann, 2009). The lower cerebellum is also involved in some aspects of language processing (for instance, verbal interference in multilingual subjects (Filippi, et al. 2011), and we acknowledge that it would be optimal to include the entire cerebellum in every subject.
5. Conclusions
The goal of language fMRI in epilepsy surgery planning is to not only lateralize, but also localize language with the aim of minimizing post-operative language deficits. Cerebellar language laterality can be reliably established in pediatric epilepsy patients, and is linearly correlated with cerebral language laterality in subjects with left and right cerebral language dominance. The contribution of pre-operative cerebellar language laterality to surgical decision-making and language outcomes appears to warrant further investigation. We suggest that patients with cerebellar language activation that is (1) ipsilateral to cerebral language activation, (2) non-lateralized, or (3) subthreshold, should be flagged for careful review prior to surgery as they appear to be at higher risk of demonstrating atypical cerebral language organization.
Contributors
Dr. Sare Akdag (Pediatric Neuropsychologist, Department of Pediatrics, University of British Columbia) – assistance with neuropsychological data.
Dr. Randy L. Buckner (Athinoula A. Martinos Center for Biomedical Imaging, Harvard University) – provision of functional connectivity atlases
Dr. Mary Connolly (Pediatric Epileptologist Division of Neurology, University of British Columbia) – verification of patient seizure characteristics and electrophysiologic data.
Dr. William D. Gaillard (Children's National Medical Center, Washington, DC) – provision of stimuli used for fMRI language task.
Mr. Boris Kuzeljevic (Clinical Research Support Unit Consultant, Statistician/ Methodologist/ Database Developer for Child and Family Research Institute (CFRI)) – consultation on statistical methodology.
Dr. Kenneth Poskitt (Pediatric Neuroradiologist, Division of Neurology, University of British Columbia) – review of structural cerebellum neuroimaging data.
Contributor Information
Jennifer N. Gelinas, Email: jennifer.gelinas@nyumc.org.
Kevin P.V. Fitzpatrick, Email: kfitzpatrick@cw.bc.ca.
Hong Cheol Kim, Email: dkim-02@cw.bc.ca.
Bruce H. Bjornson, Email: bbjornson@cw.bc.ca.
References
- Abbott D.F. fMRI assessment of language lateralization: an objective approach. NeuroImage. 2010;50(4):1446–1455. doi: 10.1016/j.neuroimage.2010.01.059. 20097290 [DOI] [PubMed] [Google Scholar]
- Anderson D.P. FMRI lateralization of expressive language in children with cerebral lesions. Epilepsia. 2006;47(6):998–1008. doi: 10.1111/j.1528-1167.2006.00572.x. 16822246 [DOI] [PubMed] [Google Scholar]
- Ardekani B.A. Quantitative comparison of algorithms for inter-subject registration of 3D volumetric brain MRI scans. Journal of Neuroscience Methods. 2005;142(1):67–76. doi: 10.1016/j.jneumeth.2004.07.014. 15652618 [DOI] [PubMed] [Google Scholar]
- Baxendale S., Thompson P. Beyond localization: the role of traditional neuropsychological tests in an age of imaging. Epilepsia. 2010;51(11):2225–2230. doi: 10.1111/j.1528-1167.2010.02710.x. 21175602 [DOI] [PubMed] [Google Scholar]
- Beckmann C.F., Jenkinson M., Smith S.M. General multilevel linear modeling for group analysis in FMRI. NeuroImage. 2003;20(2):1052–1063. doi: 10.1016/S1053-8119(03)00435-X. 14568475 [DOI] [PubMed] [Google Scholar]
- Berl M.M. Characterization of atypical language activation patterns in focal epilepsy. Annals of Neurology. 2014;75(1):33–42. doi: 10.1002/ana.24015. 24038442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berl M.M. Regional differences in the developmental trajectory of lateralization of the language network. Human Brain Mapping. 2014;35(1):270–284. doi: 10.1002/hbm.22179. 23033058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder J.R. A comparison of five fMRI protocols for mapping speech comprehension systems. Epilepsia. 2008;49(12):1980–1997. doi: 10.1111/j.1528-1167.2008.01683.x. 18513352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder J.R. Determination of language dominance using functional MRI: a comparison with the Wada test. Neurology. 1996;46(4):978–984. doi: 10.1212/wnl.46.4.978. 8780076 [DOI] [PubMed] [Google Scholar]
- Bookheimer S. Pre-surgical language mapping with functional magnetic resonance imaging. Neuropsychology Review. 2007;17(2):145–155. doi: 10.1007/s11065-007-9026-x. 17484055 [DOI] [PubMed] [Google Scholar]
- Brázdil M. Reorganization of language-related neuronal networks in patients with left temporal lobe epilepsy — an fMRI study. European Journal of Neurology: the Official Journal of the European Federation of Neurological Societies. 2005;12(4):268–275. doi: 10.1111/j.1468-1331.2004.01127.x. 15804243 [DOI] [PubMed] [Google Scholar]
- Buckner R.L. The organization of the human cerebellum estimated by intrinsic functional connectivity. Journal of Neurophysiology. 2011;106(5):2322–2345. doi: 10.1152/jn.00339.2011. 21795627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee M.W.L. Auditory and visual word processing studied with fMRI. Human Brain Mapping. 1999;7(1):15–28. doi: 10.1002/(SICI)1097-0193(1999)7:1<15::AID-HBM2>3.0.CO;2-6. 9882087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins D.L., Holmes C.J., Peters T.M., Evans A.C. Automatic 3-D model-based neuroanatomical segmentation. Human Brain Mapping. 1995;3(3):190–208. [Google Scholar]
- Connor L.T. Cerebellar activity switches hemispheres with cerebral recovery in aphasia. Neuropsychologia. 2006;44(2):171–177. doi: 10.1016/j.neuropsychologia.2005.05.019. 16019040 [DOI] [PubMed] [Google Scholar]
- Desmond J.E. Functional MRI measurement of language lateralization in Wada-tested patients. Brain: A Journal of Neurology. 1995;118(6):1411–1419. doi: 10.1093/brain/118.6.1411. 8595473 [DOI] [PubMed] [Google Scholar]
- Diedrichsen J. A probabilistic MR atlas of the human cerebellum. Neuroimage. 2009;46(1):39–46. doi: 10.1016/j.neuroimage.2009.01.045. 19457380 [DOI] [PubMed] [Google Scholar]
- Diedrichsen J.r. A spatially unbiased atlas template of the human cerebellum. NeuroImage. 2006;33(1):127–138. doi: 10.1016/j.neuroimage.2006.05.056. 16904911 [DOI] [PubMed] [Google Scholar]
- Eickhoff S.B. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. NeuroImage. 2005;25(4):1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. 15850749 [DOI] [PubMed] [Google Scholar]
- Engel J., Jr. Surgery for seizures. New England Journal of Medicine. 1996;334(10):647–652. doi: 10.1056/NEJM199603073341008. 8592530 [DOI] [PubMed] [Google Scholar]
- Fernández G. Language mapping in less than 15 minutes: real-time functional MRI during routine clinical investigation. NeuroImage. 2001;14(3):585–594. doi: 10.1006/nimg.2001.0854. 11506532 [DOI] [PubMed] [Google Scholar]
- Fiez J.A. Impaired non-motor learning and error detection associated with cerebellar damage. A single case study. Brain: A Journal of Neurology. 1992;115(1):155–178. doi: 10.1093/brain/115.1.155. 1559151 [DOI] [PubMed] [Google Scholar]
- Filippi R. The right posterior paravermis and the control of language interference. Journal of Neuroscience: the Official Journal of the Society for Neuroscience. 2011;31(29):10732–10740. doi: 10.1523/JNEUROSCI.1783-11.2011. 21775616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitag H., Tuxhorn I. Cognitive function in preschool children after epilepsy surgery: rationale for early intervention. Epilepsia. 2005;46(4):561–567. doi: 10.1111/j.0013-9580.2005.03504.x. 15816951 [DOI] [PubMed] [Google Scholar]
- Frings M. Cerebellar involvement in verb generation: an fMRI study. Neuroscience Letters. 2006;409(1):19–23. doi: 10.1016/j.neulet.2006.08.058. 17046160 [DOI] [PubMed] [Google Scholar]
- Friston K.J. Statistical Parametric Mapping: the Analysis of Functional Brain Images. Academic Press; 2007. [Google Scholar]
- Gaillard W.D. Atypical language in lesional and nonlesional complex partial epilepsy. Neurology. 2007;69(18):1761–1771. doi: 10.1212/01.wnl.0000289650.48830.1a. 17967992 [DOI] [PubMed] [Google Scholar]
- Gaillard W.D. fMRI language task panel improves determination of language dominance. Neurology. 2004;63(8):1403–1408. doi: 10.1212/01.wnl.0000141852.65175.a7. 15505156 [DOI] [PubMed] [Google Scholar]
- Gaillard W.D. Language dominance in partial epilepsy patients identified with an fMRI reading task. Neurology. 2002;59(2):256–265. doi: 10.1212/wnl.59.2.256. 12136067 [DOI] [PubMed] [Google Scholar]
- Holmes C.J. Enhancement of MR images using registration for signal averaging. Journal of Computer Assisted Tomography. 1998;22(2):324–333. doi: 10.1097/00004728-199803000-00032. 9530404 [DOI] [PubMed] [Google Scholar]
- Hubrich-Ungureanu P. Lateralized organization of the cerebellum in a silent verbal fluency task: a functional magnetic resonance imaging study in healthy volunteers. Neuroscience Letters. 2002;319(2):91–94. doi: 10.1016/s0304-3940(01)02566-6. 11825678 [DOI] [PubMed] [Google Scholar]
- Jansen A. Crossed cerebro-cerebellar language dominance. Human Brain Mapping. 2005;24(3):165–172. doi: 10.1002/hbm.20077. 15486988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M. FSL. Neuroimage. 2012;62(2):782–790. doi: 10.1016/j.neuroimage.2011.09.015. 21979382 [DOI] [PubMed] [Google Scholar]
- Jenkinson M. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17(2):825–841. doi: 10.1016/s1053-8119(02)91132-8. 12377157 [DOI] [PubMed] [Google Scholar]
- Klein A. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. Neuroimage. 2009;46(3):786–802. doi: 10.1016/j.neuroimage.2008.12.037. 19195496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knecht S. How atypical is atypical language dominance? NeuroImage. 2003;18(4):917–927. doi: 10.1016/s1053-8119(03)00039-9. 12725767 [DOI] [PubMed] [Google Scholar]
- Kriegeskorte N. Circular analysis in systems neuroscience: the dangers of double dipping. Nature Neuroscience. 2009;12(5):535–540. doi: 10.1038/nn.2303. 19396166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krienen F.M., Buckner R.L. Segregated fronto-cerebellar circuits revealed by intrinsic functional connectivity. Cerebral Cortex (New York, N.Y.: 1991) 2009;19(10):2485–2497. doi: 10.1093/cercor/bhp135. 19592571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan P., Brodie M.J. Early identification of refractory epilepsy. New England Journal of Medicine. 2000;342(5):314–319. doi: 10.1056/NEJM200002033420503. 10660394 [DOI] [PubMed] [Google Scholar]
- Leiner H.C., Leiner A.L., Dow R.S. Does the cerebellum contribute to mental skills? Behavioral Neuroscience. 1986;100(4):443–454. doi: 10.1037//0735-7044.100.4.443. 3741598 [DOI] [PubMed] [Google Scholar]
- Lidzba K. Reorganization of the cerebro-cerebellar network of language production in patients with congenital left-hemispheric brain lesions. Brain and Language. 2008;106(3):204–210. doi: 10.1016/j.bandl.2007.11.003. 18158178 [DOI] [PubMed] [Google Scholar]
- Lux S. Crossed cerebral lateralization for verbal and visuo-spatial function in a pair of handedness discordant monozygotic twins: MRI and fMRI brain imaging. Journal of Anatomy. 2008;212(3):235–248. doi: 10.1111/j.1469-7580.2008.00855.x. 18304205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariën P. Cerebellar induced aphasia: case report of cerebellar induced prefrontal aphasic language phenomena supported by SPECT findings. Journal of the Neurological Sciences. 1996;144(1–2):34–43. doi: 10.1016/s0022-510x(96)00059-7. 8994102 [DOI] [PubMed] [Google Scholar]
- Marien P. The lateralized linguistic cerebellum: a review and a new hypothesis. Brain and Language. 2001;79(3):580–600. doi: 10.1006/brln.2001.2569. 11781058 [DOI] [PubMed] [Google Scholar]
- Mazziotta J. A probabilistic atlas and reference system for the human brain: International Consortium for Brain Mapping (ICBM) Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 2001;356(1412):1293–1322. doi: 10.1098/rstb.2001.0915. 11545704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdoch B.E. The cerebellum and language: historical perspective and review. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior. 2010;46(7):858–868. doi: 10.1016/j.cortex.2009.07.018. 19828143 [DOI] [PubMed] [Google Scholar]
- Petersen S.E. Positron emission tomographic studies of the cortical anatomy of single-word processing. Nature. 1988;331(6157):585–589. doi: 10.1038/331585a0. 3277066 [DOI] [PubMed] [Google Scholar]
- Rademacher J. Topographical variation of the human primary cortices: implications for neuroimaging, brain mapping, and neurobiology. Cerebral Cortex (New York, N.Y.: 1991) 1993;3(4):313–329. doi: 10.1093/cercor/3.4.313. 8400809 [DOI] [PubMed] [Google Scholar]
- Schmahmann J.D. From movement to thought: anatomic substrates of the cerebellar contribution to cognitive processing. Human Brain Mapping. 1996;4(3):174–198. doi: 10.1002/(SICI)1097-0193(1996)4:3<174::AID-HBM3>3.0.CO;2-0. 20408197 [DOI] [PubMed] [Google Scholar]
- Silveri M.C., Misciagna S. Language, memory, and the cerebellum. Journal of Neurolinguistics. 2000;13(2–3):129–143. [Google Scholar]
- Smith S.M. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23(Suppl. 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. 15501092 [DOI] [PubMed] [Google Scholar]
- Smith S.M. Fast robust automated brain extraction. Human Brain Mapping. 2002;17(3):143–155. doi: 10.1002/hbm.10062. 12391568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley C.J., Schmahmann J.D. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. Neuroimage. 2009;44(2):489–501. doi: 10.1016/j.neuroimage.2008.08.039. 18835452 [DOI] [PubMed] [Google Scholar]
- Stoodley C.J. The cerebellum and cognition: evidence from functional imaging studies. Cerebellum (London, England) 2012;11(2):352–365. doi: 10.1007/s12311-011-0260-7. 21373864 [DOI] [PubMed] [Google Scholar]
- Stoodley C.J., Valera E.M., Schmahmann J.D. An fMRI study of intra-individual functional topography in the human cerebellum. Behavioural Neurology. 2010;23(1–2):65–79. doi: 10.3233/BEN-2010-0268. 20714062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strick P.L., Dum R.P., Fiez J.A. Cerebellum and nonmotor function. Annual Review of Neuroscience. 2009;32:413–434. doi: 10.1146/annurev.neuro.31.060407.125606. 19555291 [DOI] [PubMed] [Google Scholar]
- Szaflarski J.P. Comprehensive presurgical functional MRI language evaluation in adult patients with epilepsy. Epilepsy & Behavior: E&B. 2008;12(1):74–83. doi: 10.1016/j.yebeh.2007.07.015. 17964221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szaflarski J.P. Language lateralization in left-handed and ambidextrous people: fMRI data. Neurology. 2002;59(2):238–244. doi: 10.1212/wnl.59.2.238. 12136064 [DOI] [PubMed] [Google Scholar]
- Wang D., Buckner R.L., Liu H. Cerebellar asymmetry and its relation to cerebral asymmetry estimated by intrinsic functional connectivity. Journal of Neurophysiology. 2013;109(1):46–57. doi: 10.1152/jn.00598.2012. 23076113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. Classification of fMRI patterns — a study of the language network segregation in pediatric localization related epilepsy. Human Brain Mapping. 2014;35(4):1446–1460. doi: 10.1002/hbm.22265. 23450847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellmer J. Cerebral lesions can impair fMRI-based language lateralization. Epilepsia. 2009;50(10):2213–2224. doi: 10.1111/j.1528-1167.2009.02102.x. 19453706 [DOI] [PubMed] [Google Scholar]
- Wilke M. An fMRI task battery for assessing hemispheric language dominance in children. NeuroImage. 2006;32(1):400–410. doi: 10.1016/j.neuroimage.2006.03.012. 16651012 [DOI] [PubMed] [Google Scholar]
- Wilke M. Comprehensive language mapping in children, using functional magnetic resonance imaging: what's missing counts. Neuroreport. 2005;16(9):915–919. doi: 10.1097/00001756-200506210-00008. 15931061 [DOI] [PubMed] [Google Scholar]
- Wilke M., Lidzba K. LI-tool: a new toolbox to assess lateralization in functional MR-data. Journal of Neuroscience Methods. 2007;163(1):128–136. doi: 10.1016/j.jneumeth.2007.01.026. 17386945 [DOI] [PubMed] [Google Scholar]
- Wood A.G. Language cortex activation in normal children. Neurology. 2004;63(6):1035–1044. doi: 10.1212/01.wnl.0000140707.61952.ca. 15452295 [DOI] [PubMed] [Google Scholar]
- Woolrich M.W. Bayesian analysis of neuroimaging data in FSL. Neuroimage. 2009;45(Suppl. 1):S173–S186. doi: 10.1016/j.neuroimage.2008.10.055. 19059349 [DOI] [PubMed] [Google Scholar]
- Worsley K. Statistical Analysis of Activation Images. Oxford Scholarship Online; 2001. [Google Scholar]
- Xiang H. Involvement of the cerebellum in semantic discrimination: an fMRI study. Human Brain Mapping. 2003;18(3):208–214. doi: 10.1002/hbm.10095. 12599279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo B.T.T. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. Journal of Neurophysiology. 2011;106(3):1125–1165. doi: 10.1152/jn.00338.2011. 21653723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- You X. A decisional space for fMRI pattern separation using the principal component analysis — a comparative study of language networks in pediatric epilepsy. Human Brain Mapping. 2013;34:2330–2342. doi: 10.1002/hbm.22069. 22461299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- You X. fMRI activation pattern recognition: a novel application of PCA in language network of pediatric localization related epilepsy. Conference Proceedings: Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Conference. 2009:5397–5400. doi: 10.1109/IEMBS.2009.5332811. 19963905 [DOI] [PubMed] [Google Scholar]
- You X. Sub-patterns of language network reorganization in pediatric localization related epilepsy: a multisite study. Human Brain Mapping. 2011;32(5):784–799. doi: 10.1002/hbm.21066. 21484949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan W. fMRI shows atypical language lateralization in pediatric epilepsy patients. Epilepsia. 2006;47(3):593–600. doi: 10.1111/j.1528-1167.2006.00474.x. 16529628 [DOI] [PMC free article] [PubMed] [Google Scholar]


