Abstract
Cassia oil (CO) from different parts of Cinnamomum cassia have different active components. Very few pharmacological properties of cassia leaf oil have been reported. This study investigated and compared effects of cassia leaf oil and cinnamaldehyde on lipopolysaccharide (LPS)-activated J774A.1 cells. Volatile compositions in cassia leaf oil were identified by gas chromatography-mass spectrometry (MS)/MS. Effects of CO and cinnamaldehyde on LPS-activated J774A.1 cells were investigated by determining nitric oxide (NO) production using Griess reaction assay; expression of pro-inflammatory cytokines, enzymes involve in inflammatory mediators; antiinflammatory cytokines, and iron exporter ferroportin1 (Fpn1) using reverse transcription-polymerase chain reaction; and production of tumor necrosis factor (TNF-α) and interleukin (IL)-10 using ELISA. The main component of CO was cinnamaldehyde. Both oils at 1-20 μg/ml markedly inhibited NO production in LPS-activated J774A.1 cells with IC50 value of 6.1 ± 0.25 and 9.97 ± 0.35 μg/ml, respectively. They similarly inhibited mRNA expression of pro-inflammatory cytokines and chemokines. These mediators included TNF-α, IL-1β, IL-6, monocyte chemoattractant protein-1, and macrophage inflammatory protein-1α in LPS-activated cells. They also significantly decreased expression of inducible enzymes inducible nitric oxide synthase, cyclooxygenase-2, microsomal prostaglandin-E synthase-1. In the opposite way, they increased mRNA expression and the production of antiinflammatory cytokines IL-10 and transforming growth factor-β. In addition, they promoted the expression of Fpn1. These results demonstrated that inhibitory effects of cassia leaf oil from C. cassia mainly came from cinnamaldehyde. This compound not only inhibited inflammatory mediators but also activated antiinflammatory mediators in LPS-activated J774A.1 cells. It may also have an effect on iron regulatory proteins in activated macrophages.
Keywords: Cinnamaldehyde, Cinnamomum cassia, J774A.1 cells, lipopolysaccharide
INTRODUCTION
Cinnamomum cassia (Lauraceae) is widely distributed in southern China, India, Bangladesh, Sri Lanka and Vietnam. Cassia oil (CO) can be extracted from the leaves or barks of this plant.[1] It has been traditionally used to treat cold, influenza, fever and other inflammatory diseases.[2,3] The main active volatile component in this oil is cinnamaldehyde that presents in a different amount in CO isolated from different parts of the plant.[4] Cinnamaldehyde demonstrated antiinflammatory effects in several in vitro and in vivo studies. It inhibited tumor necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6 and reactive oxygen species in lipopolysaccharide (LPS)-stimulated J774A.1 cells and human monocytes.[5] It also reduced TNF-α, nitric oxide (NO), inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2) and PGE2 in the carrageenan-induced mouse paw edema model.[6] Most reported biological activities of CO were from the essential oil from the barks of C. cassia.[3,7,8] There were very few reports on antiinflammatory effect and effect on iron exporter ferroportin1 (Fpn1) of CO from the leaves of C. cassia.
This study aimed to investigate antiinflammatory activity of cassia leaf oil in LPS-activated J774A.1 cells. We intended to compare this activity of CO to the activity of its main active component cinnalmaldehyde. Effects of both CO and cinnamaldehyde on the production of inflammatory mediators, antiinflammatory mediators, and on iron regulator Fpn1 were evaluated in this study.
MATERIALS AND METHODS
Cells
Mouse macrophage J774A.1 cells were obtained from American Type Culture Collection. The cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum, 100 U/ml penicillin and 100 μg/ml streptomycin, in 5% CO2 at 37°C humidified incubator.
Tested compounds
Cassia oil extracted from the leaves of C. cassia by steam distillation was obtained from Thai-China Flavours and Fragrances Industry Co., Ltd., (Bangkok, Thailand). Cinnamaldehyde was obtained from Sigma Chemical Co., (St. Louis, MO).
Analysis of essential oil
Cassia leaf oil was analyzed by gas chromatography (GC) with mass spectrometry using Finnigan Trace GC Ultra Finnigan DSQ Quadrupole detector system (Allured, Illinois, USA). The components of CO were identified by matching their mass spectra and retention times with Adams EO Mass Spectral Library and NIST05 Mass Spectral Library. The relative concentration of each compound in essential oil was computed from GC peak areas by the analysis program.
Determination of nitric oxide production
J774A.1 cells at 4 × 105 cells/ml were treated with 1.25-20 μg/ml CO or cinnamaldehyde and 100 ng/ml LPS in 96-well plate for 24 h. 0.4% ethanol and 20 μM dexamethasone, in the presence of 100 ng/ml LPS, were used as a negative and the positive controls, respectively. The amount of NO in the supernatant of the treated cells was determined by Griess reaction assay. 100 μl of the supernatant reacted with 20 μl sulfanilamide for 10 min and then with 20 μl N-1-napthylethylenediamine dihydrochloride for 10 min. The reaction mixture was measured at 540 nm. The concentrations of NO were determined as nitrite concentrations from the standard curve prepared from standard nitrite solutions.
Cell viability assay
The remaining treated cells from NO production assay were determined their viability by resazurin reduction assay. The cells were incubated in 100 μl fresh DMEM medium containing 50 μg/ml resazurin for 2 h at 37°C. The reaction mixture was measured at 570 nm against 600 nm.
Determination of the expression of tumor necrosis factor-α, interleukin (IL)-1β, IL-6, inducible nitric oxide synthase, cyclooxygenase-2, microsomal prostaglandin-E synthase-1, monocyte chemoattractant protein-1, macrophage inflammatory protein-1α, interleukin-10, TGF-β and ferroportin1 by reverse transcription-polymerase chain reaction
J774A.1 cells at 4 × 105 cells/ml were treated with 5-20 μg/ml CO or cinnamaldehyde and 100 ng/ml LPS in 24-well plate at 37°C for 4 or 24 h. Total RNA were isolated from the treated cells by TRIzol® reagent (Invitrogen, USA). cDNA was synthesized from mRNA in the RNA samples by Impromt II reverse transcription system (Promega, USA). The cDNA samples were used for amplifying mRNA of TNF-α, IL-1β, IL-6, monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein-1α (MIP-1α), IL-10, transforming growth factor (TGF)-β, iNOS, COX-2 and microsomal prostaglandin-E synthase (mPGES)-1 with suitable primers (Bio Basic, Canada) by polymerase chain reaction (PCR). The PCR products were identified by 1.5% agarose gel electrophoresis.
Measurement of tumor necrosis factor-α and interleukin-10 production by ELISA
J774A.1 cells at 4 × 105 cells/ml were treated with 5-20 μg/ml CO or cinnamaldehyde and 100 ng/ml LPS in 24-well plate at 37°C for 24 h. The amount of TNF-α and IL-10 in the supernatant of the treated cells was determined by ELISA using PeproTech's ELISA kits (PeproTech, NJ, USA).
Statistical analysis
Data from at least three independent experiments were presented as mean with a standard error of the mean. The data of the treated groups were compared with the control group by using one-way analysis of variance followed by Tukey's post-hoc test. SPSS program version 17.0 (IBM, Illinois, USA) was used to perform all statistical analysis. P < 0.05 were considered to be statistically significant.
RESULTS
Composition of cassia leaf oil
A total of 9 compounds was identified in cassia leaf oil [Table 1] and the main compound was cinnamaldehyde (78.35%).
Table 1.
Chemical compositions of cassia leaf oil
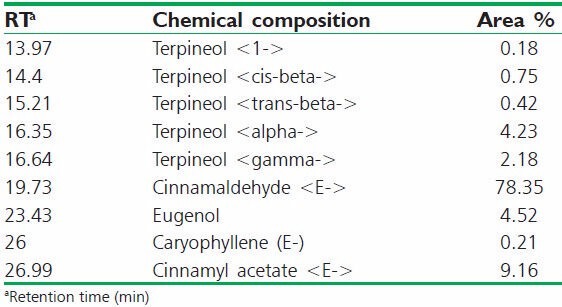
Effects of cassia oil and cinnamaldehyde on inducible nitric oxide synthase mRNA expression and nitric oxide production in lipopolysaccharide-activated J774A.1 cells
Cassia oil and cinnamaldehyde both at 1.25-20 μg/ml significantly inhibited NO production in a concentration-dependent manner with the IC50 value of 6.1 ± 0.25 and 9.97 ± 0.35 μg/ml, respectively [Figure 1a]. The inhibitory effect of CO was significantly higher than its main component cinnamaldehyde (P < 0.01). The inhibitory effects of the oil and its component did not affect the viability of J774A.1 cells [Figure 1b]. The inhibitory effect of CO and CIN on NO production was supported by the reduction of inducible nitric oxide synthase (iNOS) expression determined by reverse transcription-PCR as presented in Figure 1c. CO and cinnamaldehyde concentration-dependently attenuated iNOS mRNA expression in LPS-activated cells. Cinnamaldehyde showed fewer iNOS inhibitory effect (P < 0.01) comparing with CO in the corresponding concentration [Figure 1d].
Figure 1.
Effects of cassia oil and cinnamaldehyde on inducible nitric oxide synthase mRNA expression (c and d), cell viability (b) and nitric oxide production (a) in lipopolysaccharide (LPS)-stimulated macrophages (mean ± standard error of mean) *P < 0.05 compared with LPSstimulated control; ##P < 0.01 compared with cassia oil treated cells
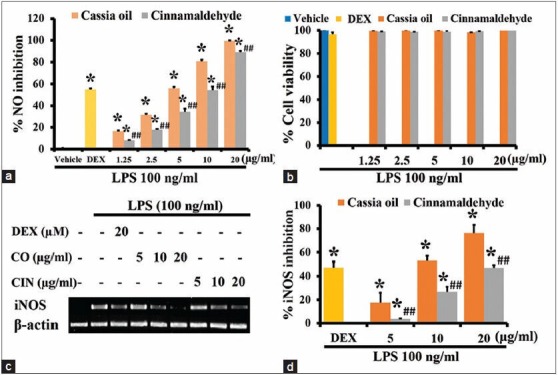
Effects of cassia oil and cinnamaldehyde on cyclooxygenase-2 and microsomal prostaglandin-E synthase-1 mRNA expression in lipopolysaccharide-activated J774A.1 cells
Inducible enzymes COX-2 and mPGES-1 were not expressed in untreated J774A.1 cells but they were highly expressed in the LPS-activated cells [Figure 2a]. CO and cinnamaldehyde at 5, 10, and 20 μg/ml significantly and similarly inhibited the mRNA expression of COX-2 enzymes as shown in Figure 2b. Cinnamaldehyde at 5 and 10 μg/ml significantly had higher inhibitory activity than CO on mPGES-1 mRNA expression [Figure 2c].
Figure 2.
Effects of cassia oil and cinnamaldehyde on mRNA expression of cyclooxygenase-2 (a and b) and microsomal prostaglandin-E synthase -1 (a and c) in lipopolysaccharide (LPS)-activated macrophages (mean ± standard error of mean) *P < 0.05 compared with LPS-stimulated control; ##P < 0.01 compared with cassia oil treated cells
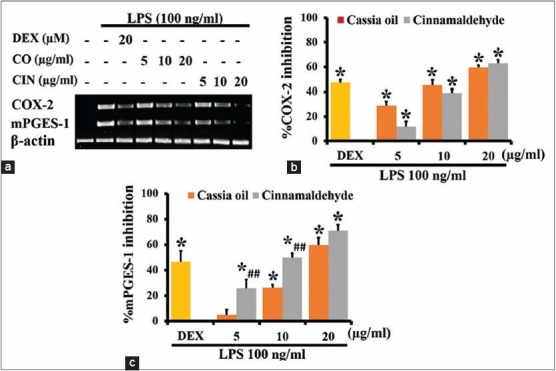
Effects of cassia oil and cinnamaldehyde on pro-inflammatory cytokine expression and production in lipopolysaccharide-activated J774A.1 cells
Pro-inflammatory cytokines, TNF-α, IL-1β and IL-6 did not express in untreated macrophage J774A.1 cells, but they were highly expressed in LPS-activated cells [Figure 3a]. Both CO and cinnamaldehyde at 5, 10 and 20 μg/ml similarly and significantly inhibited the expression of TNF-α, IL-1β and IL-6 in activated J774A.1 cells, in a concentration-dependent manner [Figure 3b–d]. They also similarly and markedly inhibited TNF-α production in a concentration-dependent manner [Figure 3e].
Figure 3.
Effects of cassia oil and cinnamaldehyde on mRNA expression (a-d) and production (e) of pro-inflammatory cytokines in lipopolysaccharide (LPS)-activated macrophages (mean ± standard error of mean) +P < 0.05 compared with untreated control; *P < 0.05 compared with LPS-stimulated control
Effects of cassia oil and cinnamaldehyde on expression of chemokines in lipopolysaccharide-activated J774A.1 cells
Cassia oil and cinnamaldehyde significantly inhibited mRNA expression of chemokines MCP-1 and MIP-1α [Figure 4a]. They have similar inhibitory effect on MIP-1α expression [Figure 4c]. At 5 and 10 μg/ml CO demonstrated higher inhibitory activity than cinnamaldehyde on MCP-1 expression [Figure 4b].
Figure 4.
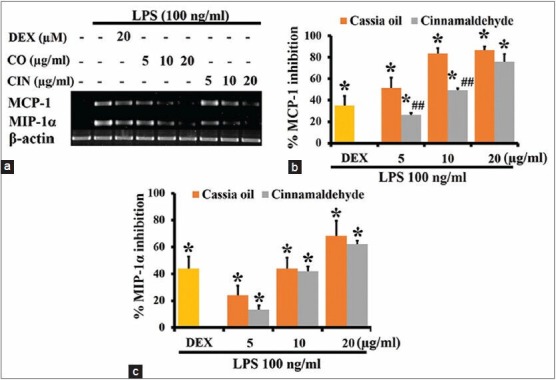
Effects of cassia oil and cinnamaldehyde on mRNA expression of chemokines (a-c) in lipopolysaccharide (LPS)-stimulated macrophages (mean ± standard error of mean) *P < 0.05 compared with LPS-stimulated control and ##P < 0.01 compared with cassia oil treated cells
Effects of cassia oil and cinnamaldehyde on antiinflammatory cytokine expression and production in lipopolysaccharide-activated J774A.1 cells
Cassia oil and cinnamaldehyde with the concentration at 10 and 20 μg/ml similarly and significantly increased the expression of antiinflammatory cytokines IL-10 and TGF-β [Figure 5a–c]. These two oils with the corresponding concentration also significantly increased IL-10 production [Figure 5d].
Figure 5.
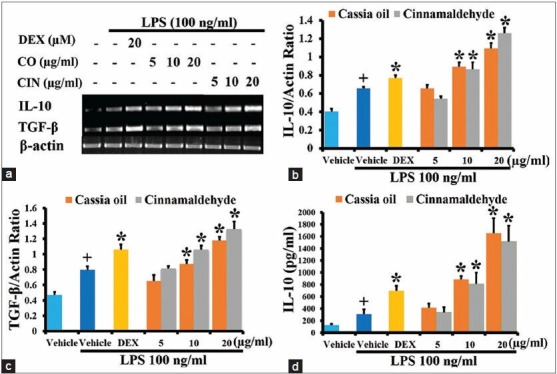
Effects of cassia oil and cinnamaldehyde on mRNA expression (a-c) and production (d) of antiinflammatory cytokines interleukin-10 and transforming growth factor-β in lipopolysaccharide (LPS)-activated macrophages (mean ± standard error of mean) +P < 0.05 compared with untreated control; *P < 0.05 compared with LPS-stimulated control
Effects of cassia oil and cinnamaldehyde on lipopolysaccharide-suppressed ferroportin1 expression
Cassia oil and cinnamaldehyde similarly and significantly increased mRNA expression of Fpn1 in LPS-activated cells [Figure 6a and b].
Figure 6.
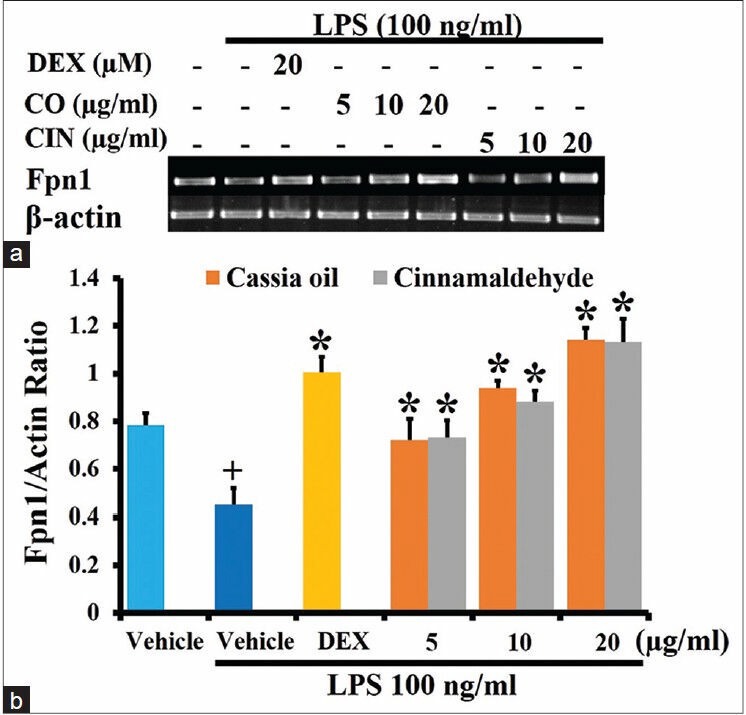
Effects of cassia oil and cinnamaldehyde on mRNA expression of ferroportin1 (a and b) in lipopolysaccharide (LPS)- stimulated macrophages (mean ± standard error of mean) +P < 0.05 compared with untreated control; *P < 0.05 compared with LPSstimulated control
DISCUSSION
Cassia leaf oil in this study contained mainly cinnamaldehyde 78.35%. Previous studies reported that the cinnamaldehyde was the main active volatile component of C. cassia bark and leaf essential oil at 91% and 30%, respectively.[9] The variation of cinnamaldehyde in each study may be due to the difference in parts of the plant, geographical region, ages of the plant, harvest seasons, and method of oil extraction. Other constituents of CO in this study were also different from reported constituents of bark CO.[1,6] We could not detect coumarin in CO used in the study.
Both CO and cinnamaldehyde significantly inhibited NO production in a concentration-dependent manner. Inhibition of NO production of these oils correlated to their inhibitory effects on iNOS expression. It is known that iNOS only expresses in macrophages when these cells are activated. This enzyme is responsible for a large amount NO production. Elevations of NO and iNOS in activated macrophages are associated with the pathological process of acute and chronic inflammatory conditions.[10] CO demonstrated more potent inhibition than cinnamaldehyde on iNOS expression and NO production. It is possible that other constituents in CO may also have an inhibitory effect along with cinnamaldehyde. Eugenol has been reported to inhibit NO and iNOS production in murine macrophage RAW 264.7 cells and human macrophages stimulated by LPS.[11,12]
Cyclooxygenase-2 and mPGES-1 play major roles in PGE2 biosynthesis from arachidonic acid in activated macrophages.[13] Expression of these enzymes is known to be induced by bacterial products, pro-inflammatory cytokines TNF-α and IL-1β and up regulation of them was detected in many chronic inflammatory diseases.[14,15,16] mPGES-1 is recently expected to be a novel therapeutic target for inhibiting inflammatory diseases due to the cardiovascular side effects of selective COX-2 inhibitors.[17,18] CO and cinnamaldehyde similarly and significantly inhibited COX-2 and mPGES-1 mRNA expression in LPS-stimulated J774A.1 cells. These results suggest that they should be able to inhibit PGE2 synthesis in activated macrophages. It has been reported that cinnamaldehyde is the only constituent in bark CO that could inhibit iNOS and COX-2 expression.[6] The effect of CIN on mPGES-1 was first evaluated in this study.
Several pro-inflammatory cytokines and chemokines from activated macrophages during chronic inflammation can cause several inflammatory diseases.[19,20] In this study, CO and cinnamaldehyde significantly inhibited expression of TNF-α, IL-1β and IL-6. They also decreased mRNA expression of chemokines MCP-1 and MIP-1α which can activate endothelial cells to express adhesion molecules resulting in enhanced the recruitment of leukocytes to the site of inflammation.[21] Cinnamaldehyde has been reported to inhibit LPS-induced pro-inflammatory cytokine production in murine macrophage RAW264.7 cells[6] and porcine alveolar macrophage.[22] Our results confirmed the previous studies and extended the information that these oils also had an inhibitory effect on chemokines MCP-1 and MIP-1α.
Activation of antiinflammatory mediators should attenuate chronic inflammatory condition.[23] CO and cinnamaldehyde up-regulated expression and production of antiinflammatory cytokines IL-10 and TGF-β while they down-regulated pro-inflammatory cytokine expression and production in LPS-activated macrophages. This study demonstrated that antiinflammatory activities of CO and cinnamaldehyde may come from inhibition of inflammatory mediator production and stimulation of antiinflammatory mediator production.
Anemia of chronic diseases (ACD) is inflammation-related pathology caused by inhibition of intestinal iron absorption and iron sequestration in macrophages by Fpn1 down-regulation. Inflammatory cytokines or LPS could down-regulate macrophage Fpn1 mRNA expression and induce iron sequestration.[24] This study also demonstrated that LPS-treated macrophages decreased mRNA expression of Fpn1. CO and cinnamaldehyde similarly and significantly increased mRNA expression of Fpn1 in LPS-activated J774A.1 cells. These two compounds can reverse LPS suppressed Fpn1 mRNA expression. Although this study did not determine the intracellular iron level, it is possible that the restoration of Fpn1 mRNA expression should increase iron release. This effect of the compounds may benefit the improvement of ACD. The effect of CO and cinnamaldehyde on an iron regulatory protein was first reported in this study.
CONCLUSION
This study demonstrated comparable antiinflammatory activity of CO and cinnamaldehyde on LPS-activated J774A.1 cells. It suggested that almost but not all activities on macrophage mediators of CO come from cinnamaldehyde. Cinnalmaldehyde exhibited antiinflammatory activity by decreasing inflammatory enzymes and mediators and by increasing antiinflammatory mediators. It may also affect the iron homeostasis in macrophages. Nevertheless, in vivo studies in animal model of inflammation and toxicity test should be performed to further validate the therapeutic effects of CO extracted from the leaves of C. cassia.
Footnotes
Source of Support: This work was financially supported by a grant from the 90th Anniversary of Chulalongkorn University Fund.
Conflict of Interest: Nil.
REFERENCES
- 1.Geng S, Cui Z, Huang X, Chen Y, Xu D, Xiong P. Variations in essential oil yield and composition during Cinnamomum cassia bark growth. Ind Crops Prod. 2011;33:248–52. [Google Scholar]
- 2.Kurokawa M, Kumeda CA, Yamamura J, Kamiyama T, Shiraki K. Antipyretic activity of cinnamyl derivatives and related compounds in influenza virus-infected mice. Eur J Pharmacol. 1998;348:45–51. doi: 10.1016/s0014-2999(98)00121-6. [DOI] [PubMed] [Google Scholar]
- 3.Nir Y, Potasman I, Stermer E, Tabak M, Neeman I. Controlled trial of the effect of cinnamon extract on Helicobacter pylori. Helicobacter. 2000;5:94–7. doi: 10.1046/j.1523-5378.2000.00014.x. [DOI] [PubMed] [Google Scholar]
- 4.Rui W, RuiJiang W, Bao Y. Extraction of essential oils from five cinnamon leaves and identification of their volatile compound compositions. Innov Food Sci Emerg Technol. 2009;10:289–92. [Google Scholar]
- 5.Chao LK, Hua KF, Hsu HY, Cheng SS, Lin IF, Chen CJ, et al. Cinnamaldehyde inhibits pro-inflammatory cytokines secretion from monocytes/macrophages through suppression of intracellular signaling. Food Chem Toxicol. 2008;46:220–31. doi: 10.1016/j.fct.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 6.Liao JC, Deng JS, Chiu CS, Hou WC, Huang SS, Shie PH, et al. Anti-Inflammatory activities of Cinnamomum cassia constituents in vitro and in vivo. Evid Based Complement Alternat Med. 2012;2012:429320. doi: 10.1155/2012/429320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atta AH, Alkofahi A. Anti-nociceptive and anti-inflammatory effects of some Jordanian medicinal plant extracts. J Ethnopharmacol. 1998;60:117–24. doi: 10.1016/s0378-8741(97)00137-2. [DOI] [PubMed] [Google Scholar]
- 8.Dugoua JJ, Seely D, Perri D, Cooley K, Forelli T, Mills E, et al. From type 2 diabetes to antioxidant activity: A systematic review of the safety and efficacy of common and cassia cinnamon bark. Can J Physiol Pharmacol. 2007;85:837–47. doi: 10.1139/Y07-080. [DOI] [PubMed] [Google Scholar]
- 9.Choi J, Lee KT, Ka H, Jung WT, Jung HJ, Park HJ. Constituents of the essential oil of the Cinnamomum cassia stem bark and the biological properties. Arch Pharm Res. 2001;24:418–23. doi: 10.1007/BF02975187. [DOI] [PubMed] [Google Scholar]
- 10.Coleman JW. Nitric oxide in immunity and inflammation. Int Immunopharmacol. 2001;1:1397–406. doi: 10.1016/s1567-5769(01)00086-8. [DOI] [PubMed] [Google Scholar]
- 11.Choi CY, Park KR, Lee JH, Jeon YJ, Liu KH, Oh S, et al. Isoeugenol suppression of inducible nitric oxide synthase expression is mediated by down-regulation of NF-kappaB, ERK1/2, and p38 kinase. Eur J Pharmacol. 2007;576:151–9. doi: 10.1016/j.ejphar.2007.07.034. [DOI] [PubMed] [Google Scholar]
- 12.Lee YY, Hung SL, Pai SF, Lee YH, Yang SF. Eugenol suppressed the expression of lipopolysaccharide-induced proinflammatory mediators in human macrophages. J Endod. 2007;33:698–702. doi: 10.1016/j.joen.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 13.Sampey AV, Monrad S, Crofford LJ. Microsomal prostaglandin E synthase-1: The inducible synthase for prostaglandin E2. Arthritis Res Ther. 2005;7:114–7. doi: 10.1186/ar1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harris SG, Padilla J, Koumas L, Ray D, Phipps RP. Prostaglandins as modulators of immunity. Trends Immunol. 2002;23:144–50. doi: 10.1016/s1471-4906(01)02154-8. [DOI] [PubMed] [Google Scholar]
- 15.Xiao L, Ornatowska M, Zhao G, Cao H, Yu R, Deng J, et al. Lipopolysaccharide-induced expression of microsomal prostaglandin E synthase-1 mediates late-phase PGE2 production in bone marrow derived macrophages. PLoS One. 2012;7:e50244. doi: 10.1371/journal.pone.0050244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shibata-Nozaki T, Ito H, Mitomi H, Akaogi J, Komagata T, Kanaji T, et al. Endogenous prostaglandin E2 inhibits aberrant overgrowth of rheumatoid synovial tissue and the development of osteoclast activity through EP4 receptor. Arthritis Rheum. 2011;63:2595–605. doi: 10.1002/art.30428. [DOI] [PubMed] [Google Scholar]
- 17.Norberg JK, Sells E, Chang HH, Alla SR, Zhang S, Meuillet EJ. Targeting inflammation: Multiple innovative ways to reduce prostaglandin E2. Pharm Pat Anal. 2013;2:265–88. doi: 10.4155/ppa.12.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng Y, Wang M, Yu Y, Lawson J, Funk CD, Fitzgerald GA. Cyclooxygenases, microsomal prostaglandin E synthase-1, and cardiovascular function. J Clin Invest. 2006;116:1391–9. doi: 10.1172/JCI27540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rolin J, Maghazachi AA. Implications of chemokines, chemokine receptors, and inflammatory lipids in atherosclerosis. J Leukoc Biol. 2014;95:575–85. doi: 10.1189/jlb.1113571. [DOI] [PubMed] [Google Scholar]
- 20.Eriksson C, Rantapää-Dahlqvist S. Cytokines in relation to autoantibodies before onset of symptoms for systemic lupus erythematosus. Lupus. 2014 doi: 10.1177/0961203314523869. [DOI] [PubMed] [Google Scholar]
- 21.Dawson J, Miltz W, Mir AK, Wiessner C. Targeting monocyte chemoattractant protein-1 signalling in disease. Expert Opin Ther Targets. 2003;7:35–48. doi: 10.1517/14728222.7.1.35. [DOI] [PubMed] [Google Scholar]
- 22.Liu Y, Song M, Che TM, Bravo D, Pettigrew JE. Anti-inflammatory effects of several plant extracts on porcine alveolar macrophages in vitro. J Anim Sci. 2012;90:2774–83. doi: 10.2527/jas.2011-4304. [DOI] [PubMed] [Google Scholar]
- 23.Koj A. Termination of acute-phase response: Role of some cytokines and anti-inflammatory drugs. Gen Pharmacol. 1998;31:9–18. doi: 10.1016/s0306-3623(97)00435-7. [DOI] [PubMed] [Google Scholar]
- 24.Ganz T, Nemeth E. Iron sequestration and anemia of inflammation. Semin Hematol. 2009;46:387–93. doi: 10.1053/j.seminhematol.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]


