Abstract
Lupeol is a triterpenoid, present in most of the medicinally effective plants and possess a wide range of biological activity against human diseases. The present study aims at evaluating the anticancer potentials of lupeol, isolated from the leaves of Elephantopus scaber L. and thereby explores its action on key cancer marker, Bcl-2. The effect of lupeol on the cell viability of MCF-7 was determined by MTT and lactate dehydrogenase assays at different concentrations. The efficacy of the compound to induce cell death was analyzed using AO/EtBr staining. Phase contrast microscopic analysis provided the changes in cell morphology of the compound treated normal breast cells (MCF-10A) and MCF-7 cells. The expression of Bcl-2 and Bcl-xL proteins in the normal, cancer and lupeol treated cancer cell was analyzed by western blotting. Lupeol induced an effective change in the cell viability of MCF-7 cells with IC50 concentration as 80 μM. Induction of cell death, change in cell morphology and population of the cancer cells was observed in the lupeol treated cells, but the normal cells were not affected. The compound effectively downregulated Bcl-2 and Bcl-xL protein expressions, which directly contribute for the induction of MCF-7 cell apoptosis. Conclusion: Thus, lupeol acts as an anticancer agent against MCF-7 cells and is a potent phytodrug to be explored further for its cytotoxic mechanism.
Keywords: Anti-apoptotic protein, apoptosis, cell death, lupeol
INTRODUCTION
Complementary and alternative medicines include several healing therapies and approaches, including the herbal remedies. The modern drug discovery programs are focused on screening of the plant and other natural products to identify structurally novel chemotypes possessing potent and selective biological activity.[1,2,3,4] Among cancer patients, women with breast cancer are found to be the most likely users of alternative medicine, of which a high proportion opt for herbal remedies.[5,6] This selective choice for herbal therapies is attributable toward their nature of the lack of increased side-effects when compared with the synthetic chemicals.[7] The chemotherapeutic effects of herbal extracts and the individual compounds from herbs, fruits, spices, teas, and vegetables has been extensively studied and has been proved to be the effect in inhibiting the development of cancer in the laboratory animal models.[8,9]
Elephantopus scaber L. (Asteraceae) is a medicinal plant used from the ancient times as medicine for human diseases in and around the world traditional system of medicine. The ethanolic leaf extracts of the plant was effective against the viability of the MCF-7 cells and served non-toxic to the normal breast cells, MCF-10 A.[10] Lupeol, a natural pentacyclic triterpenoid is a biologically active phytoconstituent identified to be present in the petroleum ether leaf extract of E. scaber and is proved to be an effective anti-diabetic agent on in vivo models.[11] Lupeol, present in many fruits and vegetables such as mango, olive and fig, possesses a wide variety of biological activities including anti-inflammatory and antioxidant properties.[12] Lupeol facilitates significant inhibition of growth of MDA-MB-231 cells in a dose-responsive manner and is able to induce estrogen receptor-α (ER-α) expression in this ER-α-negative breast cancer cell line.[13] It is also evident that the compound is a potential agent against other cancers such as human prostate, colorectal, skin, liver and gastric cancer.[14,15,16,17,18,19]
There are no evidences for the effectiveness of lupeol, isolated from a plant source such as E. scaber against human breast cancer. Hence, the present study was focused on the identification of the effectiveness of lupeol, isolated from the leave petroleum ether extracts of E. scaber on the growth of MCF-7 cell line. The action of the compound on the regulation of the key cancer marker Bcl-2 and Bcl-xL was analyzed via in vitro analysis.
MATERIALS AND METHODS
Plant material
Elephantopus scaber L. plants were collected from the Kerala, South India. The species was authenticated by Dr. Roseline, Department of Botany, Holy Cross College, Tiruchirappalli, India. The leaves were collected separately and washed. The voucher specimen is preserved in the herbarium of the department. The leaves were dried under shade and mechanically reduced to moderate coarse powder and sieved.
Isolation of lupeol
Lupeol was isolated from the petroleum ether extract of E. scaber L. leave powder. The isolation was carried out as by the previous protocol reported from our laboratory.[11]
Cell line
Human breast cancer cell line MCF-7 and normal human breast cell line (MCF-10A) was procured from ATCC and was cultured in Dulbecco's Modified Eagle Medium culture medium with 10% foetal bovine serum (FBS) at 5% CO2 and 37°C. Cells were passaged using Trypsin-EDTA at 70-80% confluence.
Sample preparation
One millimetre stock solution of the novel compound was prepared with DMSO. From the stock, the samples were prepared at different micromolar concentrations (10, 20, 30, 40 and 80) with serum-free medium (SFM) for the test. The concentration of DMSO was aimed not to exceed 0.01%.
Cell viability assay
MCF-7 cells were seeded in 96-well plate (5 × 103 cells/well) in medium containing 10% FBS and incubated for 24 h under 5% CO2 at 37°C for attachment. The cells were then washed with 1 × PBS, 100 μL of the prepared samples was added to the wells and 100 μL of SFM was added to the control well and incubated for 24 h. The medium was then removed and washed with PBS. Hundred microlitres of 0.5 mg/mL MTT solution was added to each well and incubated for 2-3 h. After an incubation period, 100 μL of DMSO was added for solubilization of cells and kept in the dark for 1 h. The intensity of the color developed was read at 570 nm using ELISA reader. The growth inhibition was then calculated as follows:
% of Cell viability = Absorbance of treated cells/Absorbance of control cells × 100
Five different observations were carried out, and the IC50 values were calculated.
Lactate dehydrogenase assay
MCF-7 cells were seeded in 96-well plate (5 × 103 cells/well) in medium containing 10% FBS and incubated for 24 h under 5% CO2 at 37°C for attachment. Then the cells were washed with PBS, 100 μL of the prepared samples was added to the wells, and 100 μL of SFM was added to the control well and incubated for 24 h. The cells were harvested, and the assay was carried out using the lactate dehydrogenase (LDH) kit from Agappe Diagnostics, Kerala. Hundred microliters of (10:1000) sample with working reagent was mixed and incubated for 1 min at 37°C, observed at 340 nm and calculated according to the manufacture's instruction. Five different observations were carried out, and the IC50 values were calculated.
Detection of cell death
The effect of lupeol to induce cell death in MCF-7 cells was determined by AO/EtBr dual staining. The cells were grown on the cover slip in 24-well plate with 1 × 105 cells/well, then the cells were treated with the IC50 (80 μM) concentration of the compound for 24 h. After incubation, 5 μL of AO (1 mg/mL) and 5 μL of EtBr (1 mg/mL) were added, and the induction of cell death was observed by using a fluorescence microscope.
Effect of the lupeol on cell morphology
The change in cell morphology was determined using a phase contrast microscope. MCF-7 (3 × 104) cells were seeded in six well plates and treated with lupeol (80 μM) for 24 h. After the incubation, the medium was removed; cells were washed with 1 × PBS and observed under phase contrast microscope.
Protein preparation
MCF-10A normal breast cells, MCF-7 cells and lupeol (80 μM) treated MCF-7 cells were washed with 1 × PBS. 600 μL of RIPA buffer in combination with protease inhibitor cocktail were added to the cells. The cell lysates were then subjected to centrifugation for 15 min at 10,000 rpm in 4°C. The supernatant was collected as protein samples, and the concentration of protein in the sample was determined by Lowry's method.[20]
Protein expression analysis
The protein samples (50 μg) were electrophoresed by sodium dodecyl sulfate polyacrylamide gel, and the separated proteins were transferred to a polyvinylidene difluoride membrane. The non-transferred sites in the membrane were blocked using 5% blocking solution for 1 h. After blocking the membrane was washed using Tris buffered saline (TBS) and TBS with Tween (TBST) and incubated overnight with the primary antibodies against Bcl-2 and Bcl-xL (1:1000) in TBST respectively, with β-actin as the loading control. Followed with a rinse, the membrane was incubated for 1 h with the secondary antibody-HRP conjugated (1:5000) in TBST. Protein bands were detected using enhanced chemiluminescence kit and quantified using Quantity One Software.
Statistical analysis
The data were subjected to statistical analysis using one-way Analysis of Variance, followed by Student-Newman-Keul's test to access the significance between groups at a level of P < 0.05 using SPSS 17.0 version.
RESULT AND DISCUSSION
Plants produce a wide range of secondary metabolites, and these plant-specific compounds represent very important quality traits of plants. An increasing number of triterpenoids have been reported to exhibit cytotoxicity against a variety of cancer cells without manifesting any toxicity in normal cells. They also demonstrate antitumor efficacy in preclinical animal models of cancer.[21,22,23] The present study resulted in the identification of the efficacy of lupeol [Figure 1], a triterpenoid isolated from E. scaber L. petroleum ether leaf extract. The compound provided an effective action against the growth of MCF-7 breast cancer cells with a significant change in their viability. The compound inhibited the growth of the cell with a dose-dependent increase in the cell death providing an IC50 value of the compound as 80 μM [Figure 2]. This effect of the compound was also evident from the results of LDH assay, where, the percentage of LDH leakage from the dead cells increased as the concentration of the compound increased [Figure 3]. The above results are in accordance to the report that the lupeol treatment permeabilizes the cell wall allowing the leakage of LDH in gastric cancer cells leading to cell death.[19] The nature of the compound to induce cell death in the MCF-7 cells was proved with the results of AO/EtBr staining [Figure 4]. The control untreated live cells exhibited green cells specifying the absence of cell death and the compound treated cells were found to be stained as red cells signifying the induction of cell death by the compound.
Figure 1.
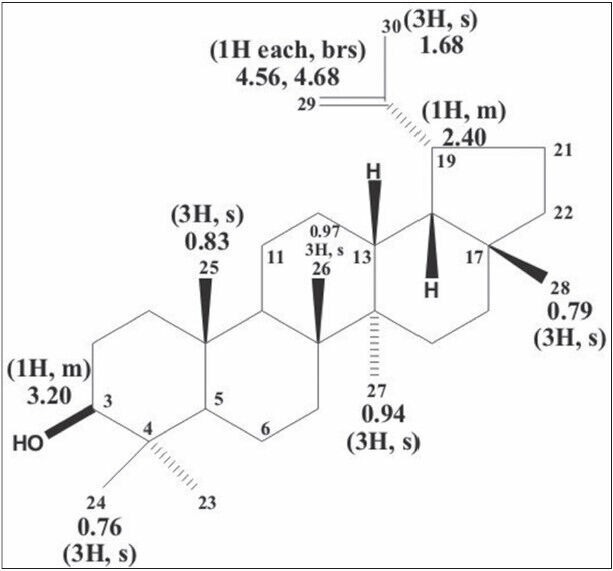
Structure of lupeol
Figure 2.
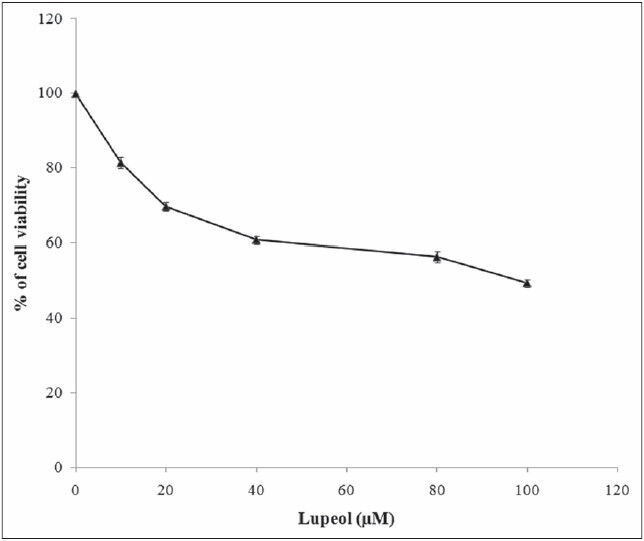
Effect of lupeol on MCF-7 cell viability. Each bar represents the mean ± standard error of the mean of five independent observations and the statistical significance between control and the treated groups at P < 0.05 level
Figure 3.
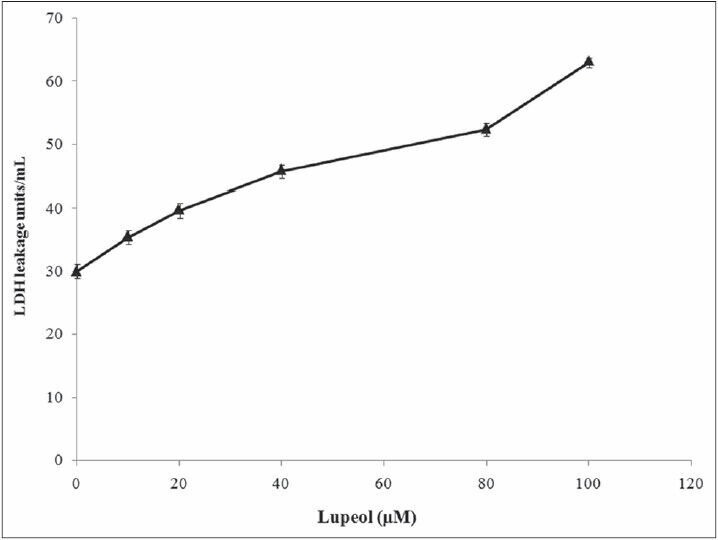
Cytotoxic effect of lupeol on MCF-7 cells. Each bar represents the mean ± standard error of the mean of five independent observations and the statistical significance between control and the treated groups at P < 0.05 level
Figure 4.
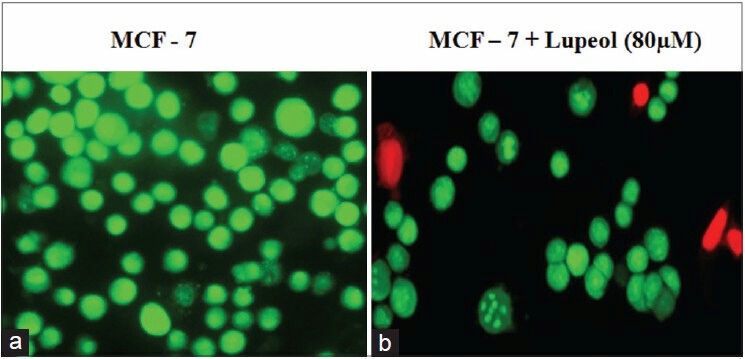
Lupeol induced cell death of MCF-7 cells. Fluorescent microscopic image of AO/EtBr staining of the MCF-7 cells. (a) Control - viable cells shows green stained nuclei. (b) 20 μM compound treated MCF-7 cells shows red dead cells
Apoptosis is the process of induction of programmed cell death, and any abnormalities in the normal pathways involved in apoptosis leads to reduced apoptosis and the resistance to apoptosis initiates enhanced cell progression, which is involved in promoting cancer.[24] During the induction of apoptosis, the cells are subjected to initiate a cascade of events that include changes in both morphological and biochemical characteristics of the cell.[25] In the present study, both normal and breast cancer cells were subjected for the analysis of morphological changes induced by lupeol. A predominant change in the cell morphology was observed in the breast cancer cells with cell shrinkage, cell wall blebbing and reduction in cell population in lupeol treated cells compared to the untreated cells. There were no such significant changes observed in the lupeol treated normal cells which indicated the non-toxic nature of the compound toward the normal breast cell [Figure 5].
Figure 5.
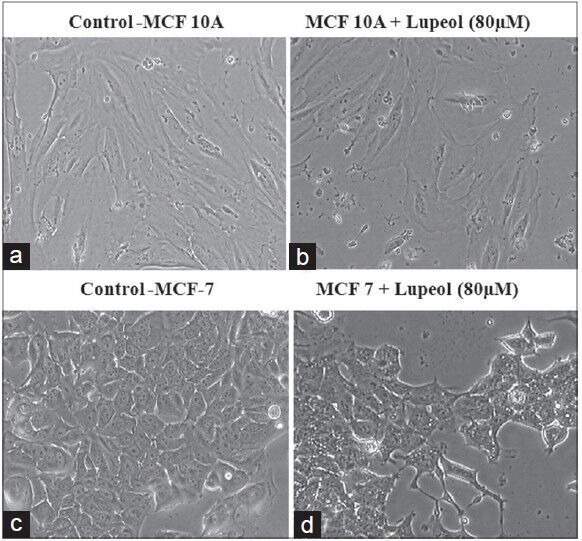
Effect of lupeol on cell morphology in MCF-7 and MCF- 10A cells. (a) Control MCF-10A cells. (b) Normal cells treated with lupeol (80 μM). (c) Control MCF-7 cells (d) Lupeol treated MCF-7 cells
The intrinsic pathway of apoptosis is the mitochondrial mediated pathway regulated by the proteins of the Bcl-2 family present in the mitochondria. The anti-apoptotic proteins Bcl-2 and Bcl-XL are prominent cancer makers, whose overexpression contribute to the enhanced cell proliferation with the inhibition of apoptosis. These proteins prevent the apoptosis-associated release of apoptogenic factors, such as cytochrome c and apoptosis-inducing factor from the mitochondrial inter-membrane space into the cytoplasm. When cytochrome c and AIF reach the cytoplasm, they directly activate caspases and thereby initiate programmed cell death.[26,27] Overexpression of Bcl-2 and Bcl-XL prevents the release of cytochrome c from mitochondria; protecting the cells from apoptosis.[28,29] Thus, it has been reported that the downregulation of Bcl-2 and Bcl-xL regulates the release of the apoptogenic molecules such as cytochrome c allowing the cells to initiate programmed cell death via the activation of caspases.[30,31] The Bcl-2 and Bcl-xL protein expression analysis of the present study also exhibited an elevated level of the protein expression in the breast cancer cells compared to that of its expression in the normal cells. The compound treated cells showed a significant decrease in the both the protein expressions indicating the mechanism of action of the compound to induce apoptosis [Figures 6 and 7]. The downregulated protein expression levels in the treated cells were found to be similar to that of the protein expressed in the normal cells.
Figure 6.
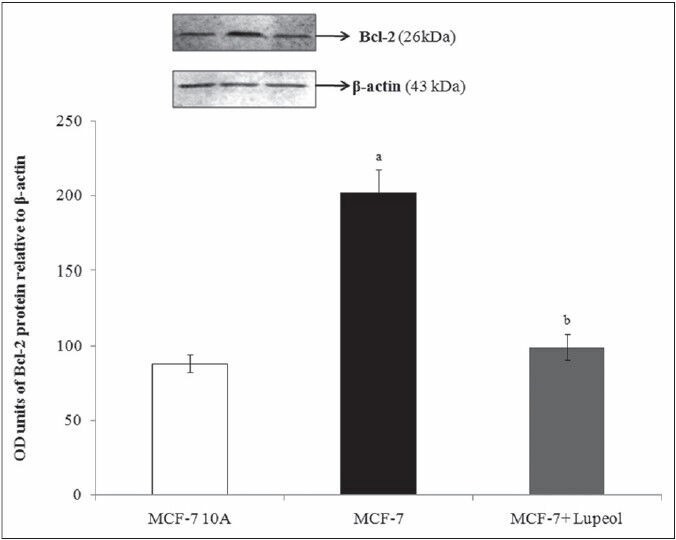
Effect of lupeol on Bcl-2 protein expressions - L1–Control MCF-10A; L2 – Control-MCF-7; L3 – 80 μM lupeol treated MCF-7. Each bar represents the mean ± standard error of the mean of threeindependent observations with statistical significance between control and the treated groups at P < 0.05 level – (a) compared with MCF-10A normal control, (b) compared with MCF-7 control
Figure 7.
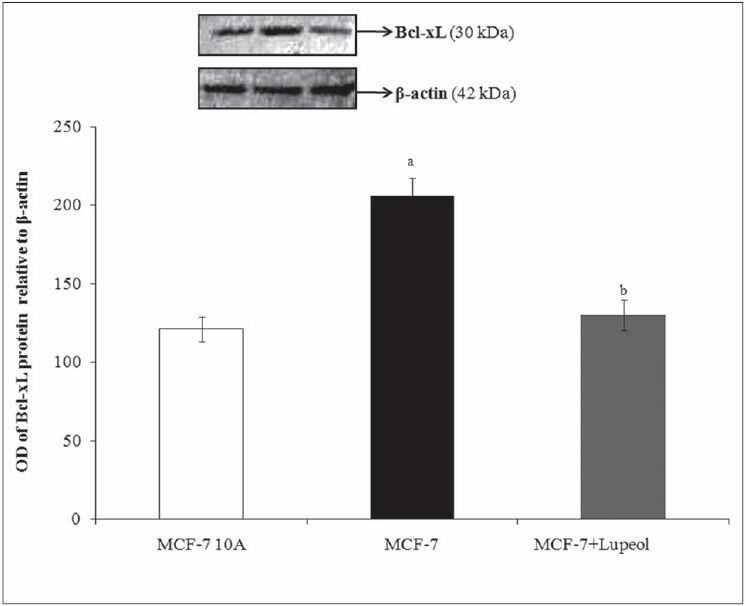
Effect of lupeol on Bcl-xL protein expressions - L1–Control MCF-10A; L2 – Control-MCF-7; L3 – 80 μM lupeol treated MCF-7. Each bar represents the mean ± standard error of the mean of three independent observations with statistical significance between control and the treated groups at P < 0.05 level – (a) compared with MCF-10A normal control, (b) compared with MCF-7 control
Thus, lupeol isolated from E. scaber L. has been identified to be an effective herbal source to induce apoptosis and act against breast cancer. Further in vitro analysis of the compound's action on the other key signaling molecules of cancer with in vivo validation may confirm and suggest the use of lupeol as an effective herbal therapeutic drug to treat breast cancer.
CONCLUSION
The present study concludes the anticancer efficacy of the plant active principle, Lupeol, isolated from the petroleum ether leaf extract of E. scaber L. The compound possess a leading effect on the growth and survival of the ER-α positive MCF-7 cells, whereas stands safe when treated on the normal breast cells. The ability of the compound to downregulate the expression of Bcl-2 and Bcl-xL anti-apoptotic protein elucidates the mechanism of action of the compound on the key cancer targets. The present study has initiated as an idea of identifying the anticancer effect of the compound and various in vitro and in vivo studies in future is required for the identification of other targets of the compound and exploration of its effect on other cancer signalling pathways may provide effective knowledge on the compound's use in breast cancer treatment.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil.
REFERENCES
- 1.Kinghorn AD, Balandrin MF, editors. Human Medicinal Agents from Plants. Washington, DC: American Chemical Society; 1993. ACS Symp. Ser. No. 534. [Google Scholar]
- 2.Gullo V, editor. Discovery of Natural Products with Therapeutic Potential. Boston: Butterworth-Heinemann; 1994. [Google Scholar]
- 3.Colegate SM, Molyneux RJ, editors. Bioactive Natural Products: Detection, Isolation, and Structural Determination. Boca Raton, FL: CRC Press; 1993. [Google Scholar]
- 4.Cragg GM, Newman DJ, Snader KM. Natural products in drug discovery and development. J Nat Prod. 1997;60:52–60. doi: 10.1021/np9604893. [DOI] [PubMed] [Google Scholar]
- 5.Morris KT, Johnson N, Homer L, Walts D. A comparison of complementary therapy use between breast cancer patients and patients with other primary tumor sites. Am J Surg. 2000;179:407–11. doi: 10.1016/s0002-9610(00)00358-5. [DOI] [PubMed] [Google Scholar]
- 6.Newman V, Rock CL, Faerber S, Flatt SW, Wright FA, Pierce JP. Dietary supplement use by women at risk for breast cancer recurrence. The Women's Healthy Eating and Living Study Group. J Am Diet Assoc. 1998;98:285–92. doi: 10.1016/s0002-8223(98)00068-6. [DOI] [PubMed] [Google Scholar]
- 7.Dubey NK, Kumar R, Tripathi P. Global promotion of herbal medicine: lndia's opportunity. Curr Sci. 2004;86:37–41. [Google Scholar]
- 8.Huang MT, Osawa T, Ho CT, Rosen RT, editors. Food Phytochemicals for Cancer Prevention I. Fruits and Vegetables. Washington, DC: American Chemical Society; 1994. ACS Symp. Ser. No. 546. [Google Scholar]
- 9.Ho CT, Osawa T, Huang MT, Rosen RT, editors. Food Phytochemicals for Cancer Prevention II. Washington, DC: American Chemical Society; 1994. Teas, Spices, and Herbs; ACS Symp. Ser. No. 547. [Google Scholar]
- 10.Wan YH, Swee KY, Chai LH, Abdul RR, Abdul AS, Noorjahan BA. Elephantopus scaber induces cytotoxicity in MCF-7 human breast cancer cells via p53-induced apoptosis. J Med Plants Res. 2011;5:5741–9. [Google Scholar]
- 11.Lilly V. Herbal lupeol as potent antidiabetic active principle on Sterptozotoin induced diabetic Wistar rats. 2011. p. 227. Available from: http://www.hdl.handle.net/10603/4775 .
- 12.Jin SE, Son YK, Min BS, Jung HA, Choi JS. Anti-inflammatory and antioxidant activities of constituents isolated from Pueraria lobata roots. Arch Pharm Res. 2012;35:823–37. doi: 10.1007/s12272-012-0508-x. [DOI] [PubMed] [Google Scholar]
- 13.Lambertini E, Lampronti I, Penolazzi L, Khan MT, Ather A, Giorgi G, et al. Expression of estrogen receptor alpha gene in breast cancer cells treated with transcription factor decoy is modulated by Bangladeshi natural plant extracts. Oncol Res. 2005;15:69–79. [PubMed] [Google Scholar]
- 14.Siddique HR, Mishra SK, Karnes RJ, Saleem M. Lupeol, a novel androgen receptor inhibitor: implications in prostate cancer therapy. Clin Cancer Res. 2011;17:5379–91. doi: 10.1158/1078-0432.CCR-11-0916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tarapore RS, Siddiqui IA, Adhami VM, Spiegelman VS, Mukhtar H. The dietary terpene lupeol targets colorectal cancer cells with constitutively active Wnt/β-catenin signaling. Mol Nutr Food Res. 2013;57:1950–8. doi: 10.1002/mnfr.201300155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saleem M, Afaq F, Adhami VM, Mukhtar H. Lupeol modulates NF-kappaB and PI3K/Akt pathways and inhibits skin cancer in CD-1 mice. Oncogene. 2004;23:5203–14. doi: 10.1038/sj.onc.1207641. [DOI] [PubMed] [Google Scholar]
- 17.Liu F, He Y, Liang Y, Wen L, Zhu Y, Wu Y, et al. PI3-kinase inhibition synergistically promoted the anti-tumor effect of lupeol in hepatocellular carcinoma. Cancer Cell Int. 2013;13:108. doi: 10.1186/1475-2867-13-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee TK, Castilho A, Cheung VC, Tang KH, Ma S, Ng IO. Lupeol targets liver tumor-initiating cells through phosphatase and tensin homolog modulation. Hepatology. 2011;53:160–70. doi: 10.1002/hep.24000. [DOI] [PubMed] [Google Scholar]
- 19.Wu XT, Liu JQ, Lu XT, Chen FX, Zhou ZH, Wang T, et al. The enhanced effect of lupeol on the destruction of gastric cancer cells by NK cells. Int Immunopharmacol. 2013;16:332–40. doi: 10.1016/j.intimp.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 20.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 21.Setzer WN, Setzer MC. Plant-derived triterpenoids as potential antineoplastic agents. Mini Rev Med Chem. 2003;3:540–56. doi: 10.2174/1389557033487854. [DOI] [PubMed] [Google Scholar]
- 22.Liby KT, Yore MM, Sporn MB. Triterpenoids and rexinoids as multifunctional agents for the prevention and treatment of cancer. Nat Rev Cancer. 2007;7:357–69. doi: 10.1038/nrc2129. [DOI] [PubMed] [Google Scholar]
- 23.Laszczyk MN. Pentacyclic triterpenes of the lupane, oleanane and ursane group as tools in cancer therapy. Planta Med. 2009;75:1549–60. doi: 10.1055/s-0029-1186102. [DOI] [PubMed] [Google Scholar]
- 24.Kerr JF, Wyllie AH, Currie AR. Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–57. doi: 10.1038/bjc.1972.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Raff MC. Social controls on cell survival and cell death. Nature. 1992;356:397–400. doi: 10.1038/356397a0. [DOI] [PubMed] [Google Scholar]
- 26.Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD. The release of cytochrome c from mitochondria: A primary site for Bcl-2 regulation of apoptosis. Science. 1997;275:1132–6. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- 27.Susin SA, Zamzami N, Castedo M, Daugas E, Wang HG, Geley S, et al. The central executioner of apoptosis: Multiple connections between protease activation and mitochondria in Fas/APO-1/CD95- and ceramide-induced apoptosis. J Exp Med. 1997;186:25–37. doi: 10.1084/jem.186.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang J, Liu X, Bhalla K, Kim CN, Ibrado AM, Cai J, et al. Prevention of apoptosis by Bcl-2: Release of cytochrome c from mitochondria blocked. Science. 1997;275:1129–32. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- 29.Shimizu S, Narita M, Tsujimoto Y. Bcl-2 family proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC. Nature. 1999;399:483–7. doi: 10.1038/20959. [DOI] [PubMed] [Google Scholar]
- 30.Susin SA, Zamzami N, Castedo M, Hirsch T, Marchetti P, Macho A, et al. Bcl-2 inhibits the mitochondrial release of an apoptogenic protease. J Exp Med. 1996;184:1331–41. doi: 10.1084/jem.184.4.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu S, Zhu H, Gu J, Zhang L, Teraishi F, Davis JJ, et al. Induction of apoptosis and down-regulation of Bcl-XL in cancer cells by a novel small molecule, 2[[3-(2,3-dichlorophenoxy) propyl] amino] ethanol. Cancer Res. 2004;64:1110–3. doi: 10.1158/0008-5472.can-03-2790. [DOI] [PubMed] [Google Scholar]


