Abstract
Background:
Within the first 3 years of life, the brain develops rapidly. Its development is characterized by critical developmental periods for speech, vision, hearing, language, balance, etc.; and alteration in any of the processes occurring in those critical periods can lead to specific delays in development.
Aims:
The present study evaluated the potential toxic effects of organic-mercury exposure from Thimerosal (49.55% mercury by weight) in childhood vaccines and its hypothesized possible relationship with specific delays in development.
Materials and Methods:
A hypothesis testing case-control study was undertaken to evaluate the relationship between exposure to Thimerosal-containing hepatitis B vaccines administered at specific intervals in the first 6 months among cases diagnosed with specific delays in development and controls born between 1991-2000, utilizing data in the Vaccine Safety Datalink database.
Results:
Cases were significantly more likely than controls to have received increased organic-mercury from Thimerosal-containing hepatitis B vaccine administered in the first, second, and sixth month of life.
Conclusion:
Though routine childhood vaccination may be an important public health tool to reduce the morbidity and mortality associated with infectious diseases, the present study supports an association between increasing organic-mercury exposure from Thimerosal-containing childhood vaccines and the subsequent risk of specific delays in development among males and females.
Keywords: Ethylmercury, Merthiolate, Thimerosal, Thiomersal, Vaccine
Introduction
Until approximately 3 years of age, the brain develops rapidly. During that period, its development is characterized by specific critical developmental periods. Brain development is extraordinarily complex, requiring many coordinated processes and events to take place.[1] These sensitive periods of elevated activity and processes can create windows of vulnerability for the developing brain. If a process of development is missed or altered, the developmental period cannot be fully recovered, leading to brain abnormalities that are brain region- and time-specific.[1,2] The brain's ability to regenerate and repair is very limited when a developmental process is suspended or delayed by an internal or external influence.[1,3] The brain's ability to recover a lost window of development and the processing that would have corresponded to it is virtually non-existent after the alteration, leading to long-lasting or permanent detrimental consequences with varying degrees of severity.[1] Studies suggest that many critical developmental periods occur postnatally, including those for vision, hearing, language, balance, etc. and that an alteration in any of these periods and the processes that occur during them can lead to specific delays in development.
At issue, then, is the dramatic rise in specific delays in development during the past two decades,[4] an alarming trend with costly consequences for the family and the society.[1] To date, there is no consensus on the cause or contributing factors related to this increase. Many questions regarding the potential contribution of genetic inheritance and susceptibility, gene/environment interaction, and epigenetic or environmental factors to specific delays in development remain unanswered.
However, studies have shown exposure to heavy metals, particularly mercury (Hg), is a risk factor for specific delays in development,[5,6] and that childhood exposure to Hg is increasing.[7] Additional studies have shown Hg levels are increasing in both the environment and in humans. For example, investigators reported on time trends on blood inorganic-Hg levels in 6,174 women, aged 18-49, in the National Health and Nutrition Examination Survey (NHANES), 1999-2006 data sets.[8] That study documented that the percentage of women with detectable levels of inorganic-Hg in the blood rose sharply from 2% in 1999-2000 to 30% in 2005-2006. In addition, the population mean inorganic-Hg concentration in the blood rose significantly over that same period, from 0.33-0.39 μg/L. The level of inorganic-Hg was significantly associated with age, suggesting bio-accumulation. The study concluded that inorganic-Hg deposition within the human body is a cumulative process, increasing in the individual with age, and in the population over time, as a result of chronic Hg exposure.
Since Hg bio-accumulates, combined exposure from different sources is of concern, especially in the face of an underlying chronic exposure that appears to be increasing. Thus, every preventable source of exposure should be assessed. Additive effects of exposures from different sources could potentially increase a child's total Hg exposure from a subclinical chronic exposure level to a clinical level in which obvious specific delays in development occur. Of particular concern is the exposure a child incurs to organic-Hg through the Hg-based compound Thimerosal, still routinely used in some vaccine formulations.
Thimerosal is an organic-Hg compound (49.55% Hg weight) added to vaccines as a preservative, typically at nominal concentrations from 0.005-0.01% (12.5 μg Hg or 25 μg Hg per 0.5 mL vaccine dose).[9] Thimerosal is known to rapidly dissociate into ethyl-Hg chloride, ethyl-Hg hydroxide, and sodium thiosalicylate in saline solutions. As a result of adherence to the recommended routine childhood vaccination schedule in the US during the 1990s, infants may have been exposed to bolus doses of Hg nominally ranging from 12.5 μg Hg to 62.5 μg Hg, collectively totaling up to nominally 200 μg Hg from Thimerosal-containing childhood vaccines during the first six months of life (> 50% of all Hg exposure when considering environmental sources of Hg).[9] This dosing pattern continues unabated in many developing nations to the present day. Even in the United States, despite a call for the removal of Thimerosal from all vaccines on July 7, 1999, by the American Academy of Pediatrics and United States Public Health Service,[10] many American children continue to receive significant doses of Hg from the routinely recommended administration of Thimerosal-containing influenza vaccines (where more than 50% of all doses of influenza vaccine continue to contain 0.01% Thimerosal), given to pregnant women, infants, and young children.[9]
The purpose of the present study was to evaluate concerns about the toxic effects of organic-Hg exposure from Thimerosal in childhood vaccines by conducting a case-control study with documented exposure to varying levels of Thimerosal from vaccinations. A hypothesis testing study was undertaken to evaluate the relationship between organic-Hg exposure from Thimerosal-containing hepatitis B vaccines, administered at specific intervals during the first 6 months of life, and the risk of diagnosed specific delays in development within the Vaccine Safety Datalink (VSD) database.
Materials and Methods
The study protocol employed was approved by the US Centers for Disease Control and Prevention (CDC), the Institutional Review Board (IRB) of Kaiser Permanente North-West (KPNW), and the IRB of Kaiser Permanente Northern California (KPNC). The data were analyzed at the secure Research Data Center of the National Center for Health Statistics in Hyattsville, MD. The views expressed in this study do not necessarily reflect those of the CDC or those of Kaiser Permanente.
Determining the population at risk
A cohort of infants enrolled in the VSD project (updated through the end of 2000) from KPNW, Kaiser Permanente Colorado (KPC), and KPNC was examined using SAS; software. The VSD project was created in 1991 by the National Immunization Program (NIP) of the CDC, and VSD's data collection and study methods have been previously described.[11,12,13,14] The project links medical event information, specific vaccine history, and selected demographic information from the computerized databases of several healthcare management organizations (HMOs). The cohort examined was comprised of individuals with non-missing date of birth and non-missing gender, who were HMO-enrolled from their date of birth.
Determining cases
The outcome files (inpatient and outpatient diagnoses) from this population were then reviewed to find the first instance of International Classification of Disease, 9th revision (ICD-9) diagnosed specific delays in development, including: reading disorder, unspecified (315.00), alexia (315.01), developmental dyslexia (315.02), specific spelling difficulty (315.09), dyscalculia (315.1), disorder of written expression (315.2), expressive language disorder (315.31), mixed receptive-expressive language disorder (315.32), speech and language developmental delay due to hearing loss (315.34), developmental articulation disorder (315.39), developmental coordination disorder (315.4), mixed development disorder (315.5), other specified delays in development (315.8), and unspecified delay in development (315.9). If there were multiple instances of the same diagnosis in a child, only the first instance was counted. In addition, to ensure the potential for an association between exposure and outcome, only individuals diagnosed with specific delays in development following administration of all the vaccines under study were included in the present analyses as cases.
A total of 5,699 cases diagnosed with specific delays in development “(males = 3,916, females = 1,783; male/female ratio = 2.2:1), born from 1991 through 2000, were identified. These individuals diagnosed with specific delays in development were evaluated to determine their mean age of initial diagnosis of specific delays in development and the standard deviation of mean age of initial diagnosis of specific delays in development (2.62 ± 1.58 years-old).
Determining controls
In order to identify controls without a diagnosis of these specific delays in development who would have only a minimal chance of subsequently receiving such a diagnosis, controls had to have been continuously enrolled from birth for at least 5.78 years (mean age of initial diagnosis of specific delays in development plus 2 times the standard deviation of mean age of initial diagnosis of specific delays in development). Applying this follow-up criterion yielded a total of 48,528 controls without specific delays in development diagnoses (males = 24,612, females = 23,915; male/female ratio = 1.03) born between 1991 through 1995.
Hepatitis B vaccine exposure
The vaccine file for cases and controls was then reviewed to determine the exact dates of hepatitis B vaccine administration. Those cases and controls receiving no doses of hepatitis B vaccine were also included in the present study. Overall, among the cases and controls, Hg exposure was assigned as 12.5 μg organic-Hg per dose for those receiving a pediatric hepatitis B vaccine or 0 μg organic-Hg per dose for those receiving either combined Haemophilus influenzae Type B (Hib)-hepatitis B vaccine or neither of the aforementioned vaccines.
Statistical analyses
The Fisher's exact test contained in the SAS; software was utilized for all statistical analyses, and a two-sided P-value < 0.05 was considered statistically significant. In the first case-control experimental group (Experiment I), the data was examined to determine the frequency of exposure to 12.5 μg organic-Hg from a Thimerosal-containing hepatitis B vaccine dose in the first month of life, in comparison to the frequency of 0 μg organic-Hg from the no-Thimerosal hepatitis B-containing vaccine dose or no vaccination for hepatitis B in the first month of life, among cases and controls. In the second experimental group (Experiment II), the data was examined to determine the frequency of receiving two Thimerosal-containing hepatitis B vaccine doses within the first 2 months of life or a total of 25 μg organic-Hg, in comparison to the frequency of receiving 0 μg organic-Hg from two no-Thimerosal hepatitis B-containing vaccine doses and/or no hepatitis B vaccinations in the first two months of life, among cases and controls. In the third experimental group (Experiment III), the data was examined to determine the frequency of receiving three Thimerosal-containing hepatitis B vaccine doses within the first six months of life, or a total of 37.5 μg organic-Hg, in comparison to the frequency of receiving 0 μg organic-Hg in the first sixth months of life, from no-Thimerosal hepatitis B-containing vaccine doses and/or no hepatitis B vaccinations, among cases and controls. In addition, because the ratio of males to females was 2.2:1, additional separate analyses were completed where male cases were compared to male controls (Experiments IV-VI) and female cases were compared to female controls (Experiments VII-IX)). The overall null hypotheses for each of these case-control experimental groups examined was that there would be no difference in the frequency of exposure to organic-Hg doses from Thimerosal-containing hepatitis B vaccines between the cases and the controls. Finally, a series of experiments were conducted to evaluate the effect of the gender of cases and increasing exposure to organic-Hg doses from Thimerosal-containing hepatitis B vaccines. Experiment X determined the frequency of exposure to 12.5 μg organic-Hg from a Thimerosal-containing hepatitis B vaccine dose in the first month of life, in comparison to the frequency of 0 μg organic-Hg from a no-Thimerosal hepatitis B-containing vaccine dose or no hepatitis B vaccination in the first month of life, among male cases in comparison to female cases. Experiment XI determined the frequency of receiving two Thimerosal-containing hepatitis B vaccine doses within the first 2 months of life or a total of 25 μg organic-Hg, in comparison to the frequency of receiving 0 μg organic-Hg from two no-Thimerosal hepatitis B-containing vaccine doses and/or no hepatitis B vaccinations in the first 2 months of life, among male cases in comparison to female cases. Experiment XII determined the frequency of receiving three Thimerosal-containing hepatitis B vaccine doses within the first 6 months of life, or a total of 37.5 μg organic-Hg, in comparison to the frequency of receiving 0 μg organic-Hg in the first 6 months of life, from no-Thimerosal hepatitis B-containing vaccine doses and/or no hepatitis B vaccinations, among male cases in comparison to female cases. The overall null hypotheses for each of these experimental groups examined was that there would be no difference in the frequency of exposure to organic-Hg doses from Thimerosal-containing hepatitis B vaccines between the male cases in comparison to female cases.
Results
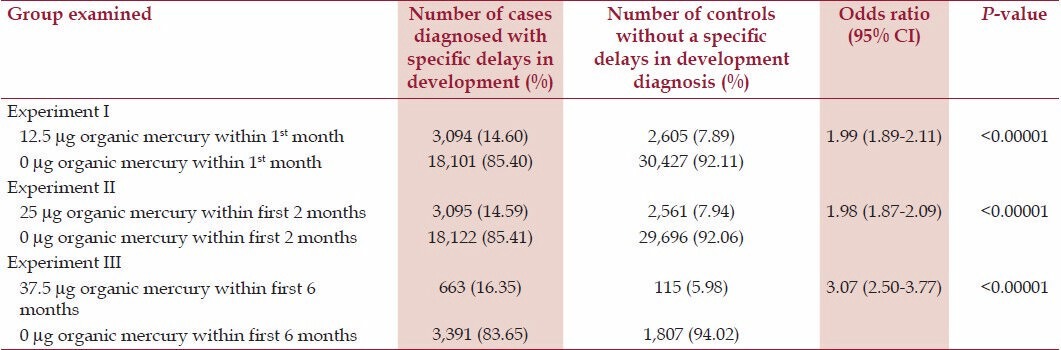
Table 1 displays the relationship between cases and controls receiving increasing doses of organic-Hg from Thimerosal-containing hepatitis B vaccines at several specific points within the first 6 months of life. Experiment I documented that cases were significantly more likely (odds ratio, OR = 1.99, P < 0.00001) than controls to have received 12.5 μg organic-Hg from a Thimerosal-containing hepatitis B vaccine dose in comparison to 0 μg organic-Hg from a no-Thimerosal hepatitis B-containing vaccine dose or no hepatitis B vaccination within the first month of life. Experiment II documented that cases were significantly more likely (OR = 1.98, P < 0.00001) than controls to have received 25 μg organic-Hg from two Thimerosal-containing hepatitis B vaccine doses in comparison to 0 μg organic-Hg from no-Thimerosal hepatitis B-containing vaccine doses and/or no hepatitis B vaccinations within the first 2 months of life. Finally, in Experiment III, cases were significantly more likely (odds ratio = 3.07, P < 0.00001) than controls to have received 37.5 μg organic-Hg from three Thimerosal-containing hepatitis B vaccine doses in comparison to 0 μg organic-Hg from three no-Thimerosal hepatitis B-containing vaccine doses and/or no hepatitis B vaccinations within the first 6 months of life.
Table 1.
A summary of exposure to organic-Hg from Thimerosal-containing hepatitis B vaccine administration among cases diagnosed with specific delays in development in comparison to controls within the VSD database
Tables 2 and 3 display the relationship between cases and controls receiving increasing doses of organic-Hg from Thimerosal-containing hepatitis B vaccine doses at several specific points within the first 6 months of life among male and female genders, respectively. Table 2 reveals in Experiment IV that male cases were significantly more likely (odds ratio = 2.05, P < 0.00001) than male controls to have received 12.5 μg organic-Hg from a Thimerosal-containing hepatitis B vaccine dose in comparison to 0 μg organic-Hg from a no-Thimerosal hepatitis B-containing vaccine dose or no hepatitis B vaccination within the first month of life. Experiment V documented that male cases were significantly more likely (odds ratio = 2.03, P < 0.00001) than male controls to have received 25 μg organic-Hg from two Thimerosal-containing hepatitis B vaccines in comparison to 0 μg organic-Hg from two no-Thimerosal hepatitis B-containing vaccine doses and/or no hepatitis B vaccinations within the first two months of life. Finally, in Experiment VI, male cases were significantly more likely (odds ratio = 3.38, P < 0.00001) than male controls to have received 37.5 μg organic-Hg from three Thimerosal-containing hepatitis B vaccine doses in comparison to 0 μg organic-Hg from three no-Thimerosal hepatitis B-containing vaccine doses and/or no hepatitis B vaccinations within the first six months of life.
Table 2.
A summary of exposure to organic-Hg from Thimerosal-containing hepatitis B vaccine administration among male cases diagnosed with specific delays in development in comparison to male controls within the VSD database
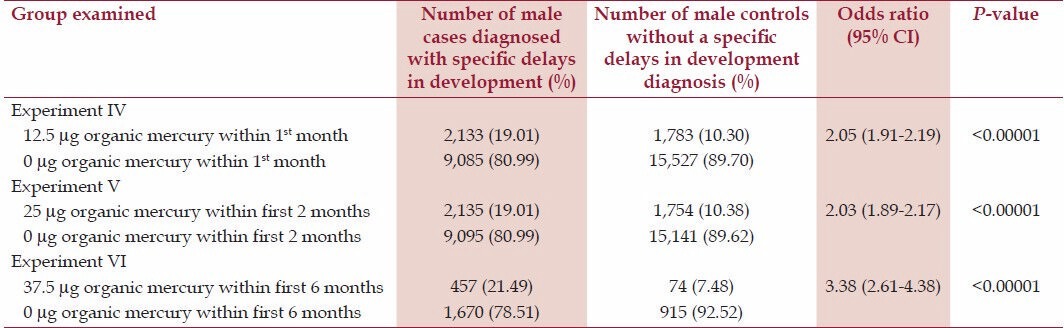
Table 3.
A summary of exposure to organic-Hg from Thimerosal-containing hepatitis B vaccine administration among female cases diagnosed with specific delays in development in comparison to female controls within the VSD database
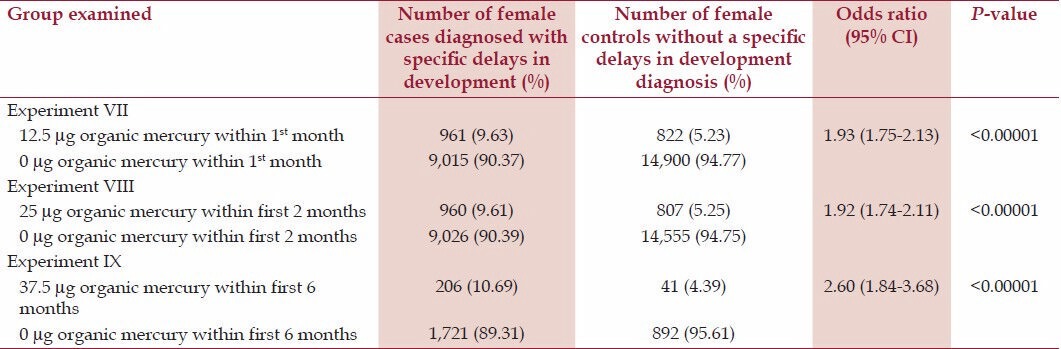
Table 3 reveals in Experiment VII that female cases were significantly more likely (odds ratio = 1.93, P < 0.00001) than female controls to have received 12.5 μg organic-Hg from a Thimerosal-containing hepatitis B vaccine dose in comparison to 0 μg organic-Hg from a no-Thimerosal hepatitis B-containing vaccine dose or no hepatitis B vaccination within the first month of life. Experiment VIII documented that female cases were significantly more likely (OR = 1.92, P < 0.00001) than female controls to have received 25 μg organic-Hg from two Thimerosal-containing hepatitis B vaccine doses in comparison to 0 μg organic-Hg from two no-Thimerosal- hepatitis B-containing vaccine doses and/or no hepatitis B vaccinations within the first 2 months of life. Finally, in Experiment IX, female cases were significantly more likely (OR = 2.60, P < 0.00001) than female controls to have received 37.5 μg organic-Hg from three Thimerosal-containing hepatitis B vaccine doses in comparison to 0 μg organic-Hg from three no-Thimerosal hepatitis B-containing vaccines and/or no hepatitis B vaccinatioins within the first 6 months of life.
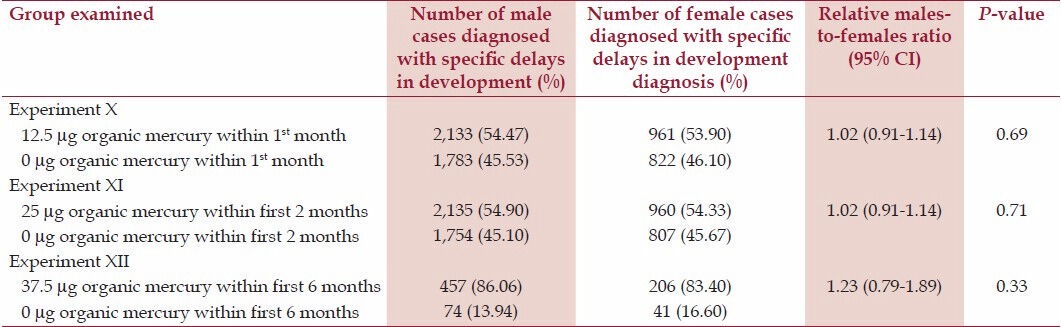
Table 4 reveals the relative relationship between increasing doses of organic-Hg exposure from Thimerosal-containing hepatitis B vaccine doses at several specific points within the first 6 months of life among the ratio of exposed-to-unexposed male cases in comparison to the ratio of exposed-to-unexposed female cases (a relative males-to-females ratio). Overall, there were no statistically significant increase in the relative males-to-females ratios for increasing organic-Hg exposure from Thimerosal-containing hepatitis B vaccine doses among the ratio of exposed-to-unexposed male cases in comparison to the ratio of exposed-to-unexposed female cases. However, there was a non-significant trend towards the ratio of exposed-to-unexposed male cases having received 37.5 μg organic-Hg from three Thimerosal-containing hepatitis B vaccine doses in comparison to 0 μg organic-Hg from three no-Thimerosal hepatitis B-containing vaccine doses and/or no hepatitis B vaccinations within the first six months of life in comparison to the ratio of exposed-to-unexposed female cases (OR = 1.23, P = 0.33).
Table 4.
A summary of exposure to organic-Hg from Thimerosal-containing hepatitis B vaccine administration among male cases in comparison to female cases within the VSD database
Discussion
Investigators from the US CDC and the US Food and Drug Administration (FDA) have repeatedly asserted that products, such as vaccines, which are intended for healthy people, must be held to a high standard of safety assurance.[15,16,17] However, the study of vaccine risks is more complex than those for therapeutic products because the exposure is virtually universal for many vaccines, ensuring the chance occurrence of many adverse outcomes in temporal association with vaccines. As a result, these investigators described using the VSD, a consortium of HMOs, to more rigorously evaluate vaccine-associated risks (hypothesis testing). The present hypothesis testing study evaluated the potential relationship between organic-Hg exposure from Thimerosal-containing childhood vaccines and specific delays in development using Fisher's exact statistical test.
The specific methods employed to evaluate the potential relationship between organic-Hg exposure from Thimerosal-containing childhood vaccines and the risk of specific delays in development in the present study were able to exploit recommendations for the timing of vaccine administration that varied widely. Specifically, differences in cumulative doses of organic-Hg received at specific intervals during the infant period were evaluated based upon the wide-ranging recommendations for routine hepatitis B vaccine administration. In 1991, the Advisory Committee on Immunization Practices (ACIP) recommended that infants should receive their hepatitis B vaccine doses as follows: First dose between birth and 2 months of age, second dose between 1-4 months of age, and third dose between 6-18 months of age.[18] Importantly, all told, it is apparent that the differences in organic-Hg exposures observed in all experiments in the present study were not the result of a small group of children receiving anomalous exposures to vaccines. Instead, the experiments assessed varying levels of organic-Hg exposure that resulted from the varying windows recommended for administration for hepatitis B vaccines during the first year of life.
The results observed in the present study also appear to offer important potential biological mechanistic insights into the relationship between the timing and cumulative effects of organic-Hg exposure from Thimerosal-containing vaccines and the risk of receiving a diagnosis of specific delays in development. For example, study subjects diagnosed with a specific delay in development were not more likely to later have received additional organic-Hg exposure from administration of a second or third Thimerosal-containing hepatitis B vaccine in a dose-proportional manner in comparison to controls. Instead, the odds ratio of receiving a “specific delays in development” diagnosis after receiving two doses of Thimerosal-containing hepatitis B vaccine (25 μg organic-Hg) by two months of age was similar to the of receiving one dose of Thimerosal-containing hepatitis B vaccine (12.5 μg organic-Hg) by 1 month of age. The OR for receiving a diagnosis for specific delays in development after three doses of Thimerosal-containing hepatitis B vaccine (37.5 μg organic-Hg) was increased relative to those children receiving one or two doses of Thimerosal-containing hepatitis B vaccine, but the increase observed was significantly below what would be expected from a cumulative perspective, without considering the variable of timing of administration. Our observation of the odds ratios being modified by time of exposure is consistent with previous observations regarding other cases of Hg intoxication, in which early exposure is associated with more significant adverse effects (that is, Hg intoxication susceptibility: Fetuses >infants >children >adults).[19]
Importantly, many recent studies support the biologically plausible role of organic-Hg exposure from Thimerosal-containing vaccines in the pathogenesis of specific delays in development.[20] Investigators have examined the distribution of organic-Hg following administration of Thimerosal to animals and infants. For example, when administering Thimerosal mimicking the US early childhood vaccination schedule of the 1990s to infant monkeys, researchers found that significant levels of Hg were present in the brain (about 40-50 parts-per-billion), a significant fraction of that Hg was present as inorganic-Hg (about 16 parts-per-billion), and this inorganic-Hg level was observed to not significantly decline 120 days following the last dose of Thimerosal.[21] Other investigators undertook further evaluations of the speciation of Hg present in rat tissues following administration of Thimerosal.[22] Interestingly, those researchers observed that administration of Thimerosal resulted in significant brain levels of Hg with 63% present in the form of inorganic-Hg, 13.5% as ethyl-Hg, and unexpectedly, 23.7% as methyl-Hg.
Disturbingly, some studies reported that Thimerosal-containing vaccine administration to human infants significantly increased the vaccinated infants' blood Hg levels (with some infants having total blood Hg levels in excess of the safety limit adopted by the US Environmental Protection Agency)[23,24,25] and also significantly increased the vaccinated infants' hair ethyl-Hg levels (with some infants having total hair Hg levels in excess of the safety limit adopted by the US Environmental Protection Agency).[26] Finally, in additional research on the distribution of Hg species within the body, investigators have recently demonstrated that ethyl-Hg is actively transported across neuronal cellular membranes to the same degree as methyl-Hg, by the L-type neutral amino acid carrier transport (LAT) system.[27]
Once Hg enters the brain, it has a plethora of effects.[28] One of its main effects is axonal degeneration, particularly of large caliber axons which tend to be long-range axons interconnecting distant parts of the brain. Once long-range axons are destroyed, they are not regenerated to restore the connection intended before the damage, in part, because the shorter the distance between the regeneration site and its distal target, the more successful regeneration of a nerve is likely to be. Postnatally, damaged mature neuronal axons only regenerate for very short distances in the central nervous system (CNS). In addition, long-range connections are dependent upon a critical prenatal and early postnatal developmental periods, during which long-range axonal connections are guided to their cortical targets by molecular signaling mechanisms.
Significantly, researchers have been able to measure the long-range connections that carry signals between distant brain regions and have shown how the development of these long-range connections is important in many processes in the brain, such as reading, hearing, coordination, and speech/language.[29,30,31,32,33,34] Abnormalities in the long-range neural tracts associated with these processes can result in specific delays in development. For example, investigators have shown that the growth pattern of long-range connections in the brain predicts how a child's reading skills will develop.[33] Those investigators stated that literacy requires the integration of activity in brain areas involved in vision, hearing, and language and, because these areas are distributed throughout the brain, it requires more speed-efficient long-range neural networks to bring about efficient communication overall between these regions. Similarly, other investigators found a lack of axonal integrity in the long-range axons of the arcuate fasciculus in children with delayed speech development.[34] Those other investigators stated that any abnormality in the neurons at the origin or termination of a tract could result in abnormal development of the tract.
In addition to Hg causing axonal degeneration, particularly of these critically important long-range axons that would require axon guidance to regenerate, studies have shown that Hg also inhibits axon guidance.[35] Furthermore, studies have found that Hg disrupts neuronal cell maturation,[35] which is another issue noted in children with reading and speech/language delay.[34]
Studies have also evaluated the potential for organic-Hg exposure from Thimerosal-containing vaccine administration to induce specific delays in development pathology or clinical symptoms in animal model systems. Those studies have yielded significant pathology or clinical symptoms in mice,[36] rats,[37,38,39] hamsters,[40] and monkeys[41] that are consistent with those observed in specific delays in development following exposure to Thimerosal-containing vaccines mimicking the US routine childhood vaccination schedule of the 1990s.
The results observed in the present study are also supported by a number of published epidemiological studies that found a significant relationship between organic-Hg exposure from Thimerosal-containing vaccines and specific delays in development, using several epidemiological methods in various databases. For example, in the VSD database, investigators, using an ecological study design, evaluated the relationship between the birth cohort prevalence of several different types of specific delays in development and birth cohort exposures to organic-Hg from Thimerosal-containing childhood vaccines.[42] Consistent with the results observed in the present study, these investigators observed that infants receiving an additional 100 μg organic-Hg from Thimerosal-containing childhood vaccines from birth to 7 months of age, had a significantly increased rate ratio of 2.27 for diagnosed developmental disorder/learning disorder, and infants receiving an additional 100 μg organic-Hg from Thimerosal-containing childhood vaccines from birth to 13 months of age, had a significantly increased rate ratio of 2.91 for diagnosed developmental disorder/learning disorder. Other investigators, using a cohort study design, evaluated the relationship between increasing organic-Hg exposure from Thimerosal-containing childhood vaccines at 1-, 2-, 3-, and 6-months of age and the eventual risk of being diagnosed with specific delays in development.[43] They observed that increasing cumulative organic-Hg exposures from Thimerosal-containing vaccines were associated with an increased risk of diagnosed unspecified developmental delay, language delay and speech delay.
As another example, investigators evaluated the relationship between the administration of three doses of Thimerosal-containing hepatitis B vaccine prior to 2000 and the subsequent risk of a child being diagnosed with a developmental disability from age 1-9 years, based upon assessment of the National Health Interview Survey (NHIS) 1999-2000 dataset.[44] They reported that boys diagnosed with a development disability in comparison to controls had a 9-fold significantly greater odds ratio for receiving three doses of Thimerosal-containing hepatitis B vaccine in comparison to those receiving no doses of Thimerosal-containing hepatitis B vaccine.
Previously, investigators reported on the results of a meta-analysis using statistical modeling to evaluate the relationship between exposure to additional doses of Hg from Thimerosal-containing childhood vaccines and neurodevelopmental disorder adverse event reports in the Vaccine Adverse Event Reporting System (VAERS).[45] This study reported that there was a statistically significant increased risk of speech disorder, mental retardation, personality disorder, thinking abnormality, and ataxia adverse event reports submitted to VAERS following the administration of additional doses of organic-Hg from Thimerosal-containing vaccine.
As yet another example, investigators evaluated neonatal exposure to organic-Hg from Thimerosal-containing vaccines and child development in the first 3 years of life among a study sample of 196 infants, born between January 2001 and March 2003.[46] These investigators observed a significant overall deficit in psychomotor development index attributable to neonatal exposure to organic-Hg from Thimerosal-containing vaccine measured during a 3-year follow-up period. Similarly, investigators assessed neurodevelopment as measured by Gesell development scores in relation to Hg exposure in infants in one urban center and two rural villages.[47] Using logistic regression analysis, these investigators observed that exposure to organic-Hg from Thimerosal-containing vaccine was negatively associated with Gesell developmental scores.
The results of the present study differ from several other studies that failed to find a consistent significant relationship between specific delays in development and organic-Hg exposure from Thimerosal-containing childhood vaccines. This may have occurred, in part, because other studies examined cohorts with significantly different childhood vaccine schedules and with different diagnostic criteria for outcomes. This difference may have also occurred because these other studies employed different epidemiological methods, especially with respect to the issue of a sufficient follow-up period for individuals in the cohorts examined. The method used to measure how a follow-up period is determined for individuals is a critical issue in all studies examining the relationship between exposures and the subsequent risk of the diagnosis of specific delays in development, especially in those instances where the exposures to all in the participants study are the same. This is the case because the risk of an individual being diagnosed with specific delays in development is not uniform throughout his/her lifetime. As observed in the present study, the initial mean age for a diagnosis of specific delays in development was 2.62 years-old, and the standard deviation of mean age for the initial diagnosis of specific delays in development was 1.58 years-old. Therefore, any follow-up method that fails to consider the lag-time between birth and the subject's age of an initial diagnosis for specific delays in development will likely not be able to observe the true relationship between exposure to Hg through vaccination and the subsequent risk of diagnosed specific delays in development.
The issue of follow-up may be of particular importance for studies, which used hazard models that assumed equal chances of an individual receiving a diagnosis of specific delays in development with each additional day of follow-up.[48,49] In one example,[49] Cox's hazard ratios were used to evaluate periods of follow-up in the cohort examined by the investigators in the General Practitioner Research Database (GPRD). These investigators observed that increased organic-Hg exposure from Thimerosal-containing vaccines was associated with significantly reduced risk for diagnosed general developmental disorders and unspecified developmental delay (although there was a significantly higher risk for diagnosed tics).
Still other investigators observed negative associations between organic-Hg from Thimerosal-containing vaccines and specific delays in development but were unable to draw conclusions based upon their results. For example, investigators evaluated neuropsychological performance 10 years after immunization in infancy with Thimerosal-containing vaccines.[50] These investigators examined children who were enrolled in an efficacy trial of pertussis vaccines in 1992-1993, and who were randomly assigned in the first year of life to one vaccine group receiving a cumulative dose of organic-Hg from Thimerosal-containing vaccines of 62.5 μg or another group with a cumulative dose of organic-Hg from Thimerosal-containing vaccines of 137.5 μg. Ten years after immunization in infancy, eleven neuropsychological tests, for a total of 24 outcomes, were administered to children during school hours. Among the 24 neuropsychological outcomes that were evaluated, 2 were significantly negatively impacted by increased organic-Hg exposure from Thimerosal-containing vaccine administration. The investigators concluded that there results might be attributable to chance, and the clinical relevance of the effects observed remain to be determined.
Similarly, investigators conducted a cohort study in the VSD to evaluate the relationship between organic-Hg exposure from Thimerosal-containing vaccines and diagnosed neurodevelopmental disorders.[48] Despite including children who were too young to have received a diagnosis of specific delays in development, these investigators still observed significantly increased risk ratios for tics and language delay with increasing doses of organic-Hg exposure from Thimerosal-containing vaccines. However, according to the investigators, these results were not consistent enough to make a determination of the potential adverse consequences of organic-Hg exposure from Thimerosal-containing vaccines. Critically, the study was significantly limited in its statistical power to detect associations between organic-Hg exposure from Thimerosal-containing vaccines and specific developmental delay diagnoses because only certain some sub-diagnostic categories of diagnosed specific developmental delays were analyzed (that is, no assessment was made for the entire category of specific developmental delays).
Strengths/Limitations
A strength of the present study was its examination of a cohort of children from the VSD database. The VSD database observations were made based upon retrospective assessment of prospectively collected medical records of patients enrolled in various HMOs. All cases examined in the present study had to be enrolled from birth and were required to be continuously enrolled until a medical diagnosis of one of the specific delays in development being studied was made, and controls had to be enrolled from birth for a sufficient time period to ensure that there was a very small chance that, during additional follow-up, any of the controls would be medically diagnosed with specific delays in development. As a result, any factors associated with enrollment (adjustment for potential independent variables between cases and controls were not necessary because enrollment was from birth) or health care-seeking behavior (adjustment for potential access/availability of healthcare was continuous among cases and controls) were minimized. In addition, cases diagnosed with specific delays in development were specifically evaluated to ensure that only those cases diagnosed with specific delays in development following vaccine administration were considered in the present analyses.
For cases diagnosed with specific delays in development, it was possible to mathematically evaluate the mean and standard deviation of age for the initial diagnosis of specific delays in development within the VSD. From this information, it was possible to estimate how many additional potential diagnoses of specific delays in development were missed. In order to ensure adequate amounts of data for our analyses while minimizing their subsequent risk of being diagnosed with a specific delay in development, a priori, controls had to be continuously enrolled in the VSD from birth until they were at least 5.78 years-old (mean age of initial diagnosis of specific delays in development plus 2 times the standard deviation of mean age of initial diagnosis of specific delays in development). Based on the data for age of initial diagnosis for the specific delays in development studied, this was a sufficient period to ensure that, with further follow-up, those controls without a diagnosis of specific delays in development would probably not subsequently receive diagnosis for the studied specific delays in development in the VSD (mathematically there is a <2.5% chance of these individuals being diagnosed with specific delays in development with additional follow-up time beyond 5.78 years).
Interestingly, by reducing the length of follow-up, and hence introducing greater uncertainty as to the correct diagnostic status of the controls examined, it was possible to reduce the adverse effects observed to be associated with organic-Hg exposure from Thimerosal-containing hepatitis B vaccine administration, but still the effect observed was so robust that significant associations were still found in each analysis. For example, by requiring that controls had to be continuously enrolled in the VSD from birth until they were at least 4.2 years-old (mean age of initial diagnosis of specific delays in development plus the standard deviation of mean age of initial diagnosis of specific delays in development), cases were still significantly more likely (OR = 2.36, P < 0.00001) than controls to have received 37.5 μg organic-Hg from three Thimerosal-containing hepatitis B vaccine doses in comparison to 0 μg organic-Hg from no-Thimerosal hepatitis B-containing vaccines and/or no hepatitis B vaccinations within the first 6 months of life. Similarly, by further reducing length of follow-up, so that controls had to be continuously enrolled in the VSD from birth until they were at least 2.62-years-old (mean age of initial diagnosis of specific delays in development), cases were again significantly more likely (OR = 2.29, P < 0.00001) than controls to have received 37.5 μg organic-Hg from three Thimerosal-containing hepatitis B vaccine doses in comparison to 0 μg organic-Hg from three no-Thimerosal hepatitis B-containing vaccines and/or no hepatitis B vaccinations within the first 6 months of life.
That the VSD data were collected independently of the study design used in the present study is another strength. The VSD data records analyzed were collected as part of the routine health care individuals received through their participation with their respective HMOs, and as such, the healthcare providers in no way were thinking about the potential association between vaccine exposures and potential health outcomes.
Another strength of the present study, was that this study investigated the potential consequences of gender on the relationship between organic-Hg exposure from Thimerosal-containing hepatitis B vaccines and the diagnosis of specific delays in development. As shown in Tables 2 and 3, even when the VSD data examined in separate gender analyses (male or female) cases diagnosed with specific delays in development were still significantly more likely than to controls to have received increased organic-Hg exposure from Thimerosal-containing hepatitis B vaccines administered at specific intervals within the first 6 months of life. The present study also evaluated exposure to Thimerosal-containing hepatitis B vaccine doses administered at specific intervals within the first 6 months of life among the ratios of the exposed-to-unexposed male cases in comparison to the ratios of exposed-to-unexposed female cases for each level of exposure. For those outcomes, overall there were no significantly increased relative males-to-females ratios for the increased organic-Hg exposure from Thimerosal-containing hepatitis B vaccine doses among male cases in comparison to female cases, but there was a non-significant trend towards the ratio of exposed-to-unexposed male cases where the exposed males received 37.5 μg organic-Hg from three Thimerosal-containing hepatitis B vaccine doses in comparison to 0 μg organic-Hg from three no-Thimerosal hepatitis B-containing vaccine doses and/or no hepatitis B vaccinations within the first 6 months of life in comparison to the same ratio for exposed-to-unexposed female cases. Future studies should be conducted to examine different amounts of exposure to organic-Hg from Thimerosal-containing vaccines and outcomes other than those examined in the present study.
However, the results of the present study may have a number of potential limitations. It is possible the results observed may have occurred from unknown biases or cofounders present in the datasets examined. This seems unlikely because other control outcomes (outcomes that are not biologically plausibly linked to postnatal organic-Hg exposure from Thimerosal-containing vaccines) were examined, such as a diagnosis of congenital anomalies (ICD-9 code: 759.9) in the VSD database, using the same methodology employed for specific delays in development. No similar patterns of significant associations were observed for these outcomes in contrast to that found for organic-Hg exposure from Thimerosal-containing hepatitis B vaccine administration and the subsequent risk of diagnosed specific delays in development. For example, congenital anomaly cases and controls were similarly exposed to 12.5 μg organic-Hg from a Thimerosal-containing hepatitis B vaccine dose administered in the first month of life in comparison to 0 μg organic-Hg from a no-Thimerosal hepatitis B-containing vaccine dose or no hepatitis B vaccination administered in the first month of life (OR = 1.03, P > 0.50).
Another potential limitation of the present study is that the results observed for specific delays in development may be the result of statistical chance. However, such a possibility would be unlikely given the limited number of statistical tests performed, the highly significant results observed (all the calculated P-values were < 0.00001), and the consistency in the direction and magnitude of the results observed.
Still, other potential limitations of the present study include the possibilities that some of the individuals in the cohorts in the VSD database examined: May have had more subtle neurological dysfunction that was not brought to the attention of their healthcare providers, healthcare providers may have misdiagnosed some individuals, or some vaccine exposures may not have been appropriately classified. While these limitations, possibly present in the data examined in the current study, should not have significantly impacted the results observed, it is unclear how differential application would have occurred to affect the study cohorts examined based upon the Thimerosal doses that the individuals received. Moreover, misclassification occurring in the data examined would tend to bias any results observed toward the null hypothesis, since such effects would result in individuals being placed in the wrong exposure and/or outcome categories examined, and this would result in decreased statistical power to determine true potential exposure-outcome relationships.
In addition, another potential limitation of the present study is that exposures to other sources of Hg were not evaluated. The individuals examined in the present study very likely incurred other organic-Hg exposures from other Thimerosal-containing childhood vaccines, breastfeeding, formula feeding, and, to a lesser extent, dental amalgams, fish, or other environmental sources. While these other sources of Hg may play a significant involvement in the pathogenesis specific delays in development, these Hg exposures, not accounted for in this study, would actually tend to bias the results observed towards the null hypothesis because they potentially would confound the specific exposure classifications of Hg examined. For example, individuals classified as having lower organic-Hg exposure from Thimerosal-containing vaccines may have actually received high doses of Hg from other sources, and individuals having higher organic-Hg exposure from Thimerosal-containing vaccines may have actually received low doses of Hg from other sources, with the net result tending to minimize the magnitude of the associations observed. In addition, the current study suffers from the potential limitation that analyses were not conducted to further explore the precise timing and cumulative doses of organic-Hg from all Thimerosal-containing childhood vaccines associated with maximum adverse consequences. In future studies, it would be worthwhile to explore these precise timing and cumulative-dose phenomena.
Finally, the present study is limited in the types of neurodevelopmental outcomes examined, as well as other covariates such as race, birth weight, etc., that may affect the magnitude of the adverse effects found. It would be of significant interest in future studies to explore other neurodevelopmental outcomes and other covariates.
Conclusion
The present study provides compelling new epidemiological evidence supporting a significant relationship between increasing organic-Hg exposure from Thimerosal-containing childhood vaccines and the subsequent risk of a diagnosis for specific delays in development among both males and females. Many recent studies support the biologically plausible role of organic-Hg exposure from Thimerosal-containing vaccines in the pathogenesis of specific delays in development. The specific ICD-9 code examined in the current study included specific delays in development involving speech/language, coordination, hearing, and reading disorders. Hg is a known developmental and neurotoxin, and its specificity in targeting long-range axons, as the evidence here would suggest, possibly contributes to the abnormal long-range tracts that are found in children diagnosed with specific delays in development, such as reading, hearing, coordination and speech/language.
In summary, using a hypothesis-testing, epidemiological analytical methodology in the VSD database, organic-Hg exposure from Thimerosal-containing childhood vaccines was determined to be a significant risk factor for the subsequent diagnosis of specific delays in development among males and females. In addition, the present study placed special emphasis on requiring an adequate follow-up period in the analysis. Thus, the cases and controls were followed for a sufficient, evidenced-based interval of time, to ensure that they were appropriately classified with respect to their exposures and outcomes. This carefully- determined period of follow-up thus helped to avoid the potential for a cause-and-effect relationship between exposure and outcome to be biased or confounded. Future studies should be completed to evaluate the possible relationship between organic-Hg exposure from Thimerosal-containing childhood vaccines and other chronic disorders, and to assess the timing of organic-Hg exposure from Thimerosal-containing vaccine administration associated with adverse outcomes within specific subpopulations.
As mentioned previously, since Hg bio-accumulates, combined exposure from different sources is a serious concern, especially in the face of an underlying chronic exposure that appears to be increasing and detrimental. Thus, every preventable source of exposure should be evaluated and avoided. Additive effects from different sources could potentially increase a child's Hg-exposure from a subclinical chronic exposure level to a clinical level in which obvious neurodevelopmental consequences occur. Routine childhood vaccination may be an important public health tool to reduce the morbidity and mortality associated with certain infectious diseases. However, it is also a public health imperative to end the unnecessary addition of organic-Hg to vaccines in the form of Thimerosal used as a preservative, based on data showing an association between its administration and adverse outcomes.
Acknowledgement
This study was financially supported by the Dwoskin Family Foundation and the Selz Foundation.
Footnotes
Source of Support: This study was financially supported by the Dwoskin Family Foundation and the Selz Foundation.
Conflict of Interest: All of the investigators on the present study have been involved in vaccine/biologic litigation.
References
- 1.Kalia M. Brain development: Anatomy, connectivity, adaptive plasticity, and toxicity. Metabolism. 2008;57(Suppl 2):S2–5. doi: 10.1016/j.metabol.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 2.Migliarini S, Pacini G, Pelosi B, Lunardi G, Pasqualetti M. Lack of brain serotonin affects postnatal development and serotonergic neuronal circuitry formation. Mol Psychiatry. 2013;18:1106–18. doi: 10.1038/mp.2012.128. [DOI] [PubMed] [Google Scholar]
- 3.LeBlanc JJ, Fagiolini M. Autism: A “critical period” disorder? Neural Plast. 2011;2011:921680. doi: 10.1155/2011/921680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyle CA, Boulet S, Schieve LA, Cohen RA, Blumberg SJ, Yeargin-Allsopp M, et al. Trends in the prevalence of developmental disabilities in US children, 1997-2008. Pediatrics. 2011;127:1034–42. doi: 10.1542/peds.2010-2989. [DOI] [PubMed] [Google Scholar]
- 5.Arroyo HA, Fernandez MC. Environmental toxic and its effect on neurodevelopment. Medicina (B Aires) 2013;73(Suppl 1):93–102. [PubMed] [Google Scholar]
- 6.Roberts AL, Lyall K, Hart JE, Laden F, Just AC, Bobb JF, et al. Perinatal air pollutant exposures and autism spectrum disorder in the children of nurses' health study II participants. Environ Health Perspect. 2013;121:978–84. doi: 10.1289/ehp.1206187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kern JK, Geier DA, Ayzac F, Adams JB, Mehta JA, Geier MR. Toxicity biomarkers among US children compared to a similar cohort in France: A blinded study measuring urinary porphyrins. Toxicol Environ Chem. 2011;93:396–405. doi: 10.1080/02772248.2010.508609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Laks DR. Assessment of chronic mercury exposure within the U.S. population, National Health and Nutrition Examination Survey, 1999-2006. Biometals. 2009;22:1103–14. doi: 10.1007/s10534-009-9261-0. [DOI] [PubMed] [Google Scholar]
- 9.Kern JK, Haley BE, Geier DA, Sykes LK, King PG, Geier MR. Thimerosal exposure and the role of sulfation chemistry and thiol availability in autism. Int J Environ Res Public Health. 2013;10:3771–800. doi: 10.3390/ijerph10083771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ball LK, Ball R, Pratt RD. An assessment of thimerosal use in childhood vaccines. Pediatrics. 2001;107:1147–54. doi: 10.1542/peds.107.5.1147. [DOI] [PubMed] [Google Scholar]
- 11.Geier DA, Geier MR. A review of the Vaccine Adverse Event Reporting System database. Expert Opin Pharmacother. 2004;5:691–8. doi: 10.1517/14656566.5.3.691. [DOI] [PubMed] [Google Scholar]
- 12.Chen RT, DeStefano F, Davis RL, Jackson LA, Thompson RS, Mullooly JP, et al. The Vaccine Safety Datalink: Immunization research in health maintenance organizations in the USA. Bull World Health Organ. 2000;78:186–94. [PMC free article] [PubMed] [Google Scholar]
- 13.Chen RT, Glasser JW, Rhodes PH, Davis RL, Barlow WE, Thompson RS, et al. Vaccine Safety Datalink project: A new tool for improving vaccine safety monitoring in the United States. The Vaccine Safety Datalink Team. Pediatrics. 1997;99:765–73. doi: 10.1542/peds.99.6.765. [DOI] [PubMed] [Google Scholar]
- 14.Wassilak SG, Glasser JW, Chen RT, Hadler SC. Utility of large-linked databases in vaccine safety, particularly in distinguishing independent and synergistic effects. The Vaccine Safety Datalink Investigators. Ann N Y Acad Sci. 1995;754:377–82. doi: 10.1111/j.1749-6632.1995.tb44473.x. [DOI] [PubMed] [Google Scholar]
- 15.Ellenberg SS, Braun MM. Monitoring the safety of vaccines: Assessing the risks. Drug Saf. 2002;25:145–52. doi: 10.2165/00002018-200225030-00001. [DOI] [PubMed] [Google Scholar]
- 16.Varricchio F, Iskander J, Destefano F, Ball R, Pless R, Braun MM, et al. Understanding vaccine safety information from the Vaccine Adverse Event Reporting System. Pediatr Infect Dis J. 2004;23:287–94. doi: 10.1097/00006454-200404000-00002. [DOI] [PubMed] [Google Scholar]
- 17.Iskander J, Pool V, Zhou W, English-Bullard R. VAERS Team. Data mining in the US using the Vaccine Adverse Event Reporting System. Drug Saf. 2006;29:375–84. doi: 10.2165/00002018-200629050-00002. [DOI] [PubMed] [Google Scholar]
- 18.Hepatitis B virus: A comprehensive strategy for eliminating transmission in the United States through universal childhood vaccination. Recommendations of the Immunization Practices Advisory Committee (ACIP) MMWR Recomm Rep. 1991;40:1–25. [PubMed] [Google Scholar]
- 19.Clarkson TW, Nordberg GF, Sager PR. Reproductive and developmental toxicity of metals. Scand J Work Environ Health. 1985;11:145–54. doi: 10.5271/sjweh.2239. [DOI] [PubMed] [Google Scholar]
- 20.Dorea JG. Low-dose mercury exposure in early life: Relevance of thimerosal to fetuses, newborns, and infants. Curr Med Chem. 2013;20:4060–9. doi: 10.2174/09298673113209990229. [DOI] [PubMed] [Google Scholar]
- 21.Burbacher TM, Shen DD, Liberato N, Grant KS, Cernichiari E, Clarkson T. Comparison of blood and brain mercury levels in infant monkeys exposured to methylmercury or vaccines containing Thimerosal. Environ Health Perspect. 2005;113:1015–21. doi: 10.1289/ehp.7712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rodrigues JL, Sepeloni JM, Batista BL, Souza SS, Barbosa F., Jr Identification and distribution of mercury species in rat tissues following administration of Thimerosal or methylmercury. Arch Toxicol. 2010;84:891–6. doi: 10.1007/s00204-010-0538-4. [DOI] [PubMed] [Google Scholar]
- 23.Pichichero ME, Gentile A, Giglio N, Umido V, Clarkson T, Cernichiari E, et al. Mercury levels in newborns and infants after receipt of Thimerosal-containing vaccines. Pediatrics. 2008;121:e208–14. doi: 10.1542/peds.2006-3363. [DOI] [PubMed] [Google Scholar]
- 24.Pichichero ME, Gentile A, Giglio N, Alonso MM, Fernandez Mentaberri MV, Zareba G, et al. Mercury levels in premature and low birth weight newborn infants after receipt of Thimerosal-containing vaccines. J Pediatr. 2009;155:495–9. doi: 10.1016/j.jpeds.2009.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stajich GV, Lopez GP, Harry SW, Sexson WR. Iatrogenic exposure to mercury after hepatitis B vaccination in preterm infants. J Pediatr. 2000;136:679–81. doi: 10.1067/mpd.2000.105133. [DOI] [PubMed] [Google Scholar]
- 26.Marques RC, Dorea JG, Fonseca MF, Bastos WR, Malm O. Hair mercury in breast-fed infants exposed to Thimerosal-preserved vaccines. Eur J Pediatr. 2007;166:935–41. doi: 10.1007/s00431-006-0362-2. [DOI] [PubMed] [Google Scholar]
- 27.Zimmermann LT, Santos DB, Naime AA, Leal RB, Dorea JG, Barbosa F, Jr, et al. Comparative study on methyl- and ethylmercury-induced toxicity in C6 glioma cells and the potential role of LAT-1 in mediating mercurial-thiol complexes uptake. Neurotoxicology. 2013;38:1–8. doi: 10.1016/j.neuro.2013.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kern JK, Geier DA, Audhya T, King PG, Sykes LK, Geier MR. Evidence of parallels between mercury intoxication and the brain pathology of autism. Acta Neurobiol Exp (Wars) 2012;72:113–53. doi: 10.55782/ane-2012-1887. [DOI] [PubMed] [Google Scholar]
- 29.Hagmann P, Cammoun L, Gigandet X, Meuli R, Honey CJ, Wedeen VJ, et al. Mapping the structural core of human cerebral cortex. PLoS Biol. 2008;6:e159. doi: 10.1371/journal.pbio.0060159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fujioka T, Mourad N, Trainor LJ. Development of auditory-specific brain rhythm in infants. Eur J Neurosci. 2011;33:521–9. doi: 10.1111/j.1460-9568.2010.07544.x. [DOI] [PubMed] [Google Scholar]
- 31.Smith JB, Alloway KD. Functional specificity of claustrum connections in the rat: Interhemispheric communication between specific parts of motor cortex. J Neurosci. 2010;30:16832–44. doi: 10.1523/JNEUROSCI.4438-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wandell BA, Yeatman JD. Biological developing of reading circuits. Curr Opin Neurobiol. 2013;23:261–8. doi: 10.1016/j.conb.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yeatman JD, Dougherty RF, Ben-Shachar M, Wandell BA. Developing of white matter and reading skills. Proc Natl Acad Sci U S A. 2012;109:E3045–53. doi: 10.1073/pnas.1206792109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jeong JW, Sundaram SK, Kumar A, Chugani DC, Chugani HT. Aberrant diffusion and geometric properties in the left arcuate fasciculus of developmentally delayed children: A diffusion tensor imaging study. AJNR Am J Neuroradiol. 2011;32:323–30. doi: 10.3174/ajnr.A2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pallocca G, Fabbri M, Sacco MG, Gribaldo L, Pamies D, Laurenza I, et al. miRNA expression profiling in a human stem cell-based model as a tool for developmental neurotoxicity testing. Cell Biol Toxicol. 2013;29:239–57. doi: 10.1007/s10565-013-9250-5. [DOI] [PubMed] [Google Scholar]
- 36.Hornig M, Chian D, Lipkin WI. Neurotoxic effects of postnatal thimerosal are mouse strain dependent. Mol Psychiatry. 2004;9:833–45. doi: 10.1038/sj.mp.4001529. [DOI] [PubMed] [Google Scholar]
- 37.Olczak M, Duszczyk M, Mierzejewski P, Meyza K, Majewska MD. Persistent behavioral impairments and alterations of brain dopamine system after early postnatal administration of thimerosal in rats. Behav Brain Res. 2011;223:107–18. doi: 10.1016/j.bbr.2011.04.026. [DOI] [PubMed] [Google Scholar]
- 38.Chen YN, Wang J, Zhang J, Li SJ, Hel L, Shao DD, et al. Effect of thimerosal on the neurodevelopment of premature rats. World J Pediatr. 2013;9:356–60. doi: 10.1007/s12519-013-0443-z. [DOI] [PubMed] [Google Scholar]
- 39.Sulkowski ZL, Chen T, Midha S, Zavacki AM, Sajdel-Sulkowska EM. Maternal thimerosal exposure results in aberrant cerebellar oxidative stress, thyroid hormone metabolism, and motor behavior in rat pups; sex- and strain-dependent effects. Cerebellum. 2012;11:575–86. doi: 10.1007/s12311-011-0319-5. [DOI] [PubMed] [Google Scholar]
- 40.Laurente J, Remuzgo F, Avalos B, Chiquinta J, Ponce B, Avendano R, et al. Neurotoxic effects of thimerosal at vaccines doses on the encephalon and development in 7 days-old hamsters. An Fac Med Lima. 2007;68:222–37. [Google Scholar]
- 41.Hewitson L, Houser LA, Stott C, Sackett G, Tomko JL, Atwood D, et al. Delayed acquisition of neonatal reflexes in newborn premates receiving a Thimerosal-containing hepatitis B vaccine: Influence of gestational age and birth weight. J Toxicol Environ Health A. 2010;73:1298–313. doi: 10.1080/15287394.2010.484709. [DOI] [PubMed] [Google Scholar]
- 42.Young HA, Geier DA, Geier MR. Thimerosal exposure in infants and neurodevelopmental disorders: An assessment of computerized medical records in the Vaccine Safety Datalink. J Neurol Sci. 2008;271:110–8. doi: 10.1016/j.jns.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 43.Geier DA, Geier MR. A Two-phased population epidemiological study of the safety of Thimerosal-containing vaccines: A follow-up analysis. Med Sci Monit. 2005;11:CR160–70. [PubMed] [Google Scholar]
- 44.Gallagher C, Goodman M. Hepatitis B triple series vaccine and developmental disability in US children aged 1-9 years. Toxicol Environ Chem. 2008;90:997–1008. [Google Scholar]
- 45.Geier DA, Geier MR. A meta-analysis epidemiological assessment of neurodevelopmental disorders following vaccines administered from 1994 through 2000 in the United States. Neuro Endocrinol Lett. 2006;27:401–13. [PubMed] [Google Scholar]
- 46.Mrozek-Budzyn D, Majewska R, Kieltyka A, Augustyniak M. Neonatal exposure to thimerosal from vaccines and child development in the first 3 years of life. Neurotoxicol Teratol. 2012;34:592–7. doi: 10.1016/j.ntt.2012.10.001. [DOI] [PubMed] [Google Scholar]
- 47.Dorea JG, Marques RC, Isejima C. Neurodevelopment of amazonian infants: Antenatal and postnatal exposure to methyl- and ethylmercury. J Biomed Biotechnol. 2012;2012:132876. doi: 10.1155/2012/132876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Verstraeten T, Davis RL, DeStefano F, Lieu TA, Rhodes PH, Black SB, et al. Vaccine Safety Datalink Team. Safety of Thimerosal-containing vaccines: A two-phased study of computerized health maintenance organization databases. Pediatrics. 2003;112:1039–48. [PubMed] [Google Scholar]
- 49.Andrews N, Miller E, Grant A, Stowe J, Osborne V, Taylor B. Thimerosal exposure in infants and developmental disorders: A retrospective cohort study in the United Kingdom does not support a causal association. Pediatrics. 2004;114:584–91. doi: 10.1542/peds.2003-1177-L. [DOI] [PubMed] [Google Scholar]
- 50.Tozzi AE, Bisiacchi P, Tarantino V, De Mei B, D'Elia L, Chiarotti F, et al. Neuropsychological performance 10 years after immunization in infancy with thimerosal-containing vaccines. Pediatrics. 2009;123:475–82. doi: 10.1542/peds.2008-0795. [DOI] [PubMed] [Google Scholar]


