Abstract
Auditory hallucinations (AH) are the most frequent positive symptoms in patients with schizophrenia. Hallucinations have been related to emotional processing disturbances, altered functional connectivity and effective connectivity deficits. Previously, we observed that, compared to healthy controls, the limbic network responses of patients with auditory hallucinations differed when the subjects were listening to emotionally charged words. We aimed to compare the synchrony patterns and effective connectivity of task-related networks between schizophrenia patients with and without AH and healthy controls.
Schizophrenia patients with AH (n = 27) and without AH (n = 14) were compared with healthy participants (n = 31). We examined functional connectivity by analyzing correlations and cross-correlations among previously detected independent component analysis time courses. Granger causality was used to infer the information flow direction in the brain regions.
The results demonstrate that the patterns of cortico-cortical functional synchrony differentiated the patients with AH from the patients without AH and from the healthy participants. Additionally, Granger-causal relationships between the networks clearly differentiated the groups. In the patients with AH, the principal causal source was an occipital–cerebellar component, versus a temporal component in the patients without AH and the healthy controls.
These data indicate that an anomalous process of neural connectivity exists when patients with AH process emotional auditory stimuli. Additionally, a central role is suggested for the cerebellum in processing emotional stimuli in patients with persistent AH.
Keywords: Schizophrenia, Auditory hallucinations, Functional connectivity, Cerebellum, Synchrony, Effective connectivity
Abbreviations: AH, auditory hallucinations; CCTC, cortico-cerebellar–thalamic–cortical; MRI, functional magnetic resonance imaging; BPRS, Brief Psychiatric Rating Scale; PANSS, Positive and Negative Syndrome Scale; PSYRATS, Psychotic Symptom Rating Scale; ICA, independent component analysis; ICA-TC, ICA-time course; SPM, statistical parametric maps; BOLD, blood oxygenation level dependent; MVAR, multivariate autoregression; CoI, component of interest; GCCA, Granger causal connectivity analysis
Graphical abstract
Highlights
-
•
Schizophrenia is thought to reflect subtle dysfunctions in connectivity.
-
•
We analyze differences in functional and effective connectivity among groups.
-
•
The patterns of cortico-cortical functional synchrony are different among groups.
-
•
Granger-causal relationships between networks also clearly differentiated the groups.
-
•
These data implicate an anomalous process of neural connectivity.
1. Introduction
Increasingly, it is hypothesized that schizophrenia reflects subtle connectivity dysfunction (Pettersson-Yeo et al., 2011). Converging neurophysiological and neuroimaging data have documented widely distributed abnormalities in brain activity and functional connectivity (Buckholtz and Meyer-Lindenberg, 2012; Friston and Frith, 1995; Pettersson-Yeo et al., 2011; Stephan et al., 2006), validating the overarching disconnection hypothesis of schizophrenia (Andreasen et al., 1998). In terms of the structural aspects, a qualitative review (Kubicki et al., 2005) and a quantitative meta-analysis (Ellison-Wright and Bullmore, 2009) have documented widespread reductions in white matter fractional anisotropy in chronic schizophrenia, which have also been observed in first-episode patients (Friedman et al., 2008). An abnormal white matter ultrastructure likely underlies abnormal cooperation among brain networks (Calhoun et al., 2009). After finding abnormal functional connectivity between the frontal and temporal regions, Friston and Frith (1995) proposed fronto-temporal disconnection as a key neurobiological feature of schizophrenia. The term “disconnection” connotes reduced connectivity. “Dysconnectivity” has been introduced to refer to abnormal integration between anatomically distinct brain regions (Stephan et al., 2006, 2009). Andreasen et al. (1998) suggested that disruption of the cortico-cerebellar–thalamic–cortical (CCTC) circuits underlies the deterioration in neural synchrony, with improper coordination of the mental processes leading to “cognitive dysmetria.” Abnormal neural synchrony (Ford et al., 2007) is hypothesized to be one of the main causes of cognitive dysfunction in schizophrenia. A recent review (Uhlhaas and Singer, 2010) concluded that altered neural oscillations and synchrony are crucial elements in the pathophysiology of schizophrenia. This conclusion was based on aberrant connectivity and synchrony findings in patients with schizophrenia (Friston and Frith, 1995; Garrity et al., 2007; Jafri et al., 2008; Kim et al., 2008), as well as differences in effective connectivity in schizophrenia patients compared to controls (Demirci et al., 2008; Diaconescu et al., 2011; Kim et al., 2008). The dysconnectivity hypothesis has been extended to explain specific symptoms in schizophrenia, particularly auditory hallucinations (AH) (Rish et al., 2013) and visual hallucinations and AH in findings by Amad et al. (2014).
AH are a hallmark of the psychotic experience. Neuroimaging studies have found abnormal activation in patients with AH, particularly in cortical regions of language processing (Allen et al., 2008). Several studies have shown abnormal structural and functional connectivity in patients with AH (Benetti et al., 2013; Mechelli et al., 2007).
Most studies have found reduced functional connectivity between the temporal, limbic and frontal areas (Hoffman et al., 2011; Vercammen et al., 2010). Few studies (e.g., Mechelli et al., 2007) have analyzed synchrony and effective connectivity in a group of patients with schizophrenia and AH.
Emotional processing disturbances have been related to the origination of AH (Sanjuán et al., 2006; Aleman and Larøi, 2008). Our group has focused on understanding emotional processing in patients with AH. In a previous study, we obtained evidence of enhanced activation of the limbic and frontal brain areas in a small group of patients with persistent AH engaged in passively listening to emotional words (Sanjuán et al., 2007). These results implicated circuits subserving the processing of emotional stimuli as a neural substrate of AH. In a subsequent work, we studied functional connectivity in response to an emotional auditory paradigm in patients with AH by conducting independent component analyses (ICA) (Escartí et al., 2010). Using this approach, the activated areas could be obtained by selecting the ICA components related to the emotional auditory paradigm. Functional connectivity is then characterized by detecting statistical dependencies among the time courses of the areas activated in response to the auditory emotional stimuli (Escartí et al., 2010). In addition to the temporal, frontal and parietal networks detected in all subjects, in the schizophrenia patients with AH, we observed a specific pattern of functional connectivity involving activation of the limbic structures (predominantly the amygdala and parahippocampal gyrus). We used an emotional auditory task in this work to analyze the synchrony patterns and Granger-causal relationships to study effective connectivity and to determine which brain regions directly influence other brain regions. We predicted the following results: compared to the controls and schizophrenia patients without AH, the schizophrenia patients with AH would 1) have abnormal synchrony between the detected networks and concrete abnormalities in the frontal cortex compared with the other brain regions and 2) have different Granger-causal interactions between the frontal, parietal, temporal, limbic and cerebellar networks.
2. Materials and methods
2.1. Participants
A total of 41 male patients with schizophrenia (27 with chronic AH and 14 without AH) were recruited. All patients fulfilled the DSM-IV diagnostic criteria for schizophrenia. The patients with chronic AH fulfilled the following selection criteria for persistent hallucinations (Sanjuán et al., 2007): (a) they heard voices that were resistant to treatment for at least 1 year; (b) the voices were present at least once a day during the last year; and (c) at least two antipsychotic drugs had been tried in the last year at doses equivalent to at least 600 mg/day of chlorpromazine. Patients with a history of traumatic brain injury, epilepsy or other neurological or psychiatric history were excluded. All patients were being treated with stable doses of antipsychotic medication. The patients were clinically assessed with the Positive and Negative Syndrome Scale (PANSS) (Kay et al., 1987), the Brief Psychiatric Rating Scale (BPRS) (Ventura et al., 1993) and the Psychotic Symptom Rating Scale (PSYRATS) for AH (Haddock et al., 1999) in the 24 h prior to scanning.
A group of 31 healthy male subjects was selected as the control group. The participants were male, right handed (as assessed with the Edinburgh Questionnaire, Oldfield, 1971) and Caucasian, and they provided written informed consent to participate in the experiment. The study was approved by the local institutional ethics committee.
The healthy controls and patients did not differ significantly in terms of sex, laterality, or ethnicity. The groups differed significantly in educational level, with a greater proportion of individuals with university and high school diplomas (or equivalence) in the control group, as expected. Table 1 shows the demographic and clinical characteristics of all participants.
Table 1.
Demographic and clinical characteristics of patients and control samples.
| Controls (n = 31) | Hallucinators (n = 27) | Non-hallucinators (n = 14) | p | |
|---|---|---|---|---|
| Age (years) | 31.34 ± 10.52 | 39.15 ± 8.76 | 42.93 ± 14.76 | < 0.002 |
| Duration of illness (years) | 15.1 ± 8.1 | 18.2 ± 11.2 | N.S. | |
| Handedness (% right) | 100 | 100 | 100 | |
| PANSSa | 65.33 ± 17.83 | 53.71 ± 11.68 | < 0.021 | |
| BPRSb | 51.11 ± 10.96 | 38.86 ± 7.96 | < 0.003 | |
| PSYRATSc | 30.33 ± 4.96 | 0 | ||
| Educational level | ||||
| Elementary school | 4 | 15 | 7 | < 0.003 |
| High school degree equivalent | 14 | 10 | 4 | |
| University | 13 | 2 | 3 | |
| Treatment (type of antipsychotic) | ||||
| First generation | 1 | 1 | N.S. | |
| Second generation | 16 | 8 | N.S. | |
| Combined (first and second generation) | 10 | 5 | N.S. | |
| Chlorpromazine dose equivalence | 792.63 ± 698.80 | 533.33 ± 410.86 | p = 0.145 |
Data are displayed as mean ± SD.
Positive and Negative Syndrome Scale.
Brief Psychiatric Rating Scale.
Psychotic Symptom Rating Scale.
2.2. Functional imaging
2.2.1. Data acquisition
A 1.5 Tesla scanner (Philips Medical Systems) was used to acquire BOLD contrasts during a stimulation paradigm (Escartí et al., 2010; Sanjuán et al., 2005). An emotional auditory paradigm was designed to evoke emotions related to the patients' hallucinatory experiences (Supplementary material 1). The participants were binaurally stimulated during two different sessions. The activation blocks in one session consisted of 13 Spanish words with high emotional content. The other session had activation blocks containing 13 words with neutral or low emotional content. Four blocks of stimuli (20 s each) were interleaved with four blocks of rest of 20 s each. The subjects were informed before the test regarding the two types of words they were going to hear and were asked to focus their attention on these words.
2.2.2. Data analysis
Pre-processing of the functional data was performed using SPM (http://www.fil.ion.ucl.ac.uk/spm) (Supplementary material 2). The ICA analysis was performed using the Group ICA approach fMRI Toolbox (GIFT, http://www.icatb.sourceforge.net). Components of interest (CoI) were selected (in terms of the individual beta values related to the emotional task), and the regressors in each component were entered into a one-sample t-test with a threshold of p < 0.01 (Escartí et al., 2010). Age was controlled for in a second analysis. A correlation test was performed to evaluate the relationship between age and the components of interest loads. Correlations were not detected.
2.3. Techniques for measuring synchrony
We used the intra-group ICA time-course (TC) correlation coefficient matrix obtained for all pair-wise combinations of ICA-TC to examine functional connectivity across the three groups (Jafri et al., 2008). To observe the relationships between every CoI pair, we calculated Pearson's correlation coefficients (Friston, 1994) (Supplementary material 3).
2.4. Techniques used for causal analysis
The data tool Granger causal connectivity analysis (GCCA) was used to analyze causality (Seth, 2010). A key challenge when analyzing such data is to study the causal connectivity using a driven-data methodology to study multivariate time series. This kind of methodology combines graph-theoretic and network-theoretic techniques that allow their quantitative characterization (Seth, 2005).
2.4.1. Causal flow
Within a given causal network, we defined the causal flow of a node, “i”, as the difference (weighted or not) between its inflows and outflows. A node with positive causal flow exerts strong causal influence on a dynamic system as a whole. This node is called a causal source. In contrast, a node with negative causal flow is called a causal sink (Seth et al., 2011).
2.4.2. Causal density
The causal density of a dynamic system provides an overall measure of causal interactivity (Seth, 2005). This causal density measure is defined as a causal graph for all pairs of elements in the system. Those interactions that are not significant are assigned zero values, as calculated by the following expression:
| (1) |
where X[ij] is a X subsystem omitting variables Xi and Xj. This value could be calculated between 0 and 1 and is based on causal density, in which all significant interactions are assigned the value 1. Causal density measures dynamic complexity because it reflects the complexity of integration and the coexistence of dynamic segregation (Sporns, 2007). High causal density indicates which system elements are globally coordinated in their activities. It facilitates the prediction of the influence of a node on the graph. However, the elements are dynamically different; therefore, diverse elements contribute in a different manner.
2.4.3. Unit causal density
The unit causal density cdn (i) of a node “i” is a value related to the causal interaction of that node i normalized by the number of nodes. A system with n-nodes has n elements with n-cdn values, is an unweighted version and is calculated by assigning all significant interactions the value 1. A node with a high value for cdn could be called a causal hub of system X.
3. Results
3.1. CoI identified in each group by ICA analysis
As previously reported (Escartí et al., 2010), the following CoI were identified in the control group: temporal (CE19), fronto-temporo-parietal (CE18), subcortical–fronto-temporal (CE09) and occipito-cerebellar (CE04). The following CoI were identified in the patient group with AH: temporal (HE17), fronto-parietal (HE14), fronto-temporal (HE11), limbic (parahippocampal–amygdalar) (HE06), and occipito-cerebellar (HE02). The following CoI were identified in the patients with schizophrenia but without chronic AH: temporal (NHE19), fronto-temporal (NHE18), subcortical–temporo-parietal–cerebellar (NHE13) and fronto-parietal (NHE12).
3.2. Synchrony
Correlation coefficients could be calculated for every pair of CoI. The possible combinations are shown in Tables 2, 3 and 4.
Table 2.
Correlation coefficients and significances R (p), for all ICA components observed in the healthy control group. The table depicts all possible pair-wise correlations for ICA time courses.
| R (p) | CE19 | CE18 | CE09 | CE04 |
|---|---|---|---|---|
| CE19 | 1.00 (0.0000) | 0.64 (0.0000) | 0.78 (0.0000) | 0.84 (0.0000) |
| CE18 | 0.64 (0.0000) | 1.00 (0.0000) | 0.42 (0.0001) | 0.50 (0.0000) |
| CE09 | 0.78 (0.0000) | 0.42 (0.0001) | 1.00 (0.0000) | 0.50 (0.0000) |
| CE04 | 0.84 (0.0000) | 0.50 (0.0000) | 0.50 (0.0000) | 1.00 (0.0000) |
Table 3.
Correlation coefficients and significances R (p), for all ICA components observed in patients with AH. The table depicts all possible pair-wise correlations for ICA time courses.
| R (p) | HE17 | HE14 | HE11 | HE06 | HE02 |
|---|---|---|---|---|---|
| HE17 | 1.00 (0.0000) | 0.82 (0.0000) | 0.61 (0.0000) | 0.35 (0.0016) | 0.07 (0.5172) |
| HE14 | 0.82 (0.0000) | 1.00 (0.0000) | 0.84 (0.0000) | 0.45 (0.0000) | 0.39 (0.0003) |
| HE11 | 0.61 (0.0000) | 0.84 (0.0000) | 1.00 (0.0000) | 0.33 (0.0029) | 0.35 (0.0013) |
| HE06 | 0.35 (0.0016) | 0.45 (0.0000) | 0.33 (0.0029) | 1.00 (0.0000) | 0.61 (0.0000) |
| HE02 | 0.07 (0.5172) | 0.39 (0.0003) | 0.35 (0.0013) | 0.61 (0.0000) | 1.00 (0.0000) |
Table 4.
Correlation coefficients and significances R (p), for all ICA components observed in patients without AH. The table depicts all possible pair-wise correlations for ICA time courses.
| R (p) | NHE19 | NHE18 | NHE13 | NHE12 |
|---|---|---|---|---|
| NHE19 | 1.00 (0.0000) | 0.52 (0.0000) | 0.70 (0.0000) | 0.68 (0.0000) |
| NHE18 | 0.52 (0.0000) | 1.00 (0.0000) | 0.33 (0.0001) | 0.72 (0.0000) |
| NHE13 | 0.70 (0.0000) | 0.33 (0.0001) | 1.00 (0.0000) | 0.47 (0.0000) |
| NHE12 | 0.68 (0.0000) | 0.72 (0.0000) | 0.47 (0.0000) | 1.00 (0.0000) |
3.2.1. Control group
As shown in Table 2 and Fig. 1A, higher correlations were found for the temporal component (CE19), particularly in the relationships to the subcortical–fronto-temporal (CE09) and occipito-cerebellar (CE04) components. The minimal correlation value was found in the relationship between the fronto-temporo-parietal (CE18) and subcortical–fronto-temporal (CE09) components. All the components were highly correlated with each one another. Fig. 1A shows that the temporal (CE19) and subcortical–fronto-temporal (CE09) components were synchronized, whereas the fronto-temporo-parietal (CE18) and occipito-cerebellar (CE04) components were delayed, compared with components (CE19, CE09). Fig. 1A (right) depicts the correlation function calculations for the control group in terms of the phaser graphs. A phasor is a vector whose modulus is the maximal value of the function. The lag at which this value is obtained is represented by the means of the angle between the vector and the horizontal axis.
Fig. 1.
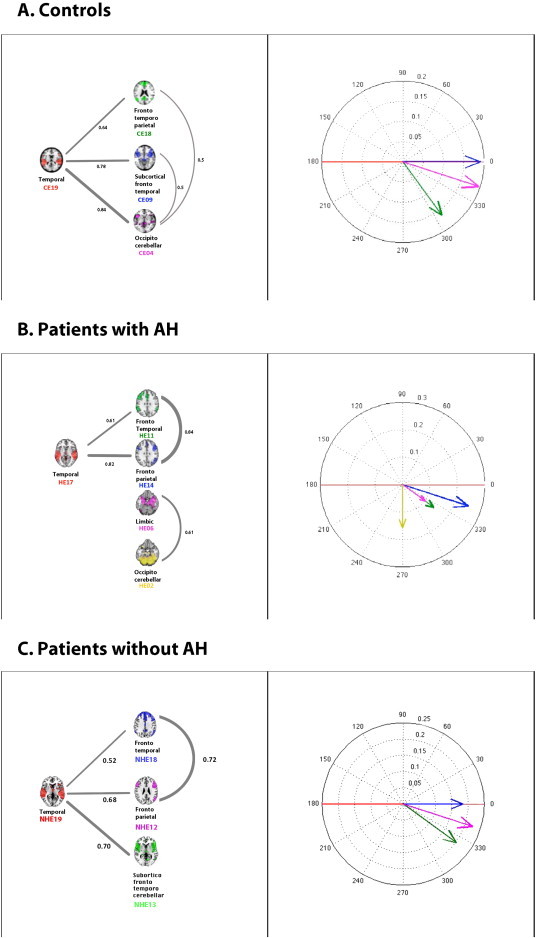
A. The network correlations in the control group. The results obtained for each pair of components with R > 0.5 (see Table 2). The correlation coefficient values are adjacent to the edges, and the thicknesses of the edges represent the magnitudes of the correlations (left). A phasorial plot, in which we used the CE19 component as a reference and compared its cross-correlation function (CCF) with those of the remaining components (right). Abbreviations: temporal (CE19), fronto-temporal–parietal (CE18), subcortical–fronto-temporal (CE09), occipito-cerebellar (CE04). B. The network correlations in the patients with schizophrenia and chronic AH. The results obtained for each pair of components with R > 0.5 (see Table 3). The correlation coefficient values are adjacent to the edges, and the thicknesses of the edges represent the magnitudes of the correlations (left). A phasorial plot, in which we used the HE17 component as a reference and compared its CCF with those of the remaining components (right). Abbreviations: temporal (HE17), fronto-parietal (HE14), fronto-temporal (HE11), limbic (parahippocampal–amygdala) (HE06), and occipito-cerebellar (HE02). C. The network correlations in the patients with schizophrenia and without chronic AH. The results obtained for each pair of components with R > 0.5 (see Table 4). The correlation coefficient values are adjacent to the edges, and the thicknesses of the edges represent the magnitudes of the correlations (left). A phasorial plot, in which we used the NHE19 component as a reference and compared its CCF with those of the remaining components (right). Abbreviations: temporal (NHE19), fronto-temporal (NHE18), subcortico-fronto-temporal–cerebellar (NHE13) and fronto-parietal (NHE12).
3.2.2. Patients with chronic AH
As shown in Table 3 and Fig. 1B, we found high correlations among the temporal (HE17), fronto-parietal (HE14) and fronto-temporal (HE11) components. Additionally, the limbic (HE06) and occipito-cerebellar (HE02) components were significantly correlated with one another; however, they were unrelated to the temporal, fronto-parietal, and fronto-temporal components (the left side of Fig. 1B).
The cross-correlation function calculations among the patients are shown in Fig. 1B. The phasers corresponding to the limbic (HE06) and occipito-cerebellar (HE02) components (the right side of Fig. 1B) showed large delays compared with the remaining components.
3.2.3. Patients without chronic AH
As shown in Table 4 and Fig. 1C, we found high significant correlations between the temporal (NHE19), fronto-parietal (NHE12), subcortico-fronto-temporal–cerebellar (NH13) and fronto-temporal (NHE18) components. The right side of Fig. 1C depicts the correlation function calculations for the patients without AH in terms of the phaser graphs. Fig. 1C shows that the temporal (NHE19) and fronto-temporal (NHE18) components were synchronized, whereas the frontal–parietal (NHE12) and subcortico-temporo-parietal–cerebellar (NHE13) components were delayed compared with the first two components.
3.3. Effective connectivity analysis
3.3.1. Control group
Based on the Granger-causality analysis, the temporal (CE19) and fronto-temporo-parietal (CE18) components were causal sources, whereas the subcortical–fronto-temporal (CE09) and occipito-cerebellar (CE04) components were causal sinks (Fig. 2A). The analysis of the causal density units showed that all nodes were highly integrated in the system, with the temporal (CE19) component being the main causal hub. The temporal (CE19) component exerted the highest Granger-causality influence on the remaining system components.
Fig. 2.
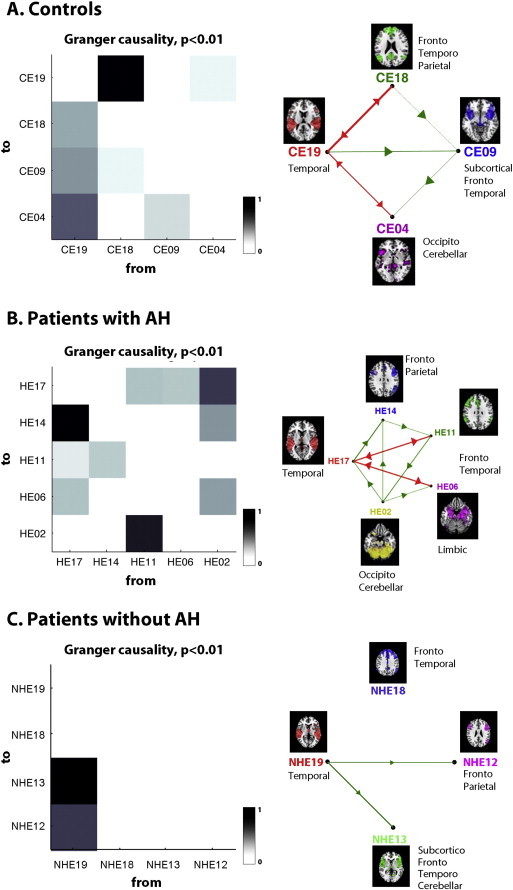
The central upper image represents the G-causality matrix between the ICA time courses, with black indicating a strong presence of a given connection across all profiles and white indicating that an interaction was never present. The right figure shows the graph representing the causality network at p < 0.01. The vertices represent the CoI, and the edges represent their causality relationships. The color red indicates bidirectional edges (high interaction), and green indicates unidirectionality. The multivariate Bayesian information criterion was calculated to determine the VAR model order, p (p = 1). A) Control group. Abbreviations: temporal (CE19), fronto-temporo-parietal (CE18), subcortical–fronto-temporal (CE09), occipito-cerebellar (CE04). B) Patients with schizophrenia and AH. Abbreviations: temporal (HE17), fronto-parietal (HE14), fronto-temporal (HE11), limbic (parahippocampal–amygdala) (HE06), and occipito-cerebellar (HE02). C) Patients with schizophrenia without auditory hallucinations. Abbreviations: temporal (NHE19), fronto-temporal (NHE18), subcortico-temporo-cerebellar (NHE13) and fronto-parietal (NHE12).
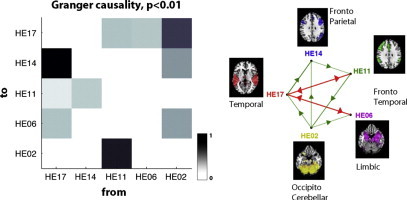
3.3.2. Patients with persistent AH
As shown in Fig. 2B, the occipito-cerebellar (HE02) component was a strong causal source, whereas the fronto-parietal (HE14) and limbic (HE06) components were causal sinks. The temporal (HE17) component was the main causal hub. The graph for the patients with AH shows a large number of interactions among the components.
3.3.3. Patients without chronic AH
We observed that the temporal component (NHE19) was a causal source, whereas the fronto-parietal (NHE12) and subcortico-fronto-temporal–cerebellar (NHE13) components were causal sinks (Fig. 2C). The temporal (NHE19) component was the main causal hub. The temporal (NHE19) component exerted the highest Granger-causality influence on the remaining system components.
4. Discussion
In a homogeneous sample of patients with schizophrenia and persistent AH, we found (1) abnormal synchrony compared to a healthy control group and to patients with schizophrenia without chronic AH, specifically between the cerebellum-limbic and frontal–temporal–parietal networks, as well as (2) a different pattern of effective connectivity between these functional networks while processing emotional auditory stimuli.
Our finding of aberrant functional connectivity between the networks (the frontal–parietal–temporal–limbic–cerebellar areas) in the patients compared to the controls aligns with reports of reduced frontal connectivity (Pettersson-Yeo et al., 2011) and disconnections between the frontal and temporal lobes (Calhoun et al., 2004; Garrity et al., 2007; Kim et al., 2008). We used the task of passively listening to emotional auditory stimuli, which elicited a different pattern of functional response between the controls and patients because we only observed activation in a network comprising subcortical–limbic areas (amygdala and parahippocampal gyrus) in the patients with AH; these results have been previously reported and discussed in depth (Escartí et al., 2010). This analysis showed that the subcortical–limbic network and an occipito-cerebellum network were more synchronized in the patients with AH (particularly in the fusiform gyrus, culmen and declive of the vermis) and desynchronized from the frontal–parietal–temporal areas.
The controls and patients without AH showed more synchronization between the networks (frontal–parietal–temporal–limbic–cerebellar areas) than the patients with AH. Our results align with the most common finding in the literature, abnormalities in the frontal lobe (Fornito et al., 2011; Salvador et al., 2010; Zalesky et al., 2010), frontal–temporal dysconnectivity (Fornito et al., 2011; Zalesky et al., 2010) and prefrontal regions connections with other brain regions, including the cerebellum and the parietal and occipital cortices. Additionally, altered regional connectivity of the parietal, temporal and occipital cortex, as well as the subcortical nuclei, has been observed (Lynall et al., 2010). It is hypothesized that language dysfunction located in the frontal and temporal regions and specific dysconnectivity between the two regions play critical roles in the core pathophysiology of schizophrenia studies, particularly in studies of auditory verbal hallucinations (Hubl et al., 2004; Seok et al., 2007; Winder et al., 2007; Wible et al., 2009).
Our second finding was that causal interactions between the functional brain networks differed among the three groups. In the control group and the patient group without AH, we found that the main causal source was the temporal lobe network component (CE19), which aligns with previous studies of emotional auditory processing (Phillips et al., 2003). In the control group, the temporal (CE19) and fronto-temporo-parietal (CE18) components were causal sources; however, in the group of patients without AH, only the temporal component (NHE19) was a casual source. In the group of patients with AH, the main causal source was the occipito-cerebellar network component (HE02), despite the processing of auditory emotional stimuli by the patients.
The cerebellum plays an important role in supporting brain synchrony. The study of synchrony could inform our understanding of the functional organization of the brain, particularly in task-evoked processes (Bartels and Zeki, 2005; McKiernan et al., 2003). Our findings support and extend the findings of numerous studies that have identified similar regions (cerebellar and limbic) associated with emotional deficits in patients with schizophrenia (Aleman and Kahn, 2005; Andreasen and Pierson, 2008). Brain synchrony is the basis for communication between different neural areas. From this perspective, the cerebellum could be considered to be crucial for synchrony (Picard et al., 2008; Schutter and Van Honk, 2005). Additionally, the cerebellum is involved in higher cognitive functions (Schmahmann, 2001; Andreasen and Pierson, 2008). The cerebellar circuits process learning (particularly error-related), timing and prediction in relation to motor and cognitive information (D'Angelo and Casali, 2012). The timing hypothesis postulates that the cerebellum is critical for representing temporal relationships among task-relevant events, which is closely related to the concept of synchrony. In this sense, the cerebellum function is analogous to a supramodal internal timing unit (such as a metronome) (Ivry et al., 2002). Hypothetically, when the cerebellar timing function is disrupted, the information processing stream becomes desynchronized, providing a nurturing environment for a diverse range of psychopathological conditions (Schutter and Van Honk, 2005). Regarding schizophrenia, the cerebellum is connected to many regions of the cerebral cortex by the cortico-cerebellar–thalamic–cortical circuits and might play a crucial role in this distributed circuit to coordinate or modulate aspects of cortical activity (Picard et al., 2008).
According to the “cognitive dysmetria” or “dysmetria of thought” models of schizophrenia, aberrant cerebellar modulation of information to the cerebral cortex is involved in the pathophysiology of the disorder (Andreasen et al., 1998; Schmahmann, 1998a). This finding is consistent with the Daskalakis et al. (2005) study, which preliminarily reported that, compared with controls, patients with schizophrenia demonstrated deficits in cerebellar inhibition. Their data are corroborated by our results demonstrating between-group differences in the causal flow of information between the cerebellar–occipital component and other regions, with an afferent direction of flow for the controls (Figs. 2A and 2C) and an efferent direction for the patients with AH (Fig. 2B).
There is evidence that neuroanatomical damage to the CCTC could be the primary pathophysiological alteration in schizophrenia (Konarski et al., 2005). It is well known that cerebellar abnormalities exist in schizophrenia patients (Andreasen and Pierson, 2008). Our findings suggest that the functional dysconnectivity of the cerebellum with the cerebrum predominantly involves the anterior and vermal areas of the cerebellum, the main areas included in the occipito-cerebellar component in the group of patients with AH. The vermis has previously been labeled as the “limbic cerebellum” because the anterior vermis is the principal cerebellar target of limbic projections (Schmahmann, 2000). Behavioral studies have supported a relationship between the cerebellar midline structures and the modulation of emotion (Heath and Harper, 1974; Stoodley and Schmahmann, 2010), and lesions of the vermis have been shown to produce affective symptoms ranging from emotional blunting and depression to disinhibition and psychotic features (Schmahmann and Sherman, 1998b).
Further evidence implicating cerebellar circuits was presented by Diederen et al. (2010), who found less activation of the left cerebellum, right insula and left parahippocampal gyrus in patients with AH. Additionally, our findings are consistent with the results of Clos et al. (2014), which showed decreased connectivity in several brain areas in patients with AH, including between the right cerebellum and left thalamus.
In this study, we relied on ICA, a driven-data method that could separate independent spatial–temporal patterns of neural activity from the fMRI data in a manner that has been helpful in studying intrinsic brain networks (Damoiseaux et al., 2006). Granger-causality was used to explore effective connectivity in fMRI data to quantify the strength of interactions between activated brain areas (Goebel et al., 2003; Demirci et al., 2009; Londei et al., 2007) The ICA and Granger-causality techniques allowed us to analyze the functional and effective connectivities, respectively. ICA allowed us to select the CoI that were candidates for the GCCA analysis of their associated time-courses. The main strength of this methodological framework was the combination of these independent techniques, which allowed us to observe whether different methods would lead to consistent results and more reliable conclusions.
This study has several limitations. First, all patients were taking antipsychotics at the time of scanning, although we did not find any significant correlations between the chlorpromazine equivalents and our imaging measures. Second, our small sample size limited our power to detect relationships with symptom severity or duration of illness, Our findings of impaired synchrony should be confirmed in larger samples, preferably with unmedicated first-episode schizophrenia patients. Third, the sample of patients without AH (n = 14) is relatively small compared with the control group (n = 31) and patients with AH (n = 27) groups. Including a subgroup of schizophrenia patients treated with the identical medications and differing only in the absence of persistent AH strengthens our confidence in our findings. Fourth, the application of the Granger-causality method to the fMRI data is somewhat controversial (Friston, 2009; Roebroeck et al., 2009). Specifically, Granger-causality analysis ignores the influence of other areas when assessing whether coupling between reference regions A and B is driven by region C (Gao et al., 2008). Accordingly, we employed GCCA (Geweke, 1984), which is based on a direct expansion of the autoregressive model to a multivariate general case, including all measured variables.
Patients with schizophrenia and AH exhibit abnormal patterns of neural synchrony, as well as different patterns of Granger-causal interaction between functional networks compared with controls and patients with schizophrenia without AH. The results indicate an anomalous process of neural connectivity in the cortico-cerebellar–thalamic–cortical circuits in patients with AH. A central role for the cerebellum in the pathological processing of emotional stimuli by patients with schizophrenic and persistent AH is suggested, perhaps reflecting deficiencies in predicting the emotional effect of a given stimuli.
Funding
Funding for this study was provided by Spanish grants from Ministry of Science and Innovation (ISCIII: FIS P.I.02/0018, P.I. 05/2332.), Spanish Mental Health Network: CIBERSAM and Combiomed Network.
Acknowledgments
This study was supported by Spanish grants from Ministry of Science and Innovation (ISCIII: FIS P.I. 02/0018, P.I. 05/2332.). The authors would also like to thank José Vicente Sancho, Marta Bleda, Francisco García, Jose M. Puig and Erika Proal for helping us and the next institutions: Spanish Mental Health Network: CIBERSAM, GIBI230 (Grupo de Investigación Biomédica en Imagen, CIBER-BBN), Instituto de Investigación Sanitaria (IIS), CEIB-CS (Centre of Excellence in Biomedical Imaging — Regional Ministry of Health in the Valencia Region), FISABIO and General Directorate of Information Systems for Health in the Valencia Region.
Contributor Information
Maria de la Iglesia-Vaya, Email: miglesia@cipf.es, delaiglesia_mar@gva.es, maigva@gmail.com.
Maria José Escartí, Email: mescartifabra@hotmail.com.
Jose Molina-Mateo, Email: jmmateo@fis.upv.es.
Luis Martí-Bonmatí, Email: luis.marti@uv.es.
Marien Gadea, Email: marien_gadea@uv.es.
Francisco Xavier Castellanos, Email: francisco.castellanos@nyumc.org.
Eduardo J. Aguilar García-Iturrospe, Email: eduardoj.aguilar@gmail.com.
Montserrat Robles, Email: mrobles@fis.upv.es.
Bharat B. Biswal, Email: bbiswal@gmail.com.
Julio Sanjuan, Email: Julio.Sanjuan@uv.es.
Appendix A. Supplementary material
Supplementary material
References
- Aleman A., Kahn R.S. Strange feelings: do amygdala abnormalities dysregulate the emotional brain in schizophrenia? Progress in Neurobiology. 2005;77:283–298. doi: 10.1016/j.pneurobio.2005.11.005. 16352388 [DOI] [PubMed] [Google Scholar]
- Aleman A., Larøi F. Hallucinations and the Brain. American Psychological Association; Washington, D.C.: 2008. [Google Scholar]
- Allen P., Larøi F., McGuire P.K., Aleman A. The hallucinating brain: a review of structural and functional neuroimaging studies of hallucinations. Neuroscience and Biobehavioral Reviews. 2008;32:175–191. doi: 10.1016/j.neubiorev.2007.07.012. 17884165 [DOI] [PubMed] [Google Scholar]
- Amad A., Cachia A., Gorwood P., Pins D., Delmaire C., Rolland B., Mondino M. The multimodal connectivity of the hippocampal complex in auditory and visual hallucinations. Molecular Psychiatry. 2014;19:184–191. doi: 10.1038/mp.2012.181. 23318999 [DOI] [PubMed] [Google Scholar]
- Andreasen N.C., Paradiso S., O’Leary D.S. "Cognitive dysmetria” as an integrative theory of schizophrenia: a dysfunction in cortical–subcortical–cerebellar circuitry? Schizophrenia Bulletin. 1998;24:203–218. doi: 10.1093/oxfordjournals.schbul.a033321. 9613621 [DOI] [PubMed] [Google Scholar]
- Andreasen N.C., Pierson R. The role of the cerebellum in schizophrenia. Biological Psychiatry. 2008;64:81–88. doi: 10.1016/j.biopsych.2008.01.003. 18395701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels A., Zeki S. Brain dynamics during natural viewing conditions — a new guide for mapping connectivity in vivo. Neuroimage. 2005;24:339–349. doi: 10.1016/j.neuroimage.2004.08.044. 15627577 [DOI] [PubMed] [Google Scholar]
- Benetti S., Pettersson-Yeo W., Mechelli A. Connectivity issues of the “hallucinating” brain. In: Jardri R., Cachia A., Thomas P., Pins D., editors. The Neuroscience of Hallucinations SE — 22. Springer; New York: 2013. pp. 417–443. [Google Scholar]
- Buckholtz J.W., Meyer-Lindenberg A. Psychopathology and the human connectome: toward a transdiagnostic model of risk for mental illness. Neuron. 2012;74:990–1004. doi: 10.1016/j.neuron.2012.06.002. 22726830 [DOI] [PubMed] [Google Scholar]
- Calhoun V.D., Kiehl K.A., Liddle P.F., Pearlson G.D. Aberrant localization of synchronous hemodynamic activity in auditory cortex reliably characterizes schizophrenia. Biological Psychiatry. 2004;55:842–849. doi: 10.1016/j.biopsych.2004.01.011. 15050866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun V.D., Eichele T., Pearlson G. Functional brain networks in schizophrenia: a review. Frontiers in Human Neuroscience. 2009;3:17. doi: 10.3389/neuro.09.017.2009. 19738925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clos M., Diederen K.M., Meijering A.L., Sommer I.E., Eickhoff S.B. Aberrant connectivity of areas for decoding degraded speech in patients with auditory verbal hallucinations. Brain Structure & Function. 2014;219:581–594. doi: 10.1007/s00429-013-0519-5. 23423461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Angelo E., Casali S. Seeking a unified framework for cerebellar function and dysfunction: from circuit operations to cognition. Frontiers in Neural Circuits. 2012;6:116. doi: 10.3389/fncir.2012.00116. 23335884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux J.S., Rombouts S.A., Barkhof F., Scheltens P., Stam C.J., Smith S.M., Beckmann C.F. Consistent resting-state networks across healthy subjects. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:13848–13853. doi: 10.1073/pnas.0601417103. 16945915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daskalakis Z.J., Christensen B.K., Fitzgerald P.B., Fountain S.I., Chen R. Reduced cerebellar inhibition in schizophrenia: A preliminary study. American Journal of Psychiatry. 2005;162:1203–1205. doi: 10.1176/appi.ajp.162.6.1203. 15930071 [DOI] [PubMed] [Google Scholar]
- Demirci O., Clark V.P., Calhoun V.D. A projection pursuit algorithm to classify individuals using fMRI data: Application to schizophrenia. Neuroimage. 2008;39:1774–1782. doi: 10.1016/j.neuroimage.2007.10.012. 18396487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demirci O., Stevens M.C., Andreasen N.C., Michael A., Liu J., White T., Pearlson G.D., Clark V.P., Calhoun V.D. Investigation of relationships between fMRI brain networks in the spectral domain using ICA and Granger causality reveals distinct differences between schizophrenia patients and healthy controls. Neuroimage. 2009;46:419–431. doi: 10.1016/j.neuroimage.2009.02.014. 19245841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaconescu A.O., Jensen J., Wang H., Willeit M., Menon M., Kapur S., Mcintosh A.R. Aberrant effective connectivity in schizophrenia patients during appetitive conditioning. Frontiers in Human Neuroscience. 2011;4:239. doi: 10.3389/fnhum.2010.00239. 21267430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diederen K.M.J., Neggers S.F.W., Daalman K., Blom J.D., Goekoop R., Kahn R.S., Sommer I.E.C. Deactivation of the parahippocampal gyrus preceding auditory hallucinations in schizophrenia. American Journal of Psychiatry. 2010;167:427–435. doi: 10.1176/appi.ajp.2009.09040456. 20123912 [DOI] [PubMed] [Google Scholar]
- Ellison-Wright I., Bullmore E. Meta-analysis of diffusion tensor imaging studies in schizophrenia. Schizophrenia Research. 2009;108:3–10. doi: 10.1016/j.schres.2008.11.021. 19128945 [DOI] [PubMed] [Google Scholar]
- Escartí M.J., De La Iglesia-Vayá M., Martí-Bonmatí L., Robles M., Carbonell J., Lull J.J., García-Martí G. Increased amygdala and parahippocampal gyrus activation in schizophrenic patients with auditory hallucinations: an fMRI study using independent component analysis. Schizophrenia Research. 2010;117:31–41. doi: 10.1016/j.schres.2009.12.028. 20071145 [DOI] [PubMed] [Google Scholar]
- Ford J.M., Krystal J.H., Mathalon D.H. Neural synchrony in schizophrenia: from networks to new treatments. Schizophrenia Bulletin. 2007;33:848–852. doi: 10.1093/schbul/sbm062. 17567628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornito A., Yoon J., Zalesky A., Bullmore E.T., Carter C.S. General and specific functional connectivity disturbances in first-episode schizophrenia during cognitive control performance. Biological Psychiatry. 2011;70(1):64–72. doi: 10.1016/j.biopsych.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman J.I., Tang C., Carpenter D., Buchsbaum M., Schmeidler J., Flanagan L., Golembo S. Diffusion tensor imaging findings in first-episode and chronic schizophrenia patients. American Journal of Psychiatry. 2008;165:1024–1032. doi: 10.1176/appi.ajp.2008.07101640. 18558643 [DOI] [PubMed] [Google Scholar]
- Friston K.J. Functional and effective connectivity in neuroimaging: a synthesis. Human Brain Mapping. 1994;2:56–78. [Google Scholar]
- Friston K. Causal modelling and brain connectivity in functional magnetic resonance imaging. P.L.O.S. Biology. 2009;7:6. doi: 10.1371/journal.pbio.1000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K.J., Frith C.D. Schizophrenia: a disconnection syndrome? Clinical Neuroscience (New York, N.Y.) 1995;3:89–97. 7583624 [PubMed] [Google Scholar]
- Gao Q., Chen H., Gong Q. Evaluation of the effective connectivity of the dominant primary motor cortex during bimanual movement using Granger causality. Neuroscience Letters. 2008;443:1–6. doi: 10.1016/j.neulet.2008.07.036. 18656524 [DOI] [PubMed] [Google Scholar]
- Garrity A.G., Pearlson G.D., McKiernan K., Lloyd D., Kiehl K.A., Calhoun V.D. Aberrant “default mode” functional connectivity in schizophrenia. American Journal of Psychiatry. 2007;164:450–457. doi: 10.1176/ajp.2007.164.3.450. 17329470 [DOI] [PubMed] [Google Scholar]
- Geweke J.F. Measures of conditional linear dependence and feedback between time series. Journal of the American Statistical Association. 1984;79(388):709–715. [Google Scholar]
- Goebel R., Roebroeck A., Kim D.S., Formisano E. Investigating directed cortical interactions in time-resolved fMRI data using vector autoregressive modeling and Granger causality mapping. Magnetic Resonance Imaging. 2003;21:1251–1261. doi: 10.1016/j.mri.2003.08.026. 14725933 [DOI] [PubMed] [Google Scholar]
- Haddock G., McCarron J., Tarrier N., Faragher E.B. Scales to measure dimensions of hallucinations and delusions: the psychotic symptom rating scales (PSYRATS) Psychological Medicine. 1999;29:879–889. doi: 10.1017/s0033291799008661. 10473315 [DOI] [PubMed] [Google Scholar]
- Heath R.G., Harper J.W. Ascending projections of the cerebellar fastigial nucleus to the hippocampus, amygdala, and other temporal lobe sites: evoked potential and histological studies in monkeys and cats. Experimental Neurology. 1974;45:268–287. doi: 10.1016/0014-4886(74)90118-6. 4422320 [DOI] [PubMed] [Google Scholar]
- Hoffman R.E., Fernandez T., Pittman B., Hampson M. Elevated functional connectivity along a corticostriatal loop and the mechanism of auditory/verbal hallucinations in patients with schizophrenia. Biological Psychiatry. 2011;69:407–414. doi: 10.1016/j.biopsych.2010.09.050. 21145042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubl D., Koenig T. Pathways that make voices: white matter changes in auditory hallucinations. Archives of General Psychiatry. 2004;61(7):658–668. doi: 10.1001/archpsyc.61.7.658. 15237078 [DOI] [PubMed] [Google Scholar]
- Ivry R.B., Spencer R.M., Zelaznik H.N., Diedrichsen J. The cerebellum and event timing. Annals of the New York Academy of Sciences. 2002;978:302–317. doi: 10.1111/j.1749-6632.2002.tb07576.x. 12582062 [DOI] [PubMed] [Google Scholar]
- Jafri M.J., Pearlson G.D., Stevens M., Calhoun V.D. A method for functional network connectivity among spatially independent resting-state components in schizophrenia. Neuroimage. 2008;39:1666–1681. doi: 10.1016/j.neuroimage.2007.11.001. 18082428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay S.R., Fiszbein A., Opler L.A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia Bulletin. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. 3616518 [DOI] [PubMed] [Google Scholar]
- Kim D., Burge J., Lane T., Pearlson G.D., Kiehl K.A., Calhoun V.D. Hybrid ICA-Bayesian network approach reveals distinct effective connectivity differences in schizophrenia. Neuroimage. 2008;42:1560–1568. doi: 10.1016/j.neuroimage.2008.05.065. 18602482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konarski J.Z., McIntyre R.S., Grupp L.A., Kennedy S.H. Is the cerebellum relevant in the circuitry of neuropsychiatric disorders? Journal of Psychiatry & Neuroscience: JPN. 2005;30:178–186. 15944742 [PMC free article] [PubMed] [Google Scholar]
- Kubicki M., McCarley R.W., Shenton M.E. Evidence for white matter abnormalities in schizophrenia. Current Opinion in Psychiatry. 2005;18:121–134. doi: 10.1097/00001504-200503000-00004. 16639164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Londei A., D’Ausilio A., Basso D., Sestieri C., Del Gratta C., Romani G.L., Olivetti Belardinelli M. Brain network for passive word listening as evaluated with ICA and Granger causality. Brain Research Bulletin. 2007;72:284–292. doi: 10.1016/j.brainresbull.2007.01.008. 17452288 [DOI] [PubMed] [Google Scholar]
- Lynall M.E., Bassett D.S., Kerwin R., McKenna P.J., Kitzbichler M., Muller U., Bullmore E. Functional connectivity and brain networks in schizophrenia. Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2010;30:9477–9487. doi: 10.1523/JNEUROSCI.0333-10.2010. 20631176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKiernan K.A., Kaufman J.N., Kucera-Thompson J., Binder J.R. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. Journal of Cognitive Neuroscience. 2003;15:394–408. doi: 10.1162/089892903321593117. 12729491 [DOI] [PubMed] [Google Scholar]
- Mechelli A., Allen P., Amaro E., Fu C.H.Y., Williams S.C.R., Brammer M.J., Johns L.C. Misattribution of speech and impaired connectivity in patients with auditory verbal hallucinations. Human Brain Mapping. 2007;28:1213–1222. doi: 10.1002/hbm.20341. 17266108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield R.C. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. 5146491 [DOI] [PubMed] [Google Scholar]
- Pettersson-Yeo W., Allen P., Benetti S., McGuire P., Mechelli A. Dysconnectivity in schizophrenia: where are we now? Neuroscience and Biobehavioral Reviews. 2011;35:1110–1124. doi: 10.1016/j.neubiorev.2010.11.004. 21115039 [DOI] [PubMed] [Google Scholar]
- Phillips M.L., Drevets W.C., Rauch S.L., Lane R. Neurobiology of emotion perception I: the neural basis of normal emotion perception. Biological Psychiatry. 2003;54:504–514. doi: 10.1016/s0006-3223(03)00168-9. 12946879 [DOI] [PubMed] [Google Scholar]
- Picard H., Amado I., Mouchet-Mages S., Olié J.-P., Krebs M.-O. The role of the cerebellum in schizophrenia: an update of clinical, cognitive, and functional evidences. Schizophrenia Bulletin. 2008;34:155–172. doi: 10.1093/schbul/sbm049. 17562694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rish I., Cecchi G., Thyreau B., Thirion B., Plaze M., Paillere-Martinot M.L., Martelli C. Schizophrenia as a network disease: disruption of emergent brain function in patients with auditory hallucinations. PloS One. 2013;8:e50625. doi: 10.1371/journal.pone.0050625. 23349665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roebroeck A., Formisano E., Goebel R. Reply to Friston and David after comments on: The identification of interacting networks in the brain using fMRI: model selection, causality and deconvolution. Neuroimage. 2009;58:310–311. doi: 10.1016/j.neuroimage.2009.09.036. [DOI] [PubMed] [Google Scholar]
- Salvador R., Sarró S., Gomar J.J., Ortiz-Gil J., Vila F., Capdevila A., Bullmore E., McKenna P.J., Pomarol-Clotet E. Overall brain connectivity maps show cortico-subcortical abnormalities in schizophrenia. Human Brain Mapping. 2010;31:2003–2014. doi: 10.1002/hbm.20993. 20225222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanjuán J., Lull J.J., Martí-Bonmati L., Aguilar E.J., Gadea M., Moratal-Pérez D., González J.C. Emotional auditory paradigm in neuroimaging: a base for the study of psychosis. Actas Españolas de Psiquiatría. 2005;33:383–389. 16292722 [PubMed] [Google Scholar]
- Sanjuán J. [The aetiopathogenesis of auditory hallucinations in psychosis] Revista de Neurologia. 2006;43:280–286. 16941426 [PubMed] [Google Scholar]
- Sanjuán J., Lull J.J., Aguilar E.J., Martí-Bonmatí L., Moratal D., Gonzalez J.C., Robles M. Emotional words induce enhanced brain activity in schizophrenic patients with auditory hallucinations. Psychiatry Research. 2007;154:21–29. doi: 10.1016/j.pscychresns.2006.04.011. 17184978 [DOI] [PubMed] [Google Scholar]
- Schmahmann J.D. Dysmetria of thought: clinical consequences of cerebellar dysfunction on cognition and affect. Trends in Cognitive Sciences. 1998;2:362–371. doi: 10.1016/s1364-6613(98)01218-2. 21227233 [DOI] [PubMed] [Google Scholar]
- Schmahmann J.D., Sherman J.C. The cerebellar cognitive affective syndrome. Brain: A Journal of Neurology. 1998;121(4):561–579. doi: 10.1093/brain/121.4.561. 9577385 [DOI] [PubMed] [Google Scholar]
- Schmahmann J.D. The role of the cerebellum in affect and psychosis. Journal of Neurolinguistics. 2000;13:189–214. [Google Scholar]
- Schmahmann J.D. The cerebellar cognitive affective syndrome: clinical correlations of the dysmetria of thought hypothesis. International Review of Psychiatry. 2001;13:313–322. [Google Scholar]
- Schutter D.J.L.G., Van Honk J. The cerebellum on the rise in human emotion. Cerebellum (London, England) 2005;4:290–294. doi: 10.1080/14734220500348584. 16321885 [DOI] [PubMed] [Google Scholar]
- Seok J.-H., Park H.-J. White matter abnormalities associated with auditory hallucinations in schizophrenia: a combined study of voxel-based analyses of diffusion tensor imaging and structural magnetic resonance imaging. Psychiatry Research. 2007;156(2):93–104. doi: 10.1016/j.pscychresns.2007.02.002. 17884391 [DOI] [PubMed] [Google Scholar]
- Seth A.K. Causal connectivity of evolved neural networks during behavior. Network (Bristol, England) 2005;16:35–54. doi: 10.1080/09548980500238756. 16350433 [DOI] [PubMed] [Google Scholar]
- Seth A.K. A MATLAB toolbox for Granger causal connectivity analysis. Journal of Neuroscience Methods. 2010;186:262–273. doi: 10.1016/j.jneumeth.2009.11.020. 19961876 [DOI] [PubMed] [Google Scholar]
- Seth A.K., Barrett A.B., Barnett L. Causal density and integrated information as measures of conscious level. Philosophical Transactions. Series A, Mathematical, Physical, and Engineering Sciences. 2011;369:3748–3767. doi: 10.1098/rsta.2011.0079. 21893526 [DOI] [PubMed] [Google Scholar]
- Sporns O. Brain connectivity. Scholarpedia. 2007;2:4695. [Google Scholar]
- Stephan K.E., Baldeweg T., Friston K.J. Synaptic plasticity and disconnection in schizophrenia. Biological Psychiatry. 2006;59:929–939. doi: 10.1016/j.biopsych.2005.10.005. 16427028 [DOI] [PubMed] [Google Scholar]
- Stephan K.E., Friston K.J., Frith C.D. Dysconnection in schizophrenia: from abnormal synaptic plasticity to failures of self-monitoring. Schizophrenia Bulletin. 2009;35:509–527. doi: 10.1093/schbul/sbn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley C.J., Schmahmann J.D. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex; a Journal Devoted to the Study of the Nervous System and Behavior. 2010;46:831–844. doi: 10.1016/j.cortex.2009.11.008. 20152963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas P.J., Singer W. Abnormal neural oscillations and synchrony in schizophrenia. Nature Reviews. Neuroscience. 2010;11:100–113. doi: 10.1038/nrn2774. 20087360 [DOI] [PubMed] [Google Scholar]
- Ventura J., Green M.F., Shaner A., Liberman R.P. Training and quality assurance with the Brief Psychiatric Rating Scale: “the drift busters”. International Journal of Methods in Psychiatric Research. 1993;3:221–244. [Google Scholar]
- Vercammen A., Knegtering H., Den Boer J.A., Liemburg E.J., Aleman A. Auditory hallucinations in schizophrenia are associated with reduced functional connectivity of the temporo-parietal area. Biological Psychiatry. 2010;67:912–918. doi: 10.1016/j.biopsych.2009.11.017. 20060103 [DOI] [PubMed] [Google Scholar]
- Wible C.G., Lee K. fMRI activity correlated with auditory hallucinations during performance of a working memory task: data from the FBIRN consortium study. Schizophrenia Bulletin. 2009;35(1):47–57. doi: 10.1093/schbul/sbn142. 18990710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winder R., Cortes C.R. Functional connectivity in fMRI: a modeling approach for estimation and for relating to local circuits. Neuroimage. 2007;34(3):1093–1107. doi: 10.1016/j.neuroimage.2006.10.008. 17134917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalesky A., Fornito A., Bullmore E.T. Network-based statistic: identifying differences in brain networks. NeuroImage. 2010;53:1197–1207. doi: 10.1016/j.neuroimage.2010.06.041. 20600983 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material


