Abstract
Sulfiredoxin is a recently discovered member of the oxidoreductases family which plays a crucial role in thiol homoeostasis when under oxidative stress. A myriad of systemic disorders have oxidative stress and reactive oxygen species as the key components in their etiopathogenesis. Recent studies have evaluated the role of this enzyme in oxidative stress mediated diseases such as atherosclerosis, chronic obstructive pulmonary disease and a wide array of carcinomas. Its action is responsible for the normal functioning of cells under oxidative stress and the promotion of cell survival in cancerous cells. This review will highlight the cumulative effects of sulfiredoxin in various systemic disorders with a strong emphasis on its target activity and the factors influencing its expression in such conditions.
Keywords: Sulfiredoxin, Antioxidant, Oxidative stress, Reactive oxygen species, Systemic diseases
Abbreviations: Prx, peroxiredoxin; COPD, chronic obstructive pulmonary disease; TLR, toll like receptor; Nlrp3, NOD-like receptor family, pyrin domain-containing-3; Nrf2, nuclear factor erythroid 2-related factor 2; LPS, lipopolysaccharide; RANKL, receptor activator of nuclear factor kappa β ligand; RANK, receptor activator of nuclear factor kappa β
Graphical abstract
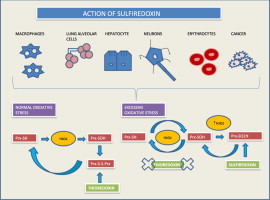
Highlights
-
•
Sulfiredoxin alleviates oxidative burden in various cell types.
-
•
Sulfiredoxin contributes to the pathogenesis of oxidative stress mediated diseases.
-
•
Influencing factors and the parameters influenced by sulfiredoxin are reviewed.
Overview-sulfiredoxin
Sulfiredoxin, a redox protein was discovered by Sun et al. in 1994 [1]. It was characterized initially in mouse epidermal JB6 cells, progressing from early to late stages of carcinogenesis. Sulfiredoxin causes activation of the mammalian peroxiredoxins, a cluster of 6 enzymes, of which it specifically acts on 2-Cys peroxiredoxins [Prx I–IV]. The peroxiredoxins are inactivated by the hyperoxidation caused due to the accumulation of hydrogen peroxide and other free radicals, thereby resulting in a molecular switch mechanism [2]. Sulfiredoxin is primarily located in the cytosol and it gets translocated to the mitochondria during increased oxidative burden [3]. Phosphorylation of the peroxiredoxin moiety is the first chemical step which can occur either by a direct transfer of the gamma phosphate of ATP to the peroxiredoxin molecule or through sulfiredoxin acting as a phosphorylated intermediate [4].
Transcriptional regulation of sulfiredoxin expression is mediated through activator protein-1 and nuclear factor erythroid-2 related factor-2 pathways. Sulfiredoxin as an AP-1 target gene was first reported in a microarray based research for glucose/cAMP regulated genes in Min6 insulin secreting cells [5]. A study on the transcriptional regulation of sulfiredoxin in neurons pinpointed the sites of regulation to two cis-acting AP-1 consensus sites [6]. The AP-1 inhibitor, TAM67 (a dominant-negative form of c-Jun) inhibited the synaptic-activity dependent induction of the sulfiredoxin promoter in neurons [7]; as well as its induction by TPA (12-O tetradecanoylphorbol-13-acetate) in mouse JB6 cells [8].
A cis-acting promoter element called antioxidant response element (ARE) recruits transcription factors such as Nrf2 and maf proteins during oxidative stress. They regulate a group of genes encoding antioxidative enzymes like sulfiredoxin and drug metabolizing enzymes. The Nrf2 pathway was ascertained with studies using Nrf2 activators such as 3H-1,2-dithiole-3-thione (D3T), sulforaphane, which showed a significant induction in the enzyme expression.
An interesting facet of sulfiredoxin is that the AP-1 and Nrf2 responsiveness is contained within the same sequence i.e the proximal conserved AP-1 site is contained within the ARE site. The apparent contradiction is that AP-1 is a target for tumour promoting agents like TPA [8], whereas Nrf2 is a target for chemopreventive compounds. So sulfiredoxin, as an Nrf2 activator has chemopreventive effects due to its antioxidant property; on the other hand, it has a diametrically opposite effect via the AP-1 pathway [9].
Sulfiredoxin causes reduction of the cysteine sulfinic acid moiety of the peroxiredoxin enzyme back to its stable thiol state. The reaction involves the utilization of ATP and magnesium by sulfiredoxin in order to repair the enzyme. Since the inactivation is caused by an increase in the ROS levels, hydrogen peroxide performs its role as an intracellular cell signalling agent. This results in the activation of sulfiredoxin which in turn exerts its action on peroxiredoxins to efficiently neutralize the hydrogen peroxide molecules and attenuate intra-cellular oxidative stress. Peroxiredoxins also exist as dimers, decamers and high molecular weight complexes, of which the highest chaperone activity is seen in the latter two forms. Hyperoxidation results in the formation of decamers and high molecular weight complexes, thereby increasing the molecular chaperone activity of this enzyme. Reversal of the hyperoxidation invokes a molecular switch mechanism from chaperone to peroxidase activity, thereby terminating the signalling function of hydrogen peroxide and at the same time protecting the cells from oxidative damage caused by its accumulation [10].
Modification of protein cysteine residues by disulphide bond formation with glutathione (glutathionylation) is a reversible post-translational modification of utmost importance in cell signalling events following oxidative or nitrosative stress. It acts as a protective mechanism for proteins from undergoing terminal modifications, when exposed to oxidative stress. When the environment becomes more reducing, deglutathionylation takes place by removal of the glutathione moiety from the protein, either in an enzymatic or non-enzymatic manner. Glutathione, with two cysteine residues takes part in both the reactions. Sulfiredoxin possesses only one cysteine residue and highlights its advantage by not getting glutathionylated during the process [11]. So the other biologically relevant function of sulfiredoxin is deglutathionylation of proteins such as peroxiredoxin, actin and protein tytosine phosphatase 1B [12].
Systemic diseases and oxidative stress
Increased oxidative stress is the major etiopathogenic factor in various systemic diseases such as diabetes mellitus, obesity, atherosclerosis, hypertension, neurodegenerative disorders, inflammatory bone diseases like rheumatoid arthritis and periodontitis. The environmental or noxious stimuli from certain micro organisms could contribute to the oxidative burden imposed on these tissues.
Sulfiredoxin levels in health and disease
The assessed diseased states for this enzyme included atherosclerosis [13], chronic obstructive pulmonary disease [14] and a wide array of carcinomatous lesions. The more invasive squamous cell carcinoma, basal cell carcinoma to the less invasive small cell and large cell lung carcinoma were reviewed for the levels of sulfiredoxin.
Certain studies showed that there is a similarity in the expression of sulfiredoxin levels in a set of squamous cell [8,15] and adenocarcinomas respectively [15,16]. Renal carcinoma and the tumour adjacent renal epithelium cell lines were evaluated for sulfiredoxin mRNA expression. The tumour cell lines showed a significant increase in the target protein expression when compared to the latter [49].
Correlation of immunohistochemical staining results of human cross sectional studies revealed significantly high sulfiredoxin positivity in squamous cell carcinoma (88.1%), basal cell carcinoma (74.4%), melanoma (65.2%) followed by adenocarcinoma (55%), large cell carcinoma (22.2%) and small cell carcinoma (9.1%). The rise in sulfiredoxin positivity can be directly associated with the invasive nature and the metastatic potential of the carcinoma. The current findings can be explained by the fact that this protein has been shown to increase the cell motility and invasion, which will be elaborately discussed under the factors influenced by sulfiredoxin. This increased expression might have a potential value in the diagnosis, prevention or treatment of these tumours. In benign tumours such as papilloma (33%), condyloma (0%), dermato-fibro sarcoma (0%), there is a decline in the levels of sulfiredoxin. Mild sulfiredoxin positivity has been observed in chronic inflammatory conditions (7.7%). Immunohistochemically, the localization of the sulfiredoxin protein was towards the basal cell layer of the dermis in chronic inflammation [8].
Since the lungs are the target organs of the majority of the oxidative insult, robust antioxidant functioning is required for homoeostasis. In an in vivo study, a significant decrease in the sulfiredoxin expression in lungs with chronic obstructive pulmonary disease was observed in comparison to healthy tissue samples. This implied that the increase in oxidative stress in COPD lungs could not be efficiently neutralized by the action of sulfiredoxin. Low levels of sulfiredoxin results in impaired hydrogen peroxide detoxification, thereby resulting in exarcebated oxidative burden to the lungs [14].
Atherosclerosis is the major aetiological factor underlying myocardial infarction and stroke. Nrf2, with its target genes like sulfiredoxin has been implicated in the advanced stages of atheromatous plaque formation [13]. This redox protein can play a crucial role in the pathogenesis of the disease which requires an interplay between oxidative stress and inflammatory response. Plaque associated macrophages can undergo pro (M1) and anti-inflammatory (M2) polarization based on the environmental signals [17]. High levels of sulfiredoxin could be attributed to the increased generation of reactive oxygen species due to the transition of M2–M1 subset of macrophages, which are pro-inflammatory and essential for atherogenesis. The macrophages later develop into foam cells in the atheroma plaques. Also, Nrf2 is required to support the inflammatory reaction in the plaque, by cholesterol crystal induced Nlrp3 inflammasome activation [18], mediated by mitochondrial ROS production [19]. Hence this inter-relationship is essential for the development of atherosclerosis.
The erythrocytes have an in-built oxidative stress response network due to the auto-oxidation of haemoglobin. They can also take up the oxidative stress from other tissues and diffuse it. Thus sulfiredoxin performs its role as an antioxidant and the intrinsic store remains constant even through the aging phases of the red blood cells [20].
Factors influencing sulfiredoxin expression
From the existing literature, various factors influencing the expression of sulfiredoxin can be summarized in the following order: chemotherapeutic agents, pro-oxidants, antioxidants, hormones, exogenous, endogenous and transcription factors (Table 1).
Table 1.
Summary of results of the factors influencing sulfiredoxin expression.
| No. | Factors | References | Sulfiredoxin expression |
||
|---|---|---|---|---|---|
| Increase | No change | Decrease | |||
| Chemopreventive agents | |||||
| 1 | Cinnamic aldehyde | [21] | ✓ | ||
| 2 | Maesopsin-4-O-β d-glucoside (TAT-2) | [22] | ✓ | ||
| 3 |
Nitrofurtimox, nitrofurtimox+ tetrathiomolybdate |
[23] | ✓ | ||
| Hormones | |||||
| 4 | Luteinizing hormone | [32] | ✓ | ||
| 5 | Human chorionic gonadotropin | [32] | ✓ | ||
| 6 | Adrenocorticotropic hormone | [34] | ✓ | ||
| 7 | Dexamethasone | [34] | ✓ | ||
| Pro-oxidants | |||||
| 8 | Diquat | [24] | ✓ | ||
| 9 | Ethanol | [25] | ✓ | ||
| 10 | Pyrazole | [26] | ✓ | ||
| 11 | Cadmium | [27] | ✓ | ||
| 12 | Copper | [28] | ✓ | ||
| Antioxidants | |||||
| 13 | Sulforaphane and curcumin | [29] | ✓ | ||
| 14 | Roasted coffee extracts | [30] | ✓ | ||
| 15 | 3H-1,2-dithiole-3-thione (D3T) | [7] | ✓ | ||
| 16 | Rutin | [31] | ✓ | ||
| 17 | Tiron and diphenylene iodonium chloride | [32] | ✓ | ||
| Exogenous factors | |||||
| 18 | Hyperoxia | [39] | ✓ | ||
| 19 | Interferon-γ | [35,36] | ✓ | ||
| 20 | Lipopolysaccharide | [34–37] | ✓ | ||
| 21 | Nitric oxide | [35] | ✓ | ||
| 22 | High cholesterol diet | [31] | ✓ | ||
| Endogenous factors | |||||
| 23 | Circadian rhythm | [34] | ✓ | ✓ | |
| Transcription factors | |||||
| 24 | Nrf2 | [27,29] | ✓ | ||
| 25 | Nf-κβ inhibitor (IMD-3054) | [28] | ✓ | ||
| 26 | RANKL | [29] | ✓ | ||
The targeted chemotherapeutic drugs are cinnamic aldehyde [21] and maesopsin 4-O-β d-glucoside [22], which are natural extracts from cinnamon and Artocarpus tonkinensis, respectively. The third drug is nitrofurtimox either given alone or in combination with tetrathiomolybdate [23]. These drugs function by inducing the oxidative stress response genes and thereby increasing the sulfiredoxin expression to function as an antioxidant. However, it is paradoxical to note that the tumour cells secrete increased amounts of this protein to facilitate its survival. Thus the mode of action of these drugs with respect to the alteration in oxidative stress response warrants further studies.
As the name suggests, pro-oxidants favour the increase in oxidative stress which results in the accumulation of various free radicals and reactive oxygen species. So, there is an onus on the antioxidant system to effectively scavenge these free radicals. Accordingly, the studies show that pro-oxidants such as diquat [24], ethanol [25], pyrazole [26], cadmium [27] and copper [28] show significantly high induction of the antioxidant enzyme sulfiredoxin when injected into the liver of animals such as mice and rats. Hence, sulfiredoxin could also be targeted as a biomarker in hepatotoxicity induced by chronic ethanol consumption or copper toxicity leading to Wilson’s disease.
Antioxidants interact with each other in order to fend off the oxidative burden in the tissues. The classic example is the synergistic action of Vitamin E and co-enzyme Q in lipid peroxidation. The effects of antioxidants such as sulforaphane [29], curcumin [29], roasted coffee extracts [30] and 3H-1,2-dithiole-3-thione (D3T) [7] have been studied with respect to sulfiredoxin. They induced a significantly higher expression of this antioxidant, whereas agents such as rutin [31], tiron [32] and diphenylene iodonium chloride [32] show a significant decrease in its expression. Further studies can be done to probe into the exact mechanism of action of these agents on sulfiredoxin.
There is a strong involvement of reactive oxygen species in the process of ovulation which has been demonstrated by administering antioxidant agents to mice [33]. The sex hormones such as gonadotropins and luteinizing hormone showed a marked increase in the sulfiredoxin expression in rat ovaries and follicular cells, which can be attributed to the higher oxidative burden during ovulation [32]. Prx III, followed by Prx V has been abundantly found in the adrenal cortex of mice. Accumulation of hydrogen peroxide due to the inactivation of peroxiredoxin and its subsequent activation results in a feedback mechanism which controls the adrenal corticosteroid production, apart from the hypothalamic–pituitary–adrenal axis. This mechanism is responsible for the circadian periodicity of sulfiredoxin. Stimulation with adrenocorticotropic hormone has been shown to increase the sulfiredoxin levels, while dexamethasone has a suppressive effect [34].
In vitro studies have showed the effects of bacterial lipopolysaccharide (LPS) [34–37] on sulfiredoxin levels in mouse bone marrow derived macrophages. The individual stimuli with LPS showed induction of sulfiredoxin expression. A combined stimulus of LPS and the cytokine interferon-γ almost doubled the expression of this redox protein in comparison to the individual stimuli [35,36]. This upregulation is mediated by nitric oxide which forms peroxynitrite ion which in turn is responsible for killing of the invading micro organisms [38]. Peroxynitrite is detrimental to the producing cells and nearby tissues and they can be efficiently scavenged by peroxiredoxins. Hence, it can be presumed that the nitric oxide mediated sulfiredoxin induction can be a feedback loop in preventing the excessive accumulation of peroxynitrite ions.
Further, exogenous factors such as hyperoxia [39] and high cholesterol diet [31] are involved in causing an unfavourable tilt in the redox balance. They are the pre-requisites in the causation of atherosclerosis and progressive lung damage. So they trigger the induction of antioxidant enzymes such as sulfiredoxin in order to combat the rise in the oxidative stress levels.
Sulfiredoxin expression is mediated via the transcription factors Nrf2 and AP-1. The Nrf2 null cells did not express sulfiredoxin, thereby ascertaining the pathway [27,29]. Moreover the nuclear factor κβ inhibitor IMD-3054 [28] and its ligand RANKL [29] suppressed the expression of sulfiredoxin. Oxidative stress is essential for the differentiation of osteoclasts from the monocyte cell lineage. Osteoblast and osteoclast coupling mediated by RANK–RANKL binding is essential for osteoclastogenesis and bone remodelling. So it can be assumed that in inflammatory bone diseases such as periodontitis, the oxidative stress is maintained at a higher level by the suppression of antioxidant enzymes such as sulfiredoxin. This results in the differentiation of osteoclasts and subsequent bone resorption characterized in the disease process. Further studies using human tissue samples are warranted in order to substantiate this data.
Factors influenced by sulfiredoxin
Most of the studies assessing the tumour related factors have an in vitro design wherein a silencing RNA of sulfiredoxin is introduced to nullify the production of the protein at the transcriptional level. The Srx null mice showed a lower trend in tumour incidence, and it was not statistically significant when compared to the wild type and heterozygous counterparts [44]. Also it was reported that depletion of sulfiredoxin did not affect the proliferation of cells in mouse skin tumours [45]. Another study demonstrated that the over expression of sulfiredoxin resulted in clonal expansion and cell growth with about 50% of the cells present in the G1 phase of the cell cycle, followed by 10% in S phase and 20% in G2 phase [41].
Few studies have demonstrated that sulfiredoxin negativity resulted in lack of anchorage dependent colony formation in mouse JB6 [8] and human A549 cell lines [46]. Colony formation is a hallmark of transformation and an in vitro correlate of tumorigenicity in vivo. Depleted levels of this antioxidant resulted in an increased percentage of intra tumoural apoptotic cells, thereby showcasing the role of sulfiredoxin as a protective factor in tumour cell survival. There is also a concomitant decrease in the tumour multiplicity and volume due to the increase in apoptosis. These parameters were assessed in colon carcinomas, skin tumours and in lung A549 cells [40,44,45].
In human lung A549 cells, a wound was created to assess the migration of sulfiredoxin positive and negative cells. The positive cells migrated faster and there was a significant wound fill, whereas the negative cells migrated at a slower rate [46,47]. The invasiveness of the cells increased in sulfiredoxin positive cells [47]. This is clinically relevant, as the most invasive and metastatic tumours like squamous cell carcinoma, basal cell carcinoma and melanoma showed the highest levels of sulfiredoxin expression.
Cisplatin is a chemotherapeutic drug which has been effectively used in the management of sarcomas, lymphomas and small cell lung carcinomas. An in vitro study demonstrated an average of 32% of dead cells in G1 subpopulation which was sulfiredoxin positive when 2, 3, 4 µg/ml dosage of the drug was administered, whereas only 15% of the sulfiredoxin negative cells were affected. Thus, it can be inferred that sulfiredoxin had a dose dependent increased sensitivity to cisplatin. The plausible mechanism to the drug sensitivity is elevated levels of the protein p53, which has been elicited in this study and this protein has an important role in lung cancer prognosis and drug response [41].
An elevated level of sulfiredoxin has been shown to protect tumour cells from oxidative damage by the activation of peroxiredoxins. Certain studies observed the survival rate of patients suffering from pancreatic adenocarcinoma and lung carcinomas (Stages I and II) and tried to assess the prognostic value of sulfiredoxin as a marker by correlating the survival rate with its levels. But the results from those studies showed no significant correlation with the patient survival in these conditions [16,48]. In another study, sulfiredoxin positivity in lung tumour samples was observed and this was associated with a decreased survival of patients receiving cytostatic drugs or radiation therapy [15].
A sulfiredoxin null environment showed a marked increase in oxidative stress markers like reactive oxygen species [40], protein carbonylation products [26], and malondialdehyde (lipid peroxidation product) [26], thereby confirming its antioxidant status.
In the presence of sulfiredoxin, elevated levels of plasma corticosterone and a concomitant decrease in adrenocorticotropic hormone were observed, and this has a bearing on the adrenal steroidogenesis [34]. Also in another study, its presence has shown to increase the survival rate of mice, especially females when exposed to LPS mediated endotoxic shock [42]. Sulfiredoxin may influence sepsis by controlling the strength of TLR signalling [43] through its effect on peroxiredoxins.
The afore mentioned factors have been collectively summated in Table 2.
Table 2.
Summary of results of the factors influenced by sulfiredoxin.
| No. | Factors | References | Sulfiredoxin |
How is it influenced? | |
|---|---|---|---|---|---|
| Srx+/+ | Srx−/− | ||||
| Survival rate | |||||
| 1 | Patient survival | [15,16,48] | ✓ | Srx positivity did not have a correlation with the patient survival in pancreatic adenocarcinoma and lung carcinoma (Stages I and II). Worse survival in patients receiving cytostatic drugs and radiation therapy | |
| 2 | Animal survival | [42] | ✓ | Presence of Srx had a positive impact on the animal survival when induced with LPS to produce endotoxic shock. | |
| Tumour related | |||||
| 3 | Incidence | [44] | ✓ | Srx null mice showed a lower trend in tumour incidence and it was not statistically significant when compared to wild type. | |
| 4 | Colony formation | [8,46] | ✓ | Lack of colony formation in Srx null cells has been observed in mouse JB6 and human A549 cells. | |
| 5 | Proliferation | [45] | ✓ | Depletion of Srx did not affect the cell proliferation in skin tumours in mice. | |
| 6 | Clonal expansion and cell growth | [41] | ✓ | Over expression of Srx promoted clonal expansion and cell growth in human lung carcinoma A549 cells. | |
| 7 | Apoptotic cells | [40,45] | ✓ | Increase in intratumoural apoptotic cells in mouse skin tumours and human A549 cells with reduced expression of Srx. | |
| 8 | Multiplicity | [44,45] | ✓ | In Srx depleted state, tumour multiplicity is reduced by 2 fold in colon carcinomas induced in mice. | |
| 9 | Volume | [45] | ✓ | Reduced tumour volume in Srx null mice with skin tumours. | |
| 10 | Cell migration | [46,47] | ✓ | Migration of Srx null cells was significantly slower than wild type when assessed in human A549 cells. | |
| 11 | Invasion | [47] | ✓ | Srx null cells were less invasive when tested with human A549 cells. | |
| Oxidative stress related | |||||
| 12 |
Protein carbonylation |
[26] | ✓ | Srx null mice showed 1.6 fold increase in protein carbonylation products when compared to wild type on injection with pyrazole. | |
| 13 | Malondialdehyde | [26] | ✓ | Srx null mice showed 1.7 fold increase in lipid peroxidation marker malondialdehyde on injection with pyrazole. | |
| 14 | Reactive oxygen species | [40] | ✓ | Increased ROS levels in Srx null human A549 cells. | |
| Agents | |||||
| 15 | Corticosterone | [34] | ✓ | Sulfiredoxin positivity causes an increase in the plasma corticosterone levels in mice. | |
| 16 | Adrenocorticotropic hormone | [34] | ✓ | Sulfiredoxin levels causes a decrease in the plasma ACTH levels in mice due to the negative feedback mechanism. | |
| 17 | Cisplatin | [41] | ✓ | Sulfiredoxin positive A549 cells show a dose dependent increased sensitivity to cisplatin. | |
Conclusion
Sulfiredoxin plays a key role in maintaining the redox balance by effectively neutralizing hydrogen peroxide through peroxiredoxins. This mechanism is maintained in health whereas in cancer cells, it behaves like a double edged sword by causing the cells to be more resistant to oxidative burden. This review concludes that sulfiredoxin has a bearing on oxidative stress mediated conditions which was evidently seen in atherosclerosis, chronic obstructive pulmonary disease and malignancies. However, future longitudinal and interventional studies are necessary to provide conclusive evidence on these results. Also the research can be extended to diseases like diabetes mellitus, obesity, inflammatory bone diseases such as periodontitis and rheumatoid arthritis to know its implications. A majority of individual studies have shown the influence of factors on sulfiredoxin expression, all of which have been collectively summarized in this review. More targeted studies on assessing these regulators can yield valuable information on the mechanism of action of sulfiredoxin in various bodily functions. Among the various factors influenced by this antioxidant, this review points out that the tumour related factors have a strong susceptibility to this enzyme. Future directions in research should have a strong focus on assessing the role of this protein as a diagnostic or prognostic marker and as a therapeutic agent in various oxidative stress induced disorders.
Contributor Information
Asha Ramesh, Email: ash.periopg@gmail.com.
Sheeja S. Varghese, Email: drsheeja@rediffmail.com.
Jayakumar Doraiswamy, Email: principaldental@saveetha.com.
Sankari Malaiappan, Email: msankari@gmail.com.
References
- 1.Sun Y., Hegamyer G., Colburn N.H. Molecular cloning of five messenger RNAs differentially expressed in preneoplastic or neoplastic JB6 mouse epidermal cells: one is homologous to human tissue inhibitor of metalloproteinases-3. Cancer Research. 1994;54:1139–1144. 8118794 [PubMed] [Google Scholar]
- 2.Rhee S.G., Jeong W., Chang T.S., Woo H.A. Sulfiredoxin, the cysteine sulfinic acid reductase specific to 2-Cys peroxiredoxin: its discovery, mechanism of action, and biological significance. Kidney International: Supplement. 2007;72:S3–S8. doi: 10.1038/sj.ki.5002380. 17653208 [DOI] [PubMed] [Google Scholar]
- 3.Noh Y.H., Baek J.Y., Jeong W., Rhee S.G., Chang T.S. Sulfiredoxin translocation into mitochondria plays a crucial role in reducing hyperoxidized peroxiredoxin III. Journal of Biological Chemistry. 2009;284:8470–8477. doi: 10.1074/jbc.M808981200. 19176523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jönsson T.J., Tsang A.W., Lowther W.T., Furdui C.M. Identification of intact protein thiosulfinate intermediate in the reduction of cysteine sulfinic acid in peroxiredoxin by human sulfiredoxin. Journal of Biological Chemistry. 2008;283:22890–22894. doi: 10.1074/jbc.C800124200. 18593714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glauser D.A., Brun T., Gauthier B.R., Schlegel W. Transcriptional response of pancreatic beta cells to metabolic stimulation: large scale identification of immediate-early and secondary response genes. BMC Molecular Biology. 2007;8:54. doi: 10.1186/1471-2199-8-54. 17587450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Papadia S., Soriano F.X., Léveillé F., Martel M.A., Dakin K.A., Hansen H.H. Synaptic NMDA receptor activity boosts intrinsic antioxidant defenses. Nature Neuroscience. 2008;11:476–487. doi: 10.1038/nn2071. 18344994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soriano F.X., Léveillé F., Papadia S., Higgins L.G., Varley J., Baxter P. Induction of sulfiredoxin expression and reduction of peroxiredoxin hyperoxidation by the neuroprotective Nrf2 activator 3H-1,2-dithiole-3-thione. Journal of Neurochemistry. 2008;107:533–543. doi: 10.1111/j.1471-4159.2008.05648.x. 18761713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wei Q., Jiang H., Matthews C.P., Colburn N.H. Sulfiredoxin is an AP-1 target gene that is required for transformation and shows elevated expression in human skin malignancies. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:19738–19743. doi: 10.1073/pnas.0810676105. 19057013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soriano F.X., Baxter P., Murray L.M., Sporn M.B., Gillingwater T.H., Hardingham G.E. Transcriptional regulation of the AP-1 and Nrf2 target gene sulfiredoxin. Molecules and Cells. 2009;27:279–282. doi: 10.1007/s10059-009-0050-y. 19326073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bozonet S.M., Findlay V.J., Day A.M., Cameron J., Veal E.A., Morgan B.A. Oxidation of a eukaryotic 2-Cys peroxiredoxin is a molecular switch controlling the transcriptional response to increasing levels of hydrogen peroxide. Journal of Biological Chemistry. 2005;280:23319–23327. doi: 10.1074/jbc.M502757200. 15824112 [DOI] [PubMed] [Google Scholar]
- 11.Findlay V.J., Tapiero H., Townsend D.M. Sulfiredoxin: a potential therapeutic agent? Biomedicine and Pharmacotherapy. 2005;59:374–379. doi: 10.1016/j.biopha.2005.07.003. 16102934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Findlay V.J., Townsend D.M., Morris T.E., Fraser J.P., He L., Tew K.D. A novel role for human sulfiredoxin in the reversal of glutathionylation. Cancer Research. 2006;66:6800–6806. doi: 10.1158/0008-5472.CAN-06-0484. 16818657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harada N., Ito K., Hosoya T., Mimura J., Maruyama A., Noguchi N. Nrf2 in bone marrow-derived cells positively contributes to the advanced stage of atherosclerotic plaque formation. Free Radical Biology and Medicine. 2012;53:2256–2262. doi: 10.1016/j.freeradbiomed.2012.10.001. 23051009 [DOI] [PubMed] [Google Scholar]
- 14.Singh A., Ling G., Suhasini A.N., Zhang P., Yamamoto M., Navas-Acien A. Nrf2-dependent sulfiredoxin-1 expression protects against cigarette smoke-induced oxidative stress in lungs. Free Radical Biology and Medicine. 2009;46:376–386. doi: 10.1016/j.freeradbiomed.2008.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Merikallio H., Pääkkö P., Kinnula V.L., Harju T., Soini Y. Nuclear factor erythroid-derived 2-like 2 (Nrf2) and DJ1 are prognostic factors in lung cancer. Human Pathology. 2012;43:577–584. doi: 10.1016/j.humpath.2011.05.024. [DOI] [PubMed] [Google Scholar]
- 16.Soini Y., Eskelinen M., Juvonen P., Kärjä V., Haapasaari K.M., Saarela A. Nuclear Nrf2 expression is related to a poor survival in pancreatic adenocarcinoma. Pathology, Research and Practice. 2014;210:35–39. doi: 10.1016/j.prp.2013.10.001. 24189098 [DOI] [PubMed] [Google Scholar]
- 17.Johnson J.L., Newby A.C. Macrophage heterogeneity in atherosclerotic plaques. Current Opinion in Lipidology. 2009;20:370–378. doi: 10.1097/MOL.0b013e3283309848. 19741337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freigang S., Ampenberger F., Spohn G., Heer S., Shamshiev A.T., Kisielow J. Nrf2 is essential for cholesterol crystal-induced inflammasome activation and exacerbation of atherosclerosis. European Journal of Immunology. 2011;41:2040–2051. doi: 10.1002/eji.201041316. 21484785 [DOI] [PubMed] [Google Scholar]
- 19.Zhou R., Yazdi A.S., Menu P., Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–225. doi: 10.1038/nature09663. 21124315 [DOI] [PubMed] [Google Scholar]
- 20.Cho C.S., Lee S., Lee G.T., Woo H.A., Choi E.J., Rhee S.G. Irreversible inactivation of glutathione peroxidase 1 and reversible inactivation of peroxiredoxin II by H2O2 in red blood cells. Antioxidants and Redox Signaling. 2010;12:1235–1246. doi: 10.1089/ars.2009.2701. 20070187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cabello C.M., Bair W.B., Lamore S.D., Ley S., Bause A.S., Azimian S. The cinnamon-derived Michael acceptor cinnamic aldehyde impairs melanoma cell proliferation, invasiveness, and tumor growth. Free Radical Biology and Medicine. 2009;46:220–231. doi: 10.1016/j.freeradbiomed.2008.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pozzesi N., Pierangeli S., Vacca C., Falchi L., Pettorossi V., Martelli M.P. Maesopsin 4-O-β-D-glucoside, a natural compound isolated from the leaves of artocarpus tonkinensis , inhibits proliferation and up-regulates HMOX1, SRXN1 and BCAS3 in acute myeloid leukemia. Journal of Chemotherapy. 2011;23:150–157. doi: 10.1179/joc.2011.23.3.150. [DOI] [PubMed] [Google Scholar]
- 23.Koto K.S., Lescault P., Brard L., Kim K., Singh R.K., Bond J. Antitumor activity of nifurtimox is enhanced with tetrathiomolybdate in medulloblastoma. International Journal of Oncology. 2011;38:1329–1341. doi: 10.3892/ijo.2011.971. 21399873 [DOI] [PubMed] [Google Scholar]
- 24.Han E.S., Muller F.L., Pérez V.I., Qi W., Liang H., Xi L. The in vivo gene expression signature of oxidative stress. Physiological Genomics. 2008;34:112–126. doi: 10.1152/physiolgenomics.00239.2007. 18445702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bae S.H., Sung S.H., Cho E.J., Lee S.K., Lee H.E., Woo H.A. Concerted action of sulfiredoxin and peroxiredoxin I protects against alcohol-induced oxidative injury in mouse liver. Hepatology (Baltimore, Md.) 2011;53:945–953. doi: 10.1002/hep.24104. 21319188 [DOI] [PubMed] [Google Scholar]
- 26.Bae S.H., Sung S.H., Lee H.E., Kang H.T., Lee S.K., Oh S.Y. Peroxiredoxin III and sulfiredoxin together protect mice from pyrazole-induced oxidative liver injury. Antioxidants and Redox Signaling. 2012;17:1351–1361. doi: 10.1089/ars.2011.4334. 22490042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu K.C., Liu J.J., Klaassen C.D. Nrf2 activation prevents cadmium-induced acute liver injury. Toxicology and Applied Pharmacology. 2012;263:14–20. doi: 10.1016/j.taap.2012.05.017. 22677785 [DOI] [PubMed] [Google Scholar]
- 28.McElwee M.K., Song M.O., Freedman J.H. Copper activation of NF-kappaB signaling in HepG2 cells. Journal of Molecular Biology. 2009;393:1013–1021. doi: 10.1016/j.jmb.2009.08.077. 19747488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hyeon S., Lee H., Yang Y., Jeong W. Nrf2 deficiency induces oxidative stress and promotes RANKL-induced osteoclast differentiation. Free Radical Biology and Medicine. 2013;65:789–799. doi: 10.1016/j.freeradbiomed.2013.08.005. 23954472 [DOI] [PubMed] [Google Scholar]
- 30.Yazheng L., Kitts D.D. Activation of antioxidant response element (ARE)-dependent genes by roasted coffee extracts. Food and Function. 2012;3:950–954. doi: 10.1039/c2fo30021d. 22699814 [DOI] [PubMed] [Google Scholar]
- 31.Al-Rejaie S.S., Aleisa A.M., Sayed-Ahmed M.M., Al-Shabanah O.A., Abuohashish H.M., Ahmed M.M. Protective effect of rutin on the antioxidant genes expression in hypercholestrolemic male Westar rat. BMC Complementary and Alternative Medicine. 2013;13:136. doi: 10.1186/1472-6882-13-136. 23773725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park J.I., Jeon H.J., Jung N.K., Jang Y.J., Kim J.S., Seo Y.W. Periovulatory expression of hydrogen peroxide-induced sulfiredoxin and peroxiredoxin 2 in the rat ovary: gonadotropin regulation and potential modification. Endocrinology. 2012;153:5512–5521. doi: 10.1210/en.2012-1414. 22989627 [DOI] [PubMed] [Google Scholar]
- 33.Shkolnik K., Tadmor A., Ben-Dor S., Nevo N., Galiani D., Dekel N. Reactive oxygen species are indispensable in ovulation. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:1462–1467. doi: 10.1073/pnas.1017213108. 21220312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kil I.S., Lee S.K., Ryu K.W., Woo H.A., Hu M.C., Bae S.H. Feedback control of adrenal steroidogenesis via H2O2-dependent, reversible inactivation of peroxiredoxin III in mitochondria. Molecular Cell. 2012;46:584–594. doi: 10.1016/j.molcel.2012.05.030. [DOI] [PubMed] [Google Scholar]
- 35.Abbas K., Breton J., Planson A.G., Bouton C., Bignon J., Seguin C. Nitric oxide activates an Nrf2/sulfiredoxin antioxidant pathway in macrophages. Free Radical Biology and Medicine. 2011;51:107–114. doi: 10.1016/j.freeradbiomed.2011.03.039. 21466852 [DOI] [PubMed] [Google Scholar]
- 36.Diet A., Abbas K., Bouton C., Guillon B., Tomasello F., Fourquet S. Regulation of peroxiredoxins by nitric oxide in immunostimulated macrophages. Journal of Biological Chemistry. 2007;282:36199–36205. doi: 10.1074/jbc.M706420200. [DOI] [PubMed] [Google Scholar]
- 37.Kim H., Jung Y., Shin B.S., Kim H., Song H., Bae S.H. Redox regulation of lipopolysaccharide-mediated sulfiredoxin induction, which depends on both AP-1 and Nrf2. Journal of Biological Chemistry. 2010;285:34419–34428. doi: 10.1074/jbc.M110.126839. 20826812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ischiropoulos H., Zhu L., Beckman J.S. Peroxynitrite formation from macrophage-derived nitric oxide. Archives of Biochemistry and Biophysics. 1992;298:446–451. doi: 10.1016/0003-9861(92)90433-w. 1329657 [DOI] [PubMed] [Google Scholar]
- 39.Bae S.H., Woo H.A., Sung S.H., Lee H.E., Lee S.K., Kil I.S. Induction of sulfiredoxin via an Nrf2-dependent pathway and hyperoxidation of peroxiredoxin III in the lungs of mice exposed to hyperoxia. Antioxidants and Redox Signaling. 2009;11:937–948. doi: 10.1089/ars.2008.2325. 19086807 [DOI] [PubMed] [Google Scholar]
- 40.Baek J.Y., Han S.H., Sung S.H., Lee H.E., Kim Y.M., Noh Y.H. Sulfiredoxin protein is critical for redox balance and survival of cells exposed to low steady-state levels of H2O2. Journal of Biological Chemistry. 2012;287:81–89. doi: 10.1074/jbc.M111.316711. 22086924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lei K., Townsend D.M., Tew K.D. Protein cysteine sulfinic acid reductase (sulfiredoxin) as a regulator of cell proliferation and drug response. Oncogene. 2008;27:4877–4887. doi: 10.1038/onc.2008.132. 18454177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Planson A.G., Palais G., Abbas K., Gerard M., Couvelard L., Delaunay A. Sulfiredoxin protects mice from lipopolysaccharide-induced endotoxic shock. Antioxidants and Redox Signaling. 2011;14:2071–2080. doi: 10.1089/ars.2010.3552. 21083423 [DOI] [PubMed] [Google Scholar]
- 43.Nathan C., Ding A. Nonresolving inflammation. Cell. 2010;140:871–882. doi: 10.1016/j.cell.2010.02.029. 20303877 [DOI] [PubMed] [Google Scholar]
- 44.Wei Q., Jiang H., Baker A., Dodge L.K., Gerard M., Young M.R. Loss of sulfiredoxin renders mice resistant to azoxymethane/dextran sulfate sodium-induced colon carcinogenesis. Carcinogenesis. 2013;34:1403–1410. doi: 10.1093/carcin/bgt059. 23393226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu L., Jiang H., Chawsheen H.A., Mishra M., Young M.R., Gerard M. Tumor promoter-induced sulfiredoxin is required for mouse skin tumorigenesis. Carcinogenesis. 2014;35:1177–1184. doi: 10.1093/carcin/bgu035. 24503444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wei Q., Jiang H., Xiao Z., Baker A., Young M.R., Veenstra T.D. Sulfiredoxin-peroxiredoxin IV axis promotes human lung cancer progression through modulation of specific phosphokinase signaling. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:7004–7009. doi: 10.1073/pnas.1013012108. 21487000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bowers R.R., Manevich Y., Townsend D.M., Tew K.D. Sulfiredoxin redox-sensitive interaction with S100a4 and non-muscle myosin IIA regulates cancer cell motility. Biochemistry. 2012;51:7740–7754. doi: 10.1021/bi301006w. 22934964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim Y.S., Lee H.L., Lee K.B., Park J.H., Chung W.Y., Lee K.S. Nuclear factor E2-related factor 2 dependent overexpression of sulfiredoxin and peroxiredoxin III in human lung cancer. Korean Journal of Internal Medicine. 2011;26:304–313. doi: 10.3904/kjim.2011.26.3.304. 22016591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seliger B., Dressler S.P., Massa C., Recktenwald C.V., Altenberend F., Bukur J. Identification and characterization of human leukocyte antigen class I ligands in renal cell carcinoma cells. Proteomics. 2011;11:2528–2541. doi: 10.1002/pmic.201000486. 21595034 [DOI] [PMC free article] [PubMed] [Google Scholar]


