Abstract
Chronic low back pain is a common neurological disorder. The periaqueductal gray (PAG) plays a key role in the descending modulation of pain. In this study, we investigated brain resting state PAG functional connectivity (FC) differences between patients with chronic low back pain (cLBP) in low pain or high pain condition and matched healthy controls (HCs). PAG seed based functional connectivity (FC) analysis of the functional MR imaging data was performed to investigate the difference among the connectivity maps in the cLBP in the low or high pain condition and HC groups as well as within the cLBP at differing endogenous back pain intensities. Results showed that FC between the PAG and the ventral medial prefrontal cortex (vmPFC)/rostral anterior cingulate cortex (rACC) increased in cLBP patients compared to matched controls. In addition, we also found significant negative correlations between pain ratings and PAG–vmPFC/rACC FC in cLBP patients after pain-inducing maneuver. The duration of cLBP was negatively correlated with PAG–insula and PAG–amygdala FC before pain-inducing maneuver in the patient group. These findings are in line with the impairments of the descending pain modulation reported in patients with cLBP. Our results provide evidence showing that cLBP patients have abnormal FC in PAG centered pain modulation network during rest.
Keywords: Chronic low back pain, fMRI, Functional connectivity, Periaqueductal gray
Perspective
Our results provide evidence showing that cLBP patients have abnormal FC in PAG centered pain modulation network during rest, which might have important treatment implications.
Highlights
-
•
cLBP patients exhibited enhanced PAG–mPFC coupling.
-
•
cLBP duration was correlated with PAG–mPFC coupling.
-
•
Pain intensity was correlated with PAG–insula and PAG–amygdala coupling.
1. Introduction
Chronic low back pain (cLBP) is one of the most common reasons for all physician visits in the USA and is a leading contributor to job-related disability and missed work (Chou and Shekelle, 2010; Hart et al., 1995). The etiology of cLBP is heterogeneous (Ehrlich, 2003). Non-specific cLBP, which represents the majority of cLBP patients, is characterized by a lack of recognizable pathology (Chou et al., 2007; Ehrlich, 2003; Savigny et al., 2009). Although cLBP is a serious health concern, treatment for cLBP has achieved limited success (Bogduk, 2004). Increasing evidence suggests a crucial role of central nervous system plasticity in the development and maintenance of non-specific cLBP. To develop more effective treatments, it is crucial to understand the underlying neurobiology of cLBP in the brain.
A prevailing theory in the pathogenesis of chronic pain is that the nociceptive afferents become sensitized in such a way that the signaling of these nociceptive afferents increases perceived pain disproportionately to the pain stimulus (Coderre et al., 1993). This process is facilitated by a dysfunction of the descending pain modulatory circuits (Woolf and Doubell, 1994; Zimmermann, 2001). The periaqueductal gray (PAG) is a key region involved in endogenous pain inhibition (Fields, 2004). Previous studies have shown that PAG stimulation can significantly inhibit behavioral responses to noxious stimuli in both animals (Mayer et al., 1971; Reynolds, 1969) and humans (Baskin et al., 1986; Hosobuchi et al., 1977). Recent studies have shown that the functional connectivity fluctuations and structural connectivity between the PAG and the ventral medial prefrontal cortex (vmPFC)/rostral anterior cingulate cortex (rACC) predicted mind wandering away from pain, i.e., spontaneous disengagement of attention from pain (Kucyi et al., 2013). The structural connectivity between these two regions also predicted individual difference in placebo analgesia (Stein et al., 2012). It is now believed that the brainstem (PAG) receives direct projections from regions within the limbic forebrain such as the anterior cingulate cortex (ACC) and limbic-related areas such as the insula and amygdala and modulates pain by descending modulation of the spinal cord neurons (Brooks and Tracey, 2005; Fields, 2004; Heinricher et al., 2009; Ploner et al., 2010; Tracey and Mantyh, 2007). A recent study has shown that the PAG is functionally connected to the vmPFC/rACC, insula and amygdala during resting state (Kong et al., 2010b).
The insula is a key region in pain process (Bernard et al., 1992; Chudler et al., 1993; Craig, 2002; Craig et al., 2000; Kong et al., 2013a; Kong et al., 2006; Schneider and Lidsky, 1981; Wiech et al., 2005). A previous study suggested that prestimulus functional connectivity between the insula and pain-modulatory brain regions (e.g., PAG) differed between physically identical trials that were rated as painful and trials perceived as non-painful (Ploner et al., 2010). The amygdala has a central role in regulating emotional responses during acute and persistent pain (Martikainen et al., 2013; Neugebauer et al., 2004). Given the close anatomical connectivity between the PAG–insula–amygdala and their role in pain perception and modulation (Ploner et al., 2010), it is possible that alterations in these pathways may also contribute to the development or maintenance of chronic pain. Recent neuroimaging studies have shown that cLBP is associated with alterations in resting state brain activity (Apkarian et al., 2009; Apkarian et al., 2004; Baliki et al., 2011; Baliki et al., 2006; Kobayashi et al., 2009; Tagliazucchi et al., 2010; Wasan et al., 2011). However, the role of the PAG and the associated networks detected by resting state fMRI in cLBP is still unclear. In the present study, we investigated PAG centered brain resting state functional connectivity (FC) differences between cLBP patients and matched HCs and FC differences when cLBP patients experienced different levels of pain intensity. We hypothesized that cLBP would be associated with abnormal FC between the PAG and other brain regions including the vmPFC, insula, and amygdala, given the close link between these regions and their role in pain modulation.
2. Materials and methods
We briefly describe the experimental procedures below. Please also see a previous published study (Kong et al., 2013b) for more details on the experimental procedure. The data have been used in this previous publication (Kong et al., 2013b), but the analytic methods used here do not overlap. In that study, we compared structure and function difference between the cLBP and controls using structural imaging data with morphometric analysis and resting state MRI data with degree centrality (DC) analysis (Kong et al., 2013b). Degree centrality is a measure of local network connectivity and identifies the most connected nodes by counting the number of direct connections to all other nodes (Buckner et al., 2009). There is no overlap on the results between the previous paper and the current paper.
2.1. Participants
Eighteen cLBP patients and 18 healthy controls, matched for age and gender, completed the study (see Table 1 for demographic details). The Institutional Review Board at Massachusetts General Hospital approved the study and all subjects gave written informed consent. All participants received compensation for their participation.
Table 1.
Demographics and clinical characteristics for cLBP patients and controls.
| Patients |
Controls |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ID | Gender | Age | Race | BDI | Duration (years) | Pain intensity (Low pain) | Pain intensity (High pain) | BPI (avg) | Gender | Age | Race | BDI |
| 1 | F | 48 | White | 13 | 3 | 4.5 | 3.5 | 7 | F | 47 | White | 0 |
| 2 | M | 41 | Asian | 8 | 4 | 5 | 6.5 | 6 | M | 37 | Asian | 10 |
| 3 | F | 49 | Black | 30 | 8 | 6.5 | 8.75 | 6 | F | 50 | Black | 4 |
| 4 | F | 47 | Hisp. | 7 | 3 | 10 | 9.5 | 10 | F | 49 | Black | 0 |
| 5 | F | 23 | White | 1 | 10 | 3 | 6 | 3 | F | 26 | White | 11 |
| 6 | M | 27 | White | 0 | 10 | 4 | 6.5 | 3 | M | 30 | White | 0 |
| 7 | F | 23 | White | 4 | 3 | 2 | 6 | 3 | F | 23 | White | 3 |
| 8 | M | 38 | White | 0 | 2 | 4.5 | 6 | 4 | M | 39 | White | 7 |
| 9 | M | 25 | Multi. | 0 | 5 | 4.5 | 7 | 3 | M | 27 | White | 0 |
| 10 | F | 44 | White | 9 | 12 | 2.25 | 6 | 4 | F | 45 | White | 0 |
| 11 | M | 30 | Multi. | 5 | 10 | 2 | 5.5 | 9 | M | 34 | White | 4 |
| 12 | F | 31 | Black | 1 | 2 | 5.5 | 8.5 | 6 | F | 32 | Black | 3 |
| 13 | F | 47 | Black | 3 | 5 | 0 | 9.5 | 8 | F | 47 | Black | 0 |
| 14 | F | 46 | Black | 9 | 3 | 3 | 8.5 | 6 | F | 46 | Black | 3 |
| 15 | F | 46 | White | 8 | 10 | 3 | 6.5 | 5 | F | 47 | White | 0 |
| 16 | F | 34 | Black | 10 | 3 | 7 | 8.5 | 8 | F | 34 | White | 2 |
| 17 | M | 26 | White | 0 | 1.5 | 0.5 | 3 | 2 | M | 27 | White | 0 |
| 18 | F | 25 | Asian | 9 | 0.5 | 1 | 4 | 2 | F | 28 | Asian | 9 |
BDI = Beck Depression Inventory; BPI = Brief Pain Inventory; Pain Intensity = Average self-reported pain rating before and after resting state fMRI scanning. Multi. = Multiple; Hisp. = Hispanic; SE = standard error.
All subjects were clinically diagnosed with nonspecific cLBP with a duration of at least 6 months by a clinical evaluation, including the use of X-ray/MRI reports, when available. Only those patients meeting the Quebec Low Back Pain Task Force classification criteria for Class I or II (axial LBP with possible occasional radiation to the thigh, and no sensory or motor complaints) were enrolled (Werneke and Hart, 2004). Subjects were classified as having non-specific back pain (i.e., patients with specific diagnoses, non-spinal etiology, or radicular symptoms were not included in the study). Subjects were also excluded if they reported major systemic diseases or history of head injury or coma. cLBP patients were asked to rate their current pain using a visual analog scale (0 no pain, 10 maximum imaginable pain), both before and after the resting state fMRI scan. Endogenous back pain intensity during the resting state was defined as the average pain rating before and after the scan. The Brief Pain Inventory (BPI) was used to assess the severity of pain (the sensory dimension) and the impact of pain on daily feelings and functions (the reactive dimension) in the preceding week, i.e., a week before the experiment date (Cleeland and Ryan, 1994; Tan et al., 2004). Depressive symptoms were assessed using the Beck Depression Inventory (BDI-II) for all participants (Beck et al., 1961). All questionnaires were administered immediately prior to brain scanning. Healthy controls, matched for gender, age and race, were recruited in the community. All HC subjects were screened to ensure that they did not have back pain.
2.2. Clinical maneuvers
After the first resting state scan, cLBP patients were taken out of the scanner to perform exercises for a period lasting up to 10 min to exacerbate their endogenous lower back pain (see Fig. 1A). These exercises were tailored to each patient based on their report of which movements exacerbated their pain. The exercises determined to exacerbate low back pain included a set of slow movements such as sit-ups, lumbar flexion/extension, and lumbar rotation, where the subject rotated his or her body from side to side at a self-selected speed. During the screening, all subjects were asked to confirm that they could perform these exercises. If at the end of the first resting state scan the patient's cLBP pain rating was too strong (≥7 in 0–10 scale) and the patient was reluctant to perform exercises to enhance their pain experience, they were asked to wait for 10 min in a comfortable position before starting the second half of the scanning session.
Fig. 1.
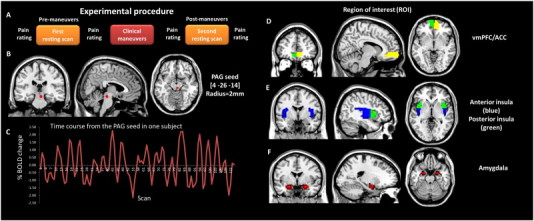
Experimental procedure and regions of interest. (A) Experimental procedure. (B) The bilateral PAG seeds used for resting state fMRI seed based functional connectivity analysis. (C) The time course from the PAG seed in one subject. (D–F) Anatomically defined regions of interest (ROIs): ACC/vmPFC, anterior insula, posterior insula, and amygdala.
During the exercises, cLBP patients were intermittently asked to report their level of pain using the 0–10 pain scale; the exercises were repeated until subjects reported an increase in pain of approximately 3 points on the pain scale. Once this level of pain was achieved, subjects were placed back in the scanner to repeat the same fMRI scans that were acquired before the pain-inducing maneuvers. All maneuvers were applied slowly at a pace that the patients feel acceptable. For healthy controls, structural and resting state scans were only collected once. Before and immediately after each 6 minute resting state MRI scan, subjects were asked to rate the intensity of their LBP using a 0–10 pain scale. The average self-reported pain rating before and after resting state fMRI scanning was used as an index of endogenous pain intensity.
2.3. Medication
Medication use per self-report was limited to non-steroidal anti-inflammatory drugs (NSAIDs, e.g., ibuprofen, Motrin, Advil, and Naproxen) and acetaminophen (e.g., Tylenol). Additional non-pharmacological methods of self-reported pain management included chiropractic massages, physical therapy, exercises, and acupuncture.
2.4. MRI data acquisition
All MRI data were acquired with a 3 T Siemens whole-body scanner with echo-planar imaging capability using a 32-channel radio-frequency head coil at the Martinos Center for Biomedical Imaging. During the resting state fMRI scan, subjects were asked to keep their eyes open and look at a darkened screen for 6 min. The BOLD fMRI scan acquisition included 47 slices with slice thickness of 3 mm, TR = 3000 ms, TE = 30 ms, and a 3 × 3 mm in-plane spatial resolution. T1-weighted MPRAGE structural images were acquired using the following parameters: voxel size 1.2 × 1.2 × 1.2 mm, TR = 2.2 s, TE = 1.54 ms, flip angle = 7°, slices = 144; field of view = 230.
2.5. PAG seed based functional connectivity analysis
The fMRI data were then preprocessed using SPM8 software (available at: http://www.fil.ion.ucl.ac.uk/spm) implemented in a MATLAB suite (MathWorks, Inc., Natick, Massachusetts). The first 10 volumes were not analyzed to allow for signal equilibration effects. Images were realigned to correct for motion, corrected for errors in slice timing, spatially transformed to standard stereotaxic space (based on the Montreal Neurologic Institute coordinate system). A recent study shows that the motion induced artifacts occur with movements on the order of a few tenths of a millimeter or less and produce systematic but spurious correlations in functional connectivity, such that long distance correlations are decreased by subject motion, whereas many short-distance correlations are increased (Power et al., 2012). ANOVA on each movement parameter with cLBP patients in high pain condition and in low pain condition and healthy control as between-subjects factor revealed no significant differences (P > 0.1). In Table 2, we show that the means for six movement parameters are small and there was no significant group difference on movement parameters. There were no participants with movement greater than 3 mm of translation or 3 degrees of rotation. There were also no significant differences between the total range of movement across any axis of translation or rotation between groups (see Table 2). The data were then smoothed with a 6-mm full-width half-maximum (FWHM) Gaussian kernel. The smooth kernel size was chosen because FWHM resolution usually equals or is greater than twice the voxel size (Mikl et al., 2008). Data were then bandpass filtered from 0.01 to 0.08 Hz to remove low frequency noise (including slow scanner drifts) and influences of higher frequencies reflecting cardiac and respiratory signals (Cordes et al., 2001).
Table 2.
Participant head motion during fMRI scans.
| cLBP patients in low pain |
cLBP patients in high pain |
Healthy controls |
||
|---|---|---|---|---|
| Motion parameters | Mean ± SE | Mean ± SE | Mean ± SE | P value |
| x | −0.0301 ± 0.0271 | −0.0396 ± 0.0436 | 0.0078 ± 0.0233 | .708 |
| y | 0.1145 ± 0.0625 | 0.0468 ± 0.0445 | 0.1046 ± 0.0272 | .297 |
| z | −0.0780 ± 0.0632 | −0.0882 ± 0.1398 | 0.0813 ± 0.0784 | .311 |
| Pitch | 0.0001 ± 0.0029 | 0.0002 ± 0.0010 | −0.0002 ± 0.0011 | .330 |
| Roll | −0.0016 ± 0.0009 | −0.0011 ± 0.0009 | 0.0007 ± 0.0007 | .176 |
| Yaw | 0.0003 ± 0.0018 | 0.0015 ± 0.0016 | 0.0002 ± 0.0005 | .913 |
Notes: SE = standard error; the six motion parameters (translation: x, y and z in mm and rotation: pitch, roll and yaw in degrees) were obtained from head movement correction for each participant. cLBP = chronic low back pain.
To address head motion concerns in resting-state fMRI analyses, we calculated the voxel-specific mean framewise displacement (FD) for accounting head motion at group-level analysis (Power et al., 2012, 2013; Power et al., 2014; Van Dijk et al., 2012). FD measure indexes the movement of the head from one volume to the next and is calculated as the sum of the absolute values of the differentiated realignment estimates (by backward differences) at every time point (Power et al., 2012). Then, we repeated the above analyses after removing frames with FD > 0.5 mm (‘scrubbing’). One time point before “bad” time points and two time points after “bad” time points were deleted.
Functional connectivity analysis was carried out by applying a seed-region approach using the right ventrolateral PAG (x = 4, y = −26, z = −14, with 2 mm radius) as the FC seed (see Fig. 1B). The rationale for choosing this location as a seed is: 1) in a previous study, we found that increased levels of heat pain can evoke a significant fMRI signal increase in this region (Kong et al., 2010a); 2) it is located within the ventrolateral PAG, which is believed to be important for opioid antinociception (Bandler and Shipley, 1994); 3) it is consistent with previous PAG seed based FC study showing that the PAG is functionally connected to the ACC/vmPFC and the insula (Kong et al., 2010b). One concern is that signals from other regions may confound the findings due to partial volume effect. We plotted the time course from the PAG seed for all subjects and found no cyclic trends in the data (see Fig. 1C for an example). Given that the PAG seed region sits adjacent to a ventricle with significant pulsatility effect, we also used a seed from the third ventricle (x = 0, y = −3, z = −7, with 2 mm radius) as a control region.
For each seed region, individual participant analyses were carried out using the General Linear Model (GLM) with the time series for the seed region as well as for the nuisance covariates (white matter, cerebrospinal fluid, and six motion parameters) as predictors. These nuisance signals are typically adjusted for in resting-state FC studies because they reflect signal fluctuations of nonneuronal origin (e.g., physiological artifacts associated with variables such as cardiac and respiratory cycles, CSF motion, and scanner drift) (Fox and Raichle, 2007).
Contrast images were generated for each subject by estimating the regression coefficient between all brain voxels and each seed's time series, respectively. These images were then included in second-level group random effect analyses, adopting a t-test design. We also used regression analyses to examine whether illness duration and/or endogenous pain intensity were related to PAG functional connectivity, when considering the cLBP participants alone. Endogenous pain intensity was defined as the average self-reported pain rating immediately before and after resting state fMRI scanning. The threshold was family-wise alpha level set to P = 0.05, family-wise error (FWE) correction for multiple comparisons for ROIs using small volume correction (svc). Based on the anatomical connection of PAG and previous studies (Brooks and Tracey, 2005; Fields, 2004; Heinricher et al., 2009; Ploner et al., 2010; Tracey and Mantyh, 2007), the ROIs include the vmPFC/ACC, anterior insula, posterior insula, and amygdala, derived from automated anatomical labeling (AAL, see Fig. 1C–E) (Tzourio-Mazoyer et al., 2002). A threshold of P < 0.05 family-wise error (FWE) corrected at the cluster level across the whole brain was used for non-ROI brain regions.
3. Results
A total of 18 cLBP patients (6 males) and 18 age- and gender-matched HCs (6 males) completed the study. Demographics, clinical assessments and characteristics for cLBP patients and HCs are presented in Table 1. The age difference between the two groups was not significant (mean ± SE 36.1 ± 2.3 in the patient group versus 37.1 ± 2.2 in the control group), P = 0.71. The duration of illness in the patient group is 5.3 ± 0.9 years. The BPI was used to measure the pain in the preceding week in the patient group, 6.5 ± 1.7.
Of the 18 patients who completed the study, one patient had strong chronic pain at baseline and thus did not perform any exercises. After lying down for 10 min, the patient felt a reduction in low back pain. The patient received the exact same set of scan procedures before and after the 10 minute rest period, comparable to the healthy control condition. This data was included in the data analysis. In this study, the self-reported endogenous LBP intensity recorded before and after resting state fMRI scanning was averaged and then used as an index of pain during scanning. After slow clinical pain-inducing maneuver, the self-reported pain intensity was significantly increased from 3.79 ± 0.58 (mean ± SE) before pain-inducing maneuver to 6.65 ± 0.46, t = 5.38, P < 0.001. In our data analysis, we define the scanning period during which patients' low back pain ratings are lower as the low pain (LP) condition and the scanning period during which patients' low back pain ratings are higher as the high pain (HP) condition.
During the LP condition (Fig. 2A) and HP condition (Fig. 2B) in the patient group and in the healthy group (Fig. 2C), we found predominantly positive correlations between PAG activity and activity in nearby structures, including the brainstem (mostly the midbrain), thalamus, parahippocampus, amygdala, and cerebellum, and with distant regions, including the anterior cingulate (not in control group) and temporal cortex. These findings were consistent with our previous PAG centered FC study applied in healthy subjects (Kong et al., 2010b) and a previous diffusion tensor imaging study on PAG (Hadjipavlou et al., 2006).
Fig. 2.
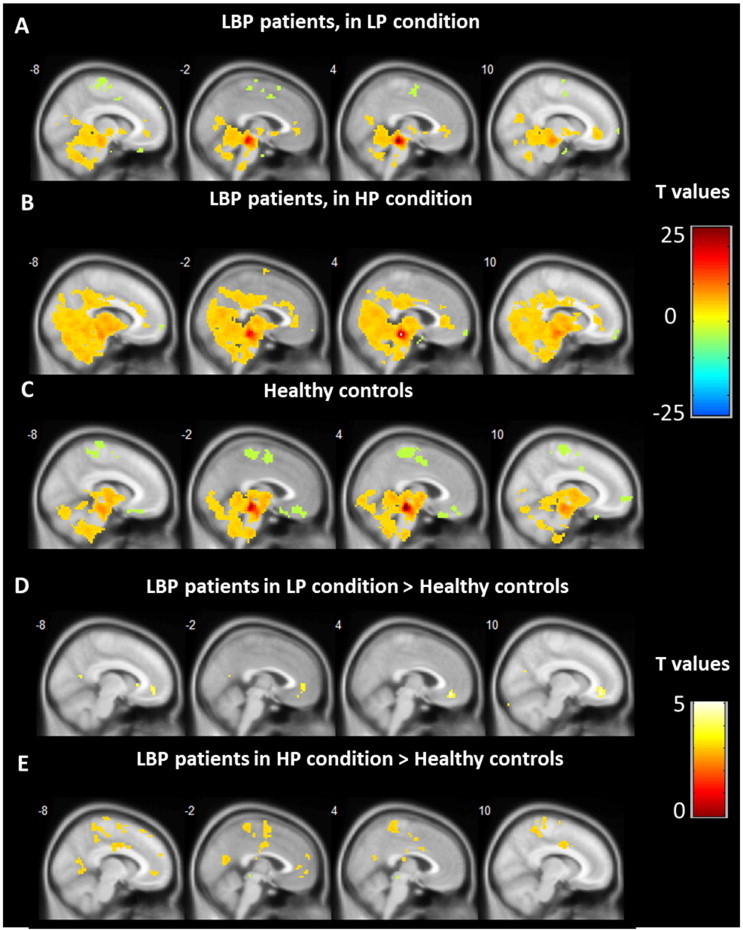
PAG centered functional connectivity. (A–C) Functional connectivity associated with the PAG in cLBP patients (in low pain condition and in high pain condition) and in the control group, respectively. (D and E) Enhanced functional connectivity associated with the PAG in cLBP patients compared with controls.
Whole brain voxel-by-voxel functional connectivity in HCs and cLBP patients during the LP condition was compared using a two-sample t-test. Results showed that cLBP patients in the LP condition had significantly greater FC between the PAG and the left vmPFC/rACC, x = −6, y = 45, z = −6, Z = 3.20, voxels = 11, PFWE < 0.05 svc and right vmPFC/ACC, x = 6, y = 42, z = −12, Z = 3.18, voxels = 36, PFWE < 0.05 svc, as well as other regions including the superior temporal gyrus and the precentral gyrus (Table 3 and Fig. 2D). The opposite contrast showed no FC differences above threshold. Compared with HCs, patients in the HP condition also showed enhanced FC between the PAG and the left vmPFC, x = −6, y = 42, z = −9, Z = 2.96, voxels = 11, PFWE < 0.05 svc, as well as other regions including the lingual gyrus, superior temporal gyrus, precentral gyrus, dorsal cingulate cortex, and posterior insula (Table 3 and Fig. 2E). The opposite contrast showed no FC differences above threshold. When we compared cLBP patients in the HP and LP conditions, we did not find a significant difference using the prior threshold (Fig. 2).
Table 3.
Main group difference results from group analysis cLBP patients in high pain and cLBP patients in low pain condition and healthy controls.
| Contrast | Voxels | Brain area | Peak coordinate (x, y, z) | Z value |
|---|---|---|---|---|
| LP > HC | 36 | R vmPFC/ACC | 6, 42, −12 | 3.18 svc |
| 11 | L vmPFC/ACC | −6, 45, −6 | 3.20 svc | |
| HC > LP | No brain region above the threshold | |||
| HP > HC | 2401 | R precentral gyrus | 39, −15, 33 | 4.68 |
| L superior temporal gyrus/operculum | −36, −6, 54 | 4.17 | ||
| R superior temporal gyrus/operculum | 57, 12, −9 | 4.09 | ||
| L dorsal cingulate cortex | −6, −9, 36 | 4.04 | ||
| R posterior insula | 48, −18, 15 | 3.86 svc | ||
| 300 | L lingual gyrus | −3, −63, 15 | 3.95 | |
| 11 | L vmPFC/ACC | −6, 43, −9 | 2.96 svc | |
| HC > HP | No brain region above the threshold | |||
| LP > HP | No brain region above the threshold | |||
| HP > LP | No brain region above the threshold | |||
| HP with pain intensity | 9 | L vmPFC | −9, 57, −12 | 3.83 svc |
| LP with illness duration | 3 | L amygdala | −27, −9, −18 | 3.18 svc |
| 19 | R posterior insula | 39, −12, 9 | 3.04 svc | |
LP = low pain before pain-inducing maneuver; HP = high pain after pain-inducing maneuver. HC = healthy controls. L = left, R = right. For regions of interest (ROIs), results were significant at PFWE < 0.05 after small volume correction (svc). Other results were significant at P < 0.05 family-wise error (FWE) corrected at the cluster level.
To test the association between FC and subjective cLBP rating at rest, we applied a regression analysis between FC and low back pain ratings using the average pain rating scores in the LP condition. We believe that this measure captures the immediate pain level during scanning since participants rated their pain before and immediately after each 6 minute resting state MRI scan. The results showed no significant correlations. For the resting state scan in the HP condition, we found significant negative correlations between FC and LBP ratings in the HP condition at the left vmPFC x = −9, y = 57, z = −12, Z = 3.83, voxels = 9, PFWE < 0.05 svc, and no positive correlations were found (Fig. 3A).
Fig. 3.
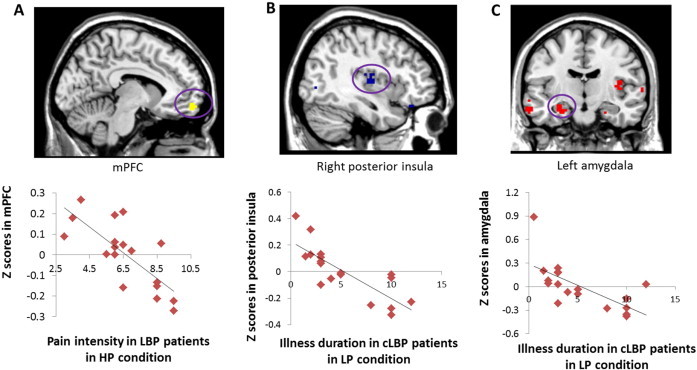
Brain–behavioral correlation results. (A) The association between PAG centered functional connectivity with the mPFC in the HP condition and endogenous pain intensity in the HP condition. (B–C) The association between PAG centered functional connectivity with the insula and amygdala in the LP condition and illness duration.
To test the association between functional connectivity with the PAG and cLBP illness duration, we applied a regression analysis between FC in the LP condition and cLBP duration. The results showed significant negative correlations between FC and cLBP illness duration at the right posterior insula, x = 39, y = −12, z = 9, Z = 3.04, voxels = 19, PFWE < 0.05 svc (Fig. 3B) and left amygdala, x = −27, y = −9, z = −18, voxels = 3, PFWE < 0.05 svc (Fig. 3C). Adding age as covariate of no interest did not change the results. No other significant brain–behavior associations were found. For resting state in the HP condition, we found no significant correlations.
Using a seed from the third ventricle (x = 0, y = −3, z = −7, with 2 mm radius) as a control region showed no group difference in FC with the prefrontal cortex even at lower threshold, P < 0.05, uncorrected. These results suggest that the ACC–PAG findings are specific to the PAG seed and are unlikely be confounded by signals from the adjacent ventricle area.
For ‘scrubbed’ data, the length of data included in analyses on average was 103.7 ± 3.9 volumes for HP condition, 107.9 ± 2.1 volumes for LP condition, and 111.3 ± 1.9 volumes for healthy controls. There was no significant difference between the HP and LP conditions (P > 0.2) and between HP and HC or LP and HC (P values > 0.2). The repeated analyses without removing frames with framewise displacement (FD) > 0.5 mm (‘scrubbing’) yielded similar results (results not reported here).
4. Discussion
In this study, we investigated differences in the PAG centered resting state functional connectivity in cLBP patients relative to age and gender matched controls. We found that PAG–vmPFC/rACC functional connectivity was enhanced in cLBP patients, compared with HCs. Interestingly, we found that the functional connectivity between the PAG and the vmPFC/rACC decreased as endogenous back pain intensity increased after pain-inducing maneuver, suggesting the dynamic character of functional connectivity at the PAG. Moreover, cLBP duration was negatively correlated with PAG–posterior insula and PAG–amygdala FC before any pain-inducing maneuver. These functional changes of PAG point out that the PAG in particular may play an important role in the pathophysiology of cLBP.
4.1. PAG–vmPFC/rACC functional connectivity
Abnormal vmPFC/rACC activity has been found in cLBP in previous studies. Compared to HCs, cLBP patients showed increased high-frequency BOLD oscillations (0.12–0.20 Hz) circumscribed mainly to the vmPFC and brain regions within the default network (Baliki et al., 2011). More recently, using arterial spin labeling, it has been found that provoked increases in endogenous LBP ratings were positively associated with statistically significant increases in regional cerebral blood flow in a widespread network of cortical areas, including the bilateral vmPFC in cLBP patients (Wasan et al., 2011). In addition, compared with healthy controls, patients demonstrated stronger default mode network connectivity to the pregenual anterior cingulate cortex, the left inferior parietal lobule, and the right insula (Loggia et al., 2013). It has been shown that the prefrontal cortex exerts active control on pain perception by modulating corticosubcortical and corticocortical pathways (Lorenz et al., 2003). The current study further highlights the role of vmPFC/rACC functional connectivity abnormality in cLBP and links the vmPFC/rACC to the PAG.
In a previous study on intrinsic connectivity patterns of the PAG using resting-state fMRI in a large cohort of 100 healthy subjects, it has been reported that PAG activity is positively correlated with surrounding subcortical brain regions as well as with cortical regions, including the anterior cingulate and anterior insula (Kong et al., 2010b). Multiple studies suggest that PAG activity tends to be connected with the rACC during pain perception and modulation (Bingel et al., 2006; Petrovic et al., 2002; Zubieta et al., 2005). Increased levels of heat pain can evoke a significant fMRI signal increase in the PAG (Kong et al., 2010a). Moreover, using midbrain/brainstem specific imaging, an increased PAG activity in response to pain has been demonstrated (Eippert et al., 2009). The involvement of the PAG in pain processing and modulation has been known for a long time. Brain imaging studies (Bushnell et al., 1999; Eippert et al., 2009; Erpelding et al., 2012; Kong et al., 2010a; Kong et al., 2006; Teutsch et al., 2008; Wasan et al., 2011) have found that the PAG is activated during the presentation of noxious stimuli as well as in association with pathological pain states such as chronic low back pain. Studies have shown that distraction tasks reduce the subjective pain sensation (Tracey et al., 2002; Valet et al., 2004). Activation in the periaqueductal gray was significantly increased during the distraction condition (Tracey et al., 2002; Valet et al., 2004), and the total increase in activation was predictive of changes in perceived pain intensity (Tracey et al., 2002).
The PAG also plays a role in pain facilitation. In accordance with a pain facilitation role, the magnitude of PAG activation has been shown to correlate with the degree of patients' neuropathic pain symptoms (Freynhagen et al., 2006). In irritable bowel syndrome (IBS) patients, activity in the vmPFC has been shown to disrupt a functional connection between the lateral PFC and the PAG, suggesting that the vmPFC and the PAG are involved in enhancing clinical pain (Mayer et al., 2005). Previous studies have shown a general hypersensitivity to painful stimuli in cLBP patients (Farasyn and Meeusen, 2005; Giesecke et al., 2004; Puta et al., 2012). Thus, the enhanced PAG–vmPFC/rACC FC in cLBP patients may indicate an enhanced pain inhibition or facilitation. Further studies are needed to clarify the role of this FC change.
4.2. Relationship between PAG functional connectivity and endogenous pain
In the present study, in the vmPFC, the strength of intrinsic connectivity with the PAG was negatively correlated with pain intensity in high pain condition. The vmPFC has been implicated in regulating affective responses by manipulating the contextual evaluation of sensory events (Rolls and Grabenhorst, 2008). vmPFC activation was associated with decreases in pain unpleasantness ratings induced by mindfulness meditation in healthy subjects (Zeidan et al., 2011). In this context, we speculate that a decrease in the intrinsic correlations between the PAG and the vmPFC in relation to the pain intensity may indicate a failed pain modulation in the brain in response to worsening of pain. An alternative explanation is that both the vmPFC and the PAG reflect arousal, which is high in HP condition. Interestingly, such correlation was significant only in the HP condition in which the pain level was enhanced by clinical pain-inducing maneuvers, suggesting that the vmPFC–PAG network is more related with higher pain states. Whether the observed association between spontaneous pain intensity and changes in PAG connectivity reflects either an adaptive mechanism or an abnormal state in cortical excitability that predisposes individuals to chronic pain needs to be further clarified. Our study suggests that regionally specific FC changes within the PAG–vmPFC networks may be a new locus of dysfunction in cLBP.
4.3. Relationship between PAG functional connectivity and illness duration
We found that illness duration was negatively correlated with PAG–posterior insula and PAG–amgydala FC, only in the LP condition. A previous study has found that the prestimulus FC between the insula and the PAG predicted perceived painfulness (Ploner et al., 2010), suggesting that these two regions interact to determine pain perception. It has been reported that activation in the contralateral posterior insula was positively correlated with temperature level, whereas subjective intensity related more to activation of the right anterior insula (Craig et al., 2000). It has been suggested that the posterior insula may provide a primary ‘interoceptive cortex’, specialized for perception of internal bodily states incorporating pain, temperature, and autonomic arousal (Craig, 2003; Critchley et al., 2002). We found that the longer an individual is in the cLBP state, the weaker the functional connectivity between the PAG and the posterior insula. We speculate that this may suggest that after long-term cLBP suffering, the body is adapted to the situation, and thus the modulation mechanism is somehow weakened. A previous study also found that verum acupuncture induced a higher level of correlations among the amygdala-associated network including the insula and the PAG (Qin et al., 2008), suggesting that this network may be involved in pain modulation. However, it is currently unknown whether altered FC with the PAG is the consequence of or the cause of cLBP. A longitudinal study would be required to determine the order of events. Interestingly, we did not observe significant correlation between PAG–insula FC after pain-inducing maneuver and pain duration in patients. It is possible that unlike in the natural low pain condition, the PAG–insula FC was altered in high pain condition.
4.4. Heterogeneity in pain modulation system across pain conditions
In a recent study of migraine pain patients (Mainero et al., 2011), investigators have found that patients had greater FC between the PAG and the ventral prefrontal cortex compared to the control group. Migraineurs who develop pain in response to normally innocuous stimulation (i.e., migraineurs with a history of allodynia) exhibited decreased FC with the PAG in the insula and the mPFC, compared with migraineurs without allodynia. This result is consistent with findings observed in LBP patients. In another study on a fibromyalgia patient, investigators have found that patients with fibromyalgia (a chronic pain disorder characterized by chronic widespread pain and allodynia (a heightened and painful response to pressure)) display less functional connectivity between the ACC and the PAG (Jensen et al., 2012). We speculate that this may reflect the heterogeneity of different chronic pain conditions. Taken together, these findings may suggest that different roles of pain descending control system underlie the localized chronic pain (e.g., LBP, migraine) and widespread pain (e.g., fibromyalgia pain).
4.5. Study limitations
There are some potential limitations in this study worth mentioning. The first potential limitation is the order effects between the high endogenous LBP and low endogenous LBP conditions. One challenge of cLBP studies is that once LBP is provoked, it is hard to control without any pharmacological intervention. In this study, we used exercise to provoke the patients' LBP; thus, the high pain condition tended to follow the low pain condition. Secondly, our fMRI data was not acquired with cardiac-gating which minimizes physiological motion artifact due to pressure wave pulsatility in arteries within and around the brain (Napadow et al., 2009). Future studies may compare the fMRI data with and without cardiac gating. Third, a control for the movement exercises corresponding to the pain-inducing maneuvers is missing since healthy controls underwent only one resting state session. The goal of clinical maneuvers is to induce pain in patients with cLBP. The pain-inducing maneuvers include a set of slow movements such as sit-ups, lumbar flexion/extension, and lumbar rotation. These daily life activities are not intense for healthy controls, although they can elicit pain in patients with cLBP. Thus, it is unlikely that movement exercises in healthy controls would evoke low back pain or induce functional connectivity changes in pain modulation network since there would be no pain expected in healthy controls. Previous studies also indicate that the intrinsic resting state functional connectivity is reliable across different sessions (Birn et al., 2013; Liao et al., 2013; Shehzad et al., 2009). Nevertheless, the contrast between HP and LP conditions should be interpreted with caution. Fourth, the seed region is relatively small. One concern is that the signal to noise ratio (SNR) might not be good enough to detect PAG activity. However, previous resting state MRI studies have successfully used small seed regions, such as amygdala subregions (Roy et al., 2009), nucleus accumbens (Cauda et al., 2011), and red nucleus (Nioche et al., 2009), and found valid FC results. Finally, we did not include medication in the model, and although it is unlikely that medication exposure accounts for our results, we cannot completely rule out the effect of medication. It is important to note, however, that we excluded all patients using opioids in the study, as a previous study found that daily oral administration of morphine for 1 month can cause anatomical changes in the brain (Younger et al., 2011).
In summary, the present study showed that cLBP patients have increased PAG–vmPFC FC and that the FC between the PAG and the vmPFC decreases as endogenous pain intensity increases in high pain condition. These findings may not only deepen our understanding of pain modulation and the development of chronic pain but also ultimately help inform mechanism-based therapies for treating different types of acute and chronic pain.
Conflict of interest
There is no conflict of interest to claim for all authors.
Acknowledgments
This work was supported by R01AT006364 (NCCAM) to Jian Kong, R01AT005280 (NCCAM) to Randy Gollub, PO1-AT002048 (NCCAM) to Bruce Rosen, M01-RR-01066 and UL1 RR025758-01 for Clinical Research Center Biomedical Imaging Core from National Center for Research Resources (NCRR), and P41RR14075 for Center for Functional Neuroimaging Technologies from NCRR.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
For ‘non-scrubbed’ data, main group difference results from group analysis cLBP patients in high pain and cLBP patients in low pain condition and healthy controls.
PAG centered functional connectivity for ‘non-scrubbed’ data. (A–C) Functional connectivity associated with the PAG in cLBP patients (in low pain condition and in high pain condition) and control group, respectively. (D and E) Enhanced functional connectivity associated with the PAG in cLBP patients compared with controls.
Brain–behavioral correlation results for ‘non-scrubbed’ data. (A) The association between PAG centered functional connectivity with the mPFC in the HP condition and endogenous pain intensity in the HP condition. (B–C) The association between PAG centered functional connectivity with the insula and amygdala in the LP condition and illness duration.
References
- Apkarian A.V., Baliki M.N., Geha P.Y. Towards a theory of chronic pain. Progress in Neurobiology. 2009;87:81–97. doi: 10.1016/j.pneurobio.2008.09.018. 18952143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apkarian A.V., Sosa Y., Krauss B.R., Thomas P.S., Fredrickson B.E., Levy R.E., Harden R.N., Chialvo D.R. Chronic pain patients are impaired on an emotional decision-making task. Pain. 2004;108:129–136. doi: 10.1016/j.pain.2003.12.015. 15109516 [DOI] [PubMed] [Google Scholar]
- Baliki M.N., Baria A.T., Apkarian A.V. The cortical rhythms of chronic back pain. Journal of Neuroscience: the Official Journal of the Society for Neuroscience. 2011;31:13981–13990. doi: 10.1523/JNEUROSCI.1984-11.2011. 21957259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baliki M.N., Chialvo D.R., Geha P.Y., Levy R.M., Harden R.N., Parrish T.B., Apkarian A.V. Chronic pain and the emotional brain: specific brain activity associated with spontaneous fluctuations of intensity of chronic back pain. Journal of Neuroscience: the Official Journal of the Society for Neuroscience. 2006;26:12165–12173. doi: 10.1523/JNEUROSCI.3576-06.2006. 17122041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandler R., Shipley M.T. Columnar organization in the midbrain periaqueductal gray: modules for emotional expression? Trends in Neurosciences. 1994;17:379–389. doi: 10.1016/0166-2236(94)90047-7. 7817403 [DOI] [PubMed] [Google Scholar]
- Baskin D.S., Mehler W.R., Hosobuchi Y., Richardson D.E., Adams J.E., Flitter M.A. Autopsy analysis of the safety, efficacy and cartography of electrical stimulation of the central gray in humans. Brain Research. 1986;371:231–236. doi: 10.1016/0006-8993(86)90358-6. 3486027 [DOI] [PubMed] [Google Scholar]
- Beck A.T., Ward C.H., Mendelson M., Mock J., Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. 13688369 [DOI] [PubMed] [Google Scholar]
- Bernard J.F., Huang G.F., Besson J.M. Nucleus centralis of the amygdala and the globus pallidus ventralis: electrophysiological evidence for an involvement in pain processes. Journal of Neurophysiology. 1992;68:551–569. doi: 10.1152/jn.1992.68.2.551. 1527575 [DOI] [PubMed] [Google Scholar]
- Bingel U., Lorenz J., Schoell E., Weiller C., Büchel C. Mechanisms of placebo analgesia: rACC recruitment of a subcortical antinociceptive network. Pain. 2006;120:8–15. doi: 10.1016/j.pain.2005.08.027. 16364549 [DOI] [PubMed] [Google Scholar]
- Birn R.M., Molloy E.K., Patriat R., Parker T., Meier T.B., Kirk G.R., Nair V.A., Meyerand M.E., Prabhakaran V. The effect of scan length on the reliability of resting-state fMRI connectivity estimates. Neuroimage. 2013;83:550–558. doi: 10.1016/j.neuroimage.2013.05.099. 23747458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogduk N. Pharmacological alternatives for the alleviation of back pain. Expert Opinion on Pharmacotherapy. 2004;5:2091–2098. doi: 10.1517/14656566.5.10.2091. 15461544 [DOI] [PubMed] [Google Scholar]
- Brooks J., Tracey I. From nociception to pain perception: imaging the spinal and supraspinal pathways. Journal of Anatomy. 2005;207:19–33. doi: 10.1111/j.1469-7580.2005.00428.x. 16011543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner R.L., Sepulcre J., Talukdar T., Krienen F.M., Liu H., Hedden T., Andrews-Hanna J.R., Sperling R.A., Johnson K.A. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer's disease. Journal of Neuroscience: the Official Journal of the Society for Neuroscience. 2009;29:1860–1873. doi: 10.1523/JNEUROSCI.5062-08.2009. 19211893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushnell M.C., Duncan G.H., Hofbauer R.K., Ha B., Chen J.I., Carrier B. Pain perception: is there a role for primary somatosensory cortex? Proceedings of the National Academy of Sciences of the United States of America. 1999;96:7705–7709. doi: 10.1073/pnas.96.14.7705. 10393884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauda F., Cavanna A.E., D'Agata F., Sacco K., Duca S., Geminiani G.C. Functional connectivity and coactivation of the nucleus accumbens: a combined functional connectivity and structure-based meta-analysis. Journal of Cognitive Neuroscience. 2011;23:2864–2877. doi: 10.1162/jocn.2011.21624. 21265603 [DOI] [PubMed] [Google Scholar]
- Chou R., Qaseem A., Snow V., Casey D., Cross J.T., Jr., Shekelle P., Owens D.K. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Annals of Internal Medicine. 2007;147:478–491. doi: 10.7326/0003-4819-147-7-200710020-00006. 17909209 [DOI] [PubMed] [Google Scholar]
- Chou R., Shekelle P. Will this patient develop persistent disabling low back pain? JAMA: the Journal of the American Medical Association. 2010;303:1295–1302. doi: 10.1001/jama.2010.344. 20371789 [DOI] [PubMed] [Google Scholar]
- Chudler E.H., Sugiyama K., Dong W.K. Nociceptive responses in the neostriatum and globus pallidus of the anesthetized rat. Journal of Neurophysiology. 1993;69:1890–1903. doi: 10.1152/jn.1993.69.6.1890. 8350129 [DOI] [PubMed] [Google Scholar]
- Cleeland C.S., Ryan K.M. Pain assessment: global use of the Brief Pain Inventory. Annals of the Academy of Medicine, Singapore. 1994;23:129–138. 8080219 [PubMed] [Google Scholar]
- Coderre T.J., Katz J., Vaccarino A.L., Melzack R. Contribution of central neuroplasticity to pathological pain: review of clinical and experimental evidence. Pain. 1993;52:259–285. doi: 10.1016/0304-3959(93)90161-H. 7681556 [DOI] [PubMed] [Google Scholar]
- Cordes D., Haughton V.M., Arfanakis K., Carew J.D., Turski P.A., Moritz C.H., Quigley M.A., Meyerand M.E. Frequencies contributing to functional connectivity in the cerebral cortex in “resting-state” data. AJNR. American Journal of Neuroradiology. 2001;22:1326–1333. 11498421 [PMC free article] [PubMed] [Google Scholar]
- Craig A.D. How do you feel? Interoception: the sense of the physiological condition of the body. Nature Reviews. Neuroscience. 2002;3:655–666. doi: 10.1038/nrn894. 12154366 [DOI] [PubMed] [Google Scholar]
- Craig A.D. A new view of pain as a homeostatic emotion. Trends in Neurosciences. 2003;26:303–307. doi: 10.1016/s0166-2236(03)00123-1. 12798599 [DOI] [PubMed] [Google Scholar]
- Craig A.D., Chen K., Bandy D., Reiman E.M. Thermosensory activation of insular cortex. Nature Neuroscience. 2000;3:184–190. doi: 10.1038/72131. 10649575 [DOI] [PubMed] [Google Scholar]
- Critchley H.D., Mathias C.J., Dolan R.J. Fear conditioning in humans: the influence of awareness and autonomic arousal on functional neuroanatomy. Neuron. 2002;33:653–663. doi: 10.1016/s0896-6273(02)00588-3. 11856537 [DOI] [PubMed] [Google Scholar]
- Ehrlich G.E. Low back pain. Bulletin of the World Health Organization. 2003;81:671–676. 14710509 [PMC free article] [PubMed] [Google Scholar]
- Eippert F., Bingel U., Schoell E.D., Yacubian J., Klinger R., Lorenz J., Büchel C. Activation of the opioidergic descending pain control system underlies placebo analgesia. Neuron. 2009;63:533–543. doi: 10.1016/j.neuron.2009.07.014. 19709634 [DOI] [PubMed] [Google Scholar]
- Erpelding N., Moayedi M., Davis K.D. Cortical thickness correlates of pain and temperature sensitivity. Pain. 2012;153:1602–1609. doi: 10.1016/j.pain.2012.03.012. 22516588 [DOI] [PubMed] [Google Scholar]
- Farasyn A., Meeusen R. The influence of non-specific low back pain on pressure pain thresholds and disability. European Journal of Pain (London, England) 2005;9:375–381. doi: 10.1016/j.ejpain.2004.09.005. 15979017 [DOI] [PubMed] [Google Scholar]
- Fields H. State-dependent opioid control of pain. Nature Reviews. Neuroscience. 2004;5:565–575. doi: 10.1038/nrn1431. 15208698 [DOI] [PubMed] [Google Scholar]
- Fox M.D., Raichle M.E. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature Reviews. Neuroscience. 2007;8:700–711. doi: 10.1038/nrn2201. 17704812 [DOI] [PubMed] [Google Scholar]
- Freynhagen R., Baron R., Gockel U., Tölle T.R. painDETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Current Medical Research and Opinion. 2006;22:1911–1920. doi: 10.1185/030079906X132488. 17022849 [DOI] [PubMed] [Google Scholar]
- Giesecke T., Gracely R.H., Grant M.A., Nachemson A., Petzke F., Williams D.A., Clauw D.J. Evidence of augmented central pain processing in idiopathic chronic low back pain. Arthritis and Rheumatism. 2004;50:613–623. doi: 10.1002/art.20063. 14872506 [DOI] [PubMed] [Google Scholar]
- Hadjipavlou G., Dunckley P., Behrens T.E., Tracey I. Determining anatomical connectivities between cortical and brainstem pain processing regions in humans: a diffusion tensor imaging study in healthy controls. Pain. 2006;123:169–178. doi: 10.1016/j.pain.2006.02.027. 16616418 [DOI] [PubMed] [Google Scholar]
- Hart L.G., Deyo R.A., Cherkin D.C. Physician office visits for low back pain. Frequency, clinical evaluation, and treatment patterns from a U.S. national survey. Spine. 1995;20:11–19. doi: 10.1097/00007632-199501000-00003. 7709270 [DOI] [PubMed] [Google Scholar]
- Heinricher M.M., Tavares I., Leith J.L., Lumb B.M. Descending control of nociception: specificity, recruitment and plasticity. Brain Research Reviews. 2009;60:214–225. doi: 10.1016/j.brainresrev.2008.12.009. 19146877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosobuchi Y., Adams J.E., Linchitz R. Pain relief by electrical stimulation of the central gray matter in humans and its reversal by naloxone. Science (New York, N.Y.) 1977;197:183–186. doi: 10.1126/science.301658. 301658 [DOI] [PubMed] [Google Scholar]
- Jensen K.B., Loitoile R., Kosek E., Petzke F., Carville S., Fransson P., Marcus H., Williams S.C., Choy E., Mainguy Y., Vitton O., Gracely R.H., Gollub R., Ingvar M., Kong J. Patients with fibromyalgia display less functional connectivity in the brain's pain inhibitory network. Molecular Pain. 2012;8:32. doi: 10.1186/1744-8069-8-32. 22537768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y., Kurata J., Sekiguchi M., Kokubun M., Akaishizawa T., Chiba Y., Konno S.-i., Kikuchi S.-i. Augmented cerebral activation by lumbar mechanical stimulus in chronic low back pain patients: an fMRI study. Spine. 2009;34:2431–2436. doi: 10.1097/BRS.0b013e3181b1fb76. 19789470 [DOI] [PubMed] [Google Scholar]
- Kong J., Jensen K., Loiotile R., Cheetham A., Wey H.Y., Tan Y., Rosen B., Smoller J.W., Kaptchuk T.J., Gollub R.L. Functional connectivity of the frontoparietal network predicts cognitive modulation of pain. Pain. 2013;154:459–467. doi: 10.1016/j.pain.2012.12.004. 23352757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J., Loggia M.L., Zyloney C., Tu P., Laviolette P., Gollub R.L. Exploring the brain in pain: activations, deactivations and their relation. Pain. 2010;148:257–267. doi: 10.1016/j.pain.2009.11.008. 20005043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J., Spaeth R.B., Wey H.Y., Cheetham A., Cook A.H., Jensen K., Tan Y., Liu H., Wang D., Loggia M.L., Napadow V., Smoller J.W., Wasan A.D., Gollub R.L. S1 is associated with chronic low back pain: a functional and structural MRI study. Molecular Pain. 2013;9:43. doi: 10.1186/1744-8069-9-43. 23965184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J., Tu P.C., Zyloney C., Su T.P. Intrinsic functional connectivity of the periaqueductal gray, a resting fMRI study. Behavioural Brain Research. 2010;211:215–219. doi: 10.1016/j.bbr.2010.03.042. 20347878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong J., White N.S., Kwong K.K., Vangel M.G., Rosman I.S., Gracely R.H., Gollub R.L. Using fMRI to dissociate sensory encoding from cognitive evaluation of heat pain intensity. Human Brain Mapping. 2006;27:715–721. doi: 10.1002/hbm.20213. 16342273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucyi A., Salomons T.V., Davis K.D. Mind wandering away from pain dynamically engages antinociceptive and default mode brain networks. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:18692–18697. doi: 10.1073/pnas.1312902110. 24167282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao X.H., Xia M.R., Xu T., Dai Z.J., Cao X.Y., Niu H.J., Zuo X.N., Zang Y.F., He Y. Functional brain hubs and their test–retest reliability: a multiband resting-state functional MRI study. Neuroimage. 2013;83:969–982. doi: 10.1016/j.neuroimage.2013.07.058. 23899725 [DOI] [PubMed] [Google Scholar]
- Loggia M.L., Kim J., Gollub R.L., Vangel M.G., Kirsch I., Kong J., Wasan A.D., Napadow V. Default mode network connectivity encodes clinical pain: an arterial spin labeling study. Pain. 2013;154:24–33. doi: 10.1016/j.pain.2012.07.029. 23111164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenz J., Minoshima S., Casey K.L. Keeping pain out of mind: the role of the dorsolateral prefrontal cortex in pain modulation. Brain: A Journal of Neurology. 2003;126:1079–1091. doi: 10.1093/brain/awg102. 12690048 [DOI] [PubMed] [Google Scholar]
- Mainero C., Boshyan J., Hadjikhani N. Altered functional magnetic resonance imaging resting-state connectivity in periaqueductal gray networks in migraine. Annals of Neurology. 2011;70:838–845. doi: 10.1002/ana.22537. 22162064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martikainen I.K., Peciña M., Love T.M., Nuechterlein E.B., Cummiford C.M., Green C.R., Harris R.E., Stohler C.S., Zubieta J.K. Alterations in endogenous opioid functional measures in chronic back pain. Journal of Neuroscience: the Official Journal of the Society for Neuroscience. 2013;33:14729–14737. doi: 10.1523/JNEUROSCI.1400-13.2013. 24027273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer D.J., Wolfle T.L., Akil H., Carder B., Liebeskind J.C. Analgesia from electrical stimulation in the brainstem of the rat. Science (New York, N.Y.) 1971;174:1351–1354. doi: 10.1126/science.174.4016.1351. 5167502 [DOI] [PubMed] [Google Scholar]
- Mayer E.A., Berman S., Suyenobu B., Labus J., Mandelkern M.A., Naliboff B.D., Chang L. Differences in brain responses to visceral pain between patients with irritable bowel syndrome and ulcerative colitis. Pain. 2005;115:398–409. doi: 10.1016/j.pain.2005.03.023. 15911167 [DOI] [PubMed] [Google Scholar]
- Mikl M., Marecek R., Hlustík P., Pavlicová M., Drastich A., Chlebus P., Brázdil M., Krupa P. Effects of spatial smoothing on fMRI group inferences. Magnetic Resonance Imaging. 2008;26:490–503. doi: 10.1016/j.mri.2007.08.006. 18060720 [DOI] [PubMed] [Google Scholar]
- Napadow V., Dhond R., Park K., Kim J., Makris N., Kwong K.K., Harris R.E., Purdon P.L., Kettner N., Hui K.K. Time-variant fMRI activity in the brainstem and higher structures in response to acupuncture. Neuroimage. 2009;47:289–301. doi: 10.1016/j.neuroimage.2009.03.060. 19345268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer V., Li W., Bird G.C., Han J.S. The amygdala and persistent pain. Neuroscientist: A Review Journal Bringing Neurobiology, Neurology and Psychiatry. 2004;10:221–234. doi: 10.1177/1073858403261077. 15155061 [DOI] [PubMed] [Google Scholar]
- Nioche C., Cabanis E.A., Habas C. Functional connectivity of the human red nucleus in the brain resting state at 3 T. AJNR. American Journal of Neuroradiology. 2009;30:396–403. doi: 10.3174/ajnr.A1375. 19022864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovic P., Kalso E., Petersson K.M., Ingvar M. Placebo and opioid analgesia — imaging a shared neuronal network. Science (New York, N.Y.) 2002;295:1737–1740. doi: 10.1126/science.1067176. 11834781 [DOI] [PubMed] [Google Scholar]
- Ploner M., Lee M.C., Wiech K., Bingel U., Tracey I. Prestimulus functional connectivity determines pain perception in humans. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:355–360. doi: 10.1073/pnas.0906186106. 19948949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Barnes K.A., Snyder A.Z., Schlaggar B.L., Petersen S.E. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. doi: 10.1016/j.neuroimage.2011.10.018. 22019881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Barnes K.A., Snyder A.Z., Schlaggar B.L., Petersen S.E. Steps toward optimizing motion artifact removal in functional connectivity MRI; a reply to carp. Neuroimage. 2013;76:439–441. doi: 10.1016/j.neuroimage.2012.03.017. 22440651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power J.D., Mitra A., Laumann T.O., Snyder A.Z., Schlaggar B.L., Petersen S.E. Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage. 2014;84:320–341. doi: 10.1016/j.neuroimage.2013.08.048. 23994314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puta C., Schulz B., Schoeler S., Magerl W., Gabriel B., Gabriel H.H., Miltner W.H., Weiss T. Enhanced sensitivity to punctate painful stimuli in female patients with chronic low back pain. BMC Neurology. 2012;12:98. doi: 10.1186/1471-2377-12-98. 22998460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin W., Tian J., Bai L., Pan X., Yang L., Chen P., Dai J., Ai L., Zhao B., Gong Q., Wang W., von Deneen K.M., Liu Y. FMRI connectivity analysis of acupuncture effects on an amygdala-associated brain network. Molecular Pain. 2008;4:55. doi: 10.1186/1744-8069-4-55. 19014532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds D.V. Surgery in the rat during electrical analgesia induced by focal brain stimulation. Science (New York, N.Y.) 1969;164:444–445. doi: 10.1126/science.164.3878.444. 4887743 [DOI] [PubMed] [Google Scholar]
- Rolls E.T., Grabenhorst F. The orbitofrontal cortex and beyond: from affect to decision-making. Progress in Neurobiology. 2008;86:216–244. doi: 10.1016/j.pneurobio.2008.09.001. 18824074 [DOI] [PubMed] [Google Scholar]
- Roy A.K., Shehzad Z., Margulies D.S., Kelly A.M., Uddin L.Q., Gotimer K., Biswal B.B., Castellanos F.X., Milham M.P. Functional connectivity of the human amygdala using resting state fMRI. Neuroimage. 2009;45:614–626. doi: 10.1016/j.neuroimage.2008.11.030. 19110061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savigny P., Watson P., Underwood M. Low Back Pain: Early Management of Persistent Non-specific Low Back Pain. National Collaborating Centre for Primary Care and Royal College of General Practitioners (Clinical guideline 88); London: 2009. [Google Scholar]
- Schneider J.S., Lidsky T.I. Processing of somatosensory information in striatum of behaving cats. Journal of Neurophysiology. 1981;45:841–851. doi: 10.1152/jn.1981.45.5.841. 7241172 [DOI] [PubMed] [Google Scholar]
- Shehzad Z., Kelly A.M., Reiss P.T., Gee D.G., Gotimer K., Uddin L.Q., Lee S.H., Margulies D.S., Roy A.K., Biswal B.B., Petkova E., Castellanos F.X., Milham M.P. The resting brain: unconstrained yet reliable. Cerebral Cortex (New York, N.Y.: 1991) 2009;19:2209–2229. doi: 10.1093/cercor/bhn256. 19221144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein N., Sprenger C., Scholz J., Wiech K., Bingel U. White matter integrity of the descending pain modulatory system is associated with interindividual differences in placebo analgesia. Pain. 2012;153:2210–2217. doi: 10.1016/j.pain.2012.07.010. 22959599 [DOI] [PubMed] [Google Scholar]
- Tagliazucchi E., Balenzuela P., Fraiman D., Chialvo D.R. Brain resting state is disrupted in chronic back pain patients. Neuroscience Letters. 2010;485:26–31. doi: 10.1016/j.neulet.2010.08.053. 20800649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan G., Jensen M.P., Thornby J.I., Shanti B.F. Validation of the Brief Pain Inventory for chronic nonmalignant pain. Journal of Pain: Official Journal of the American Pain Society. 2004;5:133–137. doi: 10.1016/j.jpain.2003.12.005. 15042521 [DOI] [PubMed] [Google Scholar]
- Teutsch S., Herken W., Bingel U., Schoell E., May A. Changes in brain gray matter due to repetitive painful stimulation. Neuroimage. 2008;42:845–849. doi: 10.1016/j.neuroimage.2008.05.044. 18582579 [DOI] [PubMed] [Google Scholar]
- Tracey I., Mantyh P.W. The cerebral signature for pain perception and its modulation. Neuron. 2007;55:377–391. doi: 10.1016/j.neuron.2007.07.012. 17678852 [DOI] [PubMed] [Google Scholar]
- Tracey I., Ploghaus A., Gati J.S., Clare S., Smith S., Menon R.S., Matthews P.M. Imaging attentional modulation of pain in the periaqueductal gray in humans. Journal of Neuroscience: the Official Journal of the Society for Neuroscience. 2002;22:2748–2752. doi: 10.1523/JNEUROSCI.22-07-02748.2002. 11923440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Delcroix N., Mazoyer B., Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. 11771995 [DOI] [PubMed] [Google Scholar]
- Valet M., Sprenger T., Boecker H., Willoch F., Rummeny E., Conrad B., Erhard P., Tolle T.R. Distraction modulates connectivity of the cingulo-frontal cortex and the midbrain during pain — an fMRI analysis. Pain. 2004;109:399–408. doi: 10.1016/j.pain.2004.02.033. 15157701 [DOI] [PubMed] [Google Scholar]
- Van Dijk K.R., Sabuncu M.R., Buckner R.L. The influence of head motion on intrinsic functional connectivity MRI. Neuroimage. 2012;59:431–438. doi: 10.1016/j.neuroimage.2011.07.044. 21810475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasan A.D., Loggia M.L., Chen L.Q., Napadow V., Kong J., Gollub R.L. Neural correlates of chronic low back pain measured by arterial spin labeling. Anesthesiology. 2011;115:364–374. doi: 10.1097/ALN.0b013e318220e880. 21720241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werneke M.W., Hart D.L. Categorizing patients with occupational low back pain by use of the Quebec Task Force Classification system versus pain pattern classification procedures: discriminant and predictive validity. Physical Therapy. 2004;84:243–254. 14984296 [PubMed] [Google Scholar]
- Wiech K., Seymour B., Kalisch R., Stephan K.E., Koltzenburg M., Driver J., Dolan R.J. Modulation of pain processing in hyperalgesia by cognitive demand. Neuroimage. 2005;27:59–69. doi: 10.1016/j.neuroimage.2005.03.044. 15978845 [DOI] [PubMed] [Google Scholar]
- Woolf C.J., Doubell T.P. The pathophysiology of chronic pain — increased sensitivity to low threshold A beta-fibre inputs. Current Opinion in Neurobiology. 1994;4:525–534. doi: 10.1016/0959-4388(94)90053-1. 7812141 [DOI] [PubMed] [Google Scholar]
- Younger J.W., Chu L.F., D'Arcy N.T., Trott K.E., Jastrzab L.E., Mackey S.C. Prescription opioid analgesics rapidly change the human brain. Pain. 2011;152:1803–1810. doi: 10.1016/j.pain.2011.03.028. 21531077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeidan F., Martucci K.T., Kraft R.A., Gordon N.S., McHaffie J.G., Coghill R.C. Brain mechanisms supporting the modulation of pain by mindfulness meditation. Journal of Neuroscience: the Official Journal of the Society for Neuroscience. 2011;31:5540–5548. doi: 10.1523/JNEUROSCI.5791-10.2011. 21471390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann M. Pathobiology of neuropathic pain. European Journal of Pharmacology. 2001;429:23–37. doi: 10.1016/s0014-2999(01)01303-6. 11698024 [DOI] [PubMed] [Google Scholar]
- Zubieta J.K., Bueller J.A., Jackson L.R., Scott D.J., Xu Y., Koeppe R.A., Nichols T.E., Stohler C.S. Placebo effects mediated by endogenous opioid activity on mu-opioid receptors. Journal of Neuroscience: the Official Journal of the Society for Neuroscience. 2005;25:7754–7762. doi: 10.1523/JNEUROSCI.0439-05.2005. 16120776 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For ‘non-scrubbed’ data, main group difference results from group analysis cLBP patients in high pain and cLBP patients in low pain condition and healthy controls.
PAG centered functional connectivity for ‘non-scrubbed’ data. (A–C) Functional connectivity associated with the PAG in cLBP patients (in low pain condition and in high pain condition) and control group, respectively. (D and E) Enhanced functional connectivity associated with the PAG in cLBP patients compared with controls.
Brain–behavioral correlation results for ‘non-scrubbed’ data. (A) The association between PAG centered functional connectivity with the mPFC in the HP condition and endogenous pain intensity in the HP condition. (B–C) The association between PAG centered functional connectivity with the insula and amygdala in the LP condition and illness duration.


