Abstract
Deficits in social cognition including facial affect recognition and their detrimental effects on functional outcome are well established in schizophrenia. Structured training can have substantial effects on social cognitive measures including facial affect recognition. Elucidating training effects on cortical mechanisms involved in facial affect recognition may identify causes of dysfunctional facial affect recognition in schizophrenia and foster remediation strategies. In the present study, 57 schizophrenia patients were randomly assigned to (a) computer-based facial affect training that focused on affect discrimination and working memory in 20 daily 1-hour sessions, (b) similarly intense, targeted cognitive training on auditory-verbal discrimination and working memory, or (c) treatment as usual. Neuromagnetic activity was measured before and after training during a dynamic facial affect recognition task (5 s videos showing human faces gradually changing from neutral to fear or to happy expressions). Effects on 10–13 Hz (alpha) power during the transition from neutral to emotional expressions were assessed via MEG based on previous findings that alpha power increase is related to facial affect recognition and is smaller in schizophrenia than in healthy subjects. Targeted affect training improved overt performance on the training tasks. Moreover, alpha power increase during the dynamic facial affect recognition task was larger after affect training than after treatment-as-usual, though similar to that after targeted perceptual–cognitive training, indicating somewhat nonspecific benefits. Alpha power modulation was unrelated to general neuropsychological test performance, which improved in all groups. Results suggest that specific neural processes supporting facial affect recognition, evident in oscillatory phenomena, are modifiable. This should be considered when developing remediation strategies targeting social cognition in schizophrenia.
Keywords: MEG, Brain rhythms, Alpha oscillations, Schizophrenia, Facial affect, Cognitive training
1. Introduction
Deficits in social cognitive skills in schizophrenia patients (SZ) have been demonstrated in numerous studies. Facial affect recognition (FAR) is particularly relevant for effective social interaction (Johnston et al., 2010; Sachs et al., 2012; Wölwer et al., 2012). As impaired social cognitive skills are linked to functional impairment (e.g., Poole et al., 2000; Sachs et al., 2004; Hofer et al., 2009), remediation programs have targeted social-cognitive skills including FAR (e.g., Wölwer et al., 2005; Habel et al., 2010; Mazza et al., 2010; Wölwer and Frommann, 2011; Kurtz and Richardson, 2012). In their meta-analysis of 19 studies including 692 SZ, Kurtz and Richardson affirmed moderate-to-large effects on social cognitive measures (including facial affect identification tasks) and on observer-rated social function, with training effects varying with age, duration of illness, and extent of training.
Understanding brain processes contributing to social cognition deficits should facilitate the development and evaluation of tailored remediation strategies. Numerous studies have studied cortical and subcortical correlates of emotion processing in SZ including FAR (for hemodynamic imaging evidence, see Pinkham et al., 2007; Seiferth et al., 2009; Habel et al., 2010; Li et al., 2010; for event-related brain potential evidence, Turetsky et al., 2007; Wölwer et al., 2012; Wynn et al., 2013; for oscillatory activity, Singh et al., 2011; Popov et al., 2013, 2014).
These studies provide substantial evidence of deviant brain activity related to social cognition, including FAR. Yet few studies have evaluated the effects of social-cognition training on brain activity (Habel et al., 2010; Wölwer et al., 2012; Luckhaus et al., 2013). Popov et al. (2013) proposed a neural mechanism for such disrupted facial affect processing and its remediation. The study demonstrated group differences in 10–15 Hz (alpha) neuromagnetic oscillatory power modulation in bilateral sensorimotor regions while SZ and healthy controls (HC) viewed 5 s videos of dynamic facial stimuli that changed from neutral to fear or to happy expressions. During the period prior to correct affect recognition, HC exhibited a significant alpha power increase relative to baseline, whereas the significantly smaller increase in SZ varied with poorer discrimination accuracy. Because sensorimotor alpha activity has been linked to social information processing including FAR (e.g., Singh et al., 2011), the present study employed a targeted intervention to test the hypothesis that this recruitment of neural processes facilitates the recognition of unfolding facial affect in HC and is apparently impaired in SZ. Support for the hypothesis would mean that appropriate training can address deficits in recognition skills.
The present study evaluated a new Facial Affect recognition Training (FAT) protocol, designed specifically to address mechanisms facilitating FAR, and assessed alpha-power modulation as a possible mechanism of the training effect. Because beneficial effects of specific cognitive and/or social cognitive training protocols as add-ons to general SZ remediation programs have been reported (e.g., Keefe and Harvey, 2012; Sacks et al., 2013), the present study compared FAT with a well established cognitive training protocol, Cognitive Exercises (CEs; PositScience, SF, USA) already shown to be effective in SZ (Fisher et al., 2014; see also Popov et al., 2011, 2012). CE focuses on perceptual and cognitive skills and does not include facial or emotional judgments. Thus, it served as an active control for FAT's use of a training regimen. In between-group analyses, FAT and CE were compared with the inpatient unit's treatment as usual (TAU), which provided a nonspecific control for the passage of time and general treatment efforts. Analyses addressed a series of questions: Does training affect brain dynamics, and does specific training (FAT) affect specific FAR-related oscillatory dynamics? Does training normalize brain dynamics (does FAT reduce pre-training differences in FAR-related alpha dynamics between SZ and HC to nonsignificance)? To what extent are changes dependent on an active intervention in general (does FAT do so better than TAU) or on specific FAR-focused training (does FAT do better than CE)?
2. Methods and materials
2.1. Participants
Inpatients with an ICD diagnosis of paranoid-hallucinatory schizophrenia (code number 20.0) were recruited at the regional Center for Psychiatry. Inclusion criteria were normal intellectual function and no history of any neurological condition or disorder such as epilepsy or head trauma with loss of consciousness. According to the standard treatment regimen of the Center, all patients were stably medicated at the time of the study. From the pool of eligible SZ (n = 114; see Fig. 1), n = 80 were randomly assigned to three intervention groups, of which n = 62 completed the interventions and all pre- and post-intervention assessments (symptom ratings, neuropsychological assessment, magnetoencephalographic (MEG) recording). After providing written informed consent, SZ were randomly assigned (with some adjustment to ensure balanced group sizes) to one of three groups: the two computer-based training methods, FAT or CE, or TAU. Recruitment continued until at least 20 SZ were enrolled in each intervention. At post-intervention assessment, data from one subject in each group were discarded because of MEG artifact or missing MEG data. Thus, results are reported for n = 19 patients in each group. Table 1 summarizes demographic and clinical data for patients together with statistical group differences. Symptom severity pre- and post-intervention was assessed via the Positive and Negative Symptom Scale (PANSS, Kay et al., 1987) and the Global Assessment of Functioning (GAF) scale of DSM-IV. Upon treatment assignment, groups did not differ in gender, age, educational level, IQ, symptom severity (PANSS), global function (GAF), medication (evaluated by CPZ equivalent), or neuropsychological test performance (below and Table 2). In each group 2 patients were left-handed, and 2–4 were ambidextrous, as assessed by a modified version of the Edinburgh Handedness Questionnaire (Oldfield, 1971).
Fig. 1.
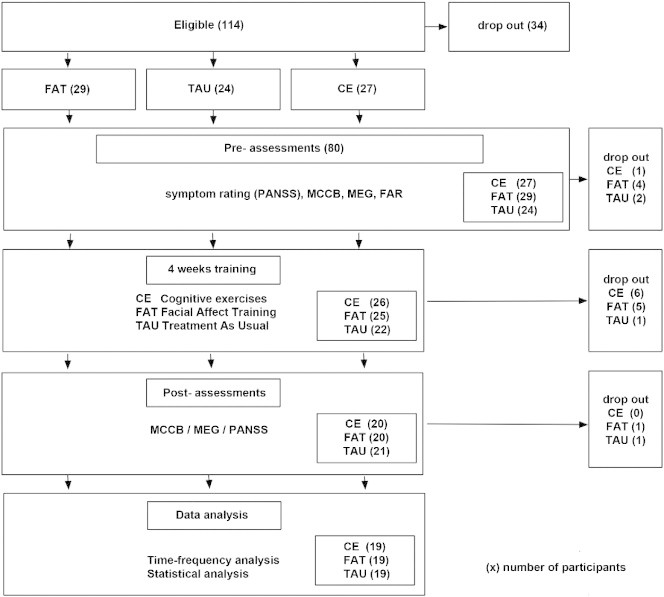
Schizophrenia patient recruitment across the study protocol, following CONSORT criteria. Number of patients per study phase in brackets. Prior to MCCB and MEG assessment, patients are randomly assigned to the three intervention groups: FAT, facial affect training; CE, cognitive exercise; TAU, treatment as usual (details in Methods and materials section). FAR: Facial affect recognition criterion task (details in Methods and materials section).
Table 1.
Demographic and clinical data (means ± standard deviation), before (pre) and after (post) treatment for schizophrenia patients per treatment group (n = 19 per group) together with statistical differences.
| Gender (m/f) | Age | Years of education | IQ | LQ | CPZ pre | CPZ post | |
|---|---|---|---|---|---|---|---|
| FAT | 11/8 | 39.6 ± 7.9 | 11.5 ± 1.7 | 108.1 ± 16.3 | 70.7 ± 59.0 | 617.2 ± 327.1 | 539.6 ± 289.2 |
| CE | 12/7 | 36.0 ± 8.5 | 10.8 ± 1.7 | 102.3 ± 13.6 | 60.7 ± 59.1 | 544.6 ± 490.1 | 506.1 ± 344.3 |
| TAU | 15/4 | 35.9 ± 10.6 | 11.3 ± 1.8 | 108.7 ± 17.4 | 59.0 ± 67.5 | 646.2 ± 393.9 | 637.9 ± 280.9 |
| Statistical difference | Chi2 = 2.0 n.s. | F2,54 = 1.04 n.s. | F2,50 < 1 n.s. | F2,54 < 1 | F2,54 < 1 | F2,53 < 1 | F2,50 < 1 |
| PANSS-P |
PANSS-N |
PANSS-G |
GAF |
|||||
|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | |
| FAT | 16.1 ± 5.2 | 14.4 ± 4.6 | 19.2 ± 6.6 | 17.9 ± 6.2 | 36.9 ± 8.4 | 33.2 ± 8.2 | 42.5 ± 12.4 | 46.3 ± 13.4 |
| CE | 15.6 ± 5.2 | 12.6 ± 4.2 | 18.0 ± 6.5 | 17.4 ± 6.8 | 35.4 ± 5.5 | 30.5 ± 7.1 | 44.7 ± 13.6 | 50.1 ± 13.5 |
| TAU | 14.7 ± 4.9 | 13.6 ± 6.0 | 19.6 ± 6.1 | 18.3 ± 6.2 | 35.1 ± 9.0 | 34.3 ± 9.9 | 41.2 ± 13.4 | 43.0 ± 12.2 |
| Statistical difference | F2,54 < 1 | F2,53 < 1 | F2,54 < 1 | F2,52 < 1 | F2,54 < 1 | F2,53 = 1.0 n.s. | F2,54 < 1 | F2,53 = 1.4 n.s. |
Note: FAT: facial affect training, CE: cognitive exercise, TAU: treatment as usual; LQ: laterality quotient; CPZ: chlorpromazine equivalents; PANSS: Positive and Negative Symptom Scale (26); GAF: Global Assessment of Functioning (DSM-IV axis V); pre: before intervention onset; post: after 4-week intervention period; n.s.: p > .1.
Table 2.
MATRICS consensus cognitive battery (MCCB) test scores (means ± standard deviation of normative T-scores) for the seven MCCB domains before (pre) and after (post) intervention period for the three schizophrenia patients group (n = 19 per group).
| Processing speed | Attentional vigilance | Working memory | Verbal learning | Visual learning | Reasoning | Social cognition | Overall composite | ||
|---|---|---|---|---|---|---|---|---|---|
| FAT | Pre | 39.5 ± 10.6 | 33.6 ± 11.5 | 44.6 ± 10.2 | 46.2 ± 9.9 | 42.8 ± 12.8 | 44.5 ± 10.7 | 39.3 ± 11.1 | 36.1 ± 10.5 |
| Post | 44.6 ± 12.2 | 38.5 ± 11.8 | 48.2 ± 9.2 | 47.1 ± 10.0 | 49.4 ± 14.0 | 47.9 ± 8.8 | 39.3 ± 10.4 | 41.6 ± 11.5 | |
| CE | Pre | 40.1 ± 14.4 | 38.8 ± 10.3 | 48.9 ± 10.7 | 45.9 ± 11.3 | 42.3 ± 17.5 | 46.3 ± 10.6 | 39.1 ± 8.5 | 38.6 ± 14.0 |
| Post | 44.2 ± 13.5 | 41.7 ± 13.7 | 48.9 ± 14.7 | 45.0 ± 12.0 | 50.3 ± 13.8 | 49.7 ± 10.4 | 38.5 ± 10.8 | 42.3 ± 15.9 | |
| TAU | Pre | 41.8 ± 11.1 | 36.6 ± 10.5 | 47.9 ± 13.0 | 48.2 ± 10.7 | 43.9 ± 13.0 | 49.3 ± 10.1 | 40.2 ± 11.3 | 39.9 ± 12.3 |
| Post | 48.2 ± 12.3 | 41.3 ± 10.1 | 50.8 ± 12.1 | 47.3 ± 10.4 | 46.5 ± 9.8 | 45.4 ± 7.7 | 42.8 ± 11.7 | 43.3 ± 10.8 | |
| Time F1,56 | 33.56** | 15.82** | n.s. | <1 | 11.99** | <1 | <1 | 28.32** | |
| Group F2,54 | <1 | <1 | <1 | <1 | <1 | <1 | <1 | <1 | |
| Group × Time F2,54 | <1 | <1 | 1.1 | <1 | <1 | 3.70* | <1 | <1 | |
Note: n.s.: p > .1.
p < .05.
p < .01.
SZ participants completed a protocol consisting of (1) assessment of clinical and demographic data, neuropsychological test performance (MATRICS Consortium Cognitive Battery, MCCB, Nuechterlein et al., 2008), and a FAR criterion task during MEG, all prior to intervention, (2) 4-week intervention (FAT, CE, or TAU), and (3) post-intervention assessment of clinical data, MCCB, and the FAR criterion task during MEG. The study was approved by the Ethics Committee of the University of Konstanz and registered as a Clinical Trial (http://ClinicalTrials.gov Registration NCT01781000). Pre-intervention MEG data from 14 of the present SZ were included in Popov et al. (2014), which did not involve training.
In addition to primary analyses comparing these three patient groups, data from two groups of healthy comparison participants (HC), each tested once, were included in targeted analyses. Following a standard protocol, HC were recruited from the community by oral advertisement and flyers and were included if they did not meet the criteria for a lifetime diagnosis of mental illness (screened with Mini International Neuropsychiatric Interview Ackenheil et al., 1999), did not report any history of head trauma with loss of consciousness, and were free of psychoactive medication. The two independent HC groups served different purposes. The HC19 group (n = 19) was recruited when SZ were provided a pre-/post-intervention comparison of oscillatory activity in the FAR outcome criterion task. The HC24 group (n = 24) was recruited after the MEG study ended, in order to provide a normative performance standard for the newly developed FAT training tasks. None of the dependent measures overlapped for the two HC groups (no FAT performance data available for HC19, and no MEG data for HC24).
The HC19 sample included 15 subjects from the HC group reported in Popov et al. (2014). Gender distribution of the HC19 (12 M, 7 F) did not differ from that of the 57 SZ, though the HC19 were younger (27.0 ± 3.7 years vs. 37.2 ± 9.1 years, F(1,74) = 22.18, p < .001) and had more years of education (13 ± 0 vs. 11.2 ± 1.7; F(1,70) = 20.82, p < .001; data on education were not available for 4 SZ). This HC19 group completed the FAR criterion task during MEG in a single session (they did not undergo training). FAR performance data for the HC19 was used twice, to confirm a pre-training abnormality in neural oscillatory activity in the SZ that would undergo FAT and to evaluate the extent of the normalization of this abnormality after this SZ group underwent training.
In the HC24 sample, gender distribution (10 M, 14 F) and education (12.0 vs. 11.7 years) did not differ from that of the 19 SZ participating in FAT, though HC24 were younger (mean age 31.8 ± 5.4 vs. 39.6 ± 7.9 years, F(1,41) = 13.99, p < .001). Comparison of the HC24 data to those from the first training session of the FAT-trained SZ group confirmed a performance deficit on the training tasks in the SZ group. Subsequent comparison of these HC24 data to those from the last training session of the FAT-trained SZ group evaluated the extent to which training normalized performance on the training tasks.
2.2. Interventions
The two active training protocols (FAT and CE) were identical with respect to computer-based exposure: 20 daily 1-hour sessions scheduled on consecutive workdays within 4 weeks, a computer algorithm providing individual adjustment of task difficulty as a function of performance, and motivating task feedback.
FAT involved four tasks, two emphasizing facial affect discrimination, and two emphasizing working memory. Emotional faces were obtained from the KDEF databank (http://www.emotionlab.se/resources/kdef) and included male and female Caucasian faces expressing one of seven emotions (sad, happy, disgusted, fear, surprised, angry, neutral). Within each task, level of difficulty was adjusted to individual performance by increasing difficulty after 6 correct (nonconsecutive) responses or decreasing difficulty after 3 consecutive errors. This algorithm ensured increasing difficulty with improving performance. Performance feedback was provided within session after 6 correct responses per level (the transition to the next level of difficulty) and at the end of each task.
FAT was developed to be comparable to the commercially available cognitive training protocol Cognitive Exercises (CEs, Posit Science, SF, USA) used in Popov et al. (2011, 2012). FAT is similarly based on principles of neuroplasticity according to Merzenich et al. (2014) and Elbert and Rockstroh (2004), such as the value of massed practice, shaping by individual adjustment of task difficulty as a function of performance within each session and task, and frequent motivating reinforcement.
FAT was not developed as a treatment alternative intended to be superior to other social-cognitive training protocols. In the present study, FAT served to examine neural correlates of training targeting facial affect discrimination, which Popov et al. (2014) found to be impaired in schizophrenia patients.
FAT differs from CE principally in that FAT includes a series of visual exercises involving human face expression. In contrast, CE includes auditory as well as visual exercises, none of which focuses on facial expressions. In particular, FAT is meant to differ only in the content of tasks: The same/different task trained the ability to discriminate whether two different posers express the same or different emotions, replacing the discrimination of two syllables/phonemes in the CE protocol. The blended emotion task addressed the identification of a target emotion in morphed faces, which Popov et al. (2014) found to be impaired in schizophrenia patients: in order to train this type of affect discrimination, each face combined two 50/50 morphed facial expressions. The participant was asked to indicate which of the two emotions in an array of seven basic Ekman emotional expressions was morphed in the presented face by clicking on the respective expression in the array of facial pictures. The emotion sequence task trained the reproduction of the sequence of a series of facial affect expressions from a single poser per trial, corresponding to the CE task of reproducing the sequence of a series of syllables/phonemes per trial. In the emotion location task patients learned to recall the location of identical pairs of poser/emotion combinations among an array of hidden faces corresponding to the CE task of recalling the location of identical pairs of syllables/phonemes, which were acoustically presented upon touching the respective cards in an array.
Performance on the four tasks was evaluated as the proportion of correct responses per level of difficulty for each task and each session. Performance change following FAT was evaluated by comparing scores for the first and the last session. The meaning of performance scores varied qualitatively for the different tasks. Therefore, change in performance scores was evaluated separately for each task, using dependent sample t-tests and effect size (Hedges' g). In addition, performance differences prior to training between SZ assigned to FAT and the HC24 group were compared by single-factor analysis of variance (ANOVA) for each task. Similar ANOVAs evaluated SZ performance after FAT against the performance of the untrained HC24 group.
CE consists of four exercises emphasizing auditory verbal discrimination and memory, not involving faces. Description of this training is provided elsewhere (Fisher et al., 2009; Popov et al., 2011).
2.3. Cognitive performance
Neuropsychological test performance was evaluated using the German version (Regents of the University of California, 2006) MCCB (Nuechterlein et al., 2008) that covers domains of cognitive function that have been shown to be impaired in schizophrenia, including processing speed, attentional vigilance, working memory, verbal learning, visual learning, reasoning, and social cognition. Raw scores were converted to T-scores based on a representative U.S.A. community sample of healthy subjects (Nuechterlein et al., 2008; German norms have not been developed). Normal distributions were verified by the Kolmogorov–Smirnov test. Intervention effects on mean T-scores for each MCCB domain were evaluated by a 3 × 2 × 7 ANOVA: the between-subject Intervention factor compared FAT, CE, and TAU groups, the within-subject Time factor compared pre- and post-training measurements, and the within-subject Domain factor compared the seven cognitive domains.
2.4. MEG data collection
Neuromagnetic activity before and after training was assessed in a dynamic FAR task that was developed and evaluated by Popov et al. (2013, 2014). These studies showed that modulation of oscillatory activity primarily in the alpha frequency range varies with facial affect recognition and that this alpha modulation is weaker in SZ than in HC. The present study used the same MEG data collection and analysis protocol, concentrating on intervention effects on alpha modulation. Eighty videos, 40 morphing from neutral to fear (NF) and 40 from neutral to happy (NH), were presented on a screen about 50 cm from the eyes. Participants were instructed to view the videos passively. Forty pictures of Caucasian individuals (20 males, 20 females, Radboud Faces Database Langner et al., 2010) showing fear, neutral, and happy expressions were selected. For each poser, two videos of the transition from neutral to emotional expression (one fear, one happy) were created. (Data from a third video, providing a transition from one poser's neutral expression to another poser's neutral expression, are not reported here.) Each video was presented for 5 s at 15 frames per second. The first second of each video was a static picture of a neutral expression, after which the image gradually morphed toward the target facial expression (fear or happy emotion). The face changed across the middle 3 s of the 5 s video. 33%, 67%, and 100% of the target expression was reached at the end of the second, third, and fourth seconds (detailed description in Popov et al., 2013, 2014). The videos were presented in a pseudo-random order with a jittered offset to onset inter-trial-interval of 5 ± 1 s and a white fixation cross in the center of the screen between videos.
MEG was recorded with a 148-channel whole-cortex magnetometer (MAGNES 2500 WH, 4D Neuroimaging, San Diego, USA) in a magnetically shielded room while participants lay on their back. Prior to each measurement, the nasion, inion, Cz, left and right ear canal, and head shape were digitized with a Polhemus 3Space Fasttrack. Subjects were instructed to passively watch the videos and avoid body movements. The continuous MEG time series was recorded with a sampling rate of 678.17 Hz and a 0.1–200 Hz bandpass filter. Trials consisted of epochs from 3 s before to 7 s after the onset of each video. Data analyses comprised (a) segmentation (time periods of 3 s before to 7 s after video onset) and artifact removal of the continuously recorded time-series, (b) single-trial time–frequency analysis of power and subsequent average over trials, (c) statistical evaluation of group differences focused on the alpha (10–15 Hz) frequency range based on a randomization approach, and (d) evaluation of potential Time (pre/post-intervention) by Emotion (fearful, happy) effects for regions of interest (ROIs, significant sensor clusters).
2.4.1. Data screening and segmentation
Prior to correcting for heart and eye-blink artifact by means of independent component analysis (ICA, Jung et al., 2001), trials containing movement artifacts and SQUID jumps were rejected based on visual inspection: topographies and time courses of ICA components were screened for signals of eye (blink and horizontal eye movements) and heart-beat-related activity, which were removed from further analysis. Across the 12 group × emotion × session cells, the average number of trials per subject retained for analyses ranged from 37.9 to 39.1 of the total of 40 trials per emotion, without significant differences between intervention groups in the number of trials retained for the two emotions (fear and happy) for either pre- or post-intervention measurement (main effects and interactions F < 1). Offline MEG processing was accomplished primarily with the MATLAB-based open-source signal processing toolbox Fieldtrip (Oostenveld et al., 2011) complemented by in-house MATLAB code.
2.4.2. MEG time–frequency analysis
Spectral analysis was computed for each sensor and each trial using a sliding time window of five consecutive cycles (Δt = 5 / f) multiplied by a Hanning taper. Resulting power estimates were averaged over trials within condition and participant. Time–frequency representations of power were calculated as the log of the ratio of the power in a given time–frequency bin to the power at that frequency during the 3 s prestimulus baseline for each emotion (happy and fear), thus decibels (dB). These power values were analyzed as 4D clusters, latency × frequency × 2D sensor position on the scalp, with dB being the entry in each cell. Time–frequency windows showing relevant effects were defined in this space using a cluster-based, independent-sample t-test with Monte Carlo randomization. This procedure effectively controls for multiple comparisons (Maris and Oostenveld, 2007) and allows the identification of clusters with significant group differences in 4D computational space. A cluster was determined to contain at least 5 neighboring sensors from 1000 randomizations for time–frequency data. The test statistic was defined as the sum of the t-statistics within the respective 3D cluster. Empirically observed clusters were labeled as statistically significant if the probability of clusters gained from permutation being larger did not exceed 5%.
2.4.3. Statistical analysis
Comparison prior to intervention of the 57 SZ with the HC19 who completed the same FAR criterion task checked for a possible pre-intervention neural abnormality in alpha power during the pre-recognition period of the FAR criterion task, via a Diagnosis (SZ, HC) × Emotion (fear, happy) ANOVA. This ANOVA was based on the respective ROIs obtained after the cluster-based approach described above. Effect sizes were characterized using Hedges' g.
2.4.4. Evaluation of intervention effects
Effects of the interventions on alpha power during the pre-recognition period of the FAR criterion task were examined with ANOVAs addressing a series of a priori questions: (1) Does FAT have an impact on brain dynamics: Emotion × Time in FAT group. (2) Does FAT normalize brain dynamics: Group (FAT pre-intervention or FAT post-intervention, respectively, vs. HC19) × Emotion. (3) Does FAT do so better than treatment as usual: Intervention (FAT, TAU) × Emotion × Time. (4) Is the impact of FAT specific: Intervention (FAT, CE) × Emotion × Time. Because score distributions were somewhat nonnormal, primary analyses relied on Winsorized data (with a 5%/95% threshold; p-values for non-Winsorized data are provided in parentheses).
Relationships between FAT-induced changes in alpha activity measures of the FAR criterion task and FAT-task performance were examined via Pearson product-moment correlations.
Reported p-values reflect Huynh–Feldt (HF) adjustment as appropriate, and significant main effects and interactions were decomposed with simple-effects ANOVAs or t-tests.
3. Results
Clinical status improved in all three SZ groups independent of type of intervention: general function (GAF F(2,53) = 16.92, p < .001), positive symptoms (PANSS-P F(2,53) = 17.92, p < .001), and general symptoms (PANSS-G F(2,53) = 10.30, p < .001; PANSS-N n.s.). There were no effects involving Intervention.
3.1. MEG during FAR criterion task prior to intervention
Fig. 2 illustrates time–frequency representation (TFR) of power during face morphing prior to intervention. The HC19 group TFR showed an increase from baseline in the 10–13 Hz range between 1 s (morph onset) and 3.5 s after stimulus onset, with maximum around 3 s. This increase was not evident in any SZ group. Statistical comparisons (Fig. 2, bottom) confirmed significant differences between HC19 and pre-intervention SZ in alpha-power increase over a central sensor cluster in the time course from 2.5 to 3.5 s after stimulus onset.
Fig. 2.
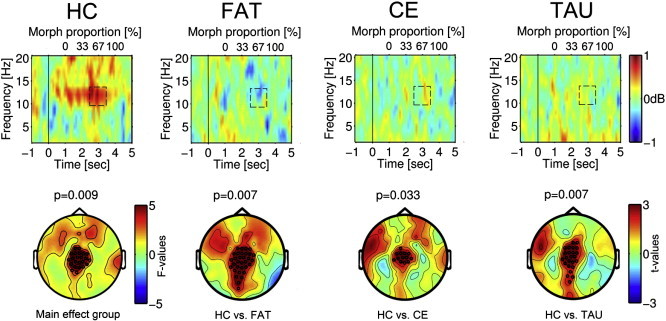
Top: Time–frequency representation of power during the 5 s video of the facial affect recognition criterion task for schizophrenia patients (SZ) prior to training and for the HC19 healthy comparison group. The video started at 0 s with presentation of a static neutral face for 1 s, which changed to an emotional expression across seconds 2–4. The face stimulus was static during the 5th sec. Change in power relative to 1 s baseline before video onset (lower abscissa: −1 to 0) is expressed in dB and indicated by color change, with warm colors representing an increase and cold colors a decrease from prestimulus baseline. The upper abscissa reflects the percentage of emotion expressed at the time indicated on the lower abscissa. In top row illustrations, time–frequency representations (TFRs) of power are averaged across fear and happy conditions. TFRs are presented separately for groups (HC19, healthy controls; FAT: SZ assigned to facial affect training; CE: SZ assigned to cognitive exercises, CE; TAU: SZ assigned to treatment as usual). Bottom: Topographic representation of statistical group differences illustrating HC vs. all SZ patients, and HC vs. each SZ group. Black circles indicate MEG sensors belonging to significant clusters (details on cluster definition in methods). Color bars reflect F- (HC vs. all SZ) or t-values (HC vs. each patient group), with warm colors indicating larger power increase in HC than in the pooled SZ group (group, F > 3, p < .01) and larger power increase in HC than in each SZ group (t > 2, p < .01).
FAT task performance was poorer in SZ during their first FAT session than in the HC24 group during their one FAT session (same-different task F(1,41) = 7.69, p = .008; blended emotion task F(1,41) = 8.24, p = .006; emotion sequence task F(1,41) = 16.11, p < .001; emotion location task F(1,41) = 16.19, p < .001; Fig. 3A). Performance improved with training on all four tasks in the FAT group, significantly in three tasks (Fig. 3B, left): same/different, F(1,18) = 4.99, p = .04; blended emotion F(1,18) = 10.17, p = .005; emotion sequence F(1,18) = 3.28, p = .09; emotion location F(1,18) = 13.12, p = .002. In the last FAT training session, SZ no longer differed significantly from HC24 on three of the four tasks. SZ still performed worse in the emotion location task (F(1,41) = 6.72, p = .01; see also effect sizes in Fig. 3B, right).
Fig. 3.
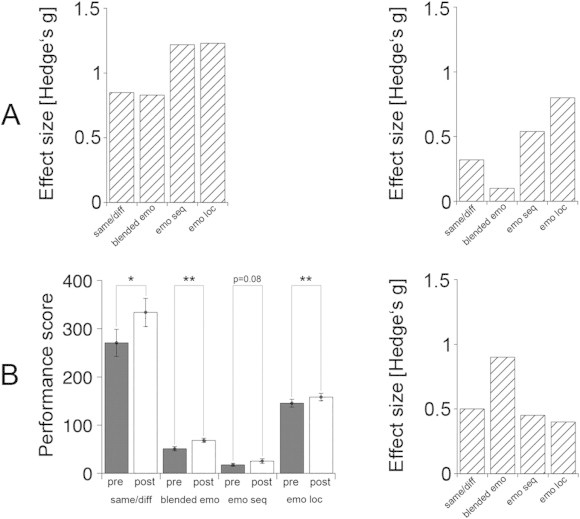
A: Performance differences on the four FAT tasks between schizophrenia groups (SZ) and the HC24 healthy comparison group expressed as effect sizes (Hedges' g). Left: hatched bars illustrate effect sizes (group differences) for SZ's first FAT session and HC24's only FAT session. Right: hatched bars illustrate effect sizes (group differences) at the end of the intervention period for SZ's last FAT session and HC24's only FAT session. B: Left: performance scores (ordinate, with higher score indicating better performance) per task (abscissa: same/different, blended emotion task, emotion sequence, emotion location task; see text for description of each task) plotted separately for pre-training (dark gray bars) and post-training (hatched bars) in the FAT group. Scores represent mean ± 1.0 SE. *p < 0.05. **p < 0.01. Right: performance difference post-training relative to pre-training expressed as effect sizes (Hedges' g) per task, bars indicating better performance after the 20-session training period.
3.2. FAT impact on MEG during FAR criterion task
An Emotion × Time analysis of the FAT group produced a main effect of Time, F(1,18) = 5.61, p = .03 (p = .02), reflecting a greater increase in alpha power from prestimulus baseline during the pre-recognition period of the FAR criterion task after training than before training (Fig. 4, left). Here and below, no effects involving Emotion emerged.
Fig. 4.
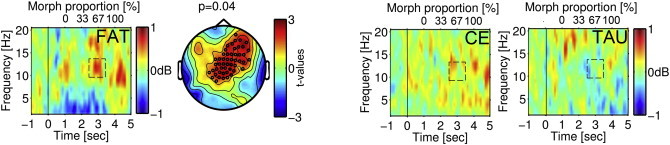
Time–frequency representation of power during the 5 s video presented as described in Fig. 3, here illustrating the difference post- minus pre-training for the fear condition for the three SZ groups. Dashed rectangles indicate the time window (2.5–3.5 s) of difference in 10–15 Hz activity as a function of training, with warm colors indicating more alpha power increase. The significant training effect in the FAT group is illustrated in the topographic representation of a fronto-central sensor cluster (black circles). Statistical effects are represented by color-coded t-values, with warm colors indicating more alpha power increase.
Pre-training, a Group (FAT pre-training vs. HC19) × Emotion analysis indicated smaller alpha power increase in SZ than in HC19 (F(1,36) = 22.05, p < .001; Fig. 3). A similar comparison of FAT post-training vs. HC19 verified this effect (Group F(1,36) = 5.73, p = .02). Effect sizes indicated larger differences in alpha power modulation prior to FAT (fearful condition g = 1.29, happy condition g = 1.08) than after FAT (fearful condition g = .66, happy condition g = .58). Thus, dysfunctional oscillatory dynamics can be improved by FAT, though not to full normalization.
3.3. Specificity of intervention effects
Fig. 4 illustrates the change in alpha power increase after intervention in the three patient groups. An Intervention (FAT vs. TAU) × Emotion × Time analysis produced a marginal effect of Time, F(1,36) = 2.80, p = .1 (p > .1), reflecting some general improvement over time in the pooled sample, and an Intervention × Time interaction, F(1,36) = 4.78, p = .04 (p = .05), reflecting improvement in the FAT group (Fig. 4, left) vs. a nonsignificant decline in the TAU group (Fig. 4, right). Thus, changes in alpha power modulation were specific to training. Here and below, no main effects of Intervention emerged. That was as anticipated, given random assignment to group, with group variance therefore expected only in Intervention × Time effects.
The final a priori ANOVA assessed whether this improvement in alpha response with active training was specific to FAT. An Intervention (FAT vs. CE) × Emotion × Time analysis produced an effect of Time, F(1,36) = 8.54, p = .006 (p = .008), reflecting improvement with training, but no Intervention × Time interaction, indicating similar improvement in the two active treatment groups (Fig. 4).
3.4. Omnibus test
An omnibus Intervention (FAT, CE, TAU) × Emotion × Time analysis is of some value as a control for experiment-wise error. Because the primary relevant hypothesis such an analysis addresses, that intervention improves brain function, is directional, a one-tailed test of the Time effect would be appropriate, or equivalently a p = .10 criterion for the ANOVA effect. The Time effect exceeded that criterion, F(1,54) = 4.81, p = .03 (p = .06), with alpha power increase from prestimulus baseline during the pre-recognition period of the FAR criterion task larger after training than before training. A secondary hypothesis that an omnibus test could address is that interventions differed in their impact, thus a nondirectional Intervention × Time effect, which was marginal, F(2,54) = 2.44, p < .10 (p = .10). However, the motivation for the present study was the series of specific, directional, a priori hypotheses provided above, which, to achieve partial experiment-wise error protection, were tested with a two-tailed criterion.
3.5. Relationship of FAT performance and alpha activity on the FAR criterion task
In the FAT group, alpha power increase in a left fronto-central sensor cluster (Fig. 5 left panel) varied with performance improvement during the blended emotion task (r = .46, p < .05; Fig. 5 right panel).
Fig. 5.
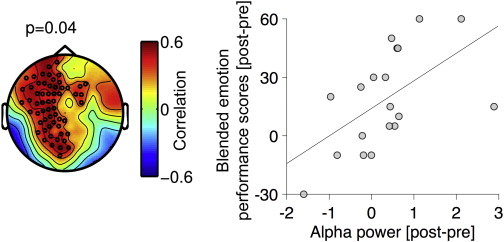
Relationship between change in performance scores on the blended emotion task (last training session minus first training session) and change in alpha power increase in the FAR criterion task (post- minus pre-FAT-training) in the FAT group. Left: topographic representation of significant (p = .04) relationship between change in performance scores and change in alpha power increase in a fronto-central ROI. The relationship is represented by color-coded correlation coefficients (for r > .5, p < .05), with warm colors indicating higher correlation. Black circles indicate MEG-sensors belonging to the fronto-central ROI of significant correlation. This ROI was used to compute alpha power scores plotted in the right panel. Right: relationship between change in performance and change in alpha power modulation in the FAT group, each circle representing an individual subject.
3.6. Neuropsychological test performance
As expected in an SZ sample (e.g., Kern et al., 2008, 2011), patient performance was below the normative T-score of 50 and varied by MCCB Domain (F(6,324) = 12.83, p < .001, HF = .94). MCCB performance generally improved after intervention (Time F(1,54) = 28.45, p < .001). A Domain × Time interaction (F(6,324) = 4.05, p = .001, HF = .91) reflected improvement in Processing Speed (F(1,56) = 33.56, p < .01), Attentional Vigilance (F(1,56) = 15.82, p < .01), and Visual Learning (F(1,56) = 11.99, p = .001) but not in Working Memory, Verbal Learning, Reasoning, or Social Cognition (see Table 2 for normative T-scores). There was a Time × Intervention effect, F(2,54) = 3.70, p = .03, resulting from significant performance improvement on Reasoning after CE, t(18) = 2.06, p = .05, not after FAT or TAU. There was no Time × Intervention × Domain effect (F(12, 324) = 1.28, p > .1). Thus, these improvements did not depend on type of treatment. Change in alpha power increase from baseline between pre- and post-intervention was not related to changes in neuropsychological test performance (MCCB) or in clinical status (symptom severity, GAF).
4. Discussion
Popov et al. (2013) identified induced alpha power modulation as a measure that varied with performance during a dynamic facial affect recognition task. This measure was proposed as indicative of neural processes involved in affect recognition. Schizophrenia patients exhibited less induced alpha power modulation than healthy subjects, corresponding to poorer behavioral performance (Popov et al., 2014). The present study addressed the power and specificity of a computer-aided intervention targeting facial affect discrimination to ameliorate this deficit in schizophrenia patients.
Present results confirmed that deficient alpha modulation can be modified by psychological intervention: poor pre-intervention alpha power modulation during the FAR criterion task improved after training in facial affect discrimination. Although neither performance on the training tasks nor alpha dynamics on the FAR criterion task reached normal levels, findings indicate that relevant brain dynamics in SZ have considerable plasticity. Similar training-induced neuroplasticity has been shown in other studies involving cognitive training (Adcock et al., 2009; Edwards et al., 2010; Subramaniam et al., 2012). Kelly and Garavan (2005) proposed principles of redistribution and reorganization as potential mechanisms of neuroplasticity. Redistribution refers to a change in activation within neural networks as a function of practice, without altering neural network structure. In contrast, reorganization is thought to reflect remodeling of structure and of functional activity related to task performance in the course of practice. Normalization via reorganization would be expected if training adequately addresses the neurobiological mechanisms of cognitive deficiency.
These mechanisms and their dysfunction in schizophrenia are still not fully understood. Present results support the conclusion that the observed changes in alpha power as a function of training result from redistribution of neuronal processing resources that support facial affect discrimination. Of course, additional changes may have resulted that the present MEG measures did not detect.
Changes in brain dynamics were larger after the two dense, computer-aided training procedures than after a treatment-as-usual program. In a meta-analysis Genevsky and colleagues (2010) concluded that, rather than fostering broad skills, efficient remediation should involve targeted, computer-aided with high-dose schedules, supplemented by psychosocial intervention. The present FAT and CE training protocols provided the first two elements, i.e., targeted, high-dose computer training, relative to TAU. Moreover, both specific training protocols targeted functions that are dysfunctional in schizophrenia such as working memory, attention, or discrimination accuracy. This is in line with the success of the Training of Affect Recognition (TAR) protocol developed by Wölwer and colleagues (Wölwer et al., 2005; Wölwer and Frommann, 2011; Sachs et al., 2012; Luckhaus et al., 2013), which includes a specific facial affect discrimination task within a broader spectrum of tasks. TAR improved social cognition indices and increased activity in various brain regions related to attention and cognitive processing. Sacks et al. (2013) combined CE with specific social cognition training and found improvements on social cognitive outcome measures in SZ.
Training protocols targeting functions known to be dysfunctional in SZ may be beneficial whether applied alone or within a broader spectrum of tasks. In the present study, training effects were not confined to FAT. The FAT and CE training protocols were similar in various ways: both targeted neuroplasticity by intensity (20 consecutive 1-hour sessions), shaping (performance-based adjustment of task demands), and motivational context (tasks embedded in computer game, frequent motivating feedback); both addressed working memory and visual learning. It is conceivable that these training protocols, though designed to target specific processes, modify more basic, general processes such as neural information sampling, readiness for information intake, and excitation–inhibition and segregation–integration balance, thus general computation mechanisms supporting diverse functions (Buzsaki, 2010; Buzsaki and Watson, 2012; Buzsaki et al., 2013). FAT may have improved item discrimination and memory in general, not only facial affect recognition. Within present data, nonspecific enhancement of visual learning did not extend to (unchanged) performance on the visual-learning domain of the MCCB. In order to distinguish specific and nonspecific effects, FAT effects on other tasks should be examined.
An effect of the specific facial affect training on the social cognition MCCB domain, examined with the MSCEIT (Mayer et al., 2003), might have been expected. Sacks et al. (2013; see also Fisher et al., 2013) reported improvement on the MSCEIT after training that combined cognitive and social cognitive elements. Similarly, the Training of Affect Recognition protocol (Wölwer and Frommann, 2011) provides various strategies for affect management and regulation. Improvement in such skills may be better reflected in MSCEIT than the effects of the present training that specifically addressed facial affect discrimination and working memory. The MSCEIT — “managing emotions” subtest, used in the measures is a multifaceted construct and may not be suitable to test effects of the more specific functions (Dawson et al., 2012) targeted by, i.e., facial affect discrimination accuracy and working memory. The extent to which modulation of brain dynamics can be directly related to complex constructs such as social cognition as measured by neuropsychological tests is a matter of debate (Miller, 2010).
For neuroplasticity-oriented training of fundamental signal discrimination, Merzenich et al. (2014) anticipated an impact of training-enhanced basic processes on higher-order cognitive processes. Similar effects might have been expected for FAT in the present study. Additional dependent measures (both specific and general) would be needed to clarify the impact of FAT on social cognitive functions in SZ. It is also possible that the present 20-hour training was not intense enough to achieve more extended effects. For example, effects of intense cognitive training on social cognitive measures and global function were prominent after 50 training sessions (Fisher et al., 2013, 2014).
Relevant to present hypotheses, training-induced change in performance on FAT varied with improvement in alpha response. The parallel change in and the correlation between FAT task performance and alpha power modulation suggest a functional relationship, thus alpha power increase as a mechanism facilitating facial affect recognition. The effect of FAT on alpha power modulation in the FAR criterion task (a) supports the hypothesis (Popov et al., 2013) that alpha power increase contributes to facial affect recognition and (b) demonstrates that a potential impairment of this mechanism can be modified by targeted training.
Conclusions from the present study are limited by several factors. First, results indicated that targeted training can affect neuronal processes believed to contribute to facial affect recognition and shown to be compromised in SZ. However, conclusions about specificity of training effects would require further evaluation of effects of different types of training on diverse tasks, for example identifying double dissociations. Moreover, specific vs. more general effects of FAT on social cognition were not comprehensively examined: performance on the FAT tasks was not assessed in the CE and TAU groups, and as noted above the MCCB social cognition domain may not appropriately interrogate the effects of specific, targeted training (FAT) on higher-order social cognitive functions. A broader range of tests of social cognitive function would be necessary to fully evaluate the specificity of training effects.
Second, the present study focused on alpha-power modulation based on the previous finding of specific modulation in the 10–15 Hz range during the process of affect recognition. Whereas SZ differed from HC in this measure, no difference was observed in other measures such as the event-related potential to face presentation, specifically the M170 component (Popov et al., 2014). This result, together with the lack of training-related changes in lower or higher frequency bands in the evaluated range (0–20 Hz, see Fig. 4), supports the proposal of alpha activity as a mechanism supporting facial recognition (Popov et al., 2013). Nevertheless, the contribution of other neuronal processes to facial affect recognition and an impact of the present training protocols on other neuronal phenomena, potentially evident in evoked high-frequency activity or event-related magnetic field variation, cannot be ruled out.
Third, conclusions are always limited by the statistical power provided by the sample size, a common challenge in clinical settings (Keefe et al., 2013).
Fourth, the two groups of healthy comparison subjects were younger than the patients. HC19 served to confirm dysfunctional alpha power modulation for the present SZ samples, as had been shown in larger, well matched samples in Popov et al. (2014). Although a matched HC group for the entire group of 57 trained SZ would have been of some interest, the present HC19 sufficed for evaluating this.
Taken together, present findings for an intervention targeting abnormal brain dynamics associated with deficient facial affect recognition in schizophrenia indicate considerable neuroplastic potential in schizophrenia. The intervention effects support the hypothesis of a neural mechanism facilitating facial affect recognition and its disruption in schizophrenia. The accessibility of this phenomenon to intervention may facilitate the development of rehabilitation strategies.
Acknowledgement
Research was supported by the Deutsche Forschungsgemeinschaft (Ro805/14-2). The authors report no conflict of interest and thank Nathan Weisz for advice on data analyses and Ursel Lommen, David Schubring, V. Hirt, A. Mühlherr, J. Kienle, M. Rack, M. Bühler, J.R. Hansen and U. Kohler for assistance in data collection and analysis. We also thank Dr. M. Odenwald, MD K. Pröpster, Dr. I. Schalinski and A. Schawohl and M. Widmann for assignment and diagnosing patients.
References
- Ackenheil M., Stotz-Ingenlath G., Dietz-Bauer R., Vossen A. M.I.N.I. Mini International Neuropsychiatric Interview, German. Version 5.0.0 DSM IV. Psychiatrische Universitätsklinik; München: 1999. [Google Scholar]
- Adcock R.A., Dale C., Fisher M., Aldebot S., Genevsky A., Simpson G.V., Nagarajan S., Vinogradov S. When top-down meets bottom-up: auditory training enhances verbal memory in schizophrenia. Schizophrenia Bulletin. 2009;35:1132–1141. doi: 10.1093/schbul/sbp068. 19745022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G. Neural syntax: cell assemblies, synapsembles, and readers. Neuron. 2010;68:362–385. doi: 10.1016/j.neuron.2010.09.023. 21040841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G., Logothetis N., Singer W. Scaling brain size, keeping timing: evolutionary preservation of brain rhythms. Neuron. 2013;80:751–764. doi: 10.1016/j.neuron.2013.10.002. 24183025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G., Watson B.O. Brain rhythms and neural syntax: implications for efficient coding of cognitive content and neuropsychiatric disease. Dialogues in Clinical Neuroscience. 2012;14:345–367. doi: 10.31887/DCNS.2012.14.4/gbuzsaki. 23393413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson S., Kettler L., Burton C., Galletly C. Do people with schizophrenia lack emotional intelligence? Schizophrenia Research and Treatment. 2012;2012 doi: 10.1155/2012/495174. 23304499 Article ID 495174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards B.G., Barch D.M., Braver T.S. Improving prefrontal cortex function in schizophrenia through focused training of cognitive control. Frontiers in Human Neuroscience. 2010;4:32. doi: 10.3389/fnhum.2010.00032. 20461148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbert T., Rockstroh B. Reorganization of human cerebral cortex: the range of changes following use and injury. Neuroscientist: A Review Journal Bringing Neurobiology, Neurology and Psychiatry. 2004;10:129–141. doi: 10.1177/1073858403262111. 15070487 [DOI] [PubMed] [Google Scholar]
- Fisher M., Holland C., Merzenich M.M., Vinogradov S. Using neuroplasticity-based auditory training to improve verbal memory in schizophrenia. American Journal of Psychiatry. 2009;166:805–811. doi: 10.1176/appi.ajp.2009.08050757. 19448187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M., Subramaniam K., Panizzutti R., Vinogradov S. Cognitive Impairment in Schizophrenia: Characteristics, Assessment and Treatment. Cambridge University Press; Cambridge: 2013. pp. 284–316. [Google Scholar]
- Fisher M., Loewy R., Carter C., Lee A., Ragland J.D., Niendam T., Schlosser D., Pham L., Miskovich T., Vinogradov S. Neuroplasticity-based auditory training via laptop computer improves cognition in young individuals with recent onset schizophrenia. Schizophrenia Bulletin. 2014 doi: 10.1093/schbul/sbt232. 24444862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genevsky A., Garrett C.T., Alexander P.P., Vinogradov S. Cognitive training in schizophrenia: a neuroscience-based approach. Dialogues in Clinical Neuroscience. 2010;12:416–421. doi: 10.31887/DCNS.2010.12.3/agenevsky. 20954435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habel U., Koch K., Kellermann T., Reske M., Frommann N., Wölwer W., Zilles K., Shah N.J., Schneider F. Training of affect recognition in schizophrenia: neurobiological correlates. Social Neuroscience. 2010;5:92–104. doi: 10.1080/17470910903170269. 19821187 [DOI] [PubMed] [Google Scholar]
- Hofer A., Benecke C., Edlinger M., Huber R., Kemmler G., Rettenbacher M.A., Schleich G., Wolfgang Fleischhacker W. Facial emotion recognition and its relationship to symptomatic, subjective, and functional outcomes in outpatients with chronic schizophrenia. European Psychiatry: the Journal of the Association of European Psychiatrists. 2009;24:27–32. doi: 10.1016/j.eurpsy.2008.06.008. 18774270 [DOI] [PubMed] [Google Scholar]
- Johnston P.J., Enticott P.G., Mayes A.K., Hoy K.E., Herring S.E., Fitzgerald P.B. Symptom correlates of static and dynamic facial affect processing in schizophrenia: evidence of a double dissociation? Schizophrenia Bulletin. 2010;36:680–687. doi: 10.1093/schbul/sbn136. 18953071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung T.P., Makeig S., McKeown M.J., Bell A.J., Lee T.W., Sejnowski T.J. Imaging brain dynamics using independent component analysis. Proceedings of the IEEE. Institute of Electrical and Electronics Engineers. 2001;89:1107–1122. doi: 10.1109/5.939827. 20824156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay S.R., Fiszbein A., Opler L.A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia Bulletin. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. 3616518 [DOI] [PubMed] [Google Scholar]
- Keefe R.S., Harvey P.D. Cognitive impairment in schizophrenia. Handbook of Experimental Pharmacology. 2012:11–37. doi: 10.1007/978-3-642-25758-2_2. 23027411 [DOI] [PubMed] [Google Scholar]
- Keefe R.S., Buchanan R.W., Marder S.R., Schooler N.R., Dugar A., Zivkov M., Stewart M. Clinical trials of potential cognitive-enhancing drugs in schizophrenia: what have we learned so far? Schizophrenia Bulletin. 2013;39(2):417–435. doi: 10.1093/schbul/sbr153. 22114098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly A.M., Garavan H. Human functional neuroimaging of brain changes associated with practice. Cerebral Cortex (New York, N.Y.: 1991) 2005;15:1089–1102. doi: 10.1093/cercor/bhi005. 15616134 [DOI] [PubMed] [Google Scholar]
- Kern R.S., Gold J.M., Dickinson D., Green M.F., Nuechterlein K.H., Baade L.E., Keefe R.S., Mesholam-Gately R.I., Seidman L.J., Lee C., Sugar C.A., Marder S.R. The MCCB impairment profile for schizophrenia outpatients: results from the MATRICS psychometric and standardization study. Schizophrenia Research. 2011;126:124–131. doi: 10.1016/j.schres.2010.11.008. 21159492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern R.S., Nuechterlein K.H., Green M.F., Baade L.E., Fenton W.S., Gold J.M., Keefe R.S., Mesholam-Gately R., Mintz J., Seidman L.J., Stover E., Marder S.R. The MATRICS consensus cognitive battery, part 2: co-norming and standardization. American Journal of Psychiatry. 2008;165:214–220. doi: 10.1176/appi.ajp.2007.07010043. 18172018 [DOI] [PubMed] [Google Scholar]
- Kurtz M.M., Richardson C.L. Social cognitive training for schizophrenia: a meta-analytic investigation of controlled research. Schizophrenia Bulletin. 2012;38:1092–1104. doi: 10.1093/schbul/sbr036. 21525166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langner O., Dotsch R., Bijlstra G., Wigboldus D.H.J., Hawk S.T., van Knippenberg A. Presentation and validation of the Radboud Faces Database. Cognition & Emotion. 2010;24:1377–1388. [Google Scholar]
- Li H., Chan R.C., McAlonan G.M., Gong Q.Y. Facial emotion processing in schizophrenia: a meta-analysis of functional neuroimaging data. Schizophrenia Bulletin. 2010;36:1029–1039. doi: 10.1093/schbul/sbn190. 19336391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckhaus C., Frommann N., Stroth S., Brinkmeyer J., Wölwer W. Training of affect recognition in schizophrenia patients with violent offences: behavioral treatment effects and electrophysiological correlates. Social Neuroscience. 2013;8:505–514. doi: 10.1080/17470919.2013.820667. 23879268 [DOI] [PubMed] [Google Scholar]
- Maris E., Oostenveld R. Nonparametric statistical testing of EEG- and MEG-data. Journal of Neuroscience Methods. 2007;164:177–190. doi: 10.1016/j.jneumeth.2007.03.024. 17517438 [DOI] [PubMed] [Google Scholar]
- Mayer J.D., Salovey P., Caruso D.R., Sitarenios G. Measuring emotional intelligence with the MSCEIT. Emotion. 2003;2:97–105. doi: 10.1037/1528-3542.3.1.97. [DOI] [PubMed] [Google Scholar]
- Mazza M., Lucci G., Pacitti F., Pino M.C., Mariano M., Casacchia M., Roncone R. Could schizophrenic subjects improve their social cognition abilities only with observation and imitation of social situations? Neuropsychological Rehabilitation. 2010;20:675–703. doi: 10.1080/09602011.2010.486284. 20714969 [DOI] [PubMed] [Google Scholar]
- Merzenich M.M., Van Vleet T.M., Nahum M. Brain plasticity-based therapeutics. Frontiers in Human Neuroscience. 2014;8:385. doi: 10.3389/fnhum.2014.00385. 25018719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G.A. Mistreating psychology in the decades of the brain. Perspectives on Psychological Science: A Journal of the Association for Psychological Science. 2010;5:716–743. doi: 10.1177/1745691610388774. 21949539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuechterlein K.H., Green M.F., Kern R.S., Baade L.E., Barch D.M., Cohen J.D., Essock S., Fenton W.S., Frese F.J., 3rd, Gold J.M., Goldberg T., Heaton R.K., Keefe R.S., Kraemer H., Mesholam-Gately R., Seidman L.J., Stover E., Weinberger D.R., Young A.S., Zalcman S., Marder S.R. The MATRICS consensus cognitive battery, part 1: test selection, reliability, and validity. American Journal of Psychiatry. 2008;165:203–213. doi: 10.1176/appi.ajp.2007.07010042. 18172019 [DOI] [PubMed] [Google Scholar]
- Oldfield R.C. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. 5146491 [DOI] [PubMed] [Google Scholar]
- Oostenveld R., Fries P., Maris E., Schoffelen J.M. Computational Intelligence and Neuroscience. Vol. 2011. 2011. 2011. p. 156869.21253357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkham A.E., Gur R.E., Gur R.C. Affect recognition deficits in schizophrenia: neural substrates and psychopharmacological implications. Expert Review of Neurotherapeutics. 2007;7:807–816. doi: 10.1586/14737175.7.7.807. 17610388 [DOI] [PubMed] [Google Scholar]
- Poole J.H., Tobias F.C., Vinogradov S. The functional relevance of affect recognition errors in schizophrenia. Journal of the International Neuropsychological Society: JINS. 2000;6:649–658. doi: 10.1017/s135561770066602x. 11011511 [DOI] [PubMed] [Google Scholar]
- Popov T., Jordanov T., Rockstroh B., Elbert T., Merzenich M.M., Miller G.A. Specific cognitive training normalizes auditory sensory gating in schizophrenia: a randomized trial. Biological Psychiatry. 2011;69:465–471. doi: 10.1016/j.biopsych.2010.09.028. 21092939 [DOI] [PubMed] [Google Scholar]
- Popov T., Miller G.A., Rockstroh B., Weisz N. Modulation of alpha power and functional connectivity during facial affect recognition. Journal of Neuroscience: the Official Journal of the Society for Neuroscience. 2013;33:6018–6026. doi: 10.1523/JNEUROSCI.2763-12.2013. 23554483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popov T., Rockstroh B., Weisz N., Elbert T., Miller G.A. Adjusting brain dynamics in schizophrenia by means of perceptual and cognitive training. PloS One. 2012;7:e39051. doi: 10.1371/journal.pone.0039051. 22815697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popov T.G., Rockstroh B.S., Popova P., Carolus A.M., Miller G.A. Dynamics of alpha oscillations elucidate facial affect recognition in schizophrenia. Cognitive, Affective & Behavioral Neuroscience. 2014;14:364–377. doi: 10.3758/s13415-013-0194-2. 23943514 [DOI] [PubMed] [Google Scholar]
- Sachs G., Steger-Wuchse D., Kryspin-Exner I., Gur R.C., Katschnig H. Facial recognition deficits and cognition in schizophrenia. Schizophrenia Research. 2004;68:27–35. doi: 10.1016/S0920-9964(03)00131-2. 15037337 [DOI] [PubMed] [Google Scholar]
- Sachs G., Winklbaur B., Jagsch R., Lasser I., Kryspin-Exner I., Frommann N., Wölwer W. Training of affect recognition (TAR) in schizophrenia — impact on functional outcome. Schizophrenia Research. 2012;138:262–267. doi: 10.1016/j.schres.2012.03.005. 22464728 [DOI] [PubMed] [Google Scholar]
- Sacks S., Fisher M., Garrett C., Alexander P., Holland C., Rose D., Hooker C., Vinogradov S. Combining computerized social cognitive training with neuroplasticity-based auditory training in schizophrenia. Clinical Schizophrenia & Related Psychoses. 2013;7:78–86A. doi: 10.3371/CSRP.SAFI.012513. 23367504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiferth N.Y., Pauly K., Kellermann T., Shah N.J., Ott G., Herpertz-Dahlmann B., Kircher T., Schneider F., Habel U. Neuronal correlates of facial emotion discrimination in early onset schizophrenia. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2009;34:477–487. doi: 10.1038/npp.2008.93. 18580874 [DOI] [PubMed] [Google Scholar]
- Singh F., Pineda J., Cadenhead K.S. Association of impaired EEG mu wave suppression, negative symptoms and social functioning in biological motion processing in first episode of psychosis. Schizophrenia Research. 2011;130:182–186. doi: 10.1016/j.schres.2011.04.004. 21549567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramaniam K., Luks T.L., Fisher M., Simpson G.V., Nagarajan S., Vinogradov S. Computerized cognitive training restores neural activity within the reality monitoring network in schizophrenia. Neuron. 2012;73:842–853. doi: 10.1016/j.neuron.2011.12.024. 22365555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turetsky B.I., Kohler C.G., Indersmitten T., Bhati M.T., Charbonnier D., Gur R.C. Facial emotion recognition in schizophrenia: when and why does it go awry? Schizophrenia Research. 2007;94:253–263. doi: 10.1016/j.schres.2007.05.001. 17583481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wölwer W., Brinkmeyer J., Stroth S., Streit M., Bechdolf A., Ruhrmann S., Wagner M., Gaebel W. Neurophysiological correlates of impaired facial affect recognition in individuals at risk for schizophrenia. Schizophrenia Bulletin. 2012;38:1021–1029. doi: 10.1093/schbul/sbr013. 21402721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wölwer W., Frommann N. Social-cognitive remediation in schizophrenia: generalization of effects of the training of affect recognition (TAR) Schizophrenia Bulletin. 2011;37(Suppl. 2):S63–S70. doi: 10.1093/schbul/sbr071. 21860049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wölwer W., Frommann N., Halfmann S., Piaszek A., Streit M., Gaebel W. Remediation of impairments in facial affect recognition in schizophrenia: efficacy and specificity of a new training program. Schizophrenia Research. 2005;80:295–303. doi: 10.1016/j.schres.2005.07.018. 16125367 [DOI] [PubMed] [Google Scholar]
- Wynn J.K., Jahshan C., Altshuler L.L., Glahn D.C., Green M.F. Event-related potential examination of facial affect processing in bipolar disorder and schizophrenia. Psychological Medicine. 2013;43:109–117. doi: 10.1017/S0033291712001006. 22583955 [DOI] [PMC free article] [PubMed] [Google Scholar]


