Abstract
Meadowfoam (Limnanthes alba L.) is a herbaceous winter-spring annual grown as a commercial oil seed crop. The meal remaining after oil extraction from the seed contains up to 4% of the glucosinolate, glucolimnanthin. Degradation of glucolimnanthin yields toxic breakdown products and therefore the meal may have potential in the management of soil-borne pathogens. To maximize the pest suppressive potential of meadowfoam seed meal, it would be beneficial to know the toxicity of individual glucolimnanthin degradation products against specific soil-borne pathogens. Meloidogyne hapla second-stage juveniles (J2) and Pythium irregulare and Verticillium dahliae mycelial cultures were exposed to glucolimnanthin as well as its degradation products. Glucolimnanthin and its degradation product, 2-(3-methoxyphenyl)acetamide, were not toxic to any of the soil-borne pathogens at concentrations up to 1.0 mg/mL. Two other degradation products, 2-(3-methoxymethyl)ethanethioamide and 3-methoxyphenylacetonitrile, were toxic to M. hapla and P. irregulare but not V. dahliae. The predominant enzyme degradation product, 3-methoxybenzyl isothiocyanate was the most toxic compound against all of the soil-borne pathogens with M. hapla being the most sensitive with EC50 values (0.0025 ± 0.0001 to 0.0027 ± 0.0001 mg/mL) 20 to 40 times lower than estimated EC50 mortality values generated for P. irregulare and V. dahliae (0.05 and 0.1 mg/mL, respectively). The potential exists to manipulate meadowfoam seed meal to promote the production of specific degradation products. The conversion of glucolimnanthin into its corresponding isothiocyanate should optimize the biopesticidal properties of meadowfoam seed meal against M. hapla, P. irregulare, and V. dahliae.
Keywords: nematicidal effect, fungicidal effect, lethal concentration, seed meal
INTRODUCTION
(Limnanthes alba L.) is an herbaceous winter-spring annual grown as a commercial oil seed crop. In the Willamette Valley of Oregon, meadowfoam fills an important niche as a winter rotation crop in grass seed production systems. In 2010, approximately 4,000 acres of meadowfoam were grown in Oregon with a value of $2M.1 Research and development of meadowfoam began in the late 1950s and commercial development began in 1980.2 When harvested, meadowfoam seeds contain 20–30% oil rich in rare long-chain 20:1 and 22:1 fatty acids.3 Meadowfoam’s unique chemical properties, which impart a high degree of stability, make it especially suitable for use in cosmetic products. After the oil has been extracted from the seeds, the seed meal constitutes approximately 70% of harvested crop yield; current commercial outlets for this byproduct are limited.
Meadowfoam seed is unique among oilseeds in that it contains several classes of plant secondary metabolites including the glucosinolate glucolimnanthin4, and minor amounts of rutin and phytoecdysteroids.5 When meadowfoam seed is physically damaged and exposed to moisture, glucolimnanthin 1 readily degrades to toxic breakdown products such as 3-methoxyphenylacetonitrile (also referred to as 3-methoxybenzyl cyanide; nitrile 2), 3-methoxybenzyl isothiocyanate (isothiocyanate 3), 2-(3-methoxyphenyl)ethanethioamide (thioamide 4), and 2-(3-methoxyphenyl)acetamide (acetamide 5) (Figure 1). This conversion is mediated by the glucosinolate-degrading enzyme, myrosinase.4 To prevent myrosinase activity during the oil extraction process, seeds are heat treated to inactivate myrosinase, thereby leaving the seed meal byproduct devoid of the active enzyme.
Figure 1.
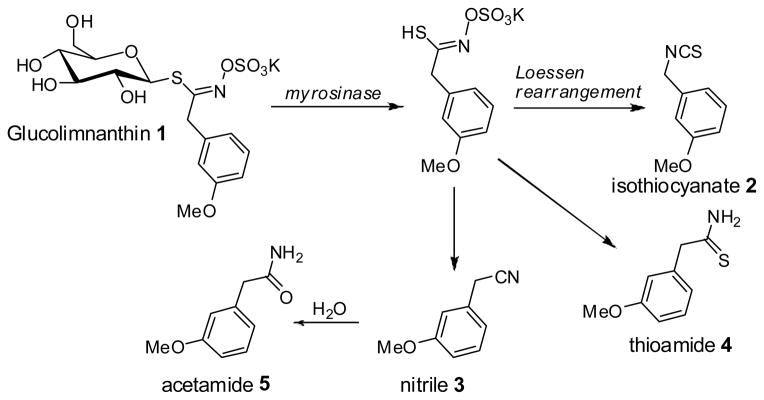
Enzymatic and chemical degradation of glucolimnanthin 1.
Degradation products from glucolimnanthin 1 are known to have biopesticidal properties. The degradation products isothiocyanate 2 and nitrile 3 inhibited radical elongation of velvetleaf (Abutilon theophrasti) and wheat (Triticum aestivum).6 Production of isothiocyanate 2, nitrile 3, and thioamide 4 from fermented meadowfoam seed meal correlated with increased herbicidal activity of the seed meal against downy brome (Bromus tectorum).7 Isothiocyanate 2 was toxic to fall army worm (Spodoptera frugiperda) larvae and European corn borer (Ostrinia nubialis) while nitrile 3 was not.8 Meadowfoam seed meal has also been shown to reduce the severity of clubroot of cauliflower caused by Plasmodiophora brassicae.9 However, the active principles responsible for this suppression were not identified in that study. The effect of meadowfoam seed meal on plant-parasitic nematodes and other soil-borne plant pathogens has not been investigated, yet there is evidence from research on other glucosinolate-containing brassicaceous seed meals that nematode suppression by meadowfoam seed meal could occur.10
Because of the inactivation of myrosinase by heat during the oil extraction process, there is a unique opportunity to direct the production of glucolimnanthin degradation products from meadowfoam seed meal depending upon how the seed meal is treated. Addition of enzyme-active seed material to meadowfoam seed meal (at a rate of 1% w/w) resulted in complete degradation of glucolimnanthin 1 and formation of isothiocyanate 2.7 Incubation of meadowfoam seed meal in an aqueous solution of FeSO4 favored the formation of isothiocyanate 2, nitrile 3, and thioamide 4.
To maximize the pest suppressive potential of meadowfoam seed meal, it would be beneficial to know the toxicity of glucolimnanthin degradation products against target microorganisms. Our research evaluated the toxicity of glucolimnathin 1 and its degradation products on several important soil-borne pathogens of high-value crops. Pests encountered in high-value cropping systems were targeted in these studies because it is likely that these cropping systems will be able to afford this type of technology to control soil-borne pests. The plant-parasitic nematode Meloidogyne hapla is commonly found in high-value crop production systems. It is distributed worldwide in cooler environments and causes damage to variety of crops.11 This nematode is a sedentary endoparasite and only the second-stage juveniles and adult males are present in soil. The fungus Verticillium dahliae is one of the most damaging and widespread soil-borne pathogens affecting the production of hundreds of susceptible vegetable, fruit, and ornamental plant species.12 The pathogen survives for years in the soil as microsclerotia and infects plants through roots. Once the plant host is infected, the pathogen may then colonize the xylem and cause symptoms of wilting, scorch, dieback, and plant death. The oomycete Pythium irregulare is also an ubiquitous soil-borne plant pathogen that infects a wide range of hosts including greenhouse floral crops and forest nursery seedlings.13–14 This pathogen persists in the soil as oospores and causes pre- and post-emergence seedling damping-off or root rot when soil moisture is abundant.
In many high value production systems, these soil-borne pathogens are managed by pre-plant soil fumigation. All of these soil-borne pathogens will become more difficult to control as soil fumigants are either removed from the market or become more regulated; therefore alternative management practices are needed. Meloidogyne hapla J2, and P. irregulare and V. dahliae mycelial cultures were exposed to glucolimnanthin 1 and its degradation products in vitro as a first step to determine the efficacy of meadowfoam seed meal in soil-borne pathogen management.
MATERIALS AND METHODS
Inoculum Production
Meloidogyne hapla, originally isolated from a vineyard near Veneta, OR and cultured on greenhouse-grown tomato (Solanum lycopersicon) ‘Rutgers’, was used in all assays. Disinfestation of eggs was conducted with sterile techniques in a laminar flow hood. Briefly, individual eggs masses were picked from roots, placed in water for 30 min, and rinsed with autoclaved water. Eggs masses were transferred to a 25 mL scintillation vial using a sterile spatula, 0.5% sodium hypochlorite was added, and the eggs were agitated for 3 min. Debris was then allowed to settle from the egg suspension for 30 s. Sterilized eggs were pipetted onto an autoclaved 500-mesh sieve and sodium hypochlorite rinsed from eggs by repeated rinsing with autoclaved water. To obtain second-stage juvenile (J2) inoculum, sterilized eggs were transferred onto a sterile hatching chamber 15 and placed in a sterile 50-mL beaker. Enough autoclaved water was added to the beaker to bring the water level up to the bottom of the filter. Meloidogyne hapla J2 were collected in water daily after 1 week and up to 2 weeks and used immediately.
Single-spore isolates of V. dahliae isolate 01-08a, originally isolated from an infected black raspberry (Rubus occidentalis) plant in a berry production field near Albany, OR, and P. irregulare isolate PR155d, originally isolated from soil in a Douglas-fir (Pseudotsuga menziesii) seedling production field near Canby, OR, were cultured on 20 mL of potato dextrose agar (PDA) amended with 250 mg/L ampicillin. Verticillium dahliae cultures were incubated for 3 weeks at 20 °C in the dark before use in microwell assays. Pythium irregulare cultures were incubated under the same conditions for 1 week before use.
Chemicals and Reagents
Glucolimnanthin 1 (CAS Registry Number: 111810-95-8; C15H20KNO10S2; S-(β-D-glucopyranosyl)-(Z)-2-(3-methoxyphenyl)-N-(sulfooxy)ethanimidothioic acid-potassium salt) was isolated from meadowfoam seed meal.7 3-Methoxybenxyl isothiocyanate (isothiocyanate 2) was obtained from Oakwood Products (West Columbia, SC). 3-Methoxyphenylacetonitrile (nitrile 3) was purchased from Sigma-Aldrich Chemicals (St. Louis, MO). 2-(3-Methoxyphenyl)ethanethioamide (thioamide 4) was purchased from Endeavour Specialty Chemicals (Daventry, Northamptonshire, U.K.) and 2-(3-methoxyphenyl)acetamide (acetamide 5) was purchased from Maybridge (Tintagel, Cornwall, UK). Chemicals (Figure 1) were prepared as 50 mg/mL stock solutions in dimethyl sulphoxide (DMSO) (Sigma-Aldrich), except for isothiocyanate 2 which was prepared as a 10 mg/mL stock solution in DMSO. Concentrations for testing were diluted from these stock solutions in DMSO.
Microwell Assays
A 96-well assay plate (Corning Inc., Corning, NY, USA) assay was used to screen chemicals against M. hapla, P. irregulare, and V. dahliae.16 For M. hapla, approximately 40 to 60 J2 were added to each well in 196 μL of water to which 4 μL of test solution in DMSO was added. For V. dahliae and P. irregulare, 3-mm-diam. colonized agar plugs (inoculum plugs) were made from culture plates using a sterile cork borer. The plugs were removed from culture plates and transferred with a sterile toothpick to wells containing 196 μL of water plus 4 μL of test solution in DMSO. The final volume in each well was 200 μL and the final concentration of DMSO to which test microorganisms were exposed was 2% v/v. Eight replicate wells were used for each chemical-concentration combination. In addition, each plate contained wells with water and 2% DMSO as control treatments. Plates were covered, sealed with Parafilm, wrapped in aluminum foil, and incubated at room temperature (25 °C).
All test microorganisms were exposed to treatments for 24 h. For M. hapla, mortality was assessed at 24 h by visually observing the nematodes (apparent mortality); they were considered alive if they exhibited constant sinusoidal movement as observed in the water and DMSO controls. Nematodes were considered dead/paralyzed if non-motile and appearing straight. Immediately after counting the nematodes, the majority of the solution in each well was removed with a pipette and replaced with water. Plates were then covered, sealed with Parafilm, wrapped in aluminum foil, and incubated at room temperature (25 °C) for another 24 h. Nematode mortality was assessed again using the same parameters described above (final mortality). For V. dahliae and P. irregulare, inoculum plugs were retrieved from wells using a sterile toothpick, rinsed with autoclaved water, blotted on a sterile paper towel, and placed on PDA amended with 250 mg/L ampicillin. Pythium irregulare and V. dahliae culture diameters were measured to the nearest mm after 48 h and 1 week of incubation at 20 °C, respectively. Inoculum plugs which did not grow were considered dead and these data (e.g., diameter = 0 mm) were not included in the culture diameter measurements. Mortality of P. irregulare and V. dahliae was determined on the basis of the number of inoculum plugs that failed to grow for each treatment. All experiments were conducted at least twice.
Statistical Analysis
For each compound and concentration assessed, M. hapla mortality (apparent and final mortality) and V. dahliae and P. irregulare culture diameters were expressed as a percentage of the water control treatment. These data were analyzed for effect of trial, concentration, and interaction using the Scheirer-Ray-Hare test, a nonparametric approach for analyzing data that do not meet normal distribution and equal variance assumptions.17 Mortality data from P. irregulare and V. dahliae were assessed among treatments using the chi-square test of independence.17 Dose-response curves were prepared for each microorganism and regressions on each data set were used to estimate the concentration that caused 50% mortality or a 50% reduction in culture diameter (EC50, 50% effective concentration). Significant differences among EC50 values were determined using analysis of variance (ANOVA). Analyses were performed using Minitab Statistical Software (release 16; Minitab Inc., State College, PA).
RESULTS
Meloidogyne hapla
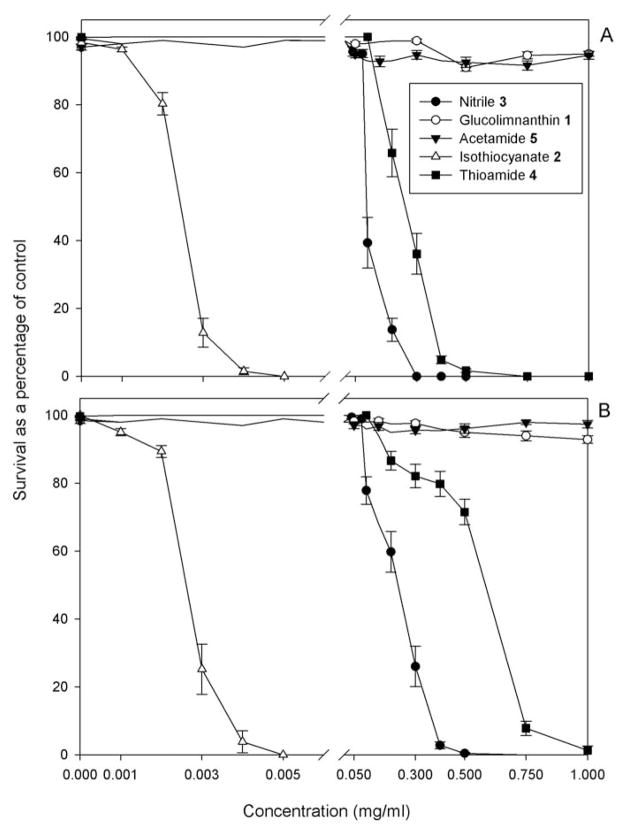
There was no difference in apparent or final nematode mortality between the water and 2% DMSO controls (data not shown), therefore only data from the water controls is presented. After a 24-h exposure, neither glucolimnanthin 1 nor acetamide 5 were toxic to M. hapla J2 (Figure 2A), therefore EC50 values could not be calculated for these compounds. Relative 24-h exposure toxicities against M. hapla for the remaining compounds (in ascending order) were thioamide 4 < nitrile 3 < isothiocyanate 2 (Table 1).
Figure 2.
Meloidogyne hapla J2 survival in glucolimnanthin (GLN) 1 and its degradation products: (A) immediately after 24 h exposure; (B) after the 24 h exposure and the subsequent 24 h rinse in water. Data are from at least two experiments, and each data point is the average of at least 16 replicates. Each error bar represents the standard error of the mean.
Table 1.
Effective Concentration Values at 50% (EC50) (mg/mL) for Meloidogyne hapla after 24 Hour Exposure (24 h) to Glucolimnanthin Degradation Products of Meadowfoam (Limnanthes alba) and After Removal of Compounds and Replacement with Water (Rinse)
| Glucosinolate degradation product | 24 h | Rinse |
|---|---|---|
| 2-(3-methoxyphenyl)ethanethioamide 4 | 0.245 ± 0.009 aA | 0.584 + 0.009 aB |
| 3-methoxyphenylacetonitrile 3 | 0.097 ± 0.001 bA | 0.221 ± 0.005 bB |
| 3-methoxybenzyl isothiocyanate 2 | 0.002 ± 0.0001 cA | 0.003 ± 0.0001 cB |
Data are from two experiments, and each value is the mean of at least 16 replicates ± the standard error of the mean. Means across compounds within a time (lower case letters) and across time within a compound (upper case letters) followed by the same letter are not significantly different according to Scheirer-Ray-Hare test (P<0.05).
There was no residual effect of glucolimanthin 1 and acetamide 5 against M. hapla after the compounds were removed, replaced with water, and assessed 24 h later (Figure 2B). After removal of nitrile 3, thioamide 4, and isothiocyanate 2 the EC50 values for all of these compounds significantly increased compared to 24-h exposure values (Table 1). Isothiocyanate 2 continued to be the most toxic compound to M. hapla followed by nitrile 3 and thioamide 4.
Pythium irregulare
There was no difference in P. irregulare culture diameter or mortality between the water and 2% DMSO controls, therefore only water control data is presented. Exposure to glucolimnanthin 1 and acetamide 5 for 24 h did not decrease culture diameter or increase mortality of P. irregulare, and EC50 values could not be calculated for these compounds. However, the remaining compounds, thioamide 4, nitrile 3, and isothiocyanate 2, all had some effect on P. irregulare culture diameter, mortality, or both.
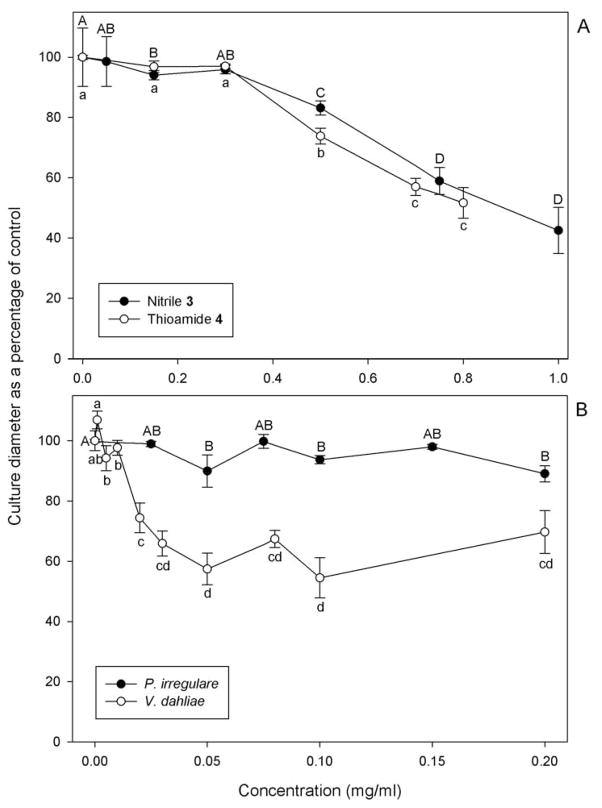
Nitrile 3 and thioamide 4 had similar effects on P. irregulare culture diameter (Figure 3A) and mortality (Table 2). Culture diameter was not affected by concentrations of either compound ≤ 0.3 mg/mL and EC50 values based upon diameter data were similar (0.85 ± 0.06 mg/mL and 0.75 ± 0.03 mg/mL, respectively, P=0.218). Mortality took place at concentrations higher than those that caused a reduction in culture diameter and was first observed at 0.75 mg/mL of nitrile 3 and 0.8 mg/mL of thioamide 4. However, estimated EC50 values based on mortality data (0.90 mg/mL and 0.76 mg/mL for nitrile and thioamide, respectively) were similar to those found for culture diameter.
Figure 3.
Percentage reduction in culture diameter: (A) of Pythium irregulare after 24 h exposure to nitrile and thioamide; (B) of P. irregulare and Verticillium dahliae after 24 h exposure to isothiocyanate 2. Data are from at least two experiments, and each data point is the average of at least 16 replicates. Each error bar represents the standard error of the mean. Means within a data series followed either by the same uppercase or lowercase letter are significantly different according to Scheirer-Ray-Hare test (P<0.05).
Table 2.
Percentage Mortality of Pythium irregulare and Verticillium dahliae Across a Range of Concentration of Glucolimnanthin Degradation Products of Meadowfoam (Limnanthes alba)
| Concentration (mg/mL) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3-methoxybenzyl isothiocyanate 2 | |||||||||||||
| 0 | 0.001 | 0.005 | 0.01 | 0.02 | 0.025 | 0.03 | 0.05 | 0.075 | 0.08 | 0.1 | 0.15 | 0.2 | |
| P. irregulare | 0 aa | - | - | - | - | 0 a | - | 54 b | 56 b | - | 63 bc | 88 c | 88 d |
| V. dahliae | 0 a | 0 a | 0 a | 0 a | 0 a | - | 0 a | 19 a | - | 7 a | 50 b | - | 69 b |
|
| |||||||||||||
| 3-methoxyphenyl nitrile 3
|
|||||||||||||
| 0 | 0.05 | 0.15 | 0.3 | 0.5 | 0.75 | 1.0 | |||||||
| P. irregulare | 0 a | 0 a | 0 a | 0 a | 0 a | 17b | 71 c | ||||||
|
| |||||||||||||
| 2-3(methoxyphenyl)ethanethioamide 4
|
|||||||||||||
| 0 | 0.05 | 0.15 | 0.3 | 0.5 | 0.7 | 0.8 | |||||||
| P. irregulare | 0 a | 0 a | 0 a | 0 a | 0 a | 0 a | 83 b | ||||||
Data are from two experiments, and each value is the mean of at least 16 replicates. Means within a compound and microorganisms followed by the same letter are not significantly different according to chi-square test of independence (P<0.05).
The response in culture diameter of P. irregulare to isothiocyanate 2 was different from that observed for thioamide 4 and nitrile 3. Regardless of the isothiocyanate 2 concentration tested, there was no significant reduction in culture diameter (P≥0.056) (Figure 3B). However, there was an effect of this compound on culture mortality with an increasing number of dead cultures occurring with increasing concentration (Table 2). The estimated EC50 value for isothiocyanate 2 against P. irregulare based upon culture morality was 0.05 mg/mL. This value was 15 times lower than those estimated for P. irregulare cultures treated with nitrile 3 and thioamide 4.
Verticillium dahliae
Similar to the other microorganisms, 2% DMSO did not affect V. dahliae culture diameter or mortality compared to the water control, therefore only water control data is presented. Regardless of the concentrations tested, glucolimnanthin 1, acetamide 5, thioamide 4, and nitrile 2 did not affect culture diameter or mortality of V. dahliae. Only isothiocyanate 2 significantly reduced culture diameter at a concentration of 0.02 mg/mL (P<0.001) (Figure 3B). However, after the initial reduction at 0.02 mg/mL, culture diameter remained relatively constant and greater isothiocyanate 2 concentrations did not significantly reduce culture diameter (P≥0.114). Isothiocyanate 2 also increased mortality of V. dahliae starting at 0.10 mg/mL. This reduction was constant up to 0.2 mg/mL isothiocyanate 2 (Table 2), and the pathogen did not survive at concentrations of compound 2 greater than 0.2 mg/mL (data not shown). The estimated EC50 value for isothiocyanate 2 against V. dahliae based upon culture mortality was 0.10 mg/mL.
DISCUSSION
Meloidogyne hapla, P. irregulare, and V. dahliae varied in sensitivity to the meadowfoam seed meal glucolimnanthin degradation products evaluated in this study. In general, V. dahliae was the least sensitive, P. irregulare was intermediate in sensitivity, and M. hapla was the most sensitive to the degradation products. Across the tested compounds, the glucolimnanthin degradation product isothiocyanate 2 was the most toxic to all of the microorganisms.
Throughout the study, we were unable to generate statistically relevant EC50 mortality values for P. irregulare and V. dahliae because the response for each culture exposed to a treatment in an individual well was binomial; either alive or dead. This was in contrast to M. hapla, where a population was exposed to a treatment in an individual well, and we were therefore able to calculate percentage J2 mortality for each well within a treatment. This obstacle could be overcome in future studies by using a larger well volume [24-well (3.5 mL volume) vs. 96-well (400 μL volume) plates] in which more than one fungal or oomycete inoculum plug could be exposed to an individual treatment. Alternatively, aliquots containing a known concentration of fungal or oomycete spores (a population) could be placed into each individual well for exposure to compounds. By using either method, the variability associated with fungal and oomycete mortality could be calculated, and the data could then be used for statistical comparison of mortality between compounds and microorganisms. Nonetheless, by expressing the number of replicate cultures that died within each treatment, we were able to estimate percentage mortality for these two microbes in order to compare EC50 values. For example, the similar EC50 values for culture diameter and mortality of P. irregulare tested with nitrile 3 and thioamide 4 demonstrated that similar concentrations were necessary to elicit a reduction in culture growth and an increase in mortality. Estimated EC50 mortality values also clearly demonstrated that V. dahliae and P. irregulare were less sensitive to isothiocyanate 2 than M. hapla.
There were similarities and differences between our study and previous studies that evaluated glucolimnanthin 1 and its degradation products against weeds and insects. Isothiocyanate 2 was toxic to fall armyworm and European corn borer, causing mortality and reductions in larval weight; in the same study nitrile 3 had little effect on these insects.8 A concentration of 0.1 mg/mL caused a reduction in larval weight while higher concentrations of 0.33 mg/mL and 1.0 mg/mL for European corn borer and fall armyworm, respectively, were necessary to cause larval mortality. However, in contrast to our study, which found no activity of glucolimnanthin 1 against soil-borne microorganisms, an equimolar amount of glucolimnanthin 1 was similar in activity to isothiocyanate 2 against these insects. In agar-based assays, isothiocyanate 2 was considerably more toxic to velvetleaf and wheat, as determined by radicle elongation, compared to nitrile 3 and glucolimnanthin 1.6 Calculated EC50 values for isothiocyanate 2 against velvetbean and wheat (0.0004 mg/mL and 0.0009 mg/mL, respectively) were much lower than those observed for M. hapla in the present study. Conversely, the relative order of toxicity of glucolimnanthin 1 and its degradation products to downy brome in soil-based assays was (in descending order) thioamide 4 > nitrile 3 > acetamide 5 > glucolimnanthin 1 > isothiocyanate 2.7 Approximate EC50 values for thioamide 4 and nitrile 3 against downy brome were 0.3 and 0.5 mg/g, respectively. These values are similar to the range of EC50 values calculated in this study.
A number of purified isothiocyanates have been tested against plant-parasitic nematodes. Isothiocyanate analogs (including allyl, 2-phenylethyl, butyl, ethyl, and acryloyl) were tested against Meloidogyne javanica and EC50 values ranged between 0.002 to 0.005 mg/mL.18 A wider range of EC50 values, 0.0002 to 0.003 mg/mL, were obtained for allyl, benzyl, butyl, ethyl, phenyl, 2-phenylethyl, and 4-methlsulfinyl(butyl) isothiocyanates tested against M. javanica and Tylenchulus semipenetrans.19 For M. hapla, a similar final mortality EC50 value was generated for isothiocyanate 2 (0.003 mg/mL) compared to several isothiocyanates tested against M. javanica.18–19
Isothiocyanates have also been tested against several species of fungi, oomycetes, and other soil microorganisms. Six isothiocyanates (methyl, propenyl, butenyl, pentenyl, benzyl, and 2-phenylethyl) were tested against five cereal root pathogens.20 Isothiocyanate EC50 values ranged from 0.001 mg/mL (for 2-propenyl isothiocyanate) to 0.01 mg/mL (for 4-pentenyl isothiocyanate). Of the pathogens evaluated Gaeumannomyces graminis var. tritici was the most sensitive, Rhizoctonia solani and Fusarium graminearum showed intermediate sensitivity, and Bipolaris sorokiniana and P. irregulare were the least sensitive. In another study, a diversity of bacteria, fungi, and oomycete species were exposed to 2-phenylethyl isothiocyanate and EC50 values ranged from 0.0005 mg/mL (for Pythium sulcatum) to 0.48 mg/mL (for Trichoderma spp.).21 In the same study, bacteria were generally more tolerant to 2-phenylethyl isothiocyanate than fungi or oomycetes. Very low concentrations of allyl isothiocyanate (0.00005 mg/ml) were needed to completely inhibit R. solani in vitro.22 In our study, 3-methoxybenzyl isothiocyanate 2 was less toxic to the P. irregulare (EC50 mortality of 0.05 mg/mL) compared to other previously tested isothiocyanates against Pythium spp.20. Verticillium dahliae was the least sensitive soil-borne organism to isothiocyanate 2 in our study, with an EC50 mortality value greater in comparison to previously tested isothiocyanates on other fungal species.20
For V. dahliae and P. irregulare, culture diameter and mortality were affected differentially depending upon the compound and range of concentrations tested. In general, a reduction in culture diameter was realized at lower concentrations of thioamide 4, nitrile 2, and isothiocyanate 2 while higher concentrations were necessary to induce mortality. A similar trend was observed for European corn borer and fall armyworm where there was an effect of isothiocyanate 2 on larval growth before insect survival was impacted.8 In our study, an exception to this was the effect of isothiocyanate 2 on P. irregulare where if the pathogen survived exposure there was no consequence on growth. For M. hapla, a proportion of the individuals that were straight and therefore considered dead (apparent mortality) after 24 h exposure recovered when the compounds were removed and replaced with water, indicating the compound was nematostatic to some extent. A question that arises is whether post-exposure fitness of fungi, oomycetes, and nematodes that are exposed to sublethal doses of glucolimnanthin degradation products is reduced or enhanced. The evolution of resistance of soil-borne microorganisms to sublethal doses has been widely documented.23 However, there may also be fitness costs that are not favorable to microorganisms. Exposure of Meloidogyne incognita J2 to a sublethal dose of benzyl isothiocyanate had a significant impact on subsequent infectivity and reproduction on plant hosts.24 A sublethal dose of diflubenzuron resulted in a lower net reproductive rate of Culex quinquefasciatus while a sublethal dose of azadirachtin did not.25 It is impossible to speculate on the long-term consequences of exposure to sublethal doses of nitrile 3, thioamide 4, and isothiocyanate 2 against M. hapla, P. irregulare, and V. dahliae in this study. However, this may be an important consideration if meadowfoam seed meal is applied to soil and only sublethal doses of glucolimnanthin degradation products are achieved.
The challenge of generating lethal doses of compounds 2–4 in soil after the application of meadowfoam seed meal is due to the complex nature of the reaction as well as the soil environment. Factors that come into consideration include: myrosinase availability, soil texture, organic matter, moisture and temperature, the chemical nature of the degradation compound, microbial communities, and the volatility of the glucosinolate degradation products.10, 26 For example, volatility played an important role in the formation and degradation of isothiocyanate 2 from an insect diet prepared from meadowfoam seed meal.8 The recovery rate of isothiocyanate 2 from freshly-prepared diet was 65% while after 9 days recovery was only 18%. Similarly, glucosinolate degradation products were monitored from Brassica napus seed meal and isothiocyanates reached maximum concentrations after 2 h and decreased by 90% within 24 h.27 Our results demonstrated that concentrations of isothiocyanate 2 required to result in a 50% reduction in soil-borne pathogen survival ranged from 0.003 to 0.10 mg/mL, and higher concentrations would be required to achieve complete control. Whether these concentrations are achievable at economically realistic rates of meadowfoam seed meal is unknown. However, with meadowfoam seed meal the ability does exist to promote the production of desired degradation products by adding intact meadowfoam seed or FeSO4 to the seed meal, with both of these methods promoting the production of isothiocyanate 2.7
Our data, as well as data from previous studies, suggests that knowledge of the target microorganism will play a role in the efficacy of meadowfoam seed meal for soil-borne pest management. All of the soil-borne pathogens tested in this study were the most sensitive to isothiocyanate 2 rather than to acetamide 5, nitrile 3, or thioamide 4. Therefore, methods that enhance the production of isothiocyanate 2 should be evaluated in order to obtain the best potential for control of M. hapla, P. irregulare, and V. dahliae by meadowfoam seed meal amendments in future studies. Once methods of isothiocyanate 2 production from meadowfoam seed meal have been optimized, experiments that evaluate the effect of seed meal amendment on soil-borne pathogen fitness in soils can be conducted.
LITERATURE CITED
- 1.Agricultural Marketing Resource Center. [Date last accessed: January 25: 2011];Meadowfoam. http://www.agmrc.org/commodities__products/grains__oilseeds/meadowfoam.cfm.
- 2.Jolliff GD, Tinsley IJ, Calhoun W, Crane J. Meadowfoam (Limnanthes alba): Its research and development as a potential new oilseed crop for the Willamette Valley of Oregon. Corvallis, OR: Oregon State University Agricultural Experiment Station; 1981. [Google Scholar]
- 3.Knapp SJ, Crane JM. Fatty acid diversity of section Inflexae Limnanthes (meadowfoam) Ind Crops Prod. 1995;4:219–227. [Google Scholar]
- 4.Stevens JF, Reed RL. Glucosinolate degradation products in fermented meadowfoam seed meal and their herbicidal activities. In: Gang DR, editor. The Biological Activity of Phytochemicals. New York: Springer; 2011. pp. 141–158. [Google Scholar]
- 5.Stevens JF, Reed RL, Morre JT. Characterization of phytoecdysteroid glycosides in meadowfoam (Limnanthes alba) seed meal by positive and negative ion LC-MS/MS. J Agric Food Chem. 2008;56:3945–3952. doi: 10.1021/jf800211k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vaughn SF, Boydston RA, Mallory-Smith CA. Isolation and identification of (3-methoxyphenyl)aceotonitrile as a phytotoxin from meadowfoam (Limnanthes alba) seedmeal. J Chem Ecol. 1996;22:1939–1949. doi: 10.1007/BF02028513. [DOI] [PubMed] [Google Scholar]
- 7.Stevens JF, Reed RL, Alber S, Pritchett L, Machado S. Herbicidal activity of glucosinoate degradation products in fermented meadowfoam (Limnanthes alba) seed meal. J Agric Food Chem. 2009;57:1821–1826. doi: 10.1021/jf8033732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bartelt RJ, Mikolajczak KL. Toxicity of compounds derived from Limnanthes alba seed to fall armyworm (Lepidoptera: Noctuidae) and European corn borer (Lepidoptera: Pyralidae) larvae. J Econ Entomol. 1989;82:1054–1060. [Google Scholar]
- 9.Deuel W, Svenson S. Field evaluation of meadowfoam seedmeal to control clubroot disease (Plasmodiophora brassicae) in cruciferous crops. In: Shenk MK, editor. IPM in Oregon: Achievements and Future Directions. Corvallis, OR: Oregon State University Extension Service; 2000. pp. 135–138. [Google Scholar]
- 10.Brown PD, Morra MJ. Control of soil-borne plant pests using glucosinolate-containing plants. Adv Agron. 1997;61:167–231. [Google Scholar]
- 11.Franklin MT. Economic importance of Meloidogyne in temperate climates. In: Lamberti F, Taylor CE, editors. Root-Knot Nematodes (Meloidogyne Species) - Systematics, Biology and Control. New York: Academic Press; 1979. pp. 331–340. [Google Scholar]
- 12.Pegg GF, Brady BL. Verticillium Wilts. CABI Publishing; New York: 2002. [Google Scholar]
- 13.Moorman GW, Kang S, Geiser DM, Kim SH. Identification and characterization of Pythium species associated with greenhouse floral crops in Pennsylvania. Plant Dis. 2002;86:1227–1231. doi: 10.1094/PDIS.2002.86.11.1227. [DOI] [PubMed] [Google Scholar]
- 14.Weiland JE. Influence of isolation method on recovery of Pythium species from forest nursery soils in Oregon and Washington. Plant Dis. 2011;95:547–553. doi: 10.1094/PDIS-04-10-0242. [DOI] [PubMed] [Google Scholar]
- 15.Zasada IA, Tenuta M. Chemical-mediated toxicity of N-Viro Soil to Heterodera glycines and Meloidogyne incognita. J Nematol. 2004;36:297–302. [PMC free article] [PubMed] [Google Scholar]
- 16.Zasada IA, Masler EP, Rogers ST, Halbrendt JM. Behavioral response of Meloidogyne incognita to benzyl isothiocyanate. Nematology. 2009;11:603–610. [Google Scholar]
- 17.Sokal RR, Rohlf FJ. Biometry. W. H. Freeman; New York: 1995. [Google Scholar]
- 18.Wu H, Wang CJ, Bian XW, Zeng SY, Lin KC, Wu B, Zhang GA, Zhang X. Nematicidal efficacy of isothiocyanates against root-knot nematode Meloidogyne javanica in cucumber. Crop Prot. 2011;30:33–37. [Google Scholar]
- 19.Zasada IA, Ferris H. Sensitivity of Meloidogyne javanica and Tylenchulus semipenetrans to isothiocyanates in laboratory assays. Phytopathology. 2003;93:747–750. doi: 10.1094/PHYTO.2003.93.6.747. [DOI] [PubMed] [Google Scholar]
- 20.Sarwar M, Kirkegaard JA, Wong PTW, Desmarchelier JM. Biofumigation potential of brassicas: III. In vitro toxicity of isothiocyanates to soil-borne fungal pathogens. Plant Soil. 1998;201:103–112. [Google Scholar]
- 21.Smith BJ, Kirkegaard JA. In vitro inhibition of soil microorganisms by 2-phenylethyl isothiocyanate. Plant Pathol. 2002;51:585–593. [Google Scholar]
- 22.Dhingra OD, Costa MLN, Silva GJ., Jr Potential of allyl isothiocyanate to control Rhizoctonia solani seedling damping off seedling blight in transplant production. J Phytopathol. 2004;152:352–357. [Google Scholar]
- 23.Gressel J. Low pesticide rates may hasten the evolution of resistance by increasing mutation frequencies. Pest Manage Sci. 2011;67:253–257. doi: 10.1002/ps.2071. [DOI] [PubMed] [Google Scholar]
- 24.Masler EP, Zasada IA, Sardanelli S, Rogers ST, Halbrendt JM. Effects of benzyl isothiocyanate on the reproduction of Meloidogyne incognita on Glycine max and Capsicum annuum. Nematology. 2010;12:693–699. [Google Scholar]
- 25.Suman DS, Parashar BD, Prakash S. Effect of sublethal dose of diflubenzuron and azadirachtin on various life table attribues of Culex quinquefasciatus (Dipertera: Culicidae) J Adhes Sci Technol. 2010;47:996–1002. doi: 10.1603/me09190. [DOI] [PubMed] [Google Scholar]
- 26.Gimsing AL, Poulsen JL, Pedersen HL, Hansen HCB. Formation and degradation kinetics of the biofumigant benzyl isothiocyanate in soil. Environ Sci Technol. 2007;41:4271–4276. doi: 10.1021/es061987t. [DOI] [PubMed] [Google Scholar]
- 27.Brown PD, Morra MJ, McCaffrey JP, Auld DL, Williams L., III Allelochemicals produced during glucosinolate degradation in soil. J Chem Ecol. 1991;17:2021–2034. doi: 10.1007/BF00992585. [DOI] [PubMed] [Google Scholar]