Abstract
Background
Contemporary trends in hospitalization patterns among perinatally HIV-infected (PHIV) patients are unknown. We describe rates and reasons for hospitalizations stratified by age group during 2003-2010 within a large cohort of PHIV patients.
Methods
579 PHIV patients engaged in care at 6 geographically-diverse pediatric HIV centers affiliated through the HIV Research Network were included. Modified Clinical Classification Software assigned primary ICD-9 codes into diagnostic categories. Analysis was performed using negative binomial regression with generalized estimating equations.
Results
There were 699 all-cause hospitalizations. The overall rate for the full cohort was 19.9 / 100 person years, and overall rates for 0-4, 5-16, and 17-24 year-olds were 25.1, 14.7, and 34.2 / 100 person years, respectively. Declines were seen in unadjusted all-cause rates for the whole group (incidence rate ratio per year, 0.93 [0.87, 0.99]) and for 5-16 (0.87 [0.76, 0.99]) and 17-24 year-olds (0.87 [0.80, 0.95]). After adjustment for CD4, HIV-1 RNA, and demographics, rates were no longer declining. Non-AIDS-defining infections and AIDS-defining illnesses together caused 349 (50%) admissions. Declines in these categories drove the overall declines in unadjusted rates. No increases over time were seen for cardiovascular, renal, or any other diagnostic categories.
Conclusions
While the declines in hospitalizations are reassuring, continued efforts are needed to address the persistently high infectious and non-infectious morbidity among PHIV patients. Innovative strategies may be most critical for 17-24 year-olds. Lack of increases in cardiovascular and renal admissions provides modest, preliminary reassurance against severe non-infectious complications from longstanding HIV infection and antiretroviral exposure.
Keywords: Perinatally HIV-Infected Patients, Hospitalizations, AIDS Defining Illnesses, Antiretroviral Therapy
During 2001-2010, declines in all-cause and AIDS defining illness (ADI) hospitalizations were seen among adults living with HIV.1, 2 These trends likely relate to improved viral suppression and immune function resulting from a combination of treatment initiation at higher CD4 counts, earlier diagnosis and linkage to care, and more potent and tolerable antiretroviral therapy (ART) regimens.3, 4
It is not known whether perinatally HIV-infected (PHIV) individuals; most of whom are young children (ages 0-4 years), school-age children (ages 5-16), or young adults (ages 17-24); have had similar declines in hospitalizations. Like non-PHIV adults, PHIV individuals have had improvements in virologic suppression over the past decade.5, 6
Compared to their younger counterparts, PHIV young adults may have particular risk for hospitalization. PHIV young adults were born prior to the advent of combination ART in 1996. They may have suffered advanced immunosuppression from the absence of effective treatment. Antiretroviral resistance acquired from exposure to sub-optimal regimens7-9 may have limited subsequent ART options to more complicated and toxic regimens. Unrelated to HIV, young adults face challenges to adherence in the context of independence from guardians, peer pressure, and low perception of risk.10
Among PHIV patients, there is reason to fear increasing renal and cardiovascular morbidity from antiretroviral toxicity and/or from HIV itself. Antiretroviral agents have been associated with renal and cardiovascular laboratory and imaging abnormalities among PHIV patients.11-15
The objective of this study was to describe trends in all cause hospitalization rates and causes among PHIV patients receiving care at pediatric HIV specialty clinics during 2003-2010. Understanding these trends is important to assessing current clinical care and to identifying opportunities for improvement.
METHODS
Study Population
We examined data from the HIV Research Network (HIVRN), a clinical cohort of patients at 6 pediatric (0-24 years-old) and 12 adult US HIV clinics.16 Our study population included all PHIV patients at the 6 pediatric sites (2 Western, 2 Southern, and 2 Northeastern). Sites prospectively collected demographic, laboratory, and medication data from electronic databases and from chart review by trained abstractors. Hospitalization data were prospectively collected from administrative databases at the study-site hospitals and from chart review for hospitalizations occurring at outside institutions. De-identified data were assembled into a uniform database. Institutional review boards at the data coordinating center at Johns Hopkins University and at each of the participating sites approved the study.
Outcome variables
The outcome of interest was all-cause hospitalization. We also stratified the outcome as cause-specific hospitalization using 19 diagnostic categories, for example, ADI (for complete list, see Supplemental Table 1).
Assigning each hospitalization to a diagnostic category involved several previously published steps.2, 17 First, the highest-listed ICD-9 code referring to neither HIV nor chronic Hepatitis C was defined as the primary ICD-9 for the hospitalization. Second, Clinical Classification Software (CCS) from the Agency for Healthcare Research and Quality was used to assign the primary ICD-9 into one of 18 categories.18 Third, we reassigned infectious conditions classified by the CCS among organ system categories to the infection category. For example pneumonia was reassigned from pulmonary to infection (Supplemental Table 1 includes all reassignments). Finally, we constructed a separate category for ADI by reassigning appropriate ICD-9 codes (Supplemental Table 2) as per the Centers for Disease Control and Prevention criteria for patients both under and over age 13 years.19, 20
To describe the two most frequent individual diagnoses within each diagnostic category, individual ICD-9 codes were explored using an online tool.21 Where appropriate, we grouped together highly similar codes, e.g. all ICD-9 codes for cellulitis at different body sites were combined into a single group called “cellulitis” (Supplemental Table 2).
Death can affect trends in hospitalization rates. The HIVRN pediatric sites routinely collect death information through medical record review and family report.
Independent Variables
Race/ethnicity was categorized as non-Hispanic black (black), non-Hispanic white (white), Hispanic, and unknown/other. Age, ART, CD4 count, and HIV-1 RNA were time-varying covariates. Age categories (0-4, 5-16, and 17-24) were assigned according to age on July 1st of each year. ART was a dichotomous variable for which simultaneous prescription of three or more antiretrovirals representing at least two antiretroviral classes was counted as “yes”. For CD4 count and HIV-1 RNA, the first measurements of each year were used.
Statistical Analysis
Person-time and hospitalization events were assessed for years of active care, defined as having at least one outpatient HIV care visit and one measured CD4 count. We estimated hospitalization unadjusted incidence rates per 100 person-years, adjusted incidence rate ratios (aIRR) and 95% confidence intervals using negative binomial regression with generalized estimating equations to allow an individual to contribute multiple hospitalization events. To assess calendar trends in hospitalizations, we modeled year linearly; we had no a priori hypothesis that the relationship would be non-linear during the study interval.
Patients who died or were lost to follow-up (and thus may possibly have died) may have had higher hospitalization rates prior to their deaths, resulting in possible declining hospitalization trends due to these patients exiting the study. To address this, we performed two sensitivity analyses of hospitalization rates and associated factors: 1) the first analysis excluded the year of patient death and the preceding year for all patients who died; 2) the second analysis excluded the same time frame for those who died and for those who were lost to follow-up. Being lost to follow-up was defined as 1) discontinuing active care not due to death or transfer to an adult site and 2) not returning to active care in a later study year.
All multivariate models included an indicator variable to adjust for HIVRN site. A 2-sided type-1 error of 5% guided statistical interpretation. All analyses were performed using STATA 12.1 (StataCorp LP, College Station, TX).22
RESULTS
From 2003 to 2010, 579 PHIV patients contributed 3,516 person-years (PY) of active outpatient care. The median number of years of active care was 7 (interquartile range (IQR)=4-8). About a third of patients (192/579) were not in active care for one or more years during the study interval. Among those who had a period without active care, 22 (11%) later returned and then remained in care, 16 (8%) died prior to 2010, and 20 (10%) transferred to adult HIV care. A total of 134 (23% of the full study population) were lost to follow-up.
Among the 20 (4%) patients who died during the study interval, the median age at death was 18.8 (IQR=14.9-20.2) years. Three deaths occurred due to ADI; 2 due to cardiovascular disease (cardiomyopathies); 1 each to non-ADI infection, metabolic, oncologic, pulmonary, and liver illness; and 10 to unknown causes.
As patients aged during the study interval, the proportions of 0-4 and 5-16 year-olds decreased (16% and 79%, respectively, in 2003 and 7% and 58%, respectively, in 2010) while the proportion of 17-24 year-olds increased (5% in 2003 and 34% in 2010) (Table 1 and Figure 1).
Table 1.
Characteristics of PHIV patients, 2003 and 2010
| 2003 (total n=429) | 2010 (total n=409) | |||||
|---|---|---|---|---|---|---|
| Age group (in years) | 0 - 4 | 5 – 16 | 17 – 24 | 0 – 4 | 5 – 16 | 17 – 24 |
|
|
|
|||||
| N (% of total n for that year) | 69 (16) | 338 (79) | 22 (5) | 29 (7) | 239 (58) | 141 (34) |
| Median age (IQR), years | 3 (1 – 4) | 11 (8 – 14) | 18 (17 – 19) | 3 (2 – 4) | 13 (10 – 15) | 20 (18 – 21) |
| Gender, n (%) | ||||||
| Male | 26 (38) | 160 (47) | 11 (50) | 15 (52) | 107 (45) | 71 (50) |
| Female | 43 (62) | 178 (53) | 11 (50) | 14 (48) | 132 (55) | 70 (50) |
| Race / Ethnicity, n (%) | ||||||
| Non-Hispanic black | 59 (86) | 230 (68) | 14 (64) | 16 (55) | 186 (78) | 105 (75) |
| Non-Hispanic white | 6 (9) | 53 (16) | 2 (9) | 6 (21) | 30 (13) | 25 (18) |
| Hispanic | 4 (6) | 51 (15) | 5 (23) | 4 (14) | 18 (8) | 10 (7) |
| Other / unknown | 0 | 4 (1) | 1 (5) | 3 (10) | 5 (2) | 1 (1) |
| Prescribed ART, n (%) | ||||||
| No | 9 (13) | 90 (27) | 8 (36) | 3 (10) | 15 (6) | 16 (11) |
| Yes | 60 (87) | 248 (73) | 14 (64) | 26 (90) | 24 (94) | 125 (89) |
| HIV-1 RNA (copies/ml) *, n (%) | ||||||
| < 400 | 18 (26) | 110 (33) | 10 (45) | 20 (69) | 182 (76) | 86 (61) |
| 400 – 49,999 | 33 (48) | 184 (54) | 8 (36) | 5 (17) | 49 (21) | 49 (35) |
| ≥ 50,000 or missing | 18 (26) | 44 (13) | 4 (18) | 4 (14) | 8 (3) | 6 (4) |
| CD4 count (cells/μl) *, n (%) | ||||||
| < 200 | 0 | 24 (7) | 8 (36) | 1 (3) | 7 (3) | 20 (14) |
| 200 – 749 | 9 (13) | 167 (49) | 9 (41) | 1 (3) | 98 (41) | 69 (49) |
| ≥ 750 | 60 (87) | 147 (43) | 5 (23) | 27 (93) | 134 (56) | 52 (37) |
| Median (IQR) | 1469 (950 – 2042) | 682 (476 – 964) | 398 (89, 731) | 1660 (1142 – 2202) | 800 (541 – 1020) | 560 (320 – 909) |
| Prescribed P. jirovecii prophylaxis, n prescribed / N indicated† (%) | 5 / 8 (63) | 14 / 21 (67) | 7 / 10 (70) | 2 / 3 (67) | 5 / 5 (100) | 14 / 16 (88) |
| Prescribed M. avium prophylaxis, n prescribed / N indicated† (%) | 0 / 0 | 8 / 10 (80) | 1 / 4 (25) | 0 / 1 (0) | 2 / 2 (100) | 8 / 9 (89) |
1st measurement of the year
Per 2009 Department of Health and Human Services age and CD4 count guidelines23
IQR, interquartile range; ART, antiretroviral therapy
Figure 1.
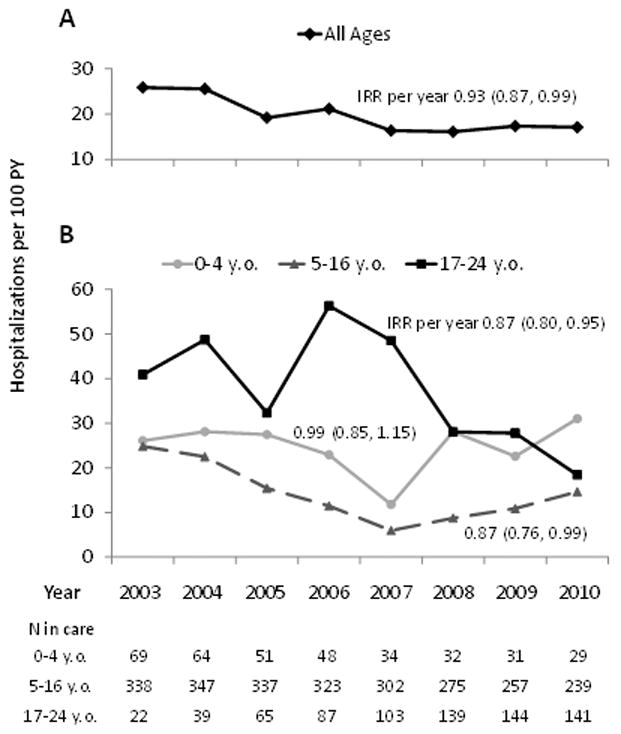
Trends in all-cause hospitalization rate, by age group.
Legend: y.o., year-old
In all years, the majority of patients in all age groups were of black race (Table 1). For all three age groups, the percentage of patients with HIV-1 RNA < 400 copies/mL on the first measurement of the year increased from ≤45% in 2003 to >60% in 2010. The percentage of 0-4 year-olds prescribed ART remained relatively constant (87% in 2003 and 90% in 2010), whereas the percentages of 5-16 and 17-24 year-olds on ART increased (73% and 64%, respectively in 2003; and 94% and 89%, respectively in 2010). Within most study years, 60-70% of patients with a CD4-based indication23 received P. jirovecii and/or M. avium prophylaxis.
All-Cause Hospitalization Rate
Over the study period, 220 patients (38%) had at least one hospitalization, for a total of 699 hospitalizations. Among those hospitalized, the median number of hospitalizations was 2 (IQR=1-4). The overall all-cause hospitalization rate was 19.9 / 100 PY. In 2003 it was 25.9 / 100 PY, and in 2010, 17.1 / 100 PY (Figure 1A), which yielded a decline in incidence of 7% per year (IRR=0.93 [0.87, 0.99]). On visual inspection, linear parameterization appeared to fit well.
All-cause crude hospitalization rates, and changes in the rates over time, differed according to age group (Figure 1B). For 0-4 year-olds, the overall rate was 25.1 / 100 PY and was generally stable (IRR=0.99 [0.85, 1.15]). The overall rate for 5-16 year-olds was 14.7 / 100 PY, and was associated with a decline of 13% per year (IRR=0.87 [0.76, 0.99]). The overall rate for 17-24 year-olds was the highest at 34.2 / 100 PY, and it declined 13% per year (IRR=0.87 [0.80, 0.95]). Although the changes in the rates over time were different by age groups, there was no statistical evidence of an interaction between time and age group (P>0.20).
Factors associated with all-cause hospitalization are shown in Table 2. In adjusted analysis, CD4 count (< 200 vs. ≥ 750 cells/μl aIRR=5.02 [3.25, 7.75]) and HIV-1 RNA (≥50,000 copies/mL or missing vs. < 400 copies/ml aIRR=3.93 [2.72, 5.69]) had the strongest associations with hospitalization. While 17-24 year-olds had a higher hospitalization rate than 5-16 year-olds in crude analysis (IRR=2.32 [1.72, 3.13]), the adjusted association was no longer significant (aIRR=1.38 [0.99, 1.93]) In contrast, 0-4 year-olds had greater hospitalization rates than 5-16 year-olds in both crude and adjusted analyses (IRR=1.71 [1.15, 2.54] and aIRR=2.26 [1.49, 3.43]).
Table 2.
Factors Associated with All-Cause Hospitalization, 2003-2010
| All Age Groups (N = 579) | Stratified by Age Group | ||||
|---|---|---|---|---|---|
| 0 – 4 year-old (n = 130) | 5 – 16 year-old (n = 509) | 17 – 24 year-old (n = 241) | |||
| IRR | aIRR | aIRR | aIRR | aIRR | |
| Age Group | |||||
| 0-4 year-old | 1.71 (1.15, 2.54) | 2.26 (1.49, 3.43) | |||
| 5-16 year-old | 1.00 (Ref) | 1.00 (Ref) | |||
| 17-24 year- old | 2.32 (1.72, 3.13) | 1.38 (0.99, 1.93) | |||
| Calendar Time | |||||
| per year, 2003-2010 | 0.93 (0.87, 0.99) | 0.96 (0.88, 1.04) | 0.97 (0.85, 1.11) | 0.96 (0.85, 1.09) | 0.95 (0.87, 1.04) |
| Gender | |||||
| Male | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Female | 0.71 (0.47, 1.09) | 0.81 (0.55, 1.21) | 1.01 (0.53, 1.90) | 0.68 (0.40, 1.15) | 0.98 (0.59, 1.64) |
| Race / Ethnicity | |||||
| Non-Hispanic black | 1.61 (1.00, 2.61) | 1.00 (0.67, 1.49) | 1.03 (0.47, 2.26) | 1.14 (0.61, 2.13) | 0.94 (0.46, 1.88) |
| Non-Hispanic white | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| Hispanic | 0.70 (0.38, 1.29) | 0.83 (0.48, 1.43) | 0.97 (0.29, 3.28) | 0.75 (0.33, 1.67) | 1.15 (0.49, 2.68) |
| Other / unknown | 0.50 (0.22, 1.17) | 0.50 (0.21, 1.19) | 0.37 (0.07, 1.98) | 0.45 (0.14, 1.50) | 3.03 (0.19, 47.34) |
| Prescribed ART | |||||
| No | 1.17 (0.74, 1.86) | 1.00 (0.58, 1.74) | 0.84 (0.34, 2.06) | 1.53 (0.70, 3.35) | 0.43 (0.24, 0.76) |
| Yes | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| HIV-1 RNA (copies/ml) | |||||
| < 400 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
| 400 – 49,999 | 2.42 (1.63, 3.60) | 1.86 (1.33, 2.60) | 1.27 (0.60, 2.69) | 1.67 (1.07, 2.60) | 1.94 (1.18, 3.18) |
| ≥ 50,000 or missing | 8.39 (6.05, 11.62) | 3.93 (2.72, 5.69) | 2.99 (1.42, 6.30) | 4.78 (2.80, 8.16) | 3.43 (1.94, 6.07) |
| CD4 count (cells/microliter) | |||||
| < 200 | 9.78 (6.73, 14.23) | 5.02 (3.25, 7.75) | 5.77 (1.70, 19.58) | 3.68 (2.02, 6.70) | 5.75 (2.66, 12.41) |
| 200 – 749 | 2.10 (1.44, 3.06) | 2.03 (1.44, 2.87) | 2.09 (1.09, 4.00) | 2.09 (1.38, 3.15) | 1.80 (0.90, 3.61) |
| ≥ 750 | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) | 1.00 (Ref) |
All adjusted models were adjusted for the covariates seen in the table as well as for HIVRN site with the exception of the adjusted model for 0-4 year-olds. This model failed to converge with the inclusion of HIVRN site.
IRR, incidence rate ratio; aIRR adjusted incidence rate ratio; ART, antiretroviral therapy
In adjusted analyses, hospitalization rates were no longer significantly declining over calendar time for the full group (aIRR=0.96 [0.88, 1.04]) or for any individual age group (Table 2).
Two sensitivity analyses of all-cause hospitalizations revealed no difference in results. Excluding 29 PY which comprised the year of death and the prior year for all persons who died yielded overall hospitalization rates similar to the main analysis (25.1, 13.8, and 29.4 / 100 PY, respectively for 0-4, 5-16, and 17-24 year-olds) and no difference in the pattern of crude and adjusted associations with hospitalization (results not shown). Excluding 163 PY to account for years before death and loss to follow-up again yielded similar overall rates (24.9, 14.0, and 31.5 / 100 PY, respectively) and an unchanged pattern of crude and adjusted associations (results not shown).
Hospitalization Rates Stratified by Diagnostic Category
Table 3 shows the number of hospitalizations and rate within each of the 10 most frequent diagnostic categories stratified by age group. Non-AIDS defining infection was the most common category, accounting for 30%, 36%, and 41% of all admissions for 0-4, 5-16, and 17-24 year-olds, respectively. For each age group, bacterial pneumonia was the first- or second-most frequent individual diagnosis among non-AIDS defining infections. For 0-4 and 17-24 year-olds, ADI was the second-most frequent category. For 5-16 year-olds, psychiatric and oncologic each had the second-highest proportion (9%) of admissions.
Table 3.
Numbers, crude incidence rates, and most frequent individual diagnoses for the 10 most frequent diagnostic categories, 2003-2010
| Diagnostic Category | 0-4 year-olds (90 total hospitalizations) | 5-16 year-olds (356 total hospitalizations) | 17-24 year-olds (253 total hospitalizations) |
|---|---|---|---|
| Non-AIDS Defining Infection | |||
| Hospitalizations, N (%) | 27 (30) | 128 (36) | 104 (41) |
| Unique patients hospitalized, N | 23 | 80 | 45 |
| Hosps / 100 PY (95% CI) | 7.54 (5.14, 11.07) | 5.29 (3.92, 7.15) | 14.05 (9.72, 20.33) * |
| Most freq. diagnosis, N | Cellulitis, 7 | Bacterial pneumonia, 31 | Bacterial pneumonia, 22 |
| 2nd most freq. diagnosis, N | Bacterial pneumonia, 6 | Varicella, 14 | Cellulitis, 12 |
| AIDS Defining Illness | |||
| Hospitalizations, N (%) | 15 (17) | 27 (8) | 48 (19) |
| Unique patients hospitalized, N | 9 | 20 | 22 |
| Hosps / 100 PY (95% CI) | 4.19 (1.87, 9.38) * | 1.12 (0.70, 1.79) | 6.49 (3.85, 10.92) * |
| Most freq. diagnosis, N | Wasting, 5 | Wasting, 9 | Cand esophagitis, 11 |
| 2nd most freq. diagnosis, N | Pneumocystosis, 4; Recurrent bacterial infection, 4 | Recurrent bacterial infection, 8 | M. avium, 10; Pneumocystosis, 10; Recurrent bacterial pneumonia, 10 |
| Symptom-Defined | |||
| Hospitalizations, N (%) | 12 (13) | 29 (8) | 9 (4) |
| Unique patients hospitalized, N | 11 | 23 | 8 |
| Hosps / 100 PY (95% CI) | 3.35 (1.86, 6.03) * | 1.20 (0.78, 1.85) | 1.22 (0.60, 2.48) |
| Most freq. diagnosis, N | Fever, 9 | Fever, 13 | Fever, 4 |
| 2nd most freq. diagnosis, N | Lymphadenopathy, 3 | Lymphadenopathy, 5 | Abdominal pain, 3 |
| Psychiatric | |||
| Hospitalizations, N (%) | 3 (3) | 32 (9) | 10 (4) |
| Unique patients hospitalized, N | 1 | 18 | 8 |
| Hosps / 100 PY (95% CI) | 0.84 (0.12, 5.85) | 1.32 (0.74, 2.37) | 1.35 (0.66, 2.76) |
| Most freq. diagnosis, N | Psychosis NOS, 3 | Depressive disorder NEC, 13 | Depressive disorder NEC, 5 |
| 2nd most freq. diagnosis, N | Major depressive disorder, 8 | Suicidal ideation, 2 | |
| Oncologic | |||
| Hospitalizations, N (%) | 2 (2) | 32 (9) | 1 (0.4) |
| Unique patients hospitalized, N | 2 | 10 | 1 |
| Hosps / 100 PY (95% CI) | 0.56 (0.14, 2.22) | 1.32 (0.55, 3.18) | 0.14 (0.02, 0.95) * |
| Most freq. diagnosis, N | Radiotherapy encounter, 2 | Bone cancer, 11 | Lymphoma NEC, 1 |
| 2nd most freq. diagnosis, N | Large cell lymphoma, 8 | ||
| Pulmonary | |||
| Hospitalizations, N (%) | 3 (3) | 10 (3) | 16 (6) |
| Unique patients hospitalized, N | 3 | 7 | 11 |
| Hosps / 100 PY (95% CI) | 0.84 (0.27, 2.56) | 0.41 (0.18, 0.95) | 2.16 (1.10, 4.24) * |
| Most freq. diagnosis, N | Asthma, 1; Nasal disease NEC, 1; Acute respiratory failure NOS, 1 | Asthma, 3 | Spontaneous pneumothorax, 7 |
| 2nd most freq. diagnosis, N | Acute respiratory failure NOS, 2 | Asthma, 4 | |
| Endocine / Metabolic / Nutritional / Immune | |||
| Hospitalizations, N (%) | 10 (11) | 10 (3) | 7 (3) |
| Unique patients hospitalized, N | 7 | 8 | 6 |
| Hosps / 100 PY (95% CI) | 2.79 (1.25, 6.23) * | 0.41 (0.19, 0.90) | 0.95 (0.41, 2.16) |
| Most freq. diagnosis, N | Failure to thrive (infant), 6 | Hypovolemia, 6 | Hypovolemia, 3 |
| 2nd most freq. diagnosis, N | Hypovolemia, 4 | Hyperkalemia, 1 | Fluid overload, 1 |
| Gastrointestinal / Liver | |||
| Hospitalizations, N (%) | 6 (7) | 13 (4) | 6 (2) |
| Unique patients hospitalized, N | 6 | 12 | 6 |
| Hosps / 100 PY (95% CI) | 1.68 (0.76, 3.69) * | 0.54 (0.30, 0.96) | 0.81 (0.37, 1.77) |
| Most freq. diagnosis, N | Non-infectious gastroenteritis NEC, 2 | Non-infectious gastroenteritis NEC, 5 | Salivary gland abscess, 2 |
| 2nd most freq. diagnosis, N | Salivary gland abscess, 1 | Appendicitis, 2 | Appendicitis, 1 |
| Cardiovascular | |||
| Hospitalizations, N (%) | 0 | 16 (4) | 6 (2) |
| Unique patients hospitalized, N | 8 | 4 | |
| Hosps / 100 PY (95% CI) | 0.66 (0.30, 1.43) | 0.81 (0.29, 2.25) | |
| Most freq. diagnosis, N | Subdural hemorrhage, 4 | Chest pain, 2 | |
| 2nd most freq. diagnosis, N | Thromboembolic stroke, 2 | Thromboembolic stroke, 1 | |
| Renal / Genitourinary | |||
| Hospitalizations, N (%) | 0 | 15 (4) | 7 (3) |
| Unique patients hospitalized, N | 8 | 5 | |
| Hosps / 100 PY (95% CI) | 0.62 (0.26, 1.46) | 0.95 (0.35, 2.59) | |
| Most freq. diagnosis, N | Hypertensive chronic renal disease, 5 | Renal failure NOS, 5 | |
| 2nd most freq. diagnosis, N | Chronic† renal disease, 3 | Chronic† renal disease, 1 |
P < 0.05 for difference in rate compared to 5 – 16 year-olds
Chronic renal disease includes end-stage renal disease
Note: For ages 0-12 years-old, recurrent bacterial infection referred to any recurrent (within 2 years) serious bacterial infection as per the Centers for Disease Control and Prevention Classification. For ages 13 and older, this AIDS defining illness was recurrent bacterial pneumonia.
NOS, not otherwise specified; NEC not elsewhere classified
Cardiovascular accounted for 0, 4%, and 2% of hospitalizations among 0-4, 5-16, and 17-24 year-olds, respectively. Three (14%) of 22 total cardiovascular hospitalizations were for thromboembolic strokes, and there were no other arterial infarctions. Renal accounted for 0, 4%, and 3% of hospitalizations among 0-4, 5-16, and 17-24 year-olds, respectively. Ten (45%) of 22 total renal hospitalizations were for renal failure NOS or for chronic (including end-stage) renal disease.
Compared to 5-16 year-olds, 17-24 year-olds had significantly higher crude hospitalization rates for non-AIDS defining infection, ADI, and pulmonary admissions, and 0-4 year-olds had significantly higher rates for ADI, symptom-defined, endocrine / metabolic / nutritional / immune, and gastrointestinal / liver admissions (Table 3).
Calendar time trends for the 10 most frequent diagnostic categories were examined stratified by age group (Supplemental Figure 1). Significantly decreasing trends were as follows: among 5-16 year-olds for non-AIDS defining infections, ADI, and cardiovascular; and among 17-24 year-olds for non-AIDS defining infections and ADI. No decline was seen for psychiatric admissions for any age group. Finally, there were no increasing time trends within any diagnostic category for any age group, including no increases for cardiovascular and renal admissions.
DISCUSSION
The declining hospitalization rates among 5-16 and 17-24 year-olds demonstrate continued improvement in morbidity among PHIV patients since the advent of combination ART.6, 24-26 There were reasons to suspect that overall morbidity may not have improved. Nationally representative data have indicated that absolute numbers of hospitalizations among HIV-infected US children were stable or even increasing during 2000-2003.24 Incidence of pneumonia, bacteremia, and several common ADI were stable 2001-2004 in a U.S. cohort of 2800 HIV-infected children.27
Few prior data are available on hospitalization trends among PHIV patients in the current ART era. Declining all-cause hospitalizations through 2003 have been evident in at least two other multicenter US cohorts and in a Spanish cohort.25, 28, 29 Data through 2006 from a large cohort of PHIV children in the United Kingdom and Ireland show an approximately 30% decline in the all-cause hospitalization rate for 2003-2006 vs. 2000-2002.6
We suspect several factors may have contributed to the declining hospitalization trends among 5-16 and 17-24 year-olds. Twelve new antiretroviral drugs, including 4 new drug classes and several drugs with pediatric indications, were licensed during 2003-2010 and may have contributed greatly to the improvement in virologic suppression.30 During the study interval, our sites employed clinical strategies that have shown efficacy in other studies; these include outreach nurses and case managers, directly observed therapy, and age-appropriate methods of engagement including peer support, text messaging, and web-based tools.31-35
The reasons for the stable hospitalization rate among 0-4 year-olds are not clear. One issue may be providers’ lower threshold to hospitalize infants and young children regardless of HIV status. For example for a fever, a provider may hospitalize an infant but pursue outpatient management for an older child.
Despite the improving trends, PHIV children and young adults continue to be hospitalized several times more frequently than occurs in the US general population, where 2010 rates (extrapolated from National Hospital Discharge Survey results) for 0-4, 5-16, and 17-24 year-olds were approximately 5.8, 2.4, and 7.2 / 100 PY, respectively.36 Also, while overall mortality was relatively low at 4%, a third of the cohort was lost to follow-up or spent at least a year out of active care, and 30% of the cohort did not have suppressed HIV-1 RNA in 2010. Hence, PHIV patients remain a group with high overall morbidity and high risk for suboptimal adherence to appointments and medications.
Rates of ADI and non-ADI infection admissions remained unacceptably high even as of 2010. All ADIs and many non-ADI infections should be preventable through early HIV diagnosis and effective ART. In particular, the 15 ADI admissions among 0-4 year-olds likely indicate late diagnosis of HIV and/or late initiation of ART and hence suggest the need to strengthen HIV case finding and care networks for high-risk infants. Use of P. jirovecii and M. avium prophylaxis was sub-optimal, and indicates an area for improvement. Similar to our results, non-ADI infections and ADIs have been the leading reasons for hospitalization in several studies in the current ART era.24, 37, 38
The high overall hospitalization rates for PHIV young adults indicate that intensive efforts and age-appropriate strategies may be particularly important for this age group. In the general population, slightly higher hospitalization rates may be expected for 17-24 year-olds vs. 5-16 year-olds, due, largely, to injuries, mental health and substance abuse, and pregnancy.36, 39 In our sample, non-ADI infections and ADI were the main drivers of the higher hospitalization rates for 17-24 year-olds. Our finding of declining morbidity over time indicates that the diverse risk factors among 17-24 year-olds (antiretroviral resistance, pill burden, risk-taking behavior, independence from parents/guardians) are not insurmountable. Efforts in this group should be maintained and strengthened.
Psychiatric admissions may represent another opportunity for improvement, particularly for PHIV school-aged children. Psychiatric admissions, often for depression, have been frequent in other studies in the current ART era.24, 38 Increased depression screening and access to outpatient treatment may help prevent admissions.
The constancy of cardiovascular and renal hospitalization rates may be slightly reassuring against early severe morbidity from prolonged HIV infection and ART exposure. However, we had relatively few patients, we were unable to track individual medications, and we were unable to assess for complications fully managed in the outpatient setting. Further surveillance for severe cardiovascular and renal complications is warranted given evidence of hospitalizations among adults2, 40 and that PHIV individuals may expect lifelong exposure to HIV and ART.
This study has several limitations. First, our cohort is small compared to adult HIV cohorts. It afforded enough events and accumulated person-time to be able to detect a 47% difference in the incidence of hospitalizations for 0-4 year-olds and a 33% difference for 17-24 year-olds compared to 5-16 year-olds with 80% power (using a two-sample, Poisson-distributed outcome, a 5% Type-1 error, and a two-sided test statistic.) Our cohort is, however, one of the largest available contemporary PHIV cohorts.6, 11, 27-29, 41 Future research may combine existing PHIV cohorts to address questions of trends in diagnostic categories. Second, our results are limited to PHIV who are engaged in outpatient HIV care. Because we do not reliably capture data for out-of-care years, we do not know whether hospitalization patterns in such years would be similar. Third, data capture from healthcare facilities outside of HIVRN treatment sites may be incomplete. However, at one adult HIVRN site, state-wide insurance claims revealed that 91% of all admissions occurred at the home hospital.17 The possible lack of data from outside hospitals would likely create a slight underestimation of hospitalization rates, but it would not likely affect the pattern across diagnostic categories.
Conclusions
Progress in the form of declining non-ADI infections and ADIs among school-age children and young adults is encouraging, but continued efforts are needed to further reduce these still-prevalent conditions. Particular attention should be given to PHIV young adults. Management of mental illness among PHIV patients may also deserve special focus. Finally, while we saw no evidence of increases in cardiovascular and renal disease, further surveillance is warranted.
Supplementary Material
Generation of diagnostic categories
ICD-9 codes used to classify individual diagnoses within diagnostic categories
Trends in hospitalization rates by diagnostic category
Acknowledgments
Sources of Funding: This work was supported by AHRQ (HHSA290201100007C) and the Health Resources and Services Administration (HHSH250201200008C). SAB is supported by the National Institutes of Health (NIH, K23 AI084854); KAG has been a consultant and received research support from Tibotec, has been a consultant for Bristol Myers Squibb, and has received payment for expert testimony to the US government; KNA is supported by the NIH (K01 AI093197); BRY is supported by the NIH (K23 MH097647); PTK is supported by the NIH (K23 DA019809); ALA is supported by the NIH (K23 AI084549);
APPENDIX: HIVRN DETAILS
HIVRN Sites
Alameda County Medical Center, Oakland, California (Howard Edelstein, M.D.)
Children’s Hospital of Philadelphia, Philadelphia, Pennsylvania (Richard Rutstein, M.D.)
Community Health Network, Rochester, New York (Roberto Corales, D.O.)
Drexel University, Philadelphia, Pennsylvania (Jeffrey Jacobson, M.D., Sara Allen, C.R.N.P.)
Fenway Health, Boston, Massachusetts (Stephen Boswell, M.D.)
Johns Hopkins University, Baltimore, Maryland (Kelly Gebo, M.D., Richard Moore, M.D., Allison Agwu M.D.)
Montefiore Medical Group, Bronx, New York (Robert Beil, M.D.)
Montefiore Medical Center, Bronx, New York (Lawrence Hanau, M.D.)
Oregon Health and Science University, Portland, Oregon (P. Todd Korthuis, M.D.)
Parkland Health and Hospital System, Dallas, Texas (Ank Nijhawan, M.D., Muhammad Akbar, M.D.)
St. Jude’s Children’s Hospital and University of Tennessee, Memphis, Tennessee (Aditya Gaur, M.D.)
St. Luke’s Roosevelt Hospital Center, New York, New York (Victoria Sharp, M.D., Stephen Arpadi, M.D.)
Tampa General Health Care, Tampa, Florida (Charurut Somboonwit, M.D.)
University of California, San Diego, California (W. Christopher Mathews, M.D.) Wayne State University, Detroit, Michigan (Jonathan Cohn, M.D.)
Sponsoring Agencies
Agency for Healthcare Research and Quality, Rockville, Maryland (Fred Hellinger, Ph.D., John Fleishman, Ph.D., Irene Fraser, Ph.D.)
Health Resources and Services Administration, Rockville, Maryland (Robert Mills, Ph.D., Faye Malitz, M.S.)
Data Coordinating Center
Johns Hopkins University (Richard Moore, M.D., Jeanne Keruly, C.R.N.P., Kelly Gebo, M.D., Cindy Voss, M.A.)
Footnotes
Conflicts of Interest: RMR, SAS, and RW have none to declare.
References
- 1.Buchacz K, Baker RK, Moorman AC, et al. Rates of hospitalizations and associated diagnoses in a large multisite cohort of HIV patients in the United States, 1994-2005. AIDS. 2008;22:1345–1354. doi: 10.1097/QAD.0b013e328304b38b. [DOI] [PubMed] [Google Scholar]
- 2.Berry SA, Fleishman JA, Moore RD, Gebo KA HIV Research Network. Trends in reasons for hospitalization in a multisite United States cohort of persons living with HIV, 2001-2008. J Acquir Immune Defic Syndr. 2012;59:368–375. doi: 10.1097/QAI.0b013e318246b862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Montaner J, Nosyk B, Colley G, et al. The Evolution of the Cascade of HIV Care: British Columbia, Canada 1996-2010. 2013 [Google Scholar]
- 4.Yehia BR, Fleishman JA, Metlay JP, Moore RD, Gebo KA. Sustained viral suppression in HIV-infected patients receiving antiretroviral therapy. JAMA. 2012;308:339–342. doi: 10.1001/jama.2012.5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Agwu AL, Fleishman JA, Rutstein R, Korthuis PT, Gebo KA. Changes in Advanced Immunosuppression and Detectable HIV Viremia among Perinatally HIV-Infected Youth in the Multisite United States HIV Research Network. J Pediatric Infect Dis Soc. 2013:1–9. doi: 10.1093/jpids/pit008. [DOI] [PubMed] [Google Scholar]
- 6.Judd A, Doerholt K, Tookey PA, et al. Morbidity, mortality, and response to treatment by children in the United Kingdom and Ireland with perinatally acquired HIV infection during 1996-2006: planning for teenage and adult care. Clin Infect Dis. 2007;45:918–924. doi: 10.1086/521167. [DOI] [PubMed] [Google Scholar]
- 7.Foster C, Judd A, Tookey P, et al. Young people in the United Kingdom and Ireland with perinatally acquired HIV: the pediatric legacy for adult services. AIDS Patient Care STDS. 2009;23:159–166. doi: 10.1089/apc.2008.0153. [DOI] [PubMed] [Google Scholar]
- 8.de Mulder M, Yebra G, Navas A, et al. High drug resistance prevalence among vertically HIV-infected patients transferred from pediatric care to adult units in Spain. PLoS One. 2012;7:e52155. doi: 10.1371/journal.pone.0052155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Van Dyke RB, Patel K, Siberry GK, et al. Antiretroviral treatment of US children with perinatally acquired HIV infection: temporal changes in therapy between 1991 and 2009 and predictors of immunologic and virologic outcomes. J Acquir Immune Defic Syndr. 2011;57:165–173. doi: 10.1097/QAI.0b013e318215c7b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blum RW, McNeely C, Nonnemaker J. Vulnerability, risk, and protection. J Adolesc Health. 2002;31:28–39. doi: 10.1016/s1054-139x(02)00411-1. [DOI] [PubMed] [Google Scholar]
- 11.Purswani M, Patel K, Kopp JB, et al. Tenofovir Treatment Duration Predicts Proteinuria in a Multi-Ethnic United States Cohort of Children and Adolescents with Perinatal HIV-1 Infection. Pediatr Infect Dis J. 2012 doi: 10.1097/INF.0b013e31827f4eff. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pontrelli G, Cotugno N, Amodio D, et al. Renal function in HIV-infected children and adolescents treated with tenofovir disoproxil fumarate and protease inhibitors. BMC Infect Dis. 2012;12:18. doi: 10.1186/1471-2334-12-18. 2334-12-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sims A, Frank L, Cross R, et al. Abnormal cardiac strain in children and young adults with HIV acquired in early life. J Am Soc Echocardiogr. 2012;25:741–748. doi: 10.1016/j.echo.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lipshultz SE, Mas CM, Henkel JM, Franco VI, Fisher SD, Miller TL. HAART to heart: highly active antiretroviral therapy and the risk of cardiovascular disease in HIV-infected or exposed children and adults. Expert Rev Anti Infect Ther. 2012;10:661–674. doi: 10.1586/eri.12.53. [DOI] [PubMed] [Google Scholar]
- 15.Judd A, Boyd KL, Stohr W, et al. Effect of tenofovir disoproxil fumarate on risk of renal abnormality in HIV-1-infected children on antiretroviral therapy: a nested case-control study. AIDS. 2010;24:525–534. doi: 10.1097/QAD.0b013e3283333680. [DOI] [PubMed] [Google Scholar]
- 16.Moore RD. Understanding the clinical and economic outcomes of HIV therapy: the Johns Hopkins HIV clinical practice cohort. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;17(Suppl 1):S38–41. doi: 10.1097/00042560-199801001-00011. [DOI] [PubMed] [Google Scholar]
- 17.Gebo KA, Diener-West M, Moore RD. Hospitalization rates in an urban cohort after the introduction of highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2001;27:143–152. doi: 10.1097/00126334-200106010-00009. [DOI] [PubMed] [Google Scholar]
- 18.Elixhauser A, Steiner C, Palmer L. Clinical Classifications Software (CCS) U.S. Agency for Healthcare Research and Quality; 2012. Available: http://www.hcupus.ahrq.gov/toolssoftware/ccs/ccs.jsp. [Google Scholar]
- 19.1994 Revised Classification System for Human Immunodeficiency Virus Infection in Children Less Than 13 Years of Age. MMWR Recomm Rep. 1994;43:1–24. [Google Scholar]
- 20.1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992;41:1–19. [PubMed] [Google Scholar]
- 21. [June 13, 2013];Free only searchable 2009 ICD-9-CM. 2009 icd9cm.chrisendres.com. [Google Scholar]
- 22.StataCorp. Stata Statistical Software: Release 12.1. 2012 [Google Scholar]
- 23.Mofenson LM, Brady MT, Danner SP, et al. Guidelines for the Prevention and Treatment of Opportunistic Infections among HIV-exposed and HIV-infected children: recommendations from CDC, the National Institutes of Health, the HIV Medicine Association of the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the American Academy of Pediatrics. MMWR Recomm Rep. 2009;58:1–166. [PMC free article] [PubMed] [Google Scholar]
- 24.Kourtis AP, Bansil P, Posner SF, Johnson C, Jamieson DJ. Trends in hospitalizations of HIV-infected children and adolescents in the United States: analysis of data from the 1994-2003 Nationwide Inpatient Sample. Pediatrics. 2007;120:e236–43. doi: 10.1542/peds.2006-3268. [DOI] [PubMed] [Google Scholar]
- 25.Viani RM, Araneta MR, Deville JG, Spector SA. Decrease in hospitalization and mortality rates among children with perinatally acquired HIV type 1 infection receiving highly active antiretroviral therapy. Clin Infect Dis. 2004;39:725–731. doi: 10.1086/423178. [DOI] [PubMed] [Google Scholar]
- 26.Sanchez JM, Ramos Amador JT, Fernandez de Miguel S, et al. Impact of highly active antiretroviral therapy on the morbidity and mortality in Spanish human immunodeficiency virus-infected children. Pediatr Infect Dis J. 2003;22:863–867. doi: 10.1097/01.inf.0000091282.70253.5f. [DOI] [PubMed] [Google Scholar]
- 27.Gona P, Van Dyke RB, Williams PL, et al. Incidence of opportunistic and other infections in HIV-infected children in the HAART era. JAMA. 2006;296:292–300. doi: 10.1001/jama.296.3.292. [DOI] [PubMed] [Google Scholar]
- 28.Bertolli J, Hsu HW, Sukalac T, et al. Hospitalization trends among children and youths with perinatal human immunodeficiency virus infection, 1990-2002. Pediatr Infect Dis J. 2006;25:628–633. doi: 10.1097/01.inf.0000220255.14636.b3. [DOI] [PubMed] [Google Scholar]
- 29.Resino S, Resino R, Maria Bellon J, et al. Clinical outcomes improve with highly active antiretroviral therapy in vertically HIV type-1-infected children. Clin Infect Dis. 2006;43:243–252. doi: 10.1086/505213. [DOI] [PubMed] [Google Scholar]
- 30. [Jun 12, 2013];Drugs@FDA: FDA Approved Drug Products. http://www.accessdata.fda.gov/scripts/cder/drugsatfda/
- 31.Funck-Brentano I, Dalban C, Veber F, et al. Evaluation of a peer support group therapy for HIV-infected adolescents. AIDS. 2005;19:1501–1508. doi: 10.1097/01.aids.0000183124.86335.0a. [DOI] [PubMed] [Google Scholar]
- 32.Parsons GN, Siberry GK, Parsons JK, et al. Multidisciplinary, inpatient directly observed therapy for HIV-1-infected children and adolescents failing HAART: A retrospective study. AIDS Patient Care STDS. 2006;20:275–284. doi: 10.1089/apc.2006.20.275. [DOI] [PubMed] [Google Scholar]
- 33.Berrien VM, Salazar JC, Reynolds E, McKay K. HIV Medication Adherence Intervention Group. Adherence to antiretroviral therapy in HIV-infected pediatric patients improves with home-based intensive nursing intervention. AIDS Patient Care STDS. 2004;18:355–363. doi: 10.1089/1087291041444078. [DOI] [PubMed] [Google Scholar]
- 34.Dowshen N, Kuhns LM, Gray C, Lee S, Garofalo R. Feasibility of Interactive Text message Response (ITR) as a Novel, Real-Time Measure of Adherence to Antiretroviral Therapy for HIV+ Youth. AIDS Behav. 2013;17:2237–2243. doi: 10.1007/s10461-013-0464-6. [DOI] [PubMed] [Google Scholar]
- 35.Shegog R, Markham CM, Leonard AD, Bui TC, Paul ME. “+CLICK”: pilot of a web-based training program to enhance ART adherence among HIV-positive youth. AIDS Care. 2012;24:310–318. doi: 10.1080/09540121.2011.608788. [DOI] [PubMed] [Google Scholar]
- 36.Centers for Disease Control and Prevention (CDC) [June 12, 2013];National Hospital Discharge Survey. 2010 http://www.cdc.gov/nchs/nhds/nhds_tables.htm#number.
- 37.Puthanakit T, Aurpibul L, Oberdorfer P, et al. Hospitalization and mortality among HIV-infected children after receiving highly active antiretroviral therapy. Clin Infect Dis. 2007;44:599–604. doi: 10.1086/510489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kourtis AP, Paramsothy P, Posner SF, Meikle SF, Jamieson DJ. National estimates of hospital use by children with HIV infection in the United States: analysis of data from the 2000 KIDS Inpatient Database. Pediatrics. 2006;118:e167–73. doi: 10.1542/peds.2005-2780. [DOI] [PubMed] [Google Scholar]
- 39.Pfunter A, Wier L, Stocks C. Most Frequent Conditions in U.S. Hospitals, 2010. [June 13, 2013];HCUP Statistical Brief #148. 2013 http://www.hcup-us.ahrq.gov/reports/statbriefs/sb148.pdf.
- 40.Engsig FN, Hansen AB, Gerstoft J, Kronborg G, Larsen CS, Obel N. Inpatient admissions and outpatient visits in persons with and without HIV infection in Denmark, 1995-2007. AIDS. 2010;24:457–461. doi: 10.1097/QAD.0b013e328332828d. [DOI] [PubMed] [Google Scholar]
- 41.Dollfus C, Le Chenadec J, Faye A, et al. Long-term outcomes in adolescents perinatally infected with HIV-1 and followed up since birth in the French perinatal cohort (EPF/ANRS CO10) Clin Infect Dis. 2010;51:214–224. doi: 10.1086/653674. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Generation of diagnostic categories
ICD-9 codes used to classify individual diagnoses within diagnostic categories
Trends in hospitalization rates by diagnostic category


