Abstract
Emerging evidence points to proteoglycans abnormalities in the pathophysiology of schizophrenia (SZ). In particular, markedly abnormal expression of chondroitin sulfate proteoglycans (CSPGs), key components of the extracellular matrix, was observed in the medial temporal lobe. CSPG functions, including regulation of neuronal differentiation and migration, are highly relevant to the pathophysiology of SZ. CSPGs may exert similar functions in the olfactory epithelium (OE), a continuously regenerating neural tissue that shows cell and molecular abnormalities in SZ. We tested the hypothesis that CSPG expression in OE may be altered in SZ. CSPG-positive cells in postmortem OE from nonpsychiatric control (n=9) and SZ (n=10) subjects were counted using computer-assisted light microscopy. ‘Cytoplasmic’ CSPG (c-CSPG) labeling was detected in sustentacular cells and some olfactory receptor neurons (c-CSPG+ORNs), while ‘pericellular’ CSPG (p-CSPG) labeling was found in basal cells and some ORNs (p-CSPG+ORNs). Dual labeling for CSPG and markers for mature and immature ORNs suggests that c-CSPG+ORNs correspond to mature ORNs, and p-CSPG+ORNs to immature ORNs. Previous studies in the same cohort demonstrated that densities of mature ORNs were unaltered (Arnold et al, 2001). In the present study, numerical densities of c-CSPG+ORNs were significantly decreased in SZ (p <0.025; 99.32% decrease), suggesting a reduction of CSPG expression in mature ORNs. Previous studies showed a striking increase in the ratios of immature neurons with respect to basal cells. In this study, we find that the ratio of p-CSPG+ORNs/ CSPG+ basal cells was significantly increased (p=0.03) in SZ, while numerical density changes of p-CSPG+ORNs (110.71% increase) or CSPG+ basal cells (53.71% decrease), did not reach statistical significance. Together, these results indicate that CSPG abnormalities are present in the OE of SZ and specifically point to a reduction of CSPGs expression in mature ORNs in SZ. Given the role CSPG play in OE cell differentiation and axon guidance, we suggest that altered CSPG expression may contribute to ORN lineage dysregulation, and olfactory identification abnormalities, observed in SZ.
Keywords: Schizophrenia, extracellular matrix, chondroitin sulfate proteoglycans, olfactory epitheliu, postmortem
1. INTRODUCTION
Chondroitin sulfate proteoglycans (CSPGs) play a key role in developmental and adult functions, such as axon guidance, cell adhesion, differentiation and migration, maturation of synapses and regulation of neurotransmitter receptor availability (Frischknecht et al., 2009; Maeda et al., 2010; Meyer-Puttlitz et al., 1996). These functions bear direct relevance to the pathophysiology of schizophrenia (SZ), a disease with a strong neurodevelopmental component (e.g. Arnold and Rioux, 2001; Harrison, 2007). Recently, significant CSPG expression anomalies have been detected in this disease (Buxbaum et al., 2008; Enwright et al., 2012; Mauney et al., 2013; Pantazopoulos et al., 2010). In particular, CSPG-enriched perineuronal nets were decreased in several brain regions, often in association with marked increased of CSPG-positive glial cells (Enwright et al., 2012; Mauney et al., 2013; Pantazopoulos et al., 2010). Together, these abnormalities have been postulated to disrupt neurodevelopmental processes, including neuronal migration, circuit formation and consolidation (Berretta, 2012).
CSPG developmental functions are thought to play a key role throughout life in the olfactory epithelium (OE), a neural structure in which neuronal differentiation, migration and axon outgrowth occur robustly throughout life (Clarris et al., 2000; Schwob, 2002). The adult OE contains stem cells that retain the capacity to divide and differentiate into mature olfactory receptor neurons (ORNs)(Schwob, 2002). During the course of their maturation, newly formed ORN axons join odor-specific axon bundles to reach the corresponding olfactory bulb glomeruli (Beites et al., 2005; Graziadei, 1973; Yoshihara and Mori, 1997). In the OE and olfactory bulb, development-specific patterns of CSPG expression help position ingrowing olfactory axons in the glomerular layer and maintain glomerular integrity (Clarris et al., 2000; Gonzalez and Silver, 1994). CSPG role in regulating brain cell differentiation (Purushothaman et al., 2012; Yanagisawa and Yu, 2007) suggests that they may contribute to OE basal cells differentiation and their maturation into ORNs.
OE abnormalities observed in SZ are consistent with CSPG dysregulation. Primary cell lines from OE biopsies were reported to have reduced adhesion properties and altered cell proliferation in SZ (Fan et al., 2012; Feron et al., 1999; McCurdy et al., 2006). Notably, disturbances of OE cell cycle include lower density of basal cells and increase of post-mitotic immature ORNs, providing strong support for a dysregulation of OE neuronal lineage (Arnold et al., 2001; Feron et al., 1999; McCurdy et al., 2006; Perry et al., 2002). Taken together, CSPG anomalies in several neural regions and abnormalities in the OE of SZs, suggest a disruption of key CSPG functions in this structure. We tested the hypothesis that CSPG expression may be disrupted in the OE of subjects with SZ. A broad spectrum CSPG histological marker, i.e. wisteria floribunda agglutinin (WFA) was used for group comparisons in postmortem OE tissue; antibodies raised against phosphacan and versican V0/V1, CSPGs suspected to be involved in SZ and to be expressed in the OE, were added for normal investigations on cell-specific CSPG distribution (Buxbaum et al., 2008; Clarris et al., 2000; Popp et al., 2003; Takahashi et al., 2011)(Pantazopoulos et al., unpublished observations).
2. METHODS
2.1. Human Subjects and Tissue Processing
2.1.1. Postmortem and Biopsy Human OE Tissue for Normal Study
Postmortem
A tissue block containing the OE, cribriform plate, olfactory bulbs, lateral nasal walls and septum from a healthy control subject (male, 80 years old) was obtained from National Disease Research Interchange. The tissue block was processed as previously described (Holbrook et al., 2011). Sections were cut at 10 µm and mounted on super frost plus slides.
Biopsy
Tissue samples containing the OE from two healthy controls (males – 23 and 41 years of age) were obtained by biopsy under local anesthesia. Medical history, current medical status and absence of psychiatric disorders (Structured Clinical Interview for DSM disorders, SCID) were recorded for each subject. Protocols for recruitment, consent, and biopsy were approved by the Institutional Review Boards of McLean Hospital and Massachusetts Eye and Ear Infirmary. Subjects provided written informed consent prior to their inclusion in the study. Biopsy samples were postfixed in 4% paraformaldheyde for 1 hour, cryoprotected in 30% sucrose overnight and sectioned on a cryostat (10 μm).
2.1.2. Postmortem Human OE Tissue for Group Comparisons
Postmortem OE tissue was collected from 10 chronic SZ patients and 9 age- and sex-matched non-psychiatric controls. All subjects with SZ were prospectively accrued from two state hospitals in Pennsylvania and were clinically assessed and diagnosed according to DSM-IV criteria by research psychiatrists of the University of Pennsylvania’s Schizophrenia Mental Health Clinical Research Center, Philadelphia, as previously described (Arnold et al., 2001). This involved a standardized medical record review of demographic variables, presenting and subsequent symptoms, treatment history, medical history, caregivers’ interview and laboratory and neuroimaging findings. Based on all information, diagnoses and inclusion were established by research team consensus. Non-psychiatric controls were obtained through the University of Pennsylvania’s Alzheimer Disease Core Center. Review of clinical histories found no evidence of prior major psychiatric or neurological illnesses. Gross and microscopic diagnostic neuropathologic examinations of multiple cortical and subcortical regions revelead no evidence for changes consistent with Alzheimer’s disease or cerebrovascular accidents in any of the subjects included in this cohort. At autopsy, the nasal epithelium, bony septae, and contiguous cribriform plate, was removed en bloc and processed as above (Arnold et al., 2001).
2.2. Histochemistry and Immunohistochemistry
Single Immunohistochemistry/Histochemistry for CSPGs
Tissue sections were blocked in 2% bovine albumin serum (BSA). For immunohistochemistry, sections were incubated for 48 hr in primary antibodies (see below), then for 2 hours at room temperature in biotinylated secondary antibody (1: 500 goat anti-rabbit, Vector Labs BA-1000, lot x0212); for histochemistry, sections were incubated in Wisteria Floribunda agglutinin (WFA; see also Supplementary Material) in 1% BSA for 24 h at 4 °C. For both immunocytochemistry and histochemistry, sections were then incubated in streptavidin (1:5000; Invitrogen 434323, lot 830799A). Nickel-enhanced diaminobenzidine/peroxidase reaction (0.02% diaminobenzidine (Sigma-Aldrich, St. Louis, MO), 0.08% nickel-sulfate, 0.006% hydrogen peroxide) was used to visualize the reaction product. Solutions for all the steps above were made in phosphate buffer/saline with 0.5% Triton X (PBS–Tx). Each step was followed by washes in the same solution. Antibodies: receptor tyrosine phosphate zeta (RPTPZ)/Phosphacan (1:2000; Abcam ab126497, lot# GR79986; immunogen was a synthetic peptide corresponding to amino acids 831-978 of the human RPTPZ/phosphacan sequence - made in rabbit); glycosaminoglycan beta (GAGbeta)/Versican V0/V1 (1:8000; gift from M.T. Dours-Zimmermann and D.R. Zimmermann, Univ. of Zurich, Switzerland - identifies the C-terminus amino acids 2646-3088 of human versican – made in rabbit) reported to label both the V0 and V1 versican isoforms (Dours-Zimmermann and Zimmermann, 1994). Biotinylated WFA (1:1000; Vector Labs, # B-1355, lot W0103, Burlingame, CA).
Dual Antigen Immunofluorescence
Double labeling for mature ORNs and CSPGs was carried out using a primary antibody raised in goat against olfactory marker protein (OMP), (1:12000, Wako, #544-10001, lot 1UP-1001, Richmond VA) in combination with biotinylated WFA (1:2000, Vector Labs). Immature ORNs were labeled using a primary antibody raised in mouse against growth associated protein 43 (GAP-43) (1:500, Millipore, clone 9-1E12 # MAB347, lot NG1869850, Temecula CA), in combination with WFA. After blocking in 2% BSA, sections were incubated in primary antibodies with 2% BSA at 4 °C for 48 hr, followed by a combination of either Alexa Fluor donkey anti-goat 594 (1:250, Invitrogen #A11058, lot 1003216, Grand Island, NY) and Alexa Fluor Streptavidin 488 (1:3000, Invitrogen #S-11223), or Alexa Fluor donkey anti-mouse 594 (1:250, Invitrogen A21203 lot 987237) and Streptavidin 488 (1:3000, Invitrogen #S-11223). Sections were washed with 0.1M PB and coverslipped with Prolong Gold antifade mounting media with DAPI (Invitrogen P-36931). Solutions for all the steps above were made in PBS with 0.5% Triton X.
2.3. Data collection
2.3.1. CSPG-Positive Cell Phenotype in Normal Human OE
Sections from the OE (3-4 sections per subject), dual labeled for CSPGs (WFA) in combination with OMP or GAP43 from 3 control subjects (two biopsy and one postmortem sample) were examined using a Zeiss Axioskop II plus fluorescence imaging system, interfaced with Stereo-Investigator 6.0, (MicroBrightField, Inc., Williston, VT). OE areas in each section, interspersed among respiratory epithelium (RE) patches, were identified according to established structural and cytoarchitectonic criteria (Holbrook et al., 2011; Moran et al., 1982; Morrison and Costanzo, 1990) (compare Fig. 1 A, B to Fig. 3A). OE areas were scanned at 40x throughout the extent of the x,y, and z-axes to count CSPG-positive (CSPG+) cells with cytoplasmic or pericellular labeling, and their expression, or lack thereof, of OMP or GAP43. Cytoplasmic and pericellular WFA labeling was confirmed on a subset of sections at 63x using a Leica TCS SP8 confocal imaging system (z-axis resolution: 1 µm through the extent of the tissue section; Fig. 1 C,D). Cell morphology and immunolabeling distribution of Versican V0/V1 and RPTPZ/Phosphacan (Fig. 2) were analyzed under 40x magnification.
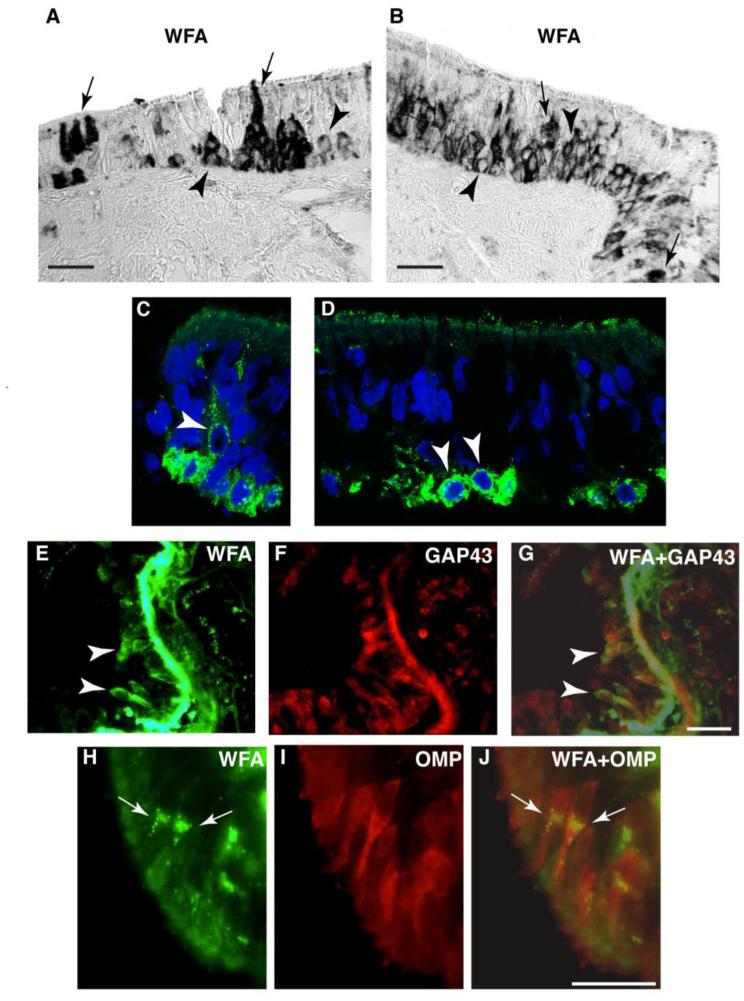
Fig. 1. Patterns of CSPG expression in the normal human OE.
In normal human OE, CSPG labeling using the broad spectrum marker WFA shows two distinct labeling patterns in OE cells: pericellular CSPG (p-CSPG) labeling (arrow heads), and cytoplasmic CSPG (c-CSPG) labeling (arrows). In A and B, light microscopy photomicrographs show examples of these two labeling patters. High resolution fluorescent confocal images of WFA labeled cells (green) and DAPI labeled nuclei (blue) confirm this pattern (C, D). In E-G, dual fluorescence labeling combining WFA with GAP43, prevalently expressed in immature ORNs, shows that p-CSPG+ ORNs correspond to GAP43+ORNs (arrow heads). In contrast, dual fluorescence labeling combining WFA with OMP, which typically labels mature ORNs, shows that c-CSPG+ ORNs correspond to OMP+ORNs (H-J; arrows). Together, these findings indicate that i) basal cells present with a pericellular pattern of CSPG/WFA; ii) immature ORNs maintain the p-CSPG expression pattern; iii) mature ORNs appear to transition to a prevalent cytoplasmic pattern of CSPG expression. Scale bar, 50 µm
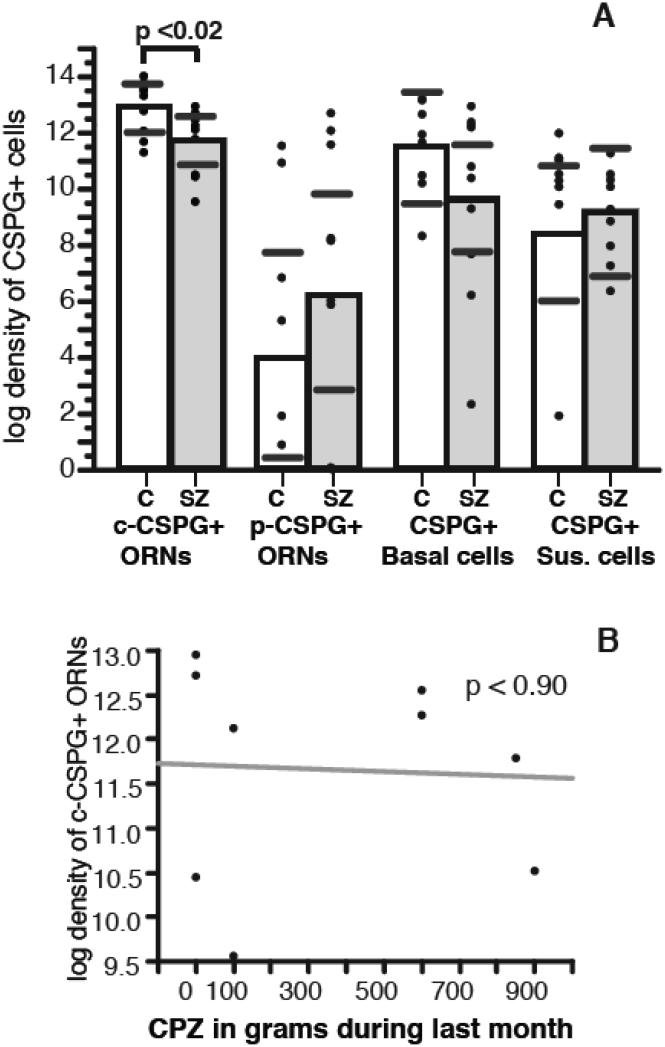
Fig. 3. Densities of c-CSPG+ORNs are decreased in the OE of SZ subjects.
Numerical densities of c-CSPG+ORNs were significantly decreased (p= 0.02) in the OE of subjects with SZ, as compared to non-psychiatric control subjects (A). Increases of p- CSPG+ORNs and decreases of CSPG+ basal cells did not reach statistical significance. Significance values are derived from stepwise linear regression models testing potential effects of confound variables. Scatterplots show the mean (black line) and 95% confidence intervals (grey lines). No significant correlation was observed between c-CSPG+ ORNs and chlorpromazine normalized (CPZ) dose of antipsychotics (B).
Fig. 2. Versican V0/V1 and phosphacan expression in normal human OE.
Light microscopy photomicrographs of normal human OE stained for versican V0/V1 and phosphacan. Versican V0/V1 showed cytoplasmic expression (arrows) similar to that detected in mature ORNs. Phosphacan predominantly showed pericellular expression patterns (arrow heads) strongly resembling that observed with WFA in immature ORNs and basal cells.
2.3.2. CSPG-positive cells in OE and RE - Group comparison
Slides were coded for analysis blind to diagnosis. Computer-assisted light microscopy interfaced with stereology quantification software (see above) was used for data collection. Two to four sections per subject were available for quantification. The borders between the OE and RE areas were drawn as described above. Sections were scanned through the extent of the x, y, and z axes within each area under 40x magnification and all CSPG-labeled cells were counted. Intra-rater (H.P.) reliability of at least 95% was established before the actual quantification process begun and assessed on a regular basis throughout the quantification process. Morphologically distinct CSPG+ cell categories were identified in the OE (see Holbrook et al., 2011; Moran et al., 1982; Morrison and Costanzo, 1990). CSPG+ ORNs were further subdivided in two groups according to their pericellular (p-CSPG+) or cytoplamic (c-CSPG+) labeling pattern (see Fig. 1 and Results). In RE, CSPG+ cells corresponded almost exclusively to basal cells (Fig. 4A).
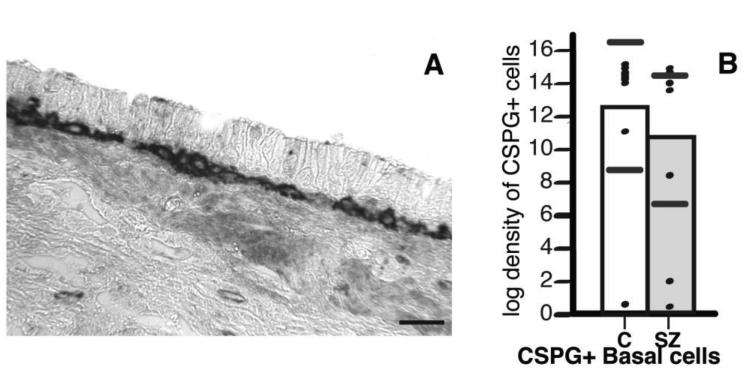
Fig. 4. Numerical densities of CSPG+ cells were not significantly different in the RE of SZ subjects.
CSPG+ cells in RE corresponded almost exclusively to basal cells. In (A), examples of RE histological stained for CSPGs (WFA) show dense black labeling. Scale bar, 50 µm. (B) No differences of numerical density of CSPG+ basal cells, and RE cells were observed between SZ and non-psychiatric control subjects. Statistical analyses were performed using stepwise linear regression models. Scatterplots show the mean (black line) and 95% confidence intervals (grey lines).
2.4. Statistical analysis
For the purpose of group comparisons, numerical densities of CSPG+ cells for each subject were the main outcome measure (sum of labeled cells divided by the sum of the areas of OE or RE). Total numbers of cells were not calculated because the anatomical characteristics of the OE, which is constituted of islands intermingled with RE (Holbrook et al., 2011), do not allow reliable total volume estimates. Statistical significance of differences between groups relative to the main outcome measures were assessed using a stepwise linear regression process. A logarithmic transformation was uniformly applied to all raw values because the data was not normally distributed. Statistical analyses were performed using JMP v5.0.1a (SAS Institute Inc., Cary, NC). Age, gender, postmortem time interval (PMI), brain weight, and daily averages of antipsychotic drugs taken during the last month of life, expressed as chlorpromazine-equivalent (CPZ) dose, were tested systematically for their effects on numerical densities and included in the model if they significantly improved the model goodness-of-fit. In addition, potential effects of exposure to antipsychotics, as well as age at the onset of the disease and duration of the illness, were tested in separate linear correlation analyses (see also Supplementary Information).
3. RESULTS
3.1. CSPG Expression in Normal Human OE
In the OE, two clearly distinguishable patterns of CSPG labeling, pericellular and intracellular, were detected. Sustentacular cells and one subgroup of ORNs (Fig. 1 A-D, H-J) showed intracellular CSPG labeling in a granular pattern within the cytoplasm, often more intense near the nucleus (Fig. 1 H-J). In contrast, basal cells and a second subgroup of ORNs presented with pericellular CSPG labeling (Fig. 1 A-D). The similarity of CSPG labeling pattern in basal cells and a subset of ORNs raised the possibility that the latter may correspond to immature, GAP43-positive, ORNs. Dual immunofluorescence labeling of normal OE confirmed this possibility. The large majority (89.4 %) of p-CSPG+ORNs expressed GAP43 (Fig. 1 E-G), a protein prevalently contained in immature ORNs (Hahn et al., 2005; Verhaagen et al., 1989), while only 1.9% of c-CSPG+ORN was GAP43-positive. Conversely 91.2% of c-CSPG+ORN expressed OMP (Fig. 1 H-J), a marker for mature ORNs (Farbman and Margolis, 1980; Hahn et al., 2005), while 15.0% of p-CSPG+ORNs were found to be OMP-positive. Finally, we tested whether the two different patterns of CSPG cellular distribution may reflect expression of distinct CSPGs. Immunolabeling for RPTPz/phosphacan showed predominant pericellular ORN and basal cell labeling (Fig. 2 B), while versican V0/V1 (GAGbeta) presented nearly exclusively with intracellular ORN labeling (Figure 2A).
3.2. CSPG-Positive Cells in the OE and RE of Subjects with SZ
In the OE, numerical densities of c-CSPG+ ORNs were significantly decreased in subjects with SZ compared to control subjects (step-wise linear regression analysis; p <0.025; 99.32% decrease; Fig. 3A). Numerical density increases of p-CSPG+ ORNs (110.71%) and decreases of CSPG+ basal cells (53.71%) in SZ subjects, did not reach statistical significance (Fig. 3A). The ratio of p-CSPG+ ORNs / CSPG+ basal cells significantly increased in subjects with SZ (p=0.038 t=2.31). Ratios of c-CSPG+ ORNs/CSPG+ basal cells and p-CSPG+ ORNs/c-CSPG+ ORNs were not altered. Numerical densities of CSPG+ sustentacular cells were not altered in subjects with SZ (Fig. 3A). In the RE, numerical densities of CSPG+ basal cells were similar in the two groups (Fig. 4B).
None of potential confounding variables tested with stepwise linear regression models showed significant effects. Antipsychotic exposure (Fig. 3B) and other potential disease-related factors, such as age at onset of the illness and duration of the illness, were also tested using linear correlation analysis (see also Supplementary Information). A positive correlation of c-CSPG+ ORNs with years of illness suggest that decreases of these cells are not due to non-specific effects of chronic illness.
4. DISCUSSION
These findings show, to our knowledge for the first time, that CSPGs are expressed in mature and immature ORNs, sustentacular cells and basal cells in the adult human OE, with a distinct, cell specific, localization pattern. Furthermore, our results represent the first evidence for CSPG abnormalities in the OE of SZ subjects. Thus, we show that these abnormalities encompass not only distinct CNS regions but also peripheral sensory structures, potential contributing to functional sensory impairment (Moberg et al., 1999; Turetsky et al., 2009). These abnormalities prevalently affect cells putatively corresponding to mature ORNs. This finding suggest that, in addition to CSPG+ glial cells and perineuronal nets anomalies previously detected in the CNS, CSPG pathology in SZ may also affect maturing neurons, disrupting ORN maturation and olfactory system connectivity and, potentially, brain development. Below we discuss the possibility that these abnormalities may reflect a disregulation of CSPG expression in the OE of subjects with SZ, and put forth the hypothesis that they may contribute to a disruption of cell lineage in OE in SZ (Arnold et al., 2001).
4.1 CSPG expression in normal human OE
The observed pericellular pattern of CSPG labeling (WFA and phosphacan) in basal cells is highly reminiscent of that of CSPG expression in neural stem/cell progenitors (NSCPs) in stem cell niches of the embryonic and adult brain (Ito et al., 2005; von Holst et al., 2006). In these cells, surface expression of CSPGs was found to be essential for proliferation, cell renewal and responsivity to growth factors that determine maturation, differentiation and migration (Sirko et al., 2010; von Holst et al., 2006). We suggest that the p-CSPG labeling in OE basal cells may mediate similar functions, such as proliferation of basal cells and their differentiation into immature ORNs. In mature ORNs, WFA/CSPG expression was instead found predominantly in the cytoplasm. Versican, which is also expressed in the cytoplasm of neurons in the brain, showed a similar pattern. Thus, our results suggest that ORN maturation involves a transition of CSPG type and distribution: from pericellular phosphacan expression to versican v0/v1 expression in the cytoplasm. The functional implications of the transition between phosphacan and versican can only be inferred on the basis of current knowledge on brain CSPG functions, because their expression in the adult OE has not been reported before. We suggest that this transition may involve a shift in the ORNs interactions with other cells and the extracellular environment (Carulli et al., 2005; Dityatev et al., 2010; Rhodes and Fawcett, 2004). While electron microscopy will determine the ultrastructural localization of CSPGs in OE cells, for the purpose of this study, we use the term ‘pericellular’ to indicate CSPG labeling at the cell periphery, as clearly distinct from CSPG labeling pattern detected in mature ORNs.
4.2 CSPG abnormalities in SZ
Comparisons between non-psychiatric control and SZ subjects show a significant decrease of c-CSPG+ ORN, putatively corresponding to mature (OMP+) ORNs. Previous results from a largely overlapping set of OE samples (Arnold et al., 2001), showed no change, or perhaps an increase, of OMP+ ORNs in the OE of subjects with SZ. Together, these findings suggest that decreased c-CSPG+ ORN densities reflect reduced CSPG expression in mature ORNs, rather than a decrease of mature ORNs. This reduction may be due to decreased synthesis and/or failure of these cells to fully transition to a mature pattern of CSPG expression. A significant increase of the p-CSPG+ORN/CSPG+basal cell ratio, consistent with increased ratio of immature ORN/basal cells reported by Arnold et al. (Arnold et al., 2001), supports the latter possibility and may offer a clue on a potential contributing mechanism. As mentioned above, cell surface CSPG expression in NSCPs is needed for cell differentiation and maturation, mediated by interactions with growth factors (Sirko et al., 2010). Altered p- CSPG+ORN/CSPG+ basal cell ratio in SZ may reflect a disruption of this process, and thus ORN’s failure to transition from p-CSPG to a mature c-CSPG expression patterns. Overall, these findings add to evidence for a disruption of OE cell lineage in SZ and raise the possibility that abnormal CSPG expression may contribute to such disruption. It should be recognized, however, that while the discrepancy between c-CSPG-ORNs decreases in this study and normal density of OMP+ORNs previously reported in the same subject cohort (Arnold et al., 2001) indicates reduced CSPG expression in these neurons, results from the 2001 study do not allow to unambiguously interpret increased ratios of pCSPG+ORNs/CSPG+basal cells in SZ as altered CSPG expression in immature ORNs and basal cells.
On a speculative level, we postulate that aberrant CSPG expression in ORNs may disrupt ORN axonal outgrowth and guidance, as suggested by preclinical studies (Clarris et al., 2000). CSPGs, and versican in particular, promote axon outgrowth (Klausmeyer et al., 2011; Maeda, 2010; Wu et al., 2004). CSPGs have been shown to play a key role in guiding ORN axons across the lamina propria and through the lamina cribrosa, and eventually forming bundles segregated by odor specificity and reaching odor specific glomeruli in the olfactory bulb (Belluscio and Katz, 2001; Gonzalez and Silver, 1994; Hayar et al., 2004; Tisay and Key, 1999; Yoshihara and Mori, 1997). It is conceivable that CSPG abnormalities in maturing ORNs may impact on axonal outgrowth and guidance resulting in mismatched and/or disorganized synaptic formation between the OE and the olfactory bulb. This phenomenon may contribute robust olfactory deficits detected in subjects with SZ (Atanasova et al., 2008; Brewer et al., 2001; Kopala et al., 1993; Moberg et al., 1999).
Caution in interpreting these results is necessary given the small sample size (9 normal control and 10 SZ subjects for the group comparison study). Although the sample size in this study is small, it is comparable to a similar published report using postmortem OE samples from SZ (13) and control (10) subjects (Arnold et al., 2001).
5. Conclusions
In summary, these results show CSPG abnormalities in the OE of SZ subjects. Given the role CSPGs play in cell differentiation and ORN neurite outgrowth and axon guidance, altered CSPG expression may contribute to cell lineage and olfactory identification abnormalities detected in SZ (Arnold et al., 2001; Turetsky et al., 2008). These results further suggest that CSPG pathology may be widespread within the nervous system and detectable in tissue, such as the OE, that can be harvested by biopsy. Future studies will use this approach to test mechanisms of CSPG abnormalities in SZ and their association with specific core clinical aspects of this disorder.
Supplementary Material
Table 1.
Sample Demographic and Descriptive Characteristics – Comparison Study
Data relative to subject cohort used for comparison studies. Brain weight expressed in grams. Abbreviations: CPZ, chlorpromazine-equivalent (CPZ) dose (expressed in average mg/day during the last month of life); Dx, diagnosis; PMI, postmortem interval (hours).
| Dx | Age | Sex | PMI | Brain weight | CPZ last month of life |
Age at onset |
Years of illness |
|---|---|---|---|---|---|---|---|
| SZ | 83 | M | 7 | 1200 | 0 | 21 | 62 |
| SZ | 74 | F | 15 | 1140 | 600 | 18 | 56 |
| SZ | 79 | F | 8 | 1040 | 0 | NK | NK |
| SZ | 75 | F | 16 | 1219 | 900 | 23 | 52 |
| SZ | 76 | M | 10 | 1322 | 600 | 20 | 56 |
| SZ | 70 | M | 17 | 1200 | 850 | 32 | 38 |
| SZ | 72 | F | 10 | 1157 | NK | 24 | 48 |
| SZ | 71 | M | 10 | 1188 | 0 | 34 | 37 |
| SZ | 74 | M | 14 | 1260 | 100 | 23 | 51 |
| SZ | 80 | F | 11 | 1060 | 100 | 22 | 58 |
| 10 |
75.4 +/−
4.2 |
5 M/
5 F |
11.8
+/−3.5 |
1178.6
+/−382.4 |
350
+/−382.4 |
24.11
+/−5.4 |
50.89
+/−8.6 |
| C | 43 | M | 30.5 | 1545 | NA | NA | NA |
| C | 73 | M | 8 | 1249 | NA | NA | NA |
| C | 74 | F | 3.5 | 1000 | NA | NA | NA |
| C | 91 | F | 11.5 | 1140 | NA | NA | NA |
| C | 74 | F | 6 | 1250 | NA | NA | NA |
| C | 69 | M | 11 | 1625 | NA | NA | NA |
| C | 63 | M | 5 | 1360 | NA | NA | NA |
| C | 67 | F | 5.5 | 1100 | NA | NA | NA |
| C | 98 | M | 15 | 1340 | NA | NA | NA |
| C | 43 | M | 30.5 | 1545 | NA | NA | NA |
| 9 |
69.5
+/− 17.6 |
5 M/
5 F |
12.7
+/−10 |
1315.4
+/−207.9 |
NA | NA | NA |
Acknowledgements
The authors are grateful to M.T. Dours-Zimmermann and D.R. Zimmermann, Univ. of Zurich, Switzerland for their generous gift of GAGbeta/Versican V0V1 antibody and to NIMH (R01MH091348 to SB) for funding this study.
This work was funded by National Institutes of Health – NIMH R01-MH091348 to Sabina Berretta, M.D.
Abbreviations
- BSA
bovine albumin serum
- CPZ
chlorpromazine-equivalent
- c-CSPG
cytoplasmic chondroitin sulfate proteoglycan
- CSPG
chondroitin sulfate proteoglycan
- GAG
glycosaminoglycan
- GAP43
growth associate protein 43
- NSCP
neural stem/cell progenitor
- OE
olfactory epithelium
- OMP
olfactory marker protein
- ORN
olfactory receptor neuron
- PBS-Tx
phosphate buffer-triton x
- p-CSPG
pericellular chondroitin sulfate proteoglycan
- PMI
postmortem time interval
- RE
respiratory epithelium
- RPTPz
receptor tyrosine phosphatase zeta
- SCID
Structured Clinical Interview for DSM Disorders
- SZ
schizophrenia
- WFA
wisteria floribunda agglutinin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
Harry Pantazopoulos: No conflict
Anne Boyer-Boiteau: No conflict
Eric H. Holbrook: No conflict
Woochan Jang: No conflict
Chang-Gyu Hahn: No conflict
Steven E. Arnold: No conflict
Sabina Berretta: No conflict
REFERENCES
- Arnold SE, Han LY, Moberg PJ, Turetsky BI, Gur RE, Trojanowski JQ, Hahn CG. Dysregulation of olfactory receptor neuron lineage in schizophrenia. Arch Gen Psychiatry. 2001;58(9):829–835. doi: 10.1001/archpsyc.58.9.829. [DOI] [PubMed] [Google Scholar]
- Arnold SE, Rioux L. Challenges, status, and opportunities for studying developmental neuropathology in adult schizophrenia. Schizophr Bull. 2001;27(3):395–416. doi: 10.1093/oxfordjournals.schbul.a006883. [DOI] [PubMed] [Google Scholar]
- Atanasova B, Graux J, El Hage W, Hommet C, Camus V, Belzung C. Olfaction: a potential cognitive marker of psychiatric disorders. Neurosci Biobehav Rev. 2008;32(7):1315–1325. doi: 10.1016/j.neubiorev.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Beites CL, Kawauchi S, Crocker CE, Calof AL. Identification and molecular regulation of neural stem cells in the olfactory epithelium. Exp Cell Res. 2005;306(2):309–316. doi: 10.1016/j.yexcr.2005.03.027. [DOI] [PubMed] [Google Scholar]
- Belluscio L, Katz LC. Symmetry, stereotypy, and topography of odorant representations in mouse olfactory bulbs. J Neurosci. 2001;21(6):2113–2122. doi: 10.1523/JNEUROSCI.21-06-02113.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berretta S. Extracellular matrix abnormalities in schizophrenia. Neuropharmacology. 2012. [DOI] [PMC free article] [PubMed]
- Brewer WJ, Pantelis C, Anderson V, Velakoulis D, Singh B, Copolov DL, McGorry PD. Stability of olfactory identification deficits in neuroleptic-naive patients with first-episode psychosis. Am J Psychiatry. 2001;158(1):107–115. doi: 10.1176/appi.ajp.158.1.107. [DOI] [PubMed] [Google Scholar]
- Buxbaum JD, Georgieva L, Young JJ, Plescia C, Kajiwara Y, Jiang Y, Moskvina V, Norton N, Peirce T, Williams H, Craddock NJ, Carroll L, Corfas G, Davis KL, Owen MJ, Harroch S, Sakurai T, O'Donovan MC. Molecular dissection of NRG1-ERBB4 signaling implicates PTPRZ1 as a potential schizophrenia susceptibility gene. Mol Psychiatry. 2008;13(2):162–172. doi: 10.1038/sj.mp.4001991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carulli D, Laabs T, Geller HM, Fawcett JW. Chondroitin sulfate proteoglycans in neural development and regeneration. Curr Opin Neurobiol. 2005;15(1):116–120. doi: 10.1016/j.conb.2005.01.014. [DOI] [PubMed] [Google Scholar]
- Clarris HJ, Rauch U, Key B. Dynamic spatiotemporal expression patterns of neurocan and phosphacan indicate diverse roles in the developing and adult mouse olfactory system. J Comp Neurol. 2000;423(1):99–111. doi: 10.1002/1096-9861(20000717)423:1<99::aid-cne8>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Dityatev A, Seidenbecher CI, Schachner M. Compartmentalization from the outside: the extracellular matrix and functional microdomains in the brain. Trends Neurosci. 2010;33(11):503–512. doi: 10.1016/j.tins.2010.08.003. [DOI] [PubMed] [Google Scholar]
- Dours-Zimmermann MT, Zimmermann DR. A novel glycosaminoglycan attachment domain identified in two alternative splice variants of human versican. J Biol Chem. 1994;269(52):32992–32998. [PubMed] [Google Scholar]
- Enwright JFI, Arion D, Fish K, Lewis D. Decreased perineuronal net labeling and altered parvalbumin immunoreactivity in the dorsal lateral prefrontal cortex of subjects with schizophrenia Society for Neuroscience. 2012.
- Fan Y, Abrahamsen G, McGrath JJ, Mackay-Sim A. Altered cell cycle dynamics in schizophrenia. Biol Psychiatry. 2012;71(2):129–135. doi: 10.1016/j.biopsych.2011.10.004. [DOI] [PubMed] [Google Scholar]
- Farbman AI, Margolis FL. Olfactory marker protein during ontogeny: immunohistochemical localization. Developmental biology. 1980;74(1):205–215. doi: 10.1016/0012-1606(80)90062-7. [DOI] [PubMed] [Google Scholar]
- Feron F, Perry C, Hirning MH, McGrath J, Mackay-Sim A. Altered adhesion, proliferation and death in neural cultures from adults with schizophrenia. Schizophr Res. 1999;40(3):211–218. doi: 10.1016/s0920-9964(99)00055-9. [DOI] [PubMed] [Google Scholar]
- Frischknecht R, Heine M, Perrais D, Seidenbecher CI, Choquet D, Gundelfinger ED. Brain extracellular matrix affects AMPA receptor lateral mobility and short-term synaptic plasticity. Nat Neurosci. 2009. [DOI] [PubMed]
- Gonzalez ML, Silver J. Axon-glia interactions regulate ECM patterning in the postnatal rat olfactory bulb. J Neurosci. 1994;14(10):6121–6131. doi: 10.1523/JNEUROSCI.14-10-06121.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziadei PP. Cell dynamics in the olfactory mucosa. Tissue Cell. 1973;5(1):113–131. doi: 10.1016/s0040-8166(73)80010-2. [DOI] [PubMed] [Google Scholar]
- Hahn CG, Han LY, Rawson NE, Mirza N, Borgmann-Winter K, Lenox RH, Arnold SE. In vivo and in vitro neurogenesis in human olfactory epithelium. J Comp Neurol. 2005;483(2):154–163. doi: 10.1002/cne.20424. [DOI] [PubMed] [Google Scholar]
- Harrison PJ. Schizophrenia susceptibility genes and their neurodevelopmental implications: focus on neuregulin 1. Novartis Found Symp. 2007;288:246–255. discussion 255-249, 276-281. [PubMed] [Google Scholar]
- Hayar A, Karnup S, Shipley MT, Ennis M. Olfactory bulb glomeruli: external tufted cells intrinsically burst at theta frequency and are entrained by patterned olfactory input. J Neurosci. 2004;24(5):1190–1199. doi: 10.1523/JNEUROSCI.4714-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holbrook EH, Wu E, Curry WT, Lin DT, Schwob JE. Immunohistochemical characterization of human olfactory tissue. Laryngoscope. 2011;121(8):1687–1701. doi: 10.1002/lary.21856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y, Hikino M, Yajima Y, Mikami T, Sirko S, von Holst A, Faissner A, Fukui S, Sugahara K. Structural characterization of the epitopes of the monoclonal antibodies 473HD, CS-56, and MO-225 specific for chondroitin sulfate D-type using the oligosaccharide library. Glycobiology. 2005;15(6):593–603. doi: 10.1093/glycob/cwi036. [DOI] [PubMed] [Google Scholar]
- Klausmeyer A, Conrad R, Faissner A, Wiese S. Influence of glial-derived matrix molecules, especially chondroitin sulfates, on neurite growth and survival of cultured mouse embryonic motoneurons. J Neurosci Res. 2011;89(2):127–141. doi: 10.1002/jnr.22531. [DOI] [PubMed] [Google Scholar]
- Kopala LC, Clark C, Hurwitz T. Olfactory deficits in neuroleptic naive patients with schizophrenia. Schizophr Res. 1993;8(3):245–250. doi: 10.1016/0920-9964(93)90022-b. [DOI] [PubMed] [Google Scholar]
- Maeda N. Structural variation of chondroitin sulfate and its roles in the central nervous system. Cent Nerv Syst Agents Med Chem. 2010;10(1):22–31. doi: 10.2174/187152410790780136. [DOI] [PubMed] [Google Scholar]
- Maeda N, Fukazawa N, Ishii M. Chondroitin sulfate proteoglycans in neural development and plasticity. Front Biosci. 2010;15:626–644. doi: 10.2741/3637. [DOI] [PubMed] [Google Scholar]
- Mauney SA, Athanas KM, Pantazopoulos H, Shaskan N, Passeri E, Berretta S, Woo T-UW. Developmental pattern of perineuronal nets in the human prefrontal cortex and their deficit in schizophrenia. Biological Psychiatry in press. 2013. [DOI] [PMC free article] [PubMed]
- McCurdy RD, Feron F, Perry C, Chant DC, McLean D, Matigian N, Hayward NK, McGrath JJ, Mackay-Sim A. Cell cycle alterations in biopsied olfactory neuroepithelium in schizophrenia and bipolar I disorder using cell culture and gene expression analyses. Schizophr Res. 2006;82(2-3):163–173. doi: 10.1016/j.schres.2005.10.012. [DOI] [PubMed] [Google Scholar]
- Meyer-Puttlitz B, Junker E, Margolis RU, Margolis RK. Chondroitin sulfate proteoglycans in the developing central nervous system. II. Immunocytochemical localization of neurocan and phosphacan. J Comp Neurol. 1996;366(1):44–54. doi: 10.1002/(SICI)1096-9861(19960226)366:1<44::AID-CNE4>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Moberg PJ, Agrin R, Gur RE, Gur RC, Turetsky BI, Doty RL. Olfactory dysfunction in schizophrenia: a qualitative and quantitative review. Neuropsychopharmacology. 1999;21(3):325–340. doi: 10.1016/S0893-133X(99)00019-6. [DOI] [PubMed] [Google Scholar]
- Moran A, Avendano C, Reinoso-Suarez F. Thalamic afferents to the motor cortex in the cat. A horseradish peroxidase study. Neurosci.Lett. 1982;33:229–233. doi: 10.1016/0304-3940(82)90376-7. [DOI] [PubMed] [Google Scholar]
- Morrison EE, Costanzo RM. Morphology of the human olfactory epithelium. J Comp Neurol. 1990;297(1):1–13. doi: 10.1002/cne.902970102. [DOI] [PubMed] [Google Scholar]
- Pantazopoulos H, Woo T-UW, Lim MP, Lange N, Berretta S. Extracellular Matrix-Glial Abnormalities in the Amygdala and Entorhinal Cortex of Subjects Diagnosed With Schizophrenia. Arch Gen Psychiatry. 2010;67(2):155–166. doi: 10.1001/archgenpsychiatry.2009.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry C, Mackay-Sim A, Feron F, McGrath J. Olfactory neural cells: an untapped diagnostic and therapeutic resource. The 2000 Ogura Lecture. Laryngoscope. 2002;112(4):603–607. doi: 10.1097/00005537-200204000-00002. [DOI] [PubMed] [Google Scholar]
- Popp S, Andersen JS, Maurel P, Margolis RU. Localization of aggrecan and versican in the developing rat central nervous system. Dev Dyn. 2003;227(1):143–149. doi: 10.1002/dvdy.10282. [DOI] [PubMed] [Google Scholar]
- Purushothaman A, Sugahara K, Faissner A. Chondroitin sulfate "wobble motifs" modulate maintenance and differentiation of neural stem cells and their progeny. J Biol Chem. 2012;287(5):2935–2942. doi: 10.1074/jbc.R111.298430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes KE, Fawcett JW. Chondroitin sulphate proteoglycans: preventing plasticity or protecting the CNS? J Anat. 2004;204(1):33–48. doi: 10.1111/j.1469-7580.2004.00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwob JE. Neural regeneration and the peripheral olfactory system. Anat Rec. 2002;269(1):33–49. doi: 10.1002/ar.10047. [DOI] [PubMed] [Google Scholar]
- Sirko S, von Holst A, Weber A, Wizenmann A, Theocharidis U, Gotz M, Faissner A. Chondroitin sulfates are required for fibroblast growth factor-2-dependent proliferation and maintenance in neural stem cells and for epidermal growth factor-dependent migration of their progeny. Stem Cells. 2010;28(4):775–787. doi: 10.1002/stem.309. [DOI] [PubMed] [Google Scholar]
- Takahashi N, Sakurai T, Bozdagi-Gunal O, Dorr NP, Moy J, Krug L, Gama-Sosa M, Elder GA, Koch RJ, Walker RH, Hof PR, Davis KL, Buxbaum JD. Increased expression of receptor phosphotyrosine phosphatase-[beta]/[zeta] is associated with molecular, cellular, behavioral and cognitive schizophrenia phenotypes. Transl Psychiatry. 2011;1:e8. doi: 10.1038/tp.2011.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisay KT, Key B. The extracellular matrix modulates olfactory neurite outgrowth on ensheathing cells. J Neurosci. 1999;19(22):9890–9899. doi: 10.1523/JNEUROSCI.19-22-09890.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turetsky BI, Hahn CG, Arnold SE, Moberg PJ. Olfactory receptor neuron dysfunction in schizophrenia. Neuropsychopharmacology. 2009;34(3):767–774. doi: 10.1038/npp.2008.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turetsky BI, Kohler CG, Gur RE, Moberg PJ. Olfactory physiological impairment in first-degree relatives of schizophrenia patients. Schizophr Res. 2008;102(1-3):220–229. doi: 10.1016/j.schres.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhaagen J, Oestreicher AB, Gispen WH, Margolis FL. The expression of the growth associated protein B50/GAP43 in the olfactory system of neonatal and adult rats. J Neurosci. 1989;9(2):683–691. doi: 10.1523/JNEUROSCI.09-02-00683.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Holst A, Sirko S, Faissner A. The unique 473HD-Chondroitinsulfate epitope is expressed by radial glia and involved in neural precursor cell proliferation. J Neurosci. 2006;26(15):4082–4094. doi: 10.1523/JNEUROSCI.0422-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Sheng W, Chen L, Dong H, Lee V, Lu F, Wong CS, Lu WY, Yang BB. Versican V1 isoform induces neuronal differentiation and promotes neurite outgrowth. Molecular biology of the cell. 2004;15(5):2093–2104. doi: 10.1091/mbc.E03-09-0667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa M, Yu RK. The expression and functions of glycoconjugates in neural stem cells. Glycobiology. 2007;17(7):57R–74R. doi: 10.1093/glycob/cwm018. [DOI] [PubMed] [Google Scholar]
- Yoshihara Y, Mori K. Basic principles and molecular mechanisms of olfactory axon pathfinding. Cell Tissue Res. 1997;290(2):457–463. doi: 10.1007/s004410050953. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.