Abstract
Aims
We investigated whether the addition of left atrial (LA) size determined by echocardiography improves cardiovascular risk prediction in young adults over and above the clinically established Framingham 10-year global CV risk score (FRS).
Methods and results
We included white and black CARDIA participants who had echocardiograms in Year-5 examination (1990–91). The combined endpoint after 20 years was incident fatal or non-fatal cardiovascular disease: myocardial infarction, heart failure, cerebrovascular disease, peripheral artery disease, and atrial fibrillation/flutter. Echocardiography-derived M-mode LA diameter (LAD; n = 4082; 149 events) and 2D four-chamber LA area (LAA; n = 2412; 77 events) were then indexed by height or body surface area (BSA). We used Cox regression, areas under the receiver operating characteristic curves (AUC), and net reclassification improvement (NRI) to assess the prediction power of LA size when added to calculated FRS or FRS covariates. The LAD and LAA cohorts had similar characteristics; mean LAD/height was 2.1 ± 0.3 mm/m and LAA/height 9.3 ± 2.0 mm2/m. After indexing by height and adjusting for FRS covariates, hazard ratios were 1.31 (95% CI 1.12, 1.60) and 1.43 (95% CI 1.13, 1.80) for LAD and LAA, respectively; AUC was 0.77 for LAD and 0.78 for LAA. When LAD and LAA were indexed to BSA, the results were similar but slightly inferior. Both LAD and LAA showed modest reclassification ability, with non-significant NRIs.
Conclusion
LA size measurements independently predict clinical outcomes. However, it only improves discrimination over clinical parameters modestly without altering risk classification. Indexing LA size by height is at least as robust as by BSA. Further research is needed to assess subgroups of young adults who may benefit from LA size information in risk stratification.
Keywords: Left atrial size, Cardiovascular events, Echocardiography, Young adults
Introduction
The assessment of cardiovascular (CV) risk is recommended in youth by using clinical parameters. However, the value of global risk scores (such as described in the Framingham Heart Study) in adults aged <30 years is unclear.1,2 Left atrial (LA) structure and function relate to ventricular function.3 CV mortality, myocardial infarction (MI), heart failure (HF), stroke, and atrial fibrillation have all been predicted by LA size in diverse populations.4 However, the additional predictive value of LA size assessed in young adults over traditional risk factors is unclear.
Atrial dilatation is the major marker of LA remodelling, an adaptive process that relates to the duration and strength of the LA exposure to stressing factors. As the atrium enlarges, the remodelling mechanism involves microstructure alterations; markedly interstitial fibrosis and myocyte hypertrophy.5 The importance of LA remodelling in young healthy adults, however, is not totally understood. In the Coronary Artery Risk Development in Young Adults (CARDIA) study of risk evolution in young adults, LA size has shown a strong relationship with traditional CV risk factors in both cross-sectional and longitudinal assessments, but relationships with incident events have not yet been reported.6–8
We hypothesized that LA size assessed in young healthy adults is associated with future CV events, independently of CV risk prediction provided by traditional risk factors. Using a large biracial cohort of the CARDIA study, we investigate the additional predictive value of LA diameter (LAD) and area (LAA) over the Framingham 10-year global CV risk score (FRS). Since the method of indexing LA size to body size has not been established, we tested the relative strength of different indexing methods on this CV event prediction.
Methods
Participant selection
As previously described, CARDIA is a prospective study that enrolled 5115 African-American and white adults (aged 18–30 years) from four US Field Centers (Birmingham, AL; Oakland, CA; Chicago, IL; and Minneapolis, MN) in 1985–86.9 The entire cohort underwent echocardiograms at follow-up Year-5 examination (1990–91); this was defined as baseline for the present study. We included participants with interpretable echocardiograms and complete data on covariates at baseline. Of the 4352 participants who attended the Year-5 examination, 109 did not have echocardiography data and one withdrew consent from the study. In the remaining 4242 participants, 132 were missing covariate data, 28 missing LAD, and 1670 missing information on LAA. LAA assessment is more complex than M-mode diameter and requires optimal 2D images. In CARDIA Year-5 examination, echocardiograms were focused to assess cardiac structure and function using an M-mode technique; this reduced the number of interpretable 2D exams. This left 4082 participants in the analytic cohort for LAD and 2412 for LAA analysis.
Echocardiography
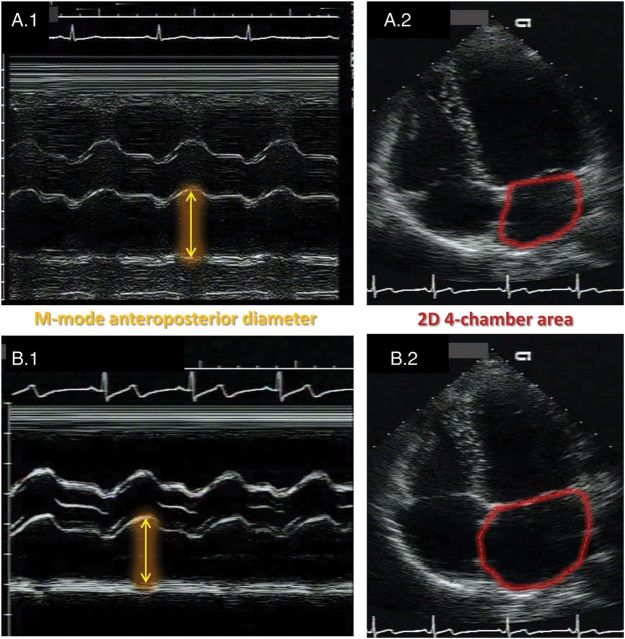
Echocardiographic exams were performed using an Acuson™ cardiac ultrasound system (Siemens Healthcare, Erlangen, Germany), as previously reported for the CARDIA study.10 The images were analysed at a single Reading Center (University of California, Irvine, CA, USA) and followed standard recommendations.11 Parasternal long-axis 2D views were used to guide the assessment of M-mode anteroposterior diameter of the LA, and the areas were acquired from a 2D four-chamber view, both measured at the point of maximum atrial volume (Figure 1). LA measurements were indexed by height or body surface area (BSA).
Figure 1.
Illustrative representation of the LA size assessment in two participants in the CARDIA follow-up Year 5. Participant A had normal findings and B showed eccentric LA remodelling. Note that the M-mode anteroposterior diameters are similar in both participants (A.1 and B.1), but the 2D areas are markedly different (A.2 and B.2).
Other variables
Assessment methods for risk factor variables have been described for the CARDIA study.12 Briefly, the use of anti-hypertensive medication and smoking status were self-reported, assessed using questionnaires. Systolic blood pressure (SBP) was the average of the last two measurements (total of three). Diabetes was defined based on CARDIA examination Years 0, 2, and 5 by the presence of one of the following criteria: history of hypoglycaemic medication use or fasting glucose ≥126 mg/dL.
Cardiovascular outcomes
To assess CV risk in young asymptomatic adults, all major CV events should be taken into account in a global assessment of risk.1 We used a combined endpoint that included CV death, non-fatal MI, HF, cerebrovascular disease (stroke or transient ischaemic attack—TIA), peripheral artery disease, and atrial fibrillation/flutter (AFib). These events include the ones described in the FRS original publication,2 adding AFib due to its high relevance in LA remodelling.5
Participants were interviewed during their scheduled study examinations and by telephone yearly regarding hospitalizations and outpatient procedures. Vital status was checked every 6 months. Inpatient and outpatient medical records and/or death certificates were requested and reviewed by two members of the endpoints committee during the process of adjudication for CV events. Atrial fibrillation or flutter cases were identified based on participants' medical records using a combination of physician documentation of atrial fibrillation or flutter, electrocardiogram tracings and reports, and cardioversion attempts, and documentation of appropriate anti-arrhythmic medication use in the setting of an arrhythmia history. All records were reviewed by two members of the endpoint committee which applied standard outcome definitions contained in a detailed adjudication manual to classify events. Committee consensus resolved eventual disagreements. For the other outcomes, the ascertainment process has been previously described in details.
Statistical analysis
We assessed the performance of LAA and LAD as predictors of CV events in multivariable analyses adjusted for traditional CV risk factors. The FRS is widely used to estimate CV risk in clinical settings using as traditional risk factors: gender, age, BMI, total cholesterol, HDL cholesterol, SBP, use of anti-hypertensive medication, diabetes status, and smoking status. To assess the independent predictive ability of LA size, we adjusted our analysis to the calculated FRS (computing the score) and also ran the same analysis using the FRS covariates independently included in multivariable models (not computing the score). To compute the FRS, we calculated the per cent of risk as first described by D'Agostino et al.,2 but modified the original calculation to include age as a continuous variable in the models (because the CARDIA participants are younger than the ones used in the FRS original publication).
The hazard ratios (HRs) of a 1 standard deviation (SD) difference of LA atrial size were assessed by a multivariable Cox regression analysis adjusted for race and1 the computed FRS plus age; or2 the FRS covariates. Receiver operating characteristic (ROC) curves were used to determine the differences in discrimination to predict CV events. The discrimination improvement for the areas under the ROC curves (AUC) was assessed using the method of DeLong et al.13 All models had good calibration as indicated by the Hosmer–Lemeshow test (data not shown). Net reclassification improvement (NRI) and integrated discrimination improvement (IDI) were calculated as first described by Pencina et al.14 to evaluate the added predictive ability for LA size to the traditional risk factors.
To calculate the reclassification performance, logistic regression models were used to define four risk groups according to FRS results with or without adding LA size information. The risk groups were defined in each model based on the predicted risk <2.5%, 2.5–5.0%, 5.0–<10.0%, and ≥10% in 20 years, in accordance with advice to select risk categories for reclassification tables that are clinically meaningful.15 NRI is strongly influenced by the cut-points used for risk stratification, and the most meaningful cut-points for LA size for young adults have not been established. Previous reports used older populations to establish clinically meaningful categories cut-points, but these were thought to be not adequate to a young healthy population as in CARDIA due to a very different base risk for events. Reclassification tables were built and risk groups (for FRS covariates with or without LA information) were cross-tabulated, according to the presence of incident event during the study follow-up period. After LA size information is added to the FRS, a correct reclassification occurs when a participant who did not have an event moves to a lower risk category or when a participant who had an event moves to a higher risk category.
Results
CARDIA participants who attended the Year-5 examination and underwent echocardiography were included in the study (Table 1). The mean ± SD values of LAD indexed to height and BSA were 2.07 ± 0.27 mm/m and 1.93 ± 0.24 mm/m2, respectively. The LAA indexed by height and BSA was, respectively, 9.25 ± 1.97 mm2/m and 8.66 ± 1.74 mm2/m2.
Table 1.
Participant characteristics at the CARDIA study examination Year 5, overall and in the analytic cohorts for LAD and LAA
| Variables | Overall cohort (n = 4352) Mean (SD) |
LAD cohort (n = 4082) Mean (SD) |
LAA cohort (n = 2412) Mean (SD) |
|---|---|---|---|
| Age (years) | 30 (4) | 30 (4) | 30 (4) |
| BMI (kg/m2) | 26 (6) | 26 (6) | 25 (5) |
| SBP (mmHg) | 108 (12) | 108 (12) | 107 (11) |
| Total chol (mg/dL) | 178 (34) | 178 (34) | 176 (33) |
| HDL-C (mg/dL) | 53 (14) | 53 (14) | 53 (14) |
| % | % | % | |
| White race | 51 | 52 | 51 |
| Male gender | 45 | 46 | 48 |
| Current smoker | 29 | 28 | 28 |
| Anti-HTN medication | 1.6 | 1.5 | 1.3 |
| Diabetes | 0.9 | 0.8 | 0.7 |
SD, standard deviation; BMI, body-mass index; SBP, systolic blood pressure; LAD, left atrial diameter assessed by M-mode echocardiography; LAA, left atrial area assessed by 2D four-chamber echocardiography; total chol, total cholesterol; HDL-C, high-density lipoprotein cholesterol; anti-HTN medication, anti-hypertensive medication.
Participants were followed in average 19.4 ± 2.3 years for those who did not have any events. Of the 4082 participants in the LAD cohort, 149 (3.7%) had events: 25.5% CV death, 24.2% non-fatal MI, 17.5% HF, 2.7% peripheral arterial disease, 5.4% TIA, 16.1% stroke, and 8.7% AFib. In the assessment of LAA as a predictor of CV events (n = 2412), 77 (3.2%) participants had events: 24.7% CV death, 28.6% non-fatal MI, 15.6% HF, 2.6% peripheral arterial disease, 3.9% TIA, 15.6% stroke, and 9.1% AFib.
Results for LA dimension
We analysed the independent ability of LAD to predict long-term CV events, adjusted for the calculated FRS or the FRS covariates (Table 2). The HRs ranged from 1.19 (95% CI 1.02, 1.39) for LAD/BSA adjusted for the calculated FRS to 1.34 (95% CI 1.12, 1.60) for LAD/height adjusted for FRS covariates. A modest increase in the AUC was found for adding LAD to FRS; in this regard, LAD/height had a slightly superior performance when compared with LAD/BSA (Table 2). Although not reaching statistical significance, a trend was found favouring AUC for LAD/height when compared with LAD/BSA (P = 0.13 for models using FRS covariates and P = 0.08 for models using the calculated FRS).
Table 2.
Cox regression HRs and AUC for LAD predicting CV events (n = 4082; 151 events)
| Predictor | FRS covariates |
Calculated FRS |
||
|---|---|---|---|---|
| HR (95% CI) | AUC (95% CI) | HR (95% CI) | AUC (95% CI) | |
| LAD/height | 1.34 (1.12, 1.60) | 0.774 (0.735, 0.812) | 1.28 (1.10, 1.50) | 0.768 (0.728, 0.808) |
| LAD/BSA | 1.26 (1.07, 1.48) | 0.770 (0.731, 0.809) | 1.19 (1.02, 1.39) | 0.761 (0.720, 0.801) |
HR refers to 1 SD increase. AUC for FRS covariates alone = 0.767 (0.727, 0.807) and for calculated FRS alone = 0.759 (0.718, 0.800). The FRS covariates includes: gender, age, BMI, total cholesterol, HDL, SBP, use of anti-hypertensive medication, diabetes status, and smoking status.
LAD, left atrial diameter; BSA, body surface area; CI, confidence interval; FRS, Framingham 10-year global CV risk score.
The reclassification tables for adding LAD indexed by height or BSA are summarized in Table 3, showing the number of participants reclassified according to the presence or absence of CV event over the 20-year follow-up period. Of the 3933 participants who did not have incident events, 344 (8.8%) were correctly down reclassified when LAD was indexed to height and 280 (7.1%) when LAD was indexed to BSA. Among the 149 participants who had events over the follow-up period, 13 (8.7%) were correctly up reclassified for LAD/height and 9 (6.0%) for LAD/BSA. No statistically significant NRI for LAD plus FRS compared with FRS covariates alone was found; the NRI values were 0.033 (P = 0.31) and 0.018 (P = 0.53) for LAD/height and LAD/BSA, respectively. A trend in significance was found for IDI, LAD/height had an IDI of 0.0053 (P = 0.09), and LAD/BSA had an IDI of 0.0040 (P = 0.08).
Table 3.
Reclassification table: absolute number of participants classified in each risk strata of FRS covariates vs. adding information on LAD
| Risk category | No event (n = 3933) |
Events (n = 149) |
||||||
|---|---|---|---|---|---|---|---|---|
| FRS + LAD/height |
FRS + LAD/height |
|||||||
| <2.5% | 2.5–4.9% | 5.0–9.9% | ≥10% | <2.5% | 2.5–4.9% | 5.0–9.9% | ≥10% | |
| FRS | ||||||||
| <2.5% | 2076 | 136 | 0 | 0 | 25 | 5 | 0 | 0 |
| 2.5–4.9% | 195 | 698 | 113 | 3 | 4 | 30 | 3 | 0 |
| 5.0–9.9% | 1 | 107 | 362 | 40 | 0 | 5 | 27 | 5 |
| ≥ 10% | 0 | 1 | 40 | 161 | 0 | 0 | 1 | 44 |
| FRS + LAD/BSA | FRS + LAD/BSA | |||||||
| FRS | ||||||||
| <2.5% | 2100 | 111 | 1 | 0 | 27 | 3 | 0 | 0 |
| 2.5–4.9% | 159 | 755 | 93 | 2 | 3 | 32 | 2 | 0 |
| 5.0–9.9% | 0 | 90 | 391 | 29 | 0 | 4 | 29 | 4 |
| ≥10% | 0 | 0 | 31 | 171 | 0 | 0 | 1 | 44 |
LAD, left atrial diameter; BSA, body surface area; FRS, Framingham 10-year global CV risk score.
Results for LAA
LAA was assessed as an independent CV event predictor, after adjustment to the calculated FRS or the FRS covariates (Table 4). The Cox regression HRs for both indexing methods were statistically significant, ranging from 1.36 (95% CI 1.09, 1.70) for BSA indexation adjusted for the calculated FRS to 1.43 (95% CI: 1.13, 1.80) for indexing LAA by height and adjusting for FRS covariates. No statistical significance was found comparing AUC for LAA indexed by height or BSA (P = 0.61 for models using FRS covariates and P = 0.57 for models using the calculated FRS).
Table 4.
Cox regression HRs and AUC for LAA predicting CV events (n = 2412; 78 events)
| Predictor | FRS covariates |
Calculated FRS |
||
|---|---|---|---|---|
| HR (95% CI) | AUC (95% CI) | HR (95% CI) | AUC (95% CI) | |
| LAA/height | 1.43 (1.13, 1.80) | 0.784 (0.734, 0.834) | 1.39 (1.12, 1.73) | 0.766 (0.712, 0.819) |
| LAA/BSA | 1.42 (1.13, 1.78) | 0.783 (0.732, 0.833) | 1.36 (1.09, 1.70) | 0.763 (0.709, 0.818) |
HR refers to 1 SD increase. In this subsample, AUC for FRS covariates alone = 0.763 (0.710, 0.817) and for calculated FRS alone = 0.749 (0.694, 0.804). The FRS covariates includes: gender, age, BMI, total cholesterol, HDL-C, SBP, use of anti-hypertensive medication, diabetes status, and smoking status.
LAA, left atrial area; BSA, body surface area; CI, confidence interval; FRS, Framingham 10-year global CV risk score.
Reclassification tables for adding LAA to risk factors are reported in Table 5. Of the 2335 participants who did not have incident events, 246 (10.5%) were correctly down reclassified by adding LAA/height to FRS and 229 (9.8%) by adding LAA/BSA. Among the 77 who had events over the follow-up period, 11 (14.3%) and 12 (15.6%) were correctly up reclassified when LAA was indexed to height and BSA, respectively. Similar to LAD, LAA did not show statistically significant NRI values; the computed NRIs were 0.050 (P = 0.40) and 0.055 (P = 0.36) for LAA indexed by height and BSA, respectively. No significant value was found for IDI regarding LAA, LAA indexed by height had IDI = 0.0047 (P = 0.26), and indexed by IDI = 0.0053 (P = 0.20).
Table 5.
Reclassification table: absolute number of participants classified in each risk strata of FRS covariates vs. adding information on LAA
| Risk category | No event (n = 2335) |
Events (n = 77) |
||||||
|---|---|---|---|---|---|---|---|---|
| FRS + LAA/height |
FRS + LAA/height |
|||||||
| <2.5% | 2.5–4.9% | 5.0–9.9% | ≥10% | <2.5% | 2.5–4.9% | 5.0–9.9% | ≥10% | |
| FRS | ||||||||
| <2.5% | 1339 | 81 | 5 | 0 | 13 | 4 | 0 | 0 |
| 2.5–4.9% | 146 | 327 | 73 | 1 | 1 | 16 | 5 | 0 |
| 5.0–9.9% | 2 | 75 | 157 | 30 | 0 | 4 | 11 | 2 |
| ≥10% | 0 | 1 | 22 | 76 | 0 | 0 | 4 | 17 |
| FRS + LAA/BSA | FRS + LAA/BSA | |||||||
| FRS | ||||||||
| <2.5% | 1337 | 83 | 4 | 1 | 11 | 6 | 0 | 0 |
| 2.5–4.9% | 138 | 336 | 72 | 1 | 1 | 17 | 4 | 0 |
| 5.0–9.9% | 0 | 73 | 160 | 31 | 0 | 4 | 11 | 2 |
| ≥10% | 0 | 1 | 17 | 81 | 0 | 0 | 4 | 17 |
LAA, left atrial area; BSA, body surface area; FRS, Framingham 10-year global CV risk score.
Discussion
We assessed the 20 prediction power for CV events of LAA and LAD in a large bi-racial cohort of young adults. As recommended by the American Heart Association, reclassification statistics were applied to estimate how LA size could aid in risk stratification for young adults.16 LA size measurements independently predicted clinical outcomes but only modestly improved discrimination and showed no improvement in risk classification. Different indexing methods for LA size were tested for event prediction. In this regard, indexing LAD and LAA by height was slightly better than those by BSA.
CV disease is a rising concern worldwide, frequently presenting as the mortality in the first manifestation of CV disease.17 The ability to identify high CV risk individuals is essential for planning primary prevention strategies.1 Young asymptomatic subjects may benefit from early CV risk stratification, but the traditional risk assessment tools have not been rigorously evaluated in this age group.1,18 We expected that LA size, as a validated predictor of CV events, would aid in risk stratification of young adults, particularly those with high risk burden that are underestimated by the FRS.18
LA size relates to left ventricular filling pressures and, therefore, diastolic dysfunction. The LA is likely to remodel early before clinical heart disease is established, since diastolic function is more likely to become impaired earlier during progression of cardiac dysfunction. In fact, LA size provides strong prognostic information in patients with established heart disease. Meris et al.19 prospectively followed 610 post-MI patients from the VALIANT echocardiography study and showed that LA volume indexed by BSA was an independent predictor of all-cause mortality or HF hospitalization. Vazquez et al.20 investigated ambulatory patients with chronic HF and showed that LA size was a strong predictor of all-cause death, pump-failure death, or sudden cardiac death.
Although the LA in many cases enlarges in a non-uniform 3D geometry, both M-mode and 2D echocardiography techniques have become established to estimate LA size.5,21 It has been shown that 2D measurements as of LAA and particularly LA volume are more accurate to assess LA size, as they account for eccentric remodelling. M-mode LA anterior–posterior diameter is a highly precise measure, probably due to the simplicity of image acquisition and interpretation. However, more steps are required to compute 2D volumes, especially when assessing biplanar LA volume, which likely affects the measurement precision.
Tsang et al. assessed American Society of Echocardiography-based categories of indexed LA volume, LA four-chamber area, and indexed LAD in 317 patients (70 years in average) in sinus rhythm who were referred for a general medical consultation and followed them over a mean period of 3.5 ± 2.3 years for new events of AFib, stroke, TIA, MI, coronary revascularization, HF, and CV death. In this elderly population of outpatients, the authors show slightly better area under the curve for LA volume categories compared with area and diameter, but failed to report superior results for outcome prediction HRs.22 Cameli et al. reported similar results after following 312 adults (71 years in average) in sinus rhythm over 3.1 ± 1.4 years. Although the assessment of LA function by speckle tracking echocardiography had the most robust results, sensitivity and specificity confidence intervals overlapped for LA volume, LAA, and LA diameter categories.23
The role of LA size predicting global CV events in early adulthood, however, is less well understood. We assessed a substantially large cohort of young healthy individuals over a 20-year follow-up period. In our study, both LAA and LAD measured in early adulthood are able to independently predict a combined endpoint of CV events. It is unlikely that LA eccentric remodelling would be significantly prevalent in our young and generally healthy population. Compared with LA volume, LAA and LAD are simpler measurements, which may reduce technical variation. Using both LAA and M-mode diameter, we acquired LA linear and 2D measurements, which we believe are adequate to the young and healthy CARDIA cohort. Moreover, the more recently recommended statistical evaluation that includes Cox regression models as well as discrimination, calibration, and reclassification16 had not been assessed until now to establish the additional predictive value of LA size in CV risk stratification.
When added to the FRS CV risk factors, LA size improved modestly discrimination in our study, as assessed by the AUC. In fact, the FRS CV risk score alone already showed powerful CV event prediction ability. This FRS good performance in our young cohort may be the major factor related to the modest increases in discrimination found in our study.24 This also may partially explain the inability to correctly reclassify risk by using NRI and IDI assessment. NRI performance for LA size may also be influenced by the lack of pathological remodelling in a young cohort of generally healthy individuals. LA size may be more useful improving risk classification in subgroups of young adults with risk factors, but the low number of events in our young population would affect the statistical power to investigate multiple subgroups of participants in CARDIA. A prospective study dedicated to a young population with comorbidities would be needed to answer this question.
The best way to index heart measures to body size is not totally clear, as indexing appears to affect the performance of cardiac parameters to predict CV events.25,26 Height seems to be the most adequate indexing method for heart parameters in mathematical models.25 Moreover, compared with BSA, indexing LA size by height was more robust to assess longitudinal changes in the CARDIA cohort.8 Although no definitive difference was reported in our study, HRs consistently favoured indexing LA size by height and we found a statistical trend in the LAD AUC models in the same direction. Indexing LA by height in young adults may also be the most appropriate method to predict long-term events. Although the reports favour LA indexation by height in the CARDIA study, these findings should be further tested in other cohorts.
Our study also showed that LAA and LAD measured in young adulthood can independently predict CV events over a 20-year period and may lead to a modest increment in discrimination compared with risk factors alone. However, these measures did not improve reclassification of participants above conventional CV risk factors. Although no definitive conclusion regarding LA indexing can be driven from our study, HRs consistently favoured indexing LA size by height and a trend in discrimination also favoured LAD/height. Further research on the value of LA size in event prediction should focus on identifying subgroups of young adults (possibly with multiple risk factors) who may benefit of the use of LA size information to better stratify CV risk.
Limitations
In this study, we used LAA and LAD assessment that is practical, low cost, and validated, but may lack accuracy to completely account for LA eccentric remodelling. At the time that the CARDIA Year 5 was performed, the M-mode technique was the standard assessment of LA size and the only 2D LA assessment was four-chamber LAA, feasible in a limited number of participants. Attempts to reassess 2D images in Year 5 are challenged by image deterioration over time (images were originally recorded in video home system tapes).
The incident CV events affected 3.7% of the cohort, which is lower than other prospective studies. It may be explained by the low base risk of this young population of healthy individuals. The relatively low number of events affects the statistical power in subgroup analyses. Therefore, we could not assess how LA size would perform in subgroups of participants with specific risk factors.
Funding
This work was supported by the National Institutes of Health (contract number HC-95241). Dr Armstrong was funded by Universidade Federal do Vale do São Francisco (Petrolina, PE, Brazil) and by the Johns Hopkins University (Baltimore, MD, USA). There are no relationships with industry in this study.
Acknowledgements
The authors thank the other investigators, the staff, and the participants of the CARDIA study for their valuable contributions.
Conflict of interest: The authors have no competing interests in this study.
References
- 1.Greenland P, Alpert JS, Beller GA, Benjamin EJ, Budoff MJ, Fayad ZA, et al. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2010;122:e584–636. doi: 10.1161/CIR.0b013e3182051b4c. [DOI] [PubMed] [Google Scholar]
- 2.D'Agostino RB, Sr, Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117:743–53. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 3.Pavlopoulos H, Nihoyannopoulos P. Left atrial size: a structural expression of abnormal left ventricular segmental relaxation evaluated by strain echocardiography. Eur J Echocardiogr. 2009;10:865–71. doi: 10.1093/ejechocard/jep093. [DOI] [PubMed] [Google Scholar]
- 4.Osranek M, Bursi F, Bailey KR, Grossardt BR, Brown RD, Jr, Kopecky SL, et al. Left atrial volume predicts cardiovascular events in patients originally diagnosed with lone atrial fibrillation: three-decade follow-up. Eur Heart J. 2005;26:2556–61. doi: 10.1093/eurheartj/ehi483. [DOI] [PubMed] [Google Scholar]
- 5.Casaclang-Verzosa G, Gersh BJ, Tsang TS. Structural and functional remodeling of the left atrium: clinical and therapeutic implications for atrial fibrillation. J Am Coll Cardiol. 2008;51:1–11. doi: 10.1016/j.jacc.2007.09.026. [DOI] [PubMed] [Google Scholar]
- 6.Gidding SS, Carnethon MR, Daniels S, Liu K, Jacobs DR, Jr, Sidney S, et al. Low cardiovascular risk is associated with favorable left ventricular mass, left ventricular relative wall thickness, and left atrial size: the CARDIA study. J Am Soc Echocardiogr. 2010;23:816–22. doi: 10.1016/j.echo.2010.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gardin JM, Iribarren C, Detrano RC, Liu K, Schreiner PJ, Loria CM, et al. Relation of echocardiographic left ventricular mass, geometry and wall stress, and left atrial dimension to coronary calcium in young adults (the CARDIA study) Am J Cardiol. 2005;95:626–9. doi: 10.1016/j.amjcard.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 8.Armstrong AC, Gidding S, Colangelo LA, Kishi S, Liu K, Sidney S, et al. Association of early adult modifiable cardiovascular risk factors with left atrial size over a 20-year follow-up period: The CARDIA study. BMJ Open. 2014;4:e004001. doi: 10.1136/bmjopen-2013-004001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Friedman GD, Cutter GR, Donahue RP, Hughes GH, Hulley SB, Jacobs DR, Jr, et al. CARDIA: study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41:1105–16. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 10.Gardin JM, Wagenknecht LE, Anton-Culver H, Flack J, Gidding S, Kurosaki T, et al. Relationship of cardiovascular risk factors to echocardiographic left ventricular mass in healthy young black and white adult men and women. The CARDIA study. Coronary Artery Risk Development in Young Adults. Circulation. 1995;92:380–7. doi: 10.1161/01.cir.92.3.380. [DOI] [PubMed] [Google Scholar]
- 11.Sahn DJ, DeMaria A, Kisslo J, Weyman A. Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation. 1978;58:1072–83. doi: 10.1161/01.cir.58.6.1072. [DOI] [PubMed] [Google Scholar]
- 12.Loria CM, Liu K, Lewis CE, Hulley SB, Sidney S, Schreiner PJ, et al. Early adult risk factor levels and subsequent coronary artery calcification: the CARDIA Study. J Am Coll Cardiol. 2007;49:2013–20. doi: 10.1016/j.jacc.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 13.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–45. [PubMed] [Google Scholar]
- 14.Pencina MJ, D'Agostino RB, Sr, D'Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–72. doi: 10.1002/sim.2929. discussion 207–12. [DOI] [PubMed] [Google Scholar]
- 15.Cook NR, Ridker PM. Advances in measuring the effect of individual predictors of cardiovascular risk: the role of reclassification measures. Ann Intern Med. 2009;150:795–802. doi: 10.7326/0003-4819-150-11-200906020-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hlatky MA, Greenland P, Arnett DK, Ballantyne CM, Criqui MH, Elkind MS, et al. Criteria for evaluation of novel markers of cardiovascular risk: a scientific statement from the American Heart Association. Circulation. 2009;119:2408–16. doi: 10.1161/CIRCULATIONAHA.109.192278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2013;380:2095–128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marma AK, Lloyd-Jones DM. Systematic examination of the updated Framingham heart study general cardiovascular risk profile. Circulation. 2009;120:384–90. doi: 10.1161/CIRCULATIONAHA.108.835470. [DOI] [PubMed] [Google Scholar]
- 19.Meris A, Amigoni M, Uno H, Thune JJ, Verma A, Kober L, et al. Left atrial remodelling in patients with myocardial infarction complicated by heart failure, left ventricular dysfunction, or both: the VALIANT Echo study. Eur Heart J. 2009;30:56–65. doi: 10.1093/eurheartj/ehn499. [DOI] [PubMed] [Google Scholar]
- 20.Vazquez R, Bayes-Genis A, Cygankiewicz I, Pascual-Figal D, Grigorian-Shamagian L, Pavon R, et al. The MUSIC Risk score: a simple method for predicting mortality in ambulatory patients with chronic heart failure. Eur Heart J. 2009;30:1088–96. doi: 10.1093/eurheartj/ehp032. [DOI] [PubMed] [Google Scholar]
- 21.Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–63. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 22.Tsang TS, Abhayaratna WP, Barnes ME, Miyasaka Y, Gersh BJ, Bailey KR, et al. Prediction of cardiovascular outcomes with left atrial size: is volume superior to area or diameter? J Am Coll Cardiol. 2006;47:1018–23. doi: 10.1016/j.jacc.2005.08.077. [DOI] [PubMed] [Google Scholar]
- 23.Cameli M, Lisi M, Focardi M, Reccia R, Natali BM, Sparla S, et al. Left atrial deformation analysis by speckle tracking echocardiography for prediction of cardiovascular outcomes. Am J Cardiol. 2012;110:264–9. doi: 10.1016/j.amjcard.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 24.Armstrong AC, Gjesdal O, Almeida A, Nacif M, Wu C, Bluemke DA, et al. Left ventricular mass and hypertrophy by echocardiography and cardiac magnetic resonance: the multi-ethnic study of atherosclerosis. Echocardiography. 2014;31:12–20. doi: 10.1111/echo.12303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dewey FE, Rosenthal D, Murphy DJ, Jr, Froelicher VF, Ashley EA. Does size matter? Clinical applications of scaling cardiac size and function for body size. Circulation. 2008;117:2279–87. doi: 10.1161/CIRCULATIONAHA.107.736785. [DOI] [PubMed] [Google Scholar]
- 26.Armstrong AC, Gjesdal O, Wu C, Gidding S, Bluemke DA, Lima JA. Left ventricular mass assessed by echocardiography and cardiac magnetic resonance, cardiovascular outcomes, and medical practice. J Am Coll Cardiol Img. 2012;5:11. doi: 10.1016/j.jcmg.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]