Abstract
Aim
Area-at-risk (AAR) measurements often rely on T2-weighted images, but subtle differences in T2 may be overlooked with this method. To determine the differences in oedema between salvaged and infarcted myocardium, we performed quantitative T2 mapping of the AAR. We also aimed to determine the impact of reperfusion time on T2 in the AAR.
Methods
Twenty-two dogs underwent 2 h of coronary occlusion followed by 4 or 48 h of reperfusion before cardiac magnetic resonance imaging at 1.5 T. Late gadolinium enhancement images were used to define the infarcted, salvaged, and remote myocardium. T2 values from T2 maps and signal intensities on T2-weighted images were measured in the corresponding areas.
Results
At both imaging time points, the T2 of the salvaged myocardium was longer than of remote (66.0 ± 6.9 vs. 51.4 ± 3.5 ms, P < 0.001 at 4 h, and 56.7 ± 7.3 vs. 48.1 ± 3.5 ms, P < 0.001 at 48 h). The T2 was also longer in the infarcted myocardium compared with remote at both 4 and 48 h (71.4 ± 7.6 ms, P < 0.01 vs. salvage and 64.0 ± 6.9 ms, P = 0.03 vs. salvage, both P < 0.001 vs. remote). The increase in T2 in the salvaged myocardium compared with remote was greater after 4 h than after 48 h (14.7 ± 5.6 vs. 8.7 ± 5.1 ms, P = 0.02).
Conclusions
T2 relaxation parameters are different in the infarcted and salvaged myocardium, and both are significantly longer than remote. Furthermore, the magnitude of increase in T2 was less in the salvaged myocardium after longer reperfusion, indicating partial resolution of oedema in the first 48 h after reperfusion.
Keywords: Magnetic resonance imaging, Acute myocardial infarction, Area-at-risk, Myocardial salvage, Myocardial ischaemia
Introduction
T2-weighted cardiac magnetic resonance imaging (CMR) of the area-at-risk (AAR) is used as a clinical tool and for determining the efficacy of novel therapeutic advances in clinical research. However, controversies still exist on the value of post-ischaemic CMR of ischaemic myocardium.1,2 Recent advances in CMR techniques may aid in clarifying the pathophysiological dynamics within the AAR and refine our understanding of underlying mechanisms that alter myocardial T2. Differentiation of T2 abnormalities within the AAR has not previously been fully explored even though salvaged and infarcted myocardium represent two different pathological processes—irreversibly (infarcted) and reversibly (salvaged) injured myocardium. It is possible that subtle T2 differences are overlooked with T2-weighted imaging and recent development of quantitative T2 mapping may bring us beyond these technical barriers.3 T2 enhancement of the AAR is thought to represent ischaemia-induced myocardial oedema.4,5 Over time, tissue oedema resolves in reversibly injured myocardium, leading to less severe T2 enhancement. Thus, the timing of imaging after myocardial infarction may also be important.
The specific aim of this study was to use quantitative T2 maps to objectively characterize the salvaged and infarcted myocardium within the AAR. We hypothesized that T2 quantification can detect differences between the salvaged and infarcted myocardium within the AAR relative to the remote myocardium in a reperfused model of acute myocardial infarction. Furthermore, we studied the impact reperfusion time has on the degree of T2 abnormality within the AAR.
Methods
Animal model
Twenty-two mongrel dogs weighing 10–20 kg were studied after approval by the Animal Care and Use Committee of the National Heart, Lung and Blood Institute of the National Institutes of Health. Anaesthesia was induced with s.c. administration of acepromazine (0.2 mg/kg) followed by i.v. administration of thiopental sodium (15 mg/kg) and maintained with inhaled sevoflurane (2–5%) after intubation. I.v. and arterial lines were established as well as a left atrial catheter for administration of microspheres. After thoracotomy at the left fifth or sixth intercostal space, the left anterior descending artery was isolated and a snare was placed around the vessel distal to the first diagonal branch. Approximately 5 × 106 fluorescent microspheres (IMT, Irvine, CA, USA) were injected during occlusion, and a reference blood sample was simultaneously drawn from an arterial line. The snare was released after 120 min of coronary occlusion. Reperfusion was maintained for at least 4 h before imaging in 11 animals, the remaining 11 animals were allowed to recover for 48 h before imaging. All animals were euthanized immediately after completion of CMR with an injection of potassium chloride.
CMR imaging
Imaging was performed on a 1.5T clinical scanner (Magnetom Avanto, Siemens Healthcare Sector, Erlangen, Germany) and an eight-channel coil. T2 prepared steady-state free precession (SSFP) and T2 mapping were performed prior to contrast administration, with coverage of the entire left ventricle. T2-weighted images were acquired using a T2-prepared SSFP sequence.6 T2 preparation time was 50 ms and spatial resolution was typically 1.7mm × 0.7 mm, slice thickness 6.0 mm. Owing to software upgrades in our lab, quantitative T2 mapping protocols differed in the two groups of animals. Specifically, in animals imaged 4 h after reperfusion, T2 mapping was performed with the following typical parameters: T2 preparations at 0, 24, 55, and 90 ms, matrix 100 × 192, field of view (FOV) 186 × 270, linear b-SSFP readout, voxel size 1.4 × 1.9 × 6 mm. The typical parameters for animals imaged after 48 h of reperfusion were T2 preparations at 5, 40, 80 ms, matrix 108 × 192, FOV 188 × 250, centric fast low angle shot (FLASH) readout, voxel size 1.3 × 1.7 × 6 mm. To adjust for a potential bias in T2 accuracy that might be derived from our two T2 mapping sequences, we report the differences in the mean T2 between the injured and remote myocardium (ΔT2) when comparing data obtained with the different mapping protocols. Late gadolinium enhancement (LGE) images were acquired using a phase sensitive inversion recovery FLASH sequence which was ECG triggered and segmented with a typical resolution of 1.1 × 1.2 × 6 mm, > 10 min after administration of a bolus (0.2 mmol/kg) of i.v. contrast (gadopentate dimeglumine, Magnevist®).7 The inversion time was manually adjusted to null normal myocardium. All imaging was done during breath holds induced by pausing the ventilator.
Histopathology and microsphere analysis
Microsphere analysis was performed to quantify the perfusion defect and to determine the severity of blood flow reduction during occlusion. After explantation, hearts were set in a 2% agarose gel and sliced on a commercial meat slicer. The infarcted myocardium was defined by triphenyltetraxolium chloride (TTC) staining. The myocardium of the left ventricle was sectioned into 16 circumferential sectors. Sectors that had visible infarction involving at least 25% of the sector were further sectioned into an infarcted (endocardial) and salvaged (epicardial) subsector. Remote sectors of normal myocardium were also chosen as a reference and further sectioned into epicardial (RSepi) and endocardial (RSendo) subsectors in order to account for differences in blood flow that might exist between the epicardium and endocardium in normal tissue. These myocardial tissue samples were sent for blood flow determination by the quantification of microspheres (IMT). Myocardial blood flow in the infarction was defined as an average of the blood flow in all the infarcted subsectors, which also was done in the salvaged and remote sectors. Landmarks such as papillary muscles and the right ventricle insertion point were chosen to match the corresponding MR images to the ex vivo sectioning.
Image analysis
Image analysis was performed on a commercially available work station (Leonardo, Siemens). Regions of interest (ROI) were manually drawn in the AAR, infarcted, salvaged, and remote myocardium. We defined these areas of the myocardium by the localization relative to LGE defined infarction instead of the 2SD or 50% threshold of abnormality, to minimize the potential influence on our results from reliance on a threshold. Thus, the infarcted myocardium was defined as the area that displayed hyperenhancement on LGE images. The salvaged myocardium was defined as the area located epicardial to the infarcted myocardium. An ROI including both of these regions was defined as the AAR. ROIs with similar spatial dimensions were placed in the corresponding areas on the T2-weighted images and T2 maps. Landmarks ensured matching of the corresponding areas. Care was taken to exclude the blood pool and image artefacts, as well as areas of microvascular obstruction and haemorrhage. Remote sectors were defined as myocardium that did not exhibit LGE or T2 abnormalities. The mean signal intensities (SI) or the mean T2 in the ROIs was reported.
Statistics
Statistical analysis was performed using MedCalc (version 12.0). Data are presented as mean ± SD unless otherwise indicated. To ensure the statistical independence of measurements, we chose one mid-ventricular slice for analysis. After confirmation of normality, paired, and unpaired t-tests were used to compare means of distributions as appropriate. Multiple comparisons were analysed with repeated measures ANOVA and Bonferroni correction. Discrimination of the infarcted and salvaged myocardium was performed with receiver operating characteristics (ROC) analyses. The ΔT2 corresponding to the Youden index J was defined as the optimal threshold value for discrimination. A P–value <0.05 was considered statistically significant.
Results
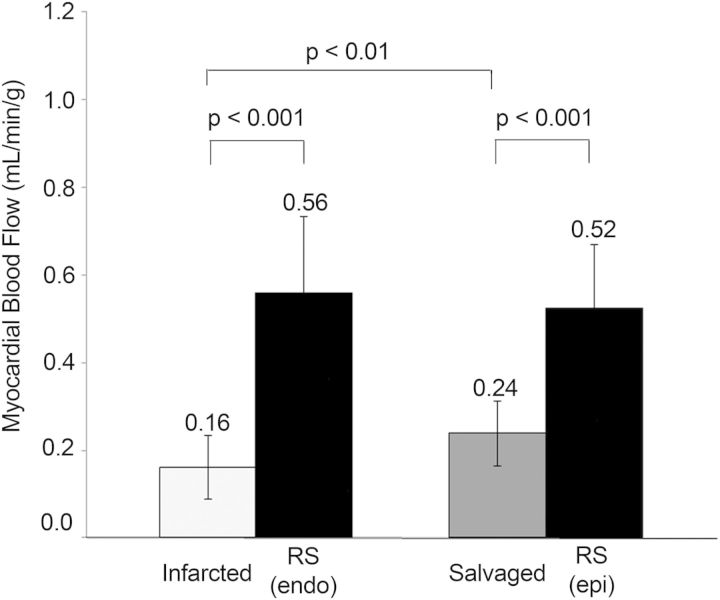
Twenty-eight animals underwent 2 h of coronary occlusion and myocardial infarction followed by 4 or 48 h of reperfusion prior to imaging. Sixteen animals underwent 4 h reperfusion, three died before imaging, and two were excluded due to severe arrhythmias during image acquisition, which resulted in poor image quality. Twelve animals underwent 48 h of reperfusion, one died before imaging. In total, 11 animals were analysed in each group. The mean reperfusion time before T2 mapping was 5.7 h (range 4.3–8.3) and 47.5 h (range 45.5–49.3) in the two groups. Figure 1 illustrates the transmural extent of T2-weighted enhancement and the T2 abnormality in the AAR compared with the remote myocardium in an animal with anterior myocardial infarction. Blood flow quantification by microspheres showed a significantly lower occlusion blood flow in the infarcted and salvaged sectors compared with the respective control areas (RSendo and RSepi) (Figure 2). The blood flow in the infarcted sectors was 0.16 ± 0.073 mL/min/g compared with the endocardial remote sector of 0.56 ± mL/min/g, P < 0.001. In the salvaged sector, the average blood flow was 48 ± 16.8% of the blood flow in the epicardial remote sector. There was no significant difference in blood flow between endocardial and epicardial remote sectors (0.56 ± 0.17 mL/min/g vs. 0.52 ± 0.15 mL/min/g, P = 1.0).
Figure 1:
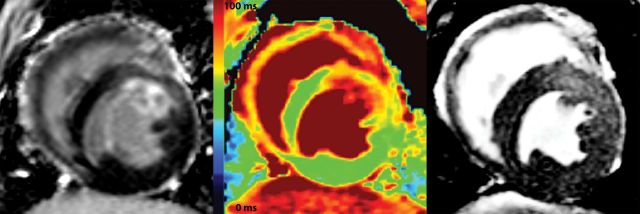
The area of T2-weighted enhancement (right panel) extends beyond the infarcted area as depicted by LGE (left panel). A difference in T2 values is also apparent in the infarcted (red), salvaged (yellow) and remote areas (green) on the T2 map (middle panel).
Figure 2:
Myocardial blood flow during occlusion as determined by microsphere analysis resulted in significantly lower blood flow in the infarcted and salvaged sectors compared with normal remote myocardium, consistent with a substantial perfusion defect. Data displayed as mean ± SD.
AAR measurements
T2 mapping and quantitative analysis was able to detect differences between salvaged, infarcted, and normal myocardium. The T2 map data are illustrated in Figures 3 and 4. At both time points, the T2 of the salvaged myocardium was longer than of the remote (66.0 ± 6.9 ms vs. 51.4 ± 3.5 ms, P < 0.001 at 4 h, and 56.8 ± 7.3 ms vs. 48.1 ± 3.5 ms, P < 0.001 at 48 h). The T2 relaxation was also longer in the infarcted myocardium compared with remote at both 4 and 48 h (71.4 ± 7.6 vs. 51.4 ± 3.5 ms, P < 0.001 and 64.0 ± 6.9 vs. 48.1 ± 3.5 ms, P < 0.001, respectively). Furthermore, the infarcted myocardium and the salvaged myocardium were statistically different from each other (P < 0.01 at 4 h and P = 0.033 at 48 h). Lines connecting measurements in individual animals indicated that all showed the same trend in T2 from the infarcted to salvaged to remote myocardium, although a few had minimal differences between infarct and salvage (see Figures 3 and 4). Also, T2 of the entire AAR was longer than remote at 4 h (69.3 ± 7.1 vs. 51.4 ± 3.5 ms, P < 0.0001) and 48 h (60.1 ± 6.0 vs. 48.1 ± 3.5 ms, P < 0.0001).
Figure 3:
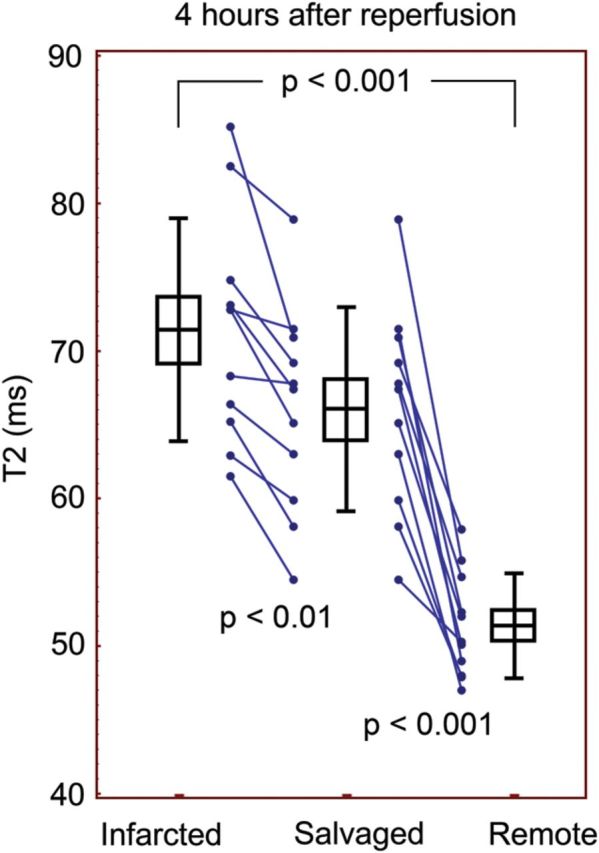
T2 mapping was able to distinguish the infarcted, salvaged, and remote myocardium 4 h after reperfusion. Boxes indicate mean and SEM and error bars indicate SD.
Figure 4:
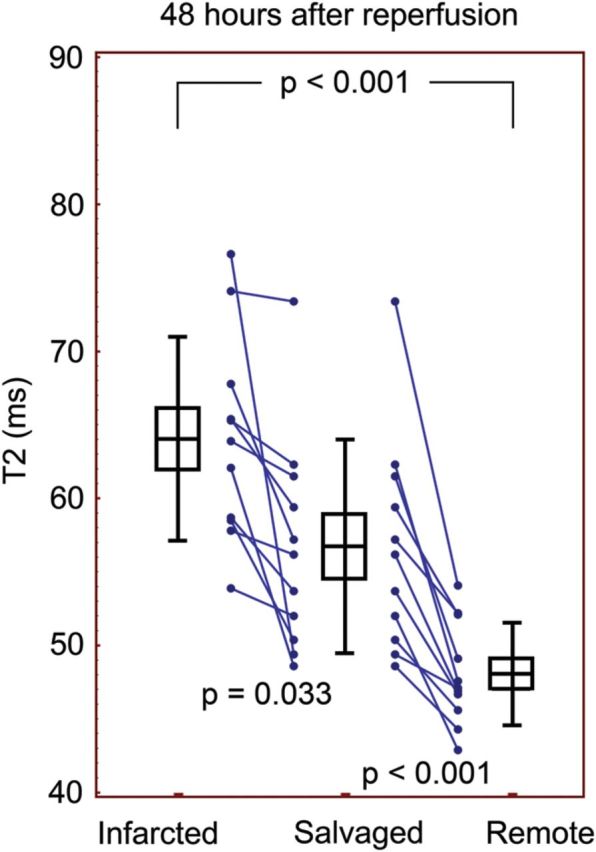
T2 quantification after 48 h of reperfusion. After longer reperfusion, the salvaged and infarcted myocardium were significantly longer than remote in all animals. Boxes indicate mean and SEM and error bars indicate SD.
Similarly, the SI of the AAR was significantly greater than the remote myocardium on T2-weighted images, both at 4 h of reperfusion (366 ± 57 vs. 253 ± 35 a.u., P < 0.0001) and 48 h (572 ± 136 vs. 436 ± 114 a.u., P < 0.001). Within the AAR, the SI of both the salvaged and the infarcted myocardium was significantly greater than the remote myocardium on T2-weighted images, as well as on T2 maps. After 4 h of reperfusion, the SI of T2-weighted images was 356 ± 64 a.u. in the salvaged myocardium and 372 ± 52 a.u. in the infarcted, vs. 253 ± 35 a.u. in remote (P < 0.001 vs. remote). At 48 h, the SI of the salvaged and infarcted myocardium was still significantly greater than remote (546 ± 136 and 597 ± 141 vs. 436 ± 114, P < 0.001 for both comparisons). Importantly, the difference between SI of the salvaged and infarcted myocardium was not statistically significant (P = 0.35) after only 4 h of reperfusion, but significantly different (P = 0.0057 for the salvaged vs. infarcted myocardium) after 48 h of reperfusion.
Discriminating between infarcted and salvaged myocardium
ROC analysis was performed to determine how well ΔT2 discriminated the salvaged from infarcted myocardium. After 4 h of reperfusion the discrimination was moderate, AUC: 0.74 (95% CI: 0.51–0.90), P = 0.03. The optimal threshold was a ΔT2 >13.1 ms which resulted in a sensitivity of 90.9 and specificity of 54.5. After 48 h of reperfusion discrimination was excellent, AUC: 0.86 (95% CI: 0.65–0.97), P < 0.001. Here, the optimal threshold was ΔT2 >9.3 ms, with a sensitivity of 100 and specificity of 72.7.
Magnitude of T2 abnormality and reperfusion times
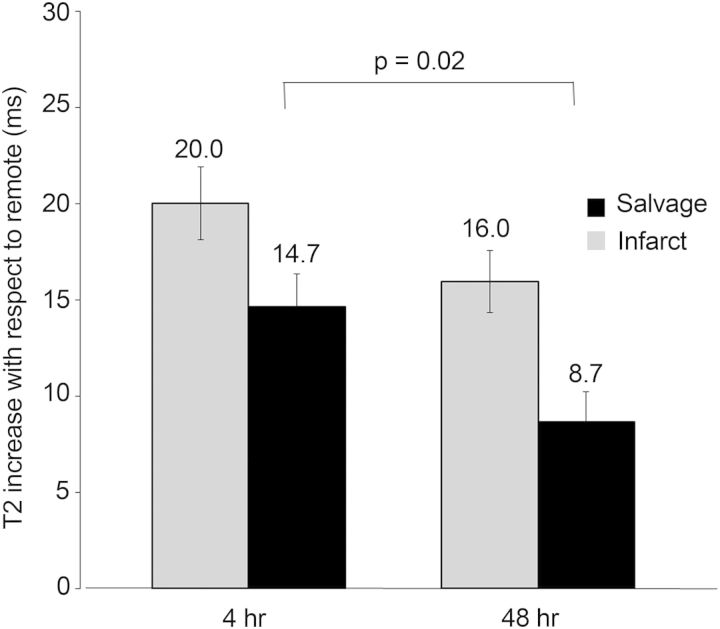
T2 relaxation times of the infarcted and salvaged myocardium were increased with respect to the remote myocardium for all animals: 18.0 ± 6.1 ms for ΔT2 in the infarcted (ΔT2infarct) and 11.7 ± 6.1 ms for salvaged (ΔT2salvage). All relaxation times were longer than remote (ΔT2infarct range: 10.9–32.9 ms, ΔT2salvage range: 2.3–23.1 ms). The ΔT2infarct was not significantly different at the two reperfusion times (20.0 ± 6.3 vs. 16.0 ± 5.4 ms, P = 0.12). Interestingly, the ΔT2salvage was significantly longer in infarcts imaged after 4 h of reperfusion compared with those where perfusion had been reestablished for 48 h (14.7 ± 5.6 vs. 8.7 ± 5.1 ms, P = 0.016), see Figure 5. After 48 h of reperfusion, the magnitude of the T2 abnormality in the salvaged myocardium was only 59% of the T2 abnormality at 4 h.
Figure 5:
ΔT2 values for the infarcted and salvaged myocardium. The magnitude of T2 abnormality is less in the salvaged myocardium after longer reperfusion. There was no significant difference in the T2 abnormality of the infarcted myocardium at the two time points.
Discussion
The main finding of this study was that quantitative T2 mapping techniques were able to show differences in T2 between different regions within the AAR in a canine model of reperfused myocardial infarction. The differences in the T2 of infarcted and salvaged myocardium vs. remote myocardium were apparent after both 4 and 48 h of reperfusion. Importantly, the T2 was longer in both the salvaged and infarcted myocardium compared with remote in all experiments and the magnitude of the difference between the salvaged and remote myocardium was greater after 4 h of reperfusion than after 48 h of reperfusion. The SI in the AAR of T2-weighted imaging was significantly greater than the remote myocardium, both in the early stages as well as 2 days after perfusion had been reestablished. This is consistent with previous findings that the extent of T1- or T2-weighted abnormality corresponds well with the zone of abnormal blood flow by microspheres.8,9 The fact that we also demonstrated a quantifiable elevation in T2 of the AAR compared with remote by T2 mapping underscores the concept that T2-weighted imaging is sensitive to more than just the infarcted myocardium. Prior work in the field that has found T2-weighted images overestimate the infarcted myocardium.8,10–13 Recently, Ugander et al.9,14 demonstrated T2-weighted abnormalities in the salvaged myocardium, as well as an increase in the extracellular volume content, which supports our current findings. Questions have been raised regarding the seemingly homogenous brightness of the AAR despite differences in underlying pathology and water content.2,15 Such controversies may now be resolved by objective measurements of T2 lengthening, which have been correlated to myocardial water content.5 Before the development of practical T1 and T2 quantitative mapping techniques, subtle differences in T1 or T2 may have been overlooked due to the specific T1 or T2 weighting or selection of window/levelling for display of images. It is important to note that the T2 of the infarcted myocardium in our study was always longer than of the salvaged, and both were significantly longer than the remote myocardium in all experiments.
Other studies have reported T2 values in porcine and canine models of infarction as well as humans by T2 mapping, with results similar to ours.3,9,16 Giri et al.3 report longer T2 values in the infarcted porcine myocardium, which may be due to a smaller sample size, species-specific effects, and differences in the T2 mapping sequence. Interestingly, the patient data are comparable with our canine data. We also found a range of values between subjects, both in the AAR and the remote zone.
Interestingly, we found that ΔT2 was moderate to excellent in discriminating between the salvaged and infarcted myocardium at both reperfusion times, with high sensitivity but lower specificity. This is an exciting finding, but should be interpreted carefully. We found a substantial inter-animal variability in the degree of T2 abnormality, why additional optimization of MRI methods may be needed to reliably discriminate infarct from the salvaged myocardium.
Lastly, we also demonstrated the importance of the timing of imaging after reperfusion. Infarct healing is a dynamic process, and imaging at different time points may highlight different stages of this process. It has been reported that T2-weighted imaging is stable in the first days after infarction and up to the first week in humans.8,17,18 We also found that the SI in T2-weighted images and T2 of the AAR was still greater than remote up to 48 h after reperfusion, but we detected some decreases in the severity of the T2 abnormality between 4 and 48 h of reperfusion. Reimer and Jennings also reported an increase in the total tissue water content in the salvaged myocardium in a reperfused canine model of ischaemia.15,19 The increase in the water content compared with remote was significant up to 24 h after reperfusion, but no longer detectable after 96 h.19 Unfortunately we do not have data from 96 h of reperfusion, but this earlier work does support our findings at 48 h. The Reimer and Jennings paper did not report the water content at time points between 24 and 96 h of reperfusion. We did not detect a significant difference in the ΔT2infarct at 4 and 48 h, but there seems to be a trend towards lower values at 48 h of reperfusion. Similarly, Kim et al.20 reported an SI in the infarct of rabbits ∼500% above normal myocardium 1 day after reperfusion that dropped to ∼ 300% at 3 days, although statistical comparison of the two time points was not reported. Whether or not these changes also occur in a similar manner in humans outside the controlled setting of a laboratory remains to be fully elucidated. Understanding these processes is important, since delineation of AAR and salvaged myocardium is already used to determine efficacy of coronary interventions.21 A substantial fading of the salvaged myocardium and the infarcted myocardium occurred between 4 and 48 h after reperfusion, and may be even greater at later time points.
Limitations
Our definition of the AAR in this study did not rely on a threshold abnormality based on microsphere flow or remote myocardium, but rather incorporated infarction defined by LGE imaging in the delineation of the AAR. Infarction is thought to progress according to the wave front phenomenon from the subendocardium to the epicardium, justifying why we used this principle to provide segmentation of the salvaged and infarcted myocardium on the T2-weighted images based on LGE images.22 Disregarding the lateral extent of the AAR is an oversimplification of the pathophysiological processes involved, but this model serves to clarify details pertaining to the salvaged myocardium without involving border zones. Reimer and Jennings also excluded the lateral border zone to avoid cross contamination in some of the fundamental experiments used to define the wavefront.23 Quantification of blood flow during occlusion revealed that the salvaged myocardium had undergone severe blood flow reduction during occlusion, but was still viable according to TTC and LGE imaging, and therefore usable as the salvaged myocardium. Furthermore, the occlusion blood flows we reported are similar to previous findings.24 In addition, this study focuses on a single slice of interest rather than whole heart coverage, data which were recently published by our group.9 Determination of the difference between the infarcted and salvaged myocardium, as presented here, requires precise localization of microsphere measurements and image analysis. As such, avoiding partial volume errors was more critical than the lateral border zone, and using only one representative slice per animal minimized these effects.
Another limitation of this study is the use of two different T2 mapping protocols. However, we compared ΔT2 rather than absolute values, which should overcome the bias associated with the upgrade of our T2 imaging protocol. Wassmuth et al.25 describe a bias in T2 due to differences in read out when compared with a phantom, the magnitude of the bias was on the order of 6–9 ms. We did observe a small difference in the T2 of the remote myocardium (51.4 ± 3.5 and 48.1 ± 3.5 ms), but this was of smaller magnitude due to the use of different T2-preparation and readout schemes. Furthermore, the bias was estimated to be less in the oedematous regions due to the influence of longer T1 values in these areas. Nonetheless, we chose to report ΔT2 salvage and ΔT2 infarct in our comparison at the two time points to minimize influence of bias in the methods. As we were interested in the magnitude of the differences between regions more than the absolute values, this was considered a reasonable approach. In addition, we did not analyse the same animals at the two time points. Serial measurements on the same subjects would have strengthened the statistical power. Though preferable, this was not possible due to logistical and financial reasons. We did, however, achieve statistical significance despite non-paired data. Additional studies including 96 h and longer periods of reperfusion would also have contributed to stronger conclusions regarding the duration of T2 abnormalities. The main limitation of this study relates to the simplicity of a canine infarct model relative to patients with varying degrees of coronary disease prior to their infarction. Also, the duration in which post-ischaemic area-at-risk measurements can reliably be performed in dogs may be shorter than that reported for humans due to species-specific effects.
In conclusion, we demonstrated that novel T2 mapping techniques could detect differences between the salvaged and infarcted myocardium compared with remote. Secondly, reperfusion time seems to have an impact on the magnitude of the T2 abnormality in the salvaged area, decreasing substantially already in the early phases of healing. Further study to determine the optimal timing for AAR imaging and progression of T2 abnormalities is warranted.
Funding
This work was supported by the Intramural Research Program of the National Heart, Lung, and Blood Institute, NIH, USA (Z01 HL006136-03 and HL004607-15).
Conflicts of interest: A.E.A. is a principal investigator on a US government Cooperative Research and Development Agreement (CRADA) with Siemens Medical Solutions (HL-CR-05–010). Other co-authors have no conflicts of interest to declare.
Acknowledgements
The authors thank Katherine Lucas and Pamela Vincent for animal care and technical support.
References
- 1.Arai AE, Leung S, Kellman P. Controversies in cardiovascular MR imaging: reasons why imaging myocardial T2 has clinical and pathophysiologic value in acute myocardial infarction. Radiology. 2012;265:23–32. doi: 10.1148/radiol.12112491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Croisille P, Kim HW, Kim RJ. Controversies in cardiovascular MR imaging: T2-weighted imaging should not be used to delineate the area at risk in ischemic myocardial injury. Radiology. 2012;265:12–22. doi: 10.1148/radiol.12111769. [DOI] [PubMed] [Google Scholar]
- 3.Giri S, Chung YC, Merchant A, Mihai G, Rajagopalan S, Raman SV, et al. T2 quantification for improved detection of myocardial edema. J Cardiovasc Magn Reson. 2009;11:56. doi: 10.1186/1532-429X-11-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Higgins CB, Herfkens R, Lipton MJ, Sievers R, Sheldon P, Kaufman L, et al. Nuclear magnetic resonance imaging of acute myocardial infarction in dogs: alterations in magnetic relaxation times. Am J Cardiol. 1983;52:184–8. doi: 10.1016/0002-9149(83)90093-0. [DOI] [PubMed] [Google Scholar]
- 5.Garcia-Dorado D, Oliveras J, Gili J, Sanz E, Perez-Villa F, Barrabes J, et al. Analysis of myocardial oedema by magnetic resonance imaging early after coronary artery occlusion with or without reperfusion. Cardiovasc Res. 1993;27:1462–9. doi: 10.1093/cvr/27.8.1462. [DOI] [PubMed] [Google Scholar]
- 6.Kellman P, Aletras AH, Mancini C, McVeigh ER, Arai AE. T2-prepared SSFP improves diagnostic confidence in edema imaging in acute myocardial infarction compared to turbo spin echo. Magn Reson Med. 2007;57:891–7. doi: 10.1002/mrm.21215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kellman P, Arai AE, McVeigh ER, Aletras AH. Phase-sensitive inversion recovery for detecting myocardial infarction using gadolinium-delayed hyperenhancement. Magn Reson Med. 2002;47:372–83. doi: 10.1002/mrm.10051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aletras AH, Tilak GS, Natanzon A, Hsu LY, Gonzalez FM, Hoyt RF, Jr, et al. Retrospective determination of the area at risk for reperfused acute myocardial infarction with T2-weighted cardiac magnetic resonance imaging: histopathological and displacement encoding with stimulated echoes (DENSE) functional validations. Circulation. 2006;113:1865–70. doi: 10.1161/CIRCULATIONAHA.105.576025. [DOI] [PubMed] [Google Scholar]
- 9.Ugander M, Bagi PS, Oki AJ, Chen B, Hsu LY, Aletras AH, et al. Myocardial edema as detected by pre-contrast T1 and T2 CMR delineates area at risk associated with acute myocardial infarction. JACC Cardiovasc Imaging. 2012;5:596–603. doi: 10.1016/j.jcmg.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tilak GS, Hsu LY, Hoyt RF, Jr, Arai AE, Aletras AH. In vivo T2-weighted magnetic resonance imaging can accurately determine the ischemic area at risk for 2-day-old nonreperfused myocardial infarction. Invest Radiol. 2008;43:7–15. doi: 10.1097/RLI.0b013e3181558822. [DOI] [PubMed] [Google Scholar]
- 11.Stork A, Lund GK, Muellerleile K, Bansmann PM, Nolte-Ernsting C, Kemper J, et al. Characterization of the peri-infarction zone using T2-weighted MRI and delayed-enhancement MRI in patients with acute myocardial infarction. Eur Radiol. 2006;16:2350–7. doi: 10.1007/s00330-006-0232-3. [DOI] [PubMed] [Google Scholar]
- 12.Friedrich MG, Abdel-Aty H, Taylor A, Schulz-Menger J, Messroghli D, Dietz R. The salvaged area at risk in reperfused acute myocardial infarction as visualized by cardiovascular magnetic resonance. J Am Coll Cardiol. 2008;51:1581–7. doi: 10.1016/j.jacc.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 13.Wright J, Adriaenssens T, Dymarkowski S, Desmet W, Bogaert J. Quantification of myocardial area at risk with T2-weighted CMR: comparison with contrast-enhanced CMR and coronary angiography. JACC Cardiovasc Imaging. 2009;2:825–31. doi: 10.1016/j.jcmg.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 14.Ugander M, Bagi P, Booker JO, Hsu LY, Oki AJ, Greiser A, et al. Understanding why edema in salvaged myocardium is difficult to detect by late gadolinium enhancement. J Cardiovasc Magn Reson. 2012;14(Suppl. 1):O63. [Google Scholar]
- 15.Jennings RB, Schaper J, Hill ML, Steenbergen C, Jr, Reimer KA. Effect of reperfusion late in the phase of reversible ischemic injury. Changes in cell volume, electrolytes, metabolites, and ultrastructure. Circ Res. 1985;56:262–78. doi: 10.1161/01.res.56.2.262. [DOI] [PubMed] [Google Scholar]
- 16.Verhaert D, Thavendiranathan P, Giri S, Mihai G, Rajagopalan S, Simonetti OP, et al. Direct T2 quantification of myocardial edema in acute ischemic injury. JACC Cardiovasc Imaging. 2011;4:269–78. doi: 10.1016/j.jcmg.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dall'Armellina E, Karia N, Lindsay AC, Karamitsos TD, Ferreira V, Robson MD, et al. Dynamic changes of edema and late gadolinium enhancement after acute myocardial infarction and their relationship to functional recovery and salvage index. Circ Cardiovasc Imaging. 2011;4:228–36. doi: 10.1161/CIRCIMAGING.111.963421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carlsson M, Ubachs JF, Hedstrom E, Heiberg E, Jovinge S, Arheden H. Myocardium at risk after acute infarction in humans on cardiac magnetic resonance: quantitative assessment during follow-up and validation with single-photon emission computed tomography. JACC Cardiovasc Imaging. 2009;2:569–76. doi: 10.1016/j.jcmg.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 19.Reimer KA, Hill ML, Jennings RB. Prolonged depletion of ATP and of the adenine nucleotide pool due to delayed resynthesis of adenine nucleotides following reversible myocardial ischemic injury in dogs. J Mol Cell Cardiol. 1981;13:229–39. doi: 10.1016/0022-2828(81)90219-4. [DOI] [PubMed] [Google Scholar]
- 20.Kim RJ, Fieno DS, Parrish TB, Harris K, Chen EL, Simonetti O, et al. Relationship of MRI delayed contrast enhancement to irreversible injury, infarct age, and contractile function. Circulation. 1999;100:1992–2002. doi: 10.1161/01.cir.100.19.1992. [DOI] [PubMed] [Google Scholar]
- 21.Francone M, Bucciarelli-Ducci C, Carbone I, Canali E, Scardala R, Calabrese FA, et al. Impact of primary coronary angioplasty delay on myocardial salvage, infarct size, and microvascular damage in patients with ST-segment elevation myocardial infarction: insight from cardiovascular magnetic resonance. J Am Coll Cardiol. 2009;54:2145–53. doi: 10.1016/j.jacc.2009.08.024. [DOI] [PubMed] [Google Scholar]
- 22.Reimer KA, Lowe JE, Rasmussen MM, Jennings RB. The wavefront phenomenon of ischemic cell death. 1. Myocardial infarct size vs duration of coronary occlusion in dogs. Circulation. 1977;56:786–94. doi: 10.1161/01.cir.56.5.786. [DOI] [PubMed] [Google Scholar]
- 23.Reimer KA, Jennings RB. The ‘wavefront phenomenon’ of myocardial ischemic cell death. II. Transmural progression of necrosis within the framework of ischemic bed size (myocardium at risk) and collateral flow. Lab Invest. 1979;40:633–44. [PubMed] [Google Scholar]
- 24.Judd RM, Lugo-Olivieri CH, Arai M, Kondo T, Croisille P, Lima JA, et al. Physiological basis of myocardial contrast enhancement in fast magnetic resonance images of 2-day-old reperfused canine infarcts. Circulation. 1995;92:1902–10. doi: 10.1161/01.cir.92.7.1902. [DOI] [PubMed] [Google Scholar]
- 25.Wassmuth R, Prothmann M, Utz W, Dieringer M, von Knobelsdorff-Brenkenhoff F, Greiser A, et al. Variability and homogeneity of cardiovascular magnetic resonance myocardial T2-mapping in volunteers compared to patients with edema. J Cardiovasc Magn Reson. 2013;15:27. doi: 10.1186/1532-429X-15-27. [DOI] [PMC free article] [PubMed] [Google Scholar]