Abstract
Preliminary evidence suggests that the neuropeptide, neurotensin (NT) may regulate fear/anxiety circuits. We investigated the effects of PD149163, a NT-1 receptor agonist, on fear-potentiated startle (FPS). Sprague Dawley rats were trained to associate a white light with a mild foot shock. In one experiment, animals were treated with either subcutaneous vehicle or PD149163 (0.01, 0.1 or 1.0 mg/kg) twenty-four hours after training. Twenty minutes later their acoustic startle response in the presence or absence of the white light was tested. In a second experiment, saline and 1.0 mg/kg PD149163 were tested using a separate group of rats. In the first experiment, PD149163 produced a non-significant decrease in baseline acoustic startle at all three doses. As expected, saline treated rats exhibited significant FPS. An ANOVA of percentage FPS revealed no significant effect of treatment group overall but the high dose group did not display FPS strongly suggesting an FPS effect at this dose. This finding was confirmed in the second experiment where the high dose of PD149163 reduced percent FPS relative to saline (P<0.05). These data suggest that systemically administered NT-1 agonists modulate the neural circuitry that regulates fear and anxiety to produce dose-dependent anxiolytic-like effects on FPS.
Introduction
Neurotensin (NT) is a tridecapeptide with a wide distribution throughout the mammalian central nervous system (Emson et al 1982). When administered directly into the brain, NT has been reported to have several antipsychotic-like behavioral and neurochemical effects including inhibition of mesolimbic dopamine function, antagonism of stimulant-induced hyperlocomotion (Kalivas et al 1984; Robledo et al 1993; Skoog et al 1986) and stimulant-induced disruption of sensorimotor gating (Feifel et al 1997). NT does not effectively reach the brain after systemic administration, nor does the C-terminal hexapeptide, NT(8-13), the smallest NT peptide fragment which contains full biological activity of the parent peptide (Kanba et al 1988; Machida et al 1993).
In order to produce viable drug candidates, several NT mimetics have been produced by chemically modifying the NT(8-13) peptide to make it more resistant to endopeptidase degradation in the periphery and thus better able to enter the central nervous system (CNS) (Cusack et al 2000; Wustrow et al 1995). PD149163 is one such NT mimetic produced by adding a reduced amide bond to NT(8-13) (Wustrow et al 1995). PD149163 has strong and selective affinity for the neurotensin-1 (NT1) receptor (Petrie et al 2004), the NT receptor type implicated in the antipsychotic-like effects of NT. PD149163 has been shown to produce robust antipsychotic-like effects (Feifel et al 1999). Recently, PD149163 has been shown to produce pro-cognitive effects in the CNS after systemic administration (Azmi et al 2006).
There is strong evidence that NT1 agonists modulate mesolimbic dopamine and forebrain acetylcholine transmission in the brain and this effect has been presumed to underlie the antipsychotic-like and pro-cognitive effects, respectively, of NT and NT mimetics such as PD149163 (Nakachi et al 1995; Szigethy and Beaudet 1987). Our laboratory discovered that NT mimetics have more diverse pharmacological effects, which raises the possibility that they may have important actions on other circuits relevant to neuropsychiatric disorders. For example, we reported that systemic administration of PD149163 blocks the behavioral effects of a serotonin-2 (5-HT2) agonist, DOI, suggesting that NT agonists also have inhibitory effects on serotoninergic transmission at 5-HT2 receptors (Feifel et al 2003b). Serotoninergic mechanisms have been strongly implicated in anxiety and depression, and inhibition of 5-HT2 receptors specifically may be a mechanism for anxiolysis and anti-depression (Mora et al 1997; Weisstaub et al 2006). Other evidence also suggests that NT agonists may regulate anxiety-relevant neurocircuitry. NT is localized in several brain regions that have been associated with fear and anxiety, such as the amygdala and hippocampus (Campeau et al 1992; Davis et al 1993; Gewirtz et al 2000; Paxinos and Watson 1997). Saiz Ruiz et al. (Saiz Ruiz et al 1992) reported that NT levels were significantly decreased in patients with anxiety and were normalized after recovery. In a fear conditioning test, duration of freezing was significantly reduced by beta lactotensin, a natural ligand for NT receptors (Yamauchi et al 2006). In addition, Shugalev et al. (2005) found that NT injections into the substantia nigra reduced fear produced by serotoninergic lesions of the dorsal raphe (Shugalev et al 2005). As these studies did not use NT receptor selective agonists, the role of the NT1 receptor in these anti-fear effects is not known. In order to investigate the role of NT1 receptors in fear circuits and to further investigate the anxiolytic potential of NT1 agonists, we tested the effects of PD149163 on fear-potentiated startle (FPS), a commonly employed animal model of anticipatory anxiety.
FPS is decreased by drugs that reduce fear and anxiety in humans such as benzodiazepines, whereas, it is increased by drugs that have anxiogenic effects, such as yohimbine (Davis et al 1993). Therefore FPS has been used extensively to test the anxiolytic potential of many compounds (Davis et al 1993; Walker and Davis 2002). In this paradigm, the magnitude of the acoustic startle reflex can be enhanced by a conditioned stimulus such as a light that has previously been associated with a shock. Fear-potentiated startle is exhibited when the startle reflex is significantly greater when the conditioned stimulus is present.
Methods
Animals
Seventy-six male Sprague Dawley rats (275 –375 grams at testing) were obtained from Harlan Laboratories, San Diego, California. Animals were housed in groups of two in clear plastic chambers in a climate-controlled room on a 12:12 hour light/dark cycle (lights on 7:00 am- 7:00 pm). The rats were handled prior to testing. All testing occurred during the light phase of the rats' circadian illumination schedule and they were allowed free access to food and water for the extent of the study, except during the actual testing. Behavioral testing was performed between 9:00 am and 4:30 pm beginning a minimum of 7 days after arrival. All studies described in this publication were carried out in accordance with the “Principles of laboratory and animal care” as described in the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the National Institutes of Health (Publication No. 85-23, revised 1985).
Drugs
PD149163 was generously made available by the NIMH Chemical Synthesis and Drug Supply Program (Washington D.C.), and SRI International (Menlo Park, California).
Startle Testing and Test Sessions
Startle testing was performed in two identical startle chambers obtained from San Diego Instruments (San Diego, California). Each chamber consisted of a clear non-restrictive Plexiglas cylinder resting on a Plexiglas platform inside a ventilated enclosure, housed in a sound-attenuated room. A continuous background noise of 65 dB, as well as the various acoustic stimuli, were produced within each chamber by a high-frequency loudspeaker (Radio Shack Supertweeter, San Diego, CA). The whole-body startle response of each animal produced vibrations of the Plexiglas cylinder, which were transduced into analog signals by a piezoelectric unit, mounted underneath the Plexiglas platform (Mansbach et al 1988). These analog signals were then digitized and stored by an interface unit connected to a microcomputer. Startle amplitude was defined as the amount of motion detected by the piezoelectric unit. The startle stimulus was a 40 ms 95 dB burst of white noise.
Baseline matching
Approximately three days before fear conditioning rats were placed in startle chambers for five minutes prior to exposure to 30 95 dB startle stimuli in the dark. This session was used to match groups of animals for similar levels of startle.
Training
For two consecutive days animals were placed in dark startle chambers for five minutes prior to exposure to 10 light-shock pairings. Each light-shock pairing consisted of a 3.7 second light (15 Watts, 6 inches from chambers) and a 0.6 mA shock delivered during the last 0.5 seconds of light exposure. The shock was generated by shockers connected to a grid (San Diego Instruments) covering the bottom of the startle chamber. The average intertrial interval was 2 minutes (ranging from 1 – 3 minutes).
Potentiated Startle Testing
On the day following startle training animals were injected with either saline, or PD149163 (0.01, 0.1 or 1.0 mg/kg). Twenty minutes later they were placed in dark startle chambers and exposed to 5 minutes of background noise followed by 10 95 dB stimuli. Rats were then presented with 20 95 dB trials (noise only) in the dark and 20 trials where the 95 dB noise bursts were presented 3.2 seconds after the onset of the light (light + noise). Interstimulus intervals were all 30 seconds. Rats were randomly assigned to receive one of two types of startle sessions. In one session type, 20 noise-only stimuli were presented followed by 20 light + noise stimuli. In the other session type, the order of trial presentation was reversed so that 20 light + noise stimuli were presented initially, followed by 20 noise-only presentations, in order to control for any order effect of the stimulus-type. We used this paradigm rather than using one session type with randomly presented noise-startle and startle-alone trials throughout the session because in parametric studies that we conducted prior to this experiment, this paradigm (non-random assignment) produced more robust FPS than random presentation. We hypothesize this is because the CS produces an emotional arousal (fear) in animals that likely lasts longer than the very short 30 second interval between the startle stimuli. Therefore, the arousal produced by CS likely “leaks” over to startle-alone presentations that may follow it. By separating CS-startle and startle-alone presentation into different halves of the startle session, the carry-over phenomenon is likely mitigated. Counterbalancing the order of presentation as we did controls for any order effect.
Two separate experiments were performed. The first experiment was a dose-response study in which thirty-six rats were administered SC injections of either saline or PD149163 (0.01, 0.1 or 1.0 mg/kg). After reviewing the results of the first experiment, we performed a second experiment to confirm an apparent effect on FPS observed with the highest dose in the first experiment. In this second experiment a new set of forty rats were treated with either saline or 1.0 mg/kg PD149163 and tested in the same FPS paradigm.
Data Analysis
The first ten 95 dB stimuli of the startle session were used to habituate animals to the 95 dB noise bursts and were not used in the data analysis. Data were reviewed and any animal that exhibited startle values less than 10 or greater than 3.0 standard deviations (SD) from the group mean (outlier) were excluded from the data analysis. In the dose-response study, one animal in the group receiving the middle dose of PD149163 and one in the high dose exhibited startle values that were less than 10 and were not included in the data analysis. One animal in the low dose group of that study exhibited startle that was greater that 3 SD from the mean and was excluded. Eight PD149163-treated animals that exhibited startle values less than 10 in the second study were excluded from data analysis. There were 16 rats in each group after eliminating the “non-startlers” in experiment #2.
A percent score for FPS was calculated for each animal by using the following formula: [(mean startle magnitude on light-noise trials – mean startle magnitude on noise alone trials) / mean startle magnitude on noise alone trials] × 100 (Walker and Davis, 2002; Winslow, Noble and Davis, 2007). We analyzed these data using percent FPS vs. absolute change in startle levels ((startle + light) – startle alone) to compensate for between group and individual differences in startle levels. Calculating FPS in each rat as a percentage increase of their own baseline startle, controls for differences in baseline startle among the rats, whether due to inherent genetic differences or differences produced by the different drug treatments (e.g., PD149163 doses versus saline). This is a common approach used, for example in studies of prepulse inhibition of startle, another modified startle measure, where percent PPI is typically measured to control for potential confounding effects of drugs on baseline startle. Paired t-tests were used to determine if the conditioned stimulus (light) significantly increased startle magnitude (i.e., produced FPS).
In the dose-response study, the effects of the PD149163 startle response (noise alone) on FPS were analyzed using a one-way ANOVA with drug treatment as a between subjects factor. Planned group-wise t-tests, corrected for multiple comparisons using the Bonferonni method, were also used to compare each PD149163 dose group with the saline-treated group. In the second experiment, in which only one saline and one dose of PD149163 were tested, an independent t-test was used to compare the startle response in saline-treated versus the PD149163-treated animals and the same approach was used for FPS data. Startle (95 dB stimuli presented after the first 10 trials) data were analyzed in a similar manner to the FPS data.
Results
Baseline Startle
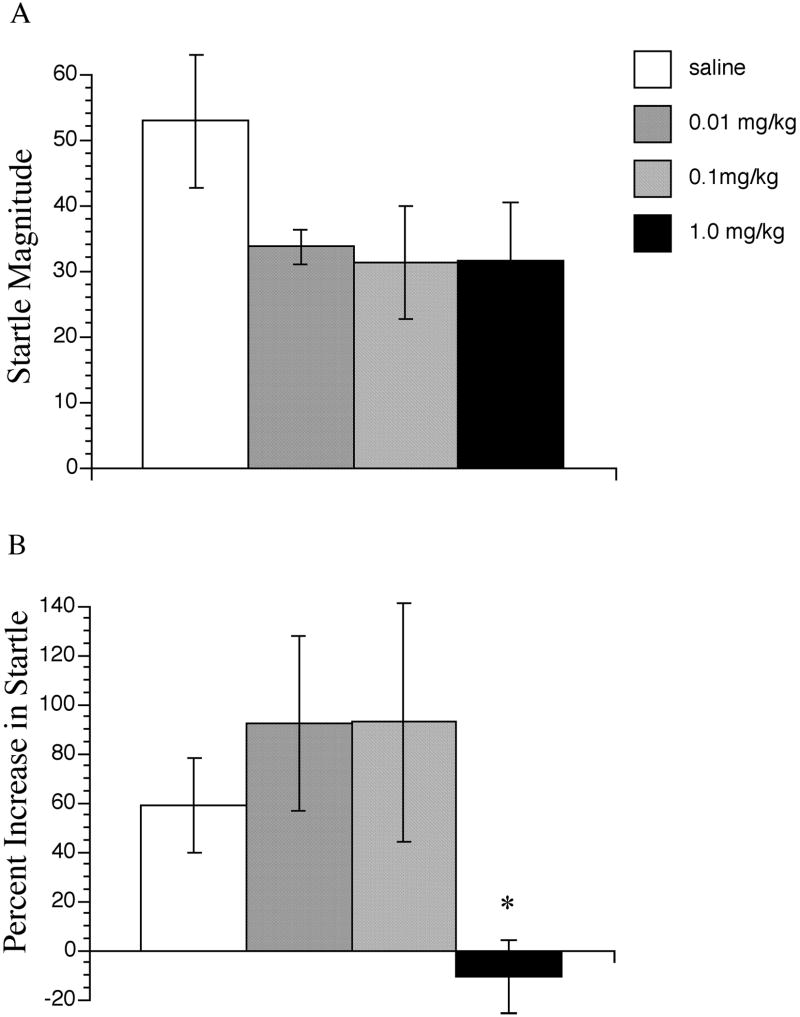
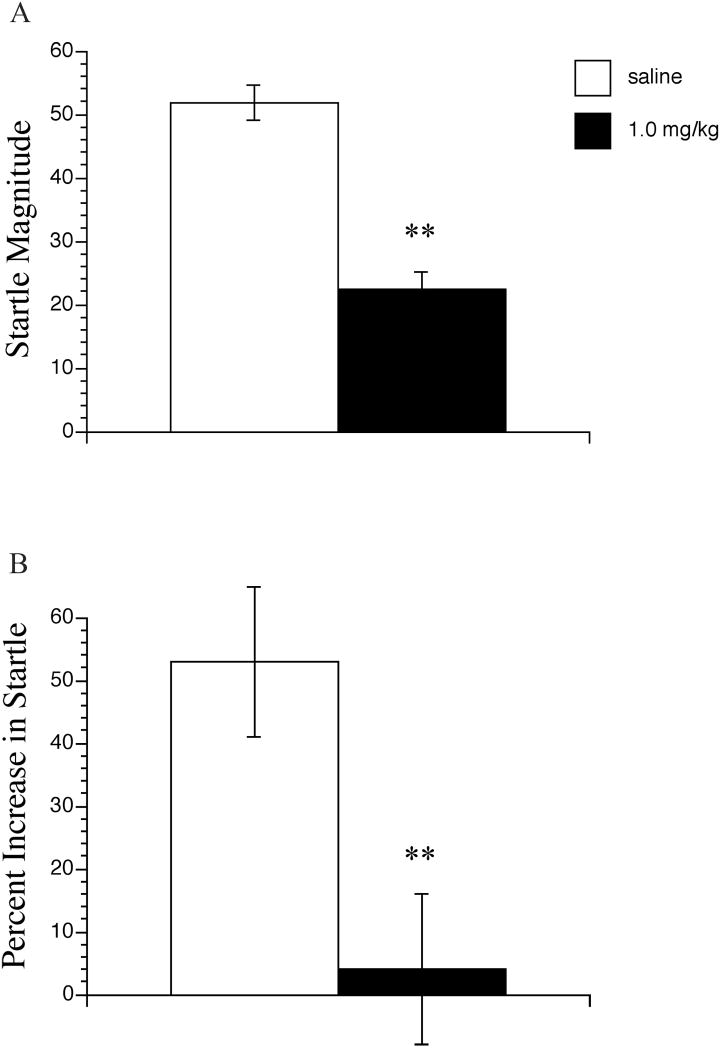
In the first experiment, PD149163 produced a non-significant trend towards a reduction in startle magnitude at all three doses, F(3, 27) = 1.70, NS (Figure 1A). In the second experiment, PD149163 significantly decreased startle magnitude (t(30)= 2.04, P < 0.01) (Figure 2A). Startle magnitude values in saline and PD149163 treated animals were comparable to those in the first experiment.
Figure 1.
The effects of PD149163 on startle magnitude (A) and fear-potentiated startle (B). Fear-potentiated startle data are represented as percent scores [(mean startle magnitude on light-noise trials – mean startle magnitude on noise alone trials) / mean startle magnitude on noise alone trials] × 100. PD149163 significantly different from saline represented by * P < 0.05. Data points represent the mean+SEM (n=7-10).
Figure 2.
The effects of PD149163 on startle magnitude (A) and fear-potentiated startle (B). Fear-potentiated startle data are represented as percent scores [(mean startle magnitude on light-noise trials – mean startle magnitude on noise alone trials) / mean startle magnitude on noise alone trials] × 100. PD149163 significantly different from saline represented by ** P < 0.01. Data points represent the mean+SEM (n=16).
Fear-Potentiated Startle
Comparison of the startle response to noise alone versus light plus noise in saline treated rats revealed that significant fear-potentiation of startle was exhibited in both the first, dose-response experiment (t(10)=1.83, P < 0.05), and the second experiment (t(15)=1.75, P<0.01). Startle was potentiated on average by 60 % and 53%, respectively in the saline-treated groups of the two experiments (Figure 1B and 2B).
In the dose-response study, ANOVA revealed no significant main effect of drug treatment on percent FPS (F(3,27) = 2.30, P= 0.100. However, visual inspection and the percent FPS data revealed that the low and middle doses of PD149163 produced a modest non-significant increase in FPS, whereas FPS was completely blocked at the highest dose of PD149163. Planned post-hoc t-tests revealed that FPS at the highest dose, but not the other two doses of PD149163 differed significantly from the FPS in the saline-treated group (t(15) = 0.502, P <0.05).
In the second experiment which was performed to corroborate the apparent effect of high dose PD149163 on FPS, animals treated with the highest dose of PD149163 exhibited significantly decreased percent FPS compared to those treated with saline, (t (30)=1.70, P < 0.01) (Figure 2B).
Discussion
Light as a conditioned fear stimulus produced a significant enhancement in the acoustic startle response demonstrating that FPS was successfully induced by the training procedures in these experiments. The findings indicate that the highest dose of systemically administered PD149163 blocked FPS in Sprague Dawley rats. Although the ANOVA used to analyze the FPS data in the first experiment did not produce a significant main effect of overall drug treatment, this was likely due to the large variance in some of the groups (i.e., low and middle dose of PD149163). In addition, individual comparisons of each PD149163 dose to saline in that experiment revealed a significant difference in the highest PD149163 dose compared to saline. This result was reproduced in the second experiment using only the highest dose and saline in different rats.
In addition to blocking FPS, PD149163 produced a reduction of startle magnitude at all three doses. It is conceivable that this reduction in baseline startle could have affected our FPS results. However, it is unlikely that the attenuation of FPS was an artifact of the decrease in startle produced by this NT1 agonist. In this respect, a lowering of the baseline startle is more likely to produce secondary enhancement of FPS rather than reduction in FPS, as it would be easier to potentiate low baseline startle than high baseline startle. Furthermore, the dose-response pattern revealed in the dose-response study provides a compelling argument against reduction in FPS as secondary to the suppression of baseline startle. All three doses of PD149163 decreased startle magnitude to a similar degree but there were distinct dose-effects on FPS. The low and middle doses produced a non-significant enhancement of FPS while the highest dose inhibited FPS. This result demonstrates a dissociation in the effects of PD149163 on startle and FPS in this experiment and that it is unlikely that inhibition of FPS by PD149163 is an artifact of the decreased startle magnitude produced by this compound. This pattern of drug effects on baseline startle and FPS has been reported with other drugs that are known anxiolytics such as diazepam (Berg and Davis, 1984). In contrast, buspirone, another known anxiolytic increases baseline startle but decreases FPS (Kehne et al, 1988; Mansbach and Geyer, 1988), providing further evidence that drug effects on baseline startle and FPS can be dissociated. Nevertheless to the extent that reduction in baseline startle is proposed as predictive of an anxiolytic, it is noteworthy that PD149163 reduced both baseline startle and FPS.
It is not totally clear where in the brain PD149163 acts to block FPS. However, a strong possibility would be the amygdala, a region implicated in a variety of anxiety disorders (Britton et al 2005; Cannistraro et al 2004; Milham et al 2005). The central amygdala (CEA) is necessary for the acquisition and expression of conditioned fear via a visual cue (Davis and Shi 2000; LeDoux, 2000). NT is co-expressed in GABAergic neurons in the CEA (Batten et al 2002) and NT1 receptors are also localized in this brain region (Alexander and Leeman 1998). Centrally administered NT increases c-fos and zif268 expression in the CEA (Lambert et al 1996) suggesting that NT activates this brain region. However, Beck and Fibiger (1995) reported that drug-induced c-fos levels in the CEA may not be associated with the attenuation of FPS (Beck and Fibiger, 1995). Further work will be needed to determine if the CEA mediates NT-induced blockade of FPS.
NT1 agonists have previously been reported to exhibit antipsychotic-like, anti-addiction and pro-cognitive effects in animal models (Azmi et al 2006; Boules et al 2005; Boules et al 2007; Feifel et al 2007; Feifel et al 2003a; Feifel et al 2003b; Feifel et al 2004; Feifel et al 1999; Fredrickson et al 2005; Richelson et al 2005; Shilling et al 2004; Shilling et al 2003). These data suggest that NT1 agonists such as PD149163 may also modulate anxiety-related circuits and the NT1 receptor system may be a novel target for anxiolytic drugs. Given that PD149163 has been shown to increase memory (Azmi et al 2006), it would be expected to increase memory of CS and therefore, increase FPS. Decreasing FPS indicates that it has a robust mitigating effect on FPS that can overcome the expected increase in FPS due to enhanced CS recall. To our knowledge only one other neuropeptide or neuropeptide-mimetic, has been shown to block FPS. In this regard, Myers et al. (2004) reported that secretin, a neurohormone neuropeptide, reduced FPS after systemic administration.
Benzodiazepines, and antidepressant drugs, particularly SSRI antidepressants, are the most widely used anti-anxiety agents with proven clinical efficacy (Rickels and Rynn 2002). Benzodiazepines generally reduce FPS after a single dose (Davis et al 1993) whereas SSRIs tend not to (Burghardt et al 2004). These effects are consistent with clinical experience that benzodiazepines produce anxiolytic effects in patients with anxiety disorders after a single injection, whereas SSRIs more typically require long-term use before producing anxiolytic effect (Feighner and Boyer 1992; Goldstein and Goodnick 1998; Goodnick and Goldstein 1998). In this respect, it is interesting that a single administration of PD149163 was sufficient to reduce FPS, a time course more similar to benzodiazepines than SSRIs.
In summary, we have provided evidence that a selective NT1 receptor agonist, PD149163, can block the expression of fear-potentiated startle suggesting that the NT1 receptor is involved in modulation of fear circuits by NT and raising the possibility that NT1 agonists may be useful for the treatment of anxiety. Additional research testing other NT1 agonists and using other animal models of anxiety (e.g., elevated plus maze) are needed to substantiate this possibility.
Acknowledgments
DF and PDS are partially funded by NIMH grant (MH62451). We thank Gilia Melendez for her excellent technical assistance.
References
- Alexander MJ, Leeman SE. Widespread expression in adult rat forebrain of mRNA encoding high-affinity neurotensin receptor. J Comp Neurol. 1998;402:475–500. [PubMed] [Google Scholar]
- Azmi N, Norman C, Spicer CH, Bennett GW. Effects of a neurotensin analogue (PD149163) and antagonist (SR142948A) on the scopolamine-induced deficits in a novel object discrimination task. Behav Pharmacol. 2006;17:357–62. doi: 10.1097/01.fbp.0000224382.63744.20. [DOI] [PubMed] [Google Scholar]
- Batten TF, Gamboa-Esteves FO, Saha S. Evidence for peptide co-transmission in retrograde- and anterograde-labelled central nucleus of amygdala neurones projecting to NTS. Auton Neurosci. 2002;98:28–32. doi: 10.1016/s1566-0702(02)00026-7. [DOI] [PubMed] [Google Scholar]
- Beck CH, Fibiger HC. Conditioned fear-induced changes in behavior and in the expression of the immediate early gene cfos: with and without diazapam. J Neurosci. 1995;15(1 Pt2):709–720. doi: 10.1523/JNEUROSCI.15-01-00709.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg WK, Davis M. Diazepam blocks fear-enhanced startle elicited electrically from the brainstem. Physiol Behav. 1984;32(2):333–6. doi: 10.1016/0031-9384(84)90148-3. [DOI] [PubMed] [Google Scholar]
- Boules M, Fredrickson P, Richelson E. Neurotensin agonists as an alternative to antipsychotics. Expert Opin Investig Drugs. 2005;14:359–69. doi: 10.1517/13543784.14.4.359. [DOI] [PubMed] [Google Scholar]
- Boules M, Shaw A, Fredrickson P, Richelson E. Neurotensin agonists: potential in the treatment of schizophrenia. CNS Drugs. 2007;21:13–23. doi: 10.2165/00023210-200721010-00002. [DOI] [PubMed] [Google Scholar]
- Britton JC, Phan KL, Taylor SF, Fig LM, Liberzon I. Corticolimbic blood flow in posttraumatic stress disorder during script-driven imagery. Biol Psychiatry. 2005;57:832–40. doi: 10.1016/j.biopsych.2004.12.025. [DOI] [PubMed] [Google Scholar]
- Burghardt NS, Sullivan GM, McEwen BS, Gorman JM, LeDoux JE. The selective serotonin reuptake inhibitor citalopram increases fear after acute treatment but reduces fear with chronic treatment: a comparison with tianeptine. Biol Psychiatry. 2004;55:1171–8. doi: 10.1016/j.biopsych.2004.02.029. [DOI] [PubMed] [Google Scholar]
- Campeau S, Miserendino MJ, Davis M. Intra-amygdala infusion of the N-methyl-D-aspartate receptor antagonist AP5 blocks acquisition but not expression of fear-potentiated startle to an auditory conditioned stimulus. Behav Neurosci. 1992;106:569–574. doi: 10.1037//0735-7044.106.3.569. [DOI] [PubMed] [Google Scholar]
- Cannistraro PA, Wright CI, Wedig MM, et al. Amygdala responses to human faces in obsessive-compulsive disorder. Biol Psychiatry. 2004;56:916–20. doi: 10.1016/j.biopsych.2004.09.029. [DOI] [PubMed] [Google Scholar]
- Cusack B, Boules M, Tyler BM, Fauq A, McCormick DJ, Richelson E. Effects of a novel neurotensin peptide analog given extracranially on CNS behaviors mediated by apomorphine and haloperidol. Brain Res. 2000;856:48–54. doi: 10.1016/s0006-8993(99)02363-x. [DOI] [PubMed] [Google Scholar]
- Davis M, Falls WA, Campeau S, Kim M. Fear-potentiated startle: a neural and pharmacological analysis. Behav Brain Res. 1993;58:175–98. doi: 10.1016/0166-4328(93)90102-v. [DOI] [PubMed] [Google Scholar]
- Davis M, Shi C. The amygdala. Curr Biol. 2000;10:R131. doi: 10.1016/s0960-9822(00)00345-6. [DOI] [PubMed] [Google Scholar]
- Emson PC, Goedert M, Horsfield P, Rioux F, St Pierre S. The regional distribution and chromatographic characterisation of neurotensin-like immunoreactivity in the rat central nervous system. J Neurochem. 1982;38:992–9. doi: 10.1111/j.1471-4159.1982.tb05340.x. [DOI] [PubMed] [Google Scholar]
- Feifel D, Melendez G, Priebe K, Shilling PD. The effects of chronic administration of established and putative antipsychotics on natural prepulse inhibition deficits in Brattleboro rats. Behav Brain Res. 2007;81:278–286. doi: 10.1016/j.bbr.2007.04.020. [DOI] [PubMed] [Google Scholar]
- Feifel D, Melendez G, Shilling PD. PD149163, a neurotensin agonist, attenuates prepulse inhibition deficits produced by dizocilpine and DOI. Neuropsychopharmacology. 2003a;28:651–653. doi: 10.1038/sj.npp.1300083. [DOI] [PubMed] [Google Scholar]
- Feifel D, Melendez G, Shilling PD. A Systemically Administered Neurotensin Agonist Blocks Disruption of Prepulse Inhibition Produced by a Serotonin-2A Agonist. Neuropsychopharmacology. 2003b;28:651–3. doi: 10.1038/sj.npp.1300083. [DOI] [PubMed] [Google Scholar]
- Feifel D, Melendez G, Shilling PD. Reversal of sensorimotor gating deficits in Brattleboro rats by acute administration of clozapine and a neurotensin agonist, but not haloperidol: a potential predictive model for novel antipsychotic effects. Neuropsychopharmacology. 2004;29:731–8. doi: 10.1038/sj.npp.1300378. [DOI] [PubMed] [Google Scholar]
- Feifel D, Minor KL, Dulawa S, Swerdlow NR. The effects of intra-accumbens neurotensin on sensorimotor gating. Brain Research. 1997;760:80–4. doi: 10.1016/s0006-8993(97)00306-5. [DOI] [PubMed] [Google Scholar]
- Feifel D, Reza TL, Wustrow DJ, Davis MD. Novel antipsychotic-like effects on prepulse inhibition of startle produced by a neurotensin agonist. Journal of Pharmacology and Experimental Therapeutics. 1999;288:710–3. [PubMed] [Google Scholar]
- Feighner JP, Boyer WF. Paroxetine in the treatment of depression: a comparison with imipramine and placebo. J Clin Psychiatry. 1992;53(Suppl):44–7. [PubMed] [Google Scholar]
- Fredrickson P, Boules M, Lin SC, Richelson E. Neurobiologic basis of nicotine addiction and psychostimulant abuse: a role for neurotensin? Psychiatr Clin North Am. 2005;28:737–51. 746. doi: 10.1016/j.psc.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Gewirtz JC, McNish KA, Davis M. Is the hippocampus necessary for contextual fear conditioning? Behav Brain Res. 2000;110:83–95. doi: 10.1016/s0166-4328(99)00187-4. [DOI] [PubMed] [Google Scholar]
- Goldstein BJ, Goodnick PJ. Selective serotonin reuptake inhibitors in the treatment of affective disorders--III. Tolerability, safety and pharmacoeconomics. J Psychopharmacol. 1998;12:S55–87. doi: 10.1177/0269881198012003041. [DOI] [PubMed] [Google Scholar]
- Goodnick PJ, Goldstein BJ. Selective serotonin reuptake inhibitors in affective disorders--II. Efficacy and quality of life. J Psychopharmacol. 1998;12:S21–54. doi: 10.1177/0269881198012003031. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Nemeroff CB, Prange AJ., Jr Neurotensin microinjection into the nucleus accumbens antagonizes dopamine-induced increase in locomotion and rearing. Neuroscience. 1984;11:919–30. doi: 10.1016/0306-4522(84)90203-3. [DOI] [PubMed] [Google Scholar]
- Kanba KS, Kanba S, Nelson A, Okazaki H, Richelson E. [3H]neurotensin(8-13) binds in human brain to the same sites as does [3H]neurotensin but with higher affinity. J Neurochem. 1988;50:131–7. doi: 10.1111/j.1471-4159.1988.tb13239.x. [DOI] [PubMed] [Google Scholar]
- Kehne JH, Cassella JV, Davis M. Anxiolytic effects of buspirone and gepirone in the fear-potentiated startle paradigm. Psychopharmacology (Berl) 1988;94(1):8–13. doi: 10.1007/BF00735872. [DOI] [PubMed] [Google Scholar]
- Lambert PD, Ely TD, Gross RE, Kilts CD. Neurotensin induces Fos and Zif268 expression in limbic nuclei of the rat brain. Neuroscience. 1996;75:1141–51. doi: 10.1016/0306-4522(96)00210-2. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–84. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Machida R, Tokumura T, Tsuchiya Y, Sasaki A, Abe K. Pharmacokinetics of novel hexapeptides with neurotensin activity in rats. Biological and Pharmaceutical Bulletin. 1993;16:43–7. doi: 10.1248/bpb.16.43. [DOI] [PubMed] [Google Scholar]
- Mansbach RS, Geyer MA, Braff DL. Dopaminergic stimulation disrupts sensorimotor gating in the rat. Psychopharmacology. 1988;94:507–14. doi: 10.1007/BF00212846. [DOI] [PubMed] [Google Scholar]
- Mansbach RS, Geyer MA. Blockade of potentiated startle responding in rats by 5-hydroxytryptamine1A receptor ligands. Eur J Pharmacol. 1988;156(3):375–83. doi: 10.1016/0014-2999(88)90283-x. [DOI] [PubMed] [Google Scholar]
- Milham MP, Nugent AC, Drevets WC, et al. Selective reduction in amygdala volume in pediatric anxiety disorders: a voxel-based morphometry investigation. Biol Psychiatry. 2005;57:961–6. doi: 10.1016/j.biopsych.2005.01.038. [DOI] [PubMed] [Google Scholar]
- Mora PO, Netto CF, Graeff FG. Role of 5-HT2A and 5-HT2C receptor subtypes in the two types of fear generated by the elevated T-maze. Pharmacol Biochem Behav. 1997;58:1051–7. doi: 10.1016/s0091-3057(97)00057-9. [DOI] [PubMed] [Google Scholar]
- Myers K, Goulet M, Rusche J, Boismenu R, Davis M. Inhibition of fear potentiated startle in rats following peripheral adminstration of secretin. Psychopharmacology (Berl) 2004;172:94–9. doi: 10.1007/s00213-003-1633-5. [DOI] [PubMed] [Google Scholar]
- Nakachi N, Yamada M, Cho T, Coleman NJ, Yamada M, Richelson E. Expression of mRNA for neurotensin in rat brain cholinergic neurons. Biol Psychiatry. 1995;37:642. [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York, New York: Academic Press; 1997. [Google Scholar]
- Petrie KA, Bubser M, Casey CD, Davis MD, Roth BL, Deutch AY. The neurotensin agonist PD149163 increases Fos expression in the prefrontal cortex of the rat. Neuropsychopharmacology. 2004;29:1878–88. doi: 10.1038/sj.npp.1300494. [DOI] [PubMed] [Google Scholar]
- Richelson E, Fredrickson PA, Boules MM. Neurotensin receptor agonists and antagonists for schizophrenia. Am J Psychiatry. 2005;162:633–4. doi: 10.1176/appi.ajp.162.3.633-b. author reply 635. [DOI] [PubMed] [Google Scholar]
- Rickels K, Rynn M. Pharmacotherapy of generalized anxiety disorder. J Clin Psychiatry. 2002;63(Suppl 14):9–16. [PubMed] [Google Scholar]
- Robledo P, Maldonado R, Koob GF. Neurotensin injected into the nucleus accumbens blocks the psychostimulant effects of cocaine but does not attenuate cocaine self-administration in the rat. Brain Research. 1993;622:105–12. doi: 10.1016/0006-8993(93)90808-z. [DOI] [PubMed] [Google Scholar]
- Saiz Ruiz J, Carrasco Perera JL, Hernanz A. Plasma neuropeptides in affective and anxiety disorders. Arch Neurobiol (Madr) 1992;55:1–5. [PubMed] [Google Scholar]
- Shilling PD, Melendez G, Priebe K, Richelson E, Feifel D. Neurotensin agonists block the prepulse inhibition deficits produced by a 5-HT2A and an alpha1 agonist. Psychopharmacology (Berl) 2004;175:353–9. doi: 10.1007/s00213-004-1835-5. [DOI] [PubMed] [Google Scholar]
- Shilling PD, Richelson E, Feifel D. NT69L, a neurotensin agonist, produces antipsychotic-like effects on prepulse inhibition of startle. Behavioural Brain Research. 2003;143:7–14. doi: 10.1016/s0166-4328(03)00037-8. [DOI] [PubMed] [Google Scholar]
- Shugalev IP, Ol'shanskii AS, Lenard L, Hartmann G. Effects of neurotensin on active and passive avoidance performance in rats with lesions of serotoninergic neurons. Zh Vyssh Nerv Deiat Im I P Pavlova. 2005;55:247–52. [PubMed] [Google Scholar]
- Skoog KM, Cain ST, Nemeroff CB. Centrally administered neurotensin suppresses locomotor hyperactivity induced by d-amphetamine but not by scopolamine or caffeine. Neuropharmacology. 1986;25:777–82. doi: 10.1016/0028-3908(86)90095-x. [DOI] [PubMed] [Google Scholar]
- Szigethy E, Beaudet A. Selective association of neurotensin receptors with cholinergic neurons in the rat basal forebrain. Neuroscience Letters. 1987;83:47–52. doi: 10.1016/0304-3940(87)90214-x. [DOI] [PubMed] [Google Scholar]
- Walker DL, Davis M. The role of amygdala glutamate receptors in fear learning, fear-potentiated startle, and extinction. Pharmacol Biochem Behav. 2002;71:379–92. doi: 10.1016/s0091-3057(01)00698-0. [DOI] [PubMed] [Google Scholar]
- Weisstaub NV, Zhou M, Lira A, et al. Cortical 5-HT2A receptor signaling modulates anxiety-like behaviors in mice. Science. 2006;313:536–40. doi: 10.1126/science.1123432. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Noble PL, Davis M. Modulation of fear-potentiated startle and vocalizations in juvenile rhesus monkeys by morphine, diazepam, and buspirone. Biol Psychiatry. 2007;61(3):389–95. doi: 10.1016/j.biopsych.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Wustrow DJ, Davis MD, Akunne HC, et al. Reduced amide bond neurotensin 8-13 mimetics with potent in vivo activity. Bioorganic & Medicinal Chemistry Letters. 1995;5:997–1002. [Google Scholar]
- Yamauchi R, Wada E, Yamada D, Yoshikawa M, Wada K. Effect of beta-lactotensin on acute stress and fear memory. Peptides. 2006;27:3176–82. doi: 10.1016/j.peptides.2006.08.009. [DOI] [PubMed] [Google Scholar]