Abstract
Background
Elevated mean platelet volume may reflect presence of active large platelets, which lead to fatal or non-fatal cardiovascular events. In recent studies, lack of nocturnal blood pressure fall was presented as an independent predictor of poor prognosis in essential hypertension. The relation of raised MPV with left ventricular hypertrophy has also been reported in hypertension.
The aim of this study was to investigate the relation between MPV, non-dipping blood pressure pattern, and left ventricular mass index (LVMI) in sustained hypertension.
Material/Methods
A total of 2500 patients, whose ambulatory blood pressure (ABP) records had been evaluated retrospectively between January 2010 and December 2012, were included. Patients were divided into 3 groups according to their ABP values: non-dipper hypertensive (n=289), dipper hypertensive (n=255), and normotensive (n=306). The MPV levels and biochemical analyses were recorded from patient files and, LVMI were automatically calculated using a regression equation.
Results
The non-dipper and dipper hypertensive groups had significantly higher MPV levels than normotensives (8.4±1 fL, 8.3±1 fL, and 8.1±0.6 fL, respectively, p<0.001). However, there was no difference among the non-dipper and dipper groups in terms of MPV level (p=0.675). Although LVMI was significantly different between non-dipper, dipper, and normotensive groups (p=0.009), no correlation was found between MPV level and LVMI in dipper and non-dipper hypertensive patients (r=−0.080, p=0.142). There was a weak correlation between MPV level and ambulatory 24-h diastolic and systolic blood pressure (r=0.076, p=0.027, and r=0.073, p=0.033, respectively).
Conclusions
We demonstrated that there was no correlation between MPV level, non-dipping pattern of blood pressure, and LVMI in sustained hypertension.
MeSH Keywords: Hypertension, Left Ventricular Hypertrophy, Mean Platelet Volume
Background
Arterial blood pressure (BP) exhibits a diurnal rhythm that is higher at daytime than at night time [1–3]. A nocturnal decline in BP of less than 10% of the daytime value has been termed as “non-dippers” [4]. Continuous 24-h ambulatory blood pressure (ABP) monitoring has been used to show the diurnal rhythm of arterial BP. The mechanisms responsible for abnormal diurnal BP variation remain unclear, but it may be associated with a deterioration of autonomic balance [5]. Erdem et al. reported a possible cardiac autonomic dysfunction in pre-hypertensive subjects with non-dipper pattern [6]. Verdecchia et al. demonstrated that lack of nocturnal BP fall in patients with essential hypertension was a good predictor of cardiovascular prognosis [7]. Furthermore, lack of nocturnal BP fall is a predictor of target organ damage, including stroke, renal failure, and adverse cardiovascular events [8–10]. Kang et al. reported that patients with CAD often had nocturnal non-dipping pattern, which might increase the risk of future coronary events, including acute coronary syndromes [11]. Zain El et al. reported that non-dipping morning blood pressure was an independent predictor of hypertensive target organ damage in elderly patients with isolated systolic hypertension [12]. Cha et al. [13] reported that masked and sustained hypertension, as well as non-dipping or reverse dipping pattern, were more common in the settings of severe renal failure and proteinuria. Karakas et al. demonstrated the relation of non-dipping blood pressure pattern with impaired left ventricular dyssynchrony in dipper and non-dipper hypertensives [14].
Previous studies have reported that increased platelet activity is associated with cardiovascular morbidity and mortality [15,16]. Mean platelet volume (MPV) is a very general and indirect marker of platelet activation that is altered in many situations, such as cigarette smoking, vascular abnormalities, hematological diseases, and atherosclerosis [17]. Elevated MPV value may reflect presence of active large platelets – which contain more dense granules that are metabolically and enzymatically more active than small ones – lead to high thrombus burden lesions that result in fatal or non-fatal cardiovascular events [17, 18]. Furthermore, increased MPV level was found to be related with cardiovascular mortality following acute myocardial infarction and restenosis [19,20].
The relation of raised MPV level with left ventricular hypertrophy has also been reported in patients with essential hypertension [21,22]. Since high MPV level, non-dipping blood pressure pattern, and left ventricular hypertrophy are known as independent risk factors for adverse cardiovascular events, we aimed to investigate the relations between MPV, non-dipping blood pressure pattern, and left ventricular mass index in patients with sustained hypertension.
Material and Methods
Study design
This study had a retrospective case-control design.
Study population
A total of 2500 patients (range, 18–80 years of age) in a large-volume tertiary hospital, whose ambulatory blood pressure (ABP) records had been evaluated retrospectively between January 2010 and December 2012, were screened. Past medical history, including cardiovascular risk factors, medications, and results of physical examination and laboratory analyses (including whole blood counts and standard biochemical parameters), were recorded from patient files. Any deficiencies in the information or missing or unavailable data about admission blood counts resulted in exclusion of the patient. By the end of the recruitment process, 840 patients remained and were included in the study.
The exclusion criteria were: secondary hypertension, masking hypertension, white-coat hypertension, active or former smoking, heart failure, recent MI, history of coronary artery bypass surgery, stroke, peripheral vascular disease, diabetes mellitus, valvular diseases, hemodynamically significant arrhythmias, history of stable and unstable angina pectoris, chronic renal failure (serum creatinine >1.5 mg/dl, blood urea nitrogen >30 mg/dl), chronic liver diseases, thromboembolic disorders, and hematological abnormalities. Patients who were already on antiplatelet therapy (acetylsalicylic acid or clopidogrel) were also excluded.
The study complies with the Declaration of Helsinki and the trial protocol was approved by the local Ethics Committee. The written informed consent was obtained from all participants.
Study protocol
Patients were divided into 3 groups according to their ambulatory BP values: non-dipper hypertensives (n=289), dipper hypertensives (n=255), and normotensives (n=306). Body mass index was calculated by dividing the weight (kg) by height squared (m2). All patients underwent trans-thoracic echocardiographic examination (General Electric Vivid 7 GE Vingmend Ultrasound AS, Horten, Norway). The value of left interventricular septal diameter in diastole (LVSd), left ventricular end-diastolic diameter (LVEDd), and left ventricular posterior wall diameter in diastole (LVPWd) were recorded from echocardiographic examination reports. The left ventricular mass index (LVMI) was calculated with the following formula:
Ambulatory blood pressure monitoring
The 24-h ABP measurement was performed using a portable compact digital recorder (Tonoport V, Milwaukee, GE Healthcare) and an analyser using customized analytical software programmed to measure blood pressures at 15-min intervals, from 07:00 until 23:00, and at 30-min intervals from 23:00 until 07:00. Daytime was defined as the time interval between the hours of 07:00 and 23:00, and night-time was defined as the time interval between the hours of 23:00 and 07:00. The patients were instructed to perform their usual daily activities, but to stay inactive during the measurements. Recordings were accepted if more than 80% of the raw data were valid.
Blood sampling
Blood samples were drawn from the antecubital vein after overnight fasting into standardized tubes containing dipotassium ethylenedinitrotetraacetic acid (EDTA) to be stored at room temperature. An automatic blood counter (Beckman Coulter, Miami, FL) was used for whole blood counts. The levels of MPV and other hematologic parameters were measured after 120 min from venipuncture. The expected values for MPV in our laboratory ranged from 6.8 to 10.8 fL. Other biochemical analyses were determined by standard methods.
Study variables
The variables of the study were as follows: age, sex, weight, height, body mass index (BMI), fasting serum glucose, plasma lipids (triglyceride, HDL cholesterol, total cholesterol, and LDL cholesterol concentrations), creatinine, MPV, LVMI, platelet count, daytime systolic blood pressure (SBP), daytime diastolic blood pressure (DBP), night-time SBP, night-time DBP, 24-h SBP, and 24-h DBP.
Diagnosis and definitions
HT was defined as an office blood pressure (OBP) of ≥140/90 mm Hg, daytime ABP ≥135/85 mm Hg, or the active use of antihypertensive drugs. Sustained HT was defined as both an office blood pressure (OBP) of ≥140/90 mm Hg and a daytime ABP of ≥135/85 mm Hg. Normotension was defined as a consistent normal BP on OBP and daytime ABP measurements in patients not receiving antihypertensive treatment (OBP <140/<90 mm Hg and daytime ABP <135/<85 mm Hg) [23]. Non-dippers were defined as those with nocturnal decrease in systolic blood pressure (SBP) of less than 10% of daytime, and dippers were defined as those with a 10% or larger decline in systolic blood pressure during the nocturnal period of diurnal blood pressure [4].
Statistical analysis
All statistical analyses were performed using SPSS version 17 (SPSS for Windows, Version 17.0, SPSS Inc., Chicago, IL). The variables were investigated using visual (histograms, probability plots) and analytical methods (Kolmogorov-Smirnov and Shapiro-Wilks test) to determine whether the variables were normally distributed. Descriptive analyses are presented as the mean±standard deviation (SD), and categorical variables are expressed as percentages. Groups were compared with one-way analysis of variance (ANOVA; age, total cholesterol, low-density lipid [LDL] cholesterol, body mass index, and hematocrit), the Kruskal-Wallis test, and the chi-square test. The Mann-Whitney U test was performed to test the significance of pairwise differences using Bonferroni correction to adjust for multiple comparisons. A Spearman correlation analysis was performed to describe the association of MPV with 24-h SBP, 24-h DBP, and LVMI. A p value <0.05 was considered to be statistically significant.
Results
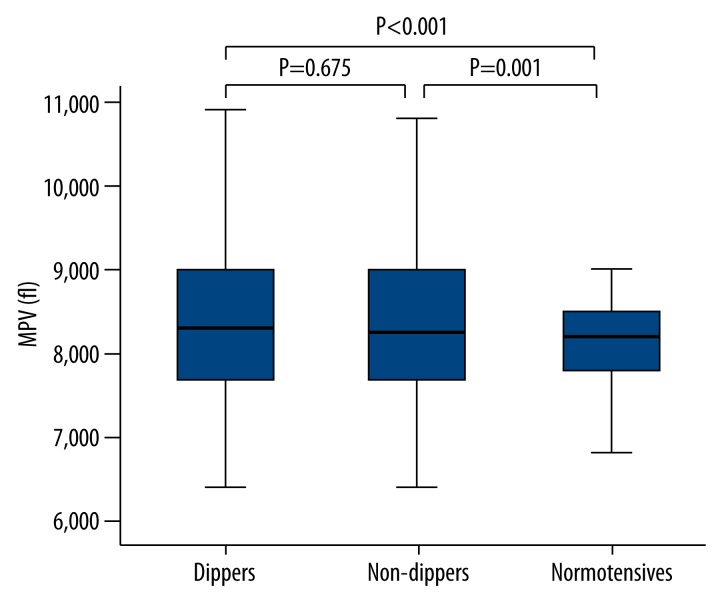
Baseline demographic, clinical, and laboratory characteristics of the study groups are presented in Table 1. There were no differences among 3 groups regarding age and male sex (p=0.690 and p=0.411, respectively). BMI was lower in the normotensive group than in non-dipper and dipper hypertensive groups, but the difference was not statistically significant (p=0.330). Moreover, the other baseline clinical and laboratory characteristics of the study groups were not significantly different between the 3 groups except for MPV levels, platelet count, and LVMI (all p values >0.05). The non-dipper and dipper hypertensive groups had significantly higher MPV levels than those of the normotensive subjects (8.4±1 fL, 8.3±1 fL, and 8.1±0.6 fL, p<0.001, respectively). However, there was no difference between the non-dipper and dipper groups in terms of MPV level (p=0.675) (Figure 1). Platelet counts were found to be significantly higher in the normotensive group than in dippers and non-dippers (275±65×106/mL, 266±722×106/mL, 54±65×106/mL, p<0.001). However, platelet counts were similar in the dippers and non-dippers groups (p=0.48). LVMI was significantly different between non-dippers, dippers, and normotensive groups (p=0.009). Also, it was found to be significantly higher in non-dippers as compared to normotensives (p=0.01).
Table 1.
Baseline demographic, clinical and laboratory characteristics of study groups.
| Non-dipper (n=289) | Dipper (n=255) | Normotensive (n=306) | p value | |
|---|---|---|---|---|
| Gender (male) n, (%) | 125 (50.8) | 117 (46.8) | 187 (45.5) | 0.411 |
| Age, years | 54±12 | 53±12 | 52±13 | 0.690 |
| BMI, kg/m2 | 31±4 | 30±5 | 27±8 | 0.330 |
| Glucose, mg/dl | 103±14 | 101±11 | 98±15 | 0.210 |
| Creatinine, mg/dl | 0.8±0.2 | 0.8±0.2 | 0.8±02 | 0.109 |
| Total-cholesterol, mg/dl | 206±45 | 205±43 | 203±41 | 0.789 |
| LDL, mg/dl | 135±33 | 132±35 | 132±35 | 0.710 |
| HDL-cholesterol, mg/dl | 46±12 | 47±12 | 49±14 | 0.067 |
| Triglycerides, mg/dl | 153±61 | 154±73 | 152±79 | 0.930 |
| ACE inh.use, (%) | 20.5 | 23.4 | 0.74 | |
| ARB use, (%) | 14.7 | 16 | 0.540 | |
| Beta-blocker use, (%) | 11.7 | 9 | 0.350 | |
| CCB use, (%) | 17.6 | 11.8 | 0.103 | |
| Diuretic use, (%) | 18.6 | 21.4 | 0.820 | |
| MPV, fl | 8.4±1 | 8.3±1 | 8.1±0.6 | <0.001* |
| Platelet count, 103/μl | 254±65 | 266±72 | 275±65 | <0.001** |
| LVMI, g/m2 | 94±25 | 92±25 | 86±27 | 0.009** |
ACE – angiotensin-converting enzyme; ARB – angiotensin receptor blocker; BMI – body mass index; CCB – Ca-channel blocker; DBP – diastolic blood pressure; HDL – high-density lipoprotein; LDL – low-density lipoprotein; LVMI – left ventricular mass index; MPV – mean platelet volume; SBP – systolic blood pressure.
p<0.01 both non-dippers and dippers vs. nornotensive;
p<0.01 non-dippers vs. normotensive.
Figure 1.
MPV levels.
The comparison of the ABP monitoring variables among the 3 groups are reported in Table 2. When the ABP values were compared among the 3 groups; daytime SBP, daytime DBP, nighttime DBP, 24-h SBP, and 24-h DBP were found to be significantly higher in non-dippers and dippers groups than in normotensives (all p values<0.001). Whereas night-time SBP and DBP in the non-dippers group were significantly higher than those of the dippers group (p<0.001), the daytime SBP and DBP were higher in dippers than in non-dippers, but the difference was not statistically significant. Twenty-four hour SBP and 24-h DBP were also found to be similar among non-dippers and dippers groups (p>0.017).
Table 2.
Comparison of the ambulatory blood pressure measurements among three groups.
| Non-dipper | Dipper | Normotensive | p value | |
|---|---|---|---|---|
| Daytime-SBP, mm Hg | 154±13 | 155±12** | 124±9* | <0.001 |
| Daytime-DBP, mm Hg | 94±11 | 98±10*** | 77±7* | <0.001 |
| Nighttime-SBP, mm Hg | 153±15 | 140±13*** | 115±11* | <0.001 |
| Nighttime-DBP, mm Hg | 92±10 | 82±9*** | 68±7* | <0.001 |
| 24-h-SBP, mm Hg | 154±13 | 151±14** | 122±9* | <0.001 |
| 24-h-DBP, mm Hg | 94±10 | 94±11** | 75±6* | <0.001 |
SBP – systolic blood pressure; DBP – diastolic blood pressure.
p<0.001 both dipper and non-dipper vs. normotensives;
p>0.017 non-dipper vs. dipper;
p<0.001 non-dipper vs. dipper.
When the relation of MPV level with ABP variables was investigated, significant correlations were only found between MPV and 24-h DBP and 24-h SBP (r=0.076, p=0.027, and r=0.073, p=0.033, respectively). Furthermore, the association of MPV with left ventricular hypertrophy represented by LVMI was evaluated among hypertensive groups in this study. There was no correlation between MPV and LVMI in dipper and non-dipper hypertensive patients (r=−0.080, p=0.142, r: −0.086, p=0.158, respectively).
Discussion
Three main findings emerged from this study. First, MPV level was significantly higher in the hypertensive group than in the normotensive group, but it did not differ between non-dippers and dippers. Second, MPV level was not associated with LVMI in patients with sustained hypertension. Third, platelet counts were higher in the normotensive group than in the non-dippers and dippers groups.
Mean platelet volume is an important platelet production index that may indirectly relate to platelet function. There are conflicting results in the literature about the relation of platelet size, measured as MPV, with platelet reactivity. Van der Loo et al. reported this positive association between them in acute coronary syndrome [25]. However, Beyan et al. did not demonstrate any relation between all of the platelet indices and platelet aggregation responses in healthy adults [26]. Moreover, De Luca et al. reported that MPV was not related to platelet reactivity and the extent of coronary artery disease among diabetic patients undergoing coronary angiography [27]. Large and hyperreactive platelets accelerate intracoronary thrombus formation, which leads to a cascade of fatal clinical events such as acute coronary syndrome [25].
Increased platelet activation and reverse effect of some antihypertensive drugs on platelet functions has been reported in patients with essential hypertension [28,29]. Shear forces, renin-angiotensin system, endothelial dysfunction, raised catecholamines levels, and presence of comorbid conditions promote increased activation of platelets in hypertensive settings [30]. Furthermore, studies have reported that MPV was significantly higher in patients with hypertension than in normotensive subjects [21,22]. Giles and Inglis reported that MPV levels were higher in patients with gestational hypertension than in normotensive pregnant subjects [31]. Also, Varol et al. reported that MPV was significantly higher in patient with prehypertension and hypertension than in controls [32]. Kaya et al. reported that the MPV level was significantly higher in the non-dippers group than in healthy controls [33]. Guven et al. demonstrated a raised level of MPV in masked and essential hypertensive subjects [34]. Taking into account the close relationship between MPV level and hypertension, we tried to elucidate this association in a very specific subgroup of hypertension termed ”sustained hypertension.” This finding, which was reported in some previous studies, was supported in our study. Although the underlying pathophysiologic mechanisms remain unclear, it may be that volume expansion or mal-suppressed sympathetic activity are important contributors to developing non-dipper hypertension [5]. The relation of MPV level between non-dipper and dipper hypertension was investigated in some clinical studies [35,36]. In contrast to our study, MPV was found to be significantly higher in the non-dippers group than in dipper hypertensives in studies by Inanc [37] and Ordu [38] et al., with a small population. This finding, which had been reported by Inanc et al., may be due to increased 24-h SBP, 24-h DBP, and 24-h average blood pressure in the non-dippers HT group compared to dippers. However, these blood pressure measurements, which were thought to have an effect on MPV level, were not different between the 2 groups in our study. This difference may arise from different study populations. The main limitation of these studies was a lack of multivariate regression analysis to determine an independent relationship between MPV and non-dipper hypertension.
LVH is one of the most important reliable indicators of target-organ damage (TOD) in hypertension. Hypertensive patients with LVH have a worse cardiovascular prognosis than those without [39,40]. There are conflicting results about the relation of LVH and non-dipping pattern of blood pressure in hypertensive subjects in the literature. Mozdzan et al. demonstrated the raised prevalence of concentric left ventricular hypertrophy in the night-time non-dipper hypertensives during ambulatory blood pressure monitoring. They also reported that dippers had lower average systolic, diastolic, and mean arterial pressure during the night-time hours [41]. Moreover, Ajayi et al. reported that the presence of LVH was associated with higher mean ABP and lower percentage nocturnal decline in systolic and diastolic blood pressure. This study confirmed the significance of the non-dipping blood pressure pattern on the development of LVH and its subsequently increased cardiovascular risk [42]. While some studies have reported that LVH is more pronounced in non-dippers, others have found no difference among hypertensive subjects [40,43]. In contrast to some clinical studies, we did not demonstrate any difference among dippers and non-dippers hypertensive groups in terms of LVMI, which is a reliable indicator of left ventricular hypertrophy, in our study. This finding may due to similar average 24-h SBP and DBP measurements in dipper and non-dipper hypertensive groups.
Furthermore, there are conflicting results in the literature regarding an association between MPV and presence of left ventricular hypertrophy [22,44,45]. While Scuteri et al. demonstrated the relation of MPV with raised left ventricular mass and interventricular septum thickness [40], Lip and Blann did not find any correlation between MPV and LVH in hypertensive patients [44]. In our study, there was no relation between MPV and LVH in patients with sustained hypertension, similar to the results of Lip and Blann.
Controversial results were reported about the relationship between MPV levels and target organ damage (TOD) in some previous studies [22,45,46]. Nadar et al. reported that hypertensive patients with TOD, such as stroke, previous myocardial infarction, angina, microalbuminuria/proteinuria, and left ventricular hypertrophy, had a higher MPV level than those of the hypertensive patients without TOD [22]. However, Bulur et al. [43] found no correlation between MPV, LVMI, and markers of TOD in patients with essential hypertension. In the present study, we did not find any association between MPV and LVMI in sustained hypertension, concordant with findings of Bulur et al. study. In addition, Gabbasov et al. found no relation between MPV and LVMI in essential hypertensive subjects, similar to our study results [47].
There are also different results regarding correlation between MPV and mean blood pressure in various clinical studies [37,45,46]. Bath et al. reported that mean BP did not correlate with MPV level in essential hypertension [45]. Moreover, Nadar et al. found no significant correlations between reduction in BP and changes in any markers of platelet activation, including beta-thromboglobulin, soluble P-selectin, and MPV [46]. Conversely, Inanc et al. [37] found a positive correlation of MPV with ADBP and ASBP in essential hypertension. In our study, a weak positive correlation was found between MPV level and 24-h ADBP and 24-h ASBP, as in the Inanc et al. study.
Platelet count was found to be higher in normotensive subjects than in hypertensives in the present study. Shear stress in hypertension causes endothelial injury, which may lead to thrombus formation. This process may promote a decrease in platelet count as a consequence of consumption and an increase in MPV level in patients with sustained hypertension. Although the relationship between MPV and platelet count is unclear, several clinical studies reported an inverse association between platelet volume and platelet count [19,20,48–50].
Study limitations
The present study has some limitations. First, this study had a non-randomized retrospective design, and is from a single center. Second, we excluded patients with clinically overt cardiovascular disease (such as coronary artery disease, cerebrovascular disease, and renal failure); therefore, our results cannot be extrapolated to all hypertensive subjects. Thirdly, our study provides no information regarding long-term clinical outcomes in patients with sustained hypertension.
Conclusions
We demonstrated that MPV level, as an indirect indicator of platelet activation, was significantly higher in sustained hypertensive patients as compared to normotensives, but there was no significant difference between dippers and non-dippers subgroups. Also, we did not find any correlation between MPV and 24-h DBP, 24-h SBP, and LVMI among the 3 groups. MPV level was not found to be correlated with the non-dipping pattern of blood pressure and LVMI in patient with sustained hypertension. Elevated MPV level may be the possible mechanism behind the raised cardiovascular risk in sustained hypertension.
Footnotes
Conflicts of interest
The authors have no relevant conflicts of interest to disclose.
Source of support: Departmental sources
References
- 1.Richardson DW, Honour AJ, Fenton GW, et al. Variation in arterial pressure throughout the day and night. Clin Sci. 1964;26:445–60. [PubMed] [Google Scholar]
- 2.Nillar-Craig NW, Bishop CV, Raftery EB. Circadian variation of blood pressure. Lancet. 1978;1:795–97. doi: 10.1016/s0140-6736(78)92998-7. [DOI] [PubMed] [Google Scholar]
- 3.Campos LA, Bader M, Baltatu OC. Brain Renin-Angiotensin system in hypertension, cardiac hypertrophy, and heart failure. Front Physiol. 2012;2:115. doi: 10.3389/fphys.2011.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Verdecchia P, Schillaci G, Guerrieri M, et al. Circadian blood pressure changes and left ventricular hypertrophy in essential hypertension. Circulation. 1990;81:528–36. doi: 10.1161/01.cir.81.2.528. [DOI] [PubMed] [Google Scholar]
- 5.Brotman DJ, Davidson MB, Boumitri M, Vidt DG. Impaired diurnal blood pressure variation and all-cause mortality. Am J Hypertens. 2008;21:92–97. doi: 10.1038/ajh.2007.7. [DOI] [PubMed] [Google Scholar]
- 6.Erdem A, Uenishi M, Küçükdurmaz Z, et al. Cardiac autonomic function measured by heart rate variability and turbulence in pre-hypertensive subjects. Clin Exp Hypertens. 2013;35:102–7. doi: 10.3109/10641963.2012.690475. [DOI] [PubMed] [Google Scholar]
- 7.Verdecchia P, Porcellati C, Schillaci G, et al. Ambulatory blood pressure. An independent predictor of prognosis in essential hypertension. Hypertension. 1994;24:793–801. doi: 10.1161/01.hyp.24.6.793. [DOI] [PubMed] [Google Scholar]
- 8.Ohkubo T, Imai Y, Tsuji I, et al. Relation between nocturnal decline in blood pressure and mortality. The Ohasama Study. Am J Hypertens. 1997;10:1201–7. doi: 10.1016/s0895-7061(97)00274-4. [DOI] [PubMed] [Google Scholar]
- 9.Lurbe E, Redon J, Kesani A, et al. Increase in nocturnal blood pressure and progression to microalbuminuria in type 1 diabetes. N Engl J Med. 2002;347:797–805. doi: 10.1056/NEJMoa013410. [DOI] [PubMed] [Google Scholar]
- 10.Davidson MB, Hix JK, Vidt DG, Brotman DJ. Association of impaired diurnal blood pressure variation with a subsequent decline in glomerular filtration rate. Arch Intern Med. 2006;166:846–52. doi: 10.1001/archinte.166.8.846. [DOI] [PubMed] [Google Scholar]
- 11.Kang YY, Li Y, Wang JG. Ambulatory blood pressure monitoring in the prediction and prevention of coronary heart disease. Curr Hypertens Rep. 2013;15:167–74. doi: 10.1007/s11906-013-0341-8. [DOI] [PubMed] [Google Scholar]
- 12.Zain-El MH, Snincak M, Pahuli K, et al. Non-dipping morning blood pressure and isolated systolic hypertension in elderly. Bratisl Lek Listy. 2013;114:150–54. doi: 10.4149/bll_2013_033. [DOI] [PubMed] [Google Scholar]
- 13.Cha RH, Kim S, Ae Yoon S, et al. Association between blood pressure and target organ damage in patients with chronic kidney disease and hypertension: results of the APrODiTe study. Hypertens Res. 2014;37:172–78. doi: 10.1038/hr.2013.127. [DOI] [PubMed] [Google Scholar]
- 14.Karakas MF, Buyukkaya E, Kurt M, et al. Assessment of left ventricular dyssynchrony in dipper and non-dipper hypertension. Blood Press. 2013;22:144–50. doi: 10.3109/08037051.2012.745224. [DOI] [PubMed] [Google Scholar]
- 15.Endler G, Klimesch A, Sunder-Plassmann H, et al. Mean platelet volume is an independent risk factor for myocardial infarction but not for coronary artery disease. Br J Haematol. 2002;117(2):399–404. doi: 10.1046/j.1365-2141.2002.03441.x. [DOI] [PubMed] [Google Scholar]
- 16.Furman MI, Barnard MR, Krueger LA, et al. Circulating platelets-monocyte aggregates are an early marker of acute myocardial infarction. J Am Coll Cardiol. 2001;38(4):1002–6. doi: 10.1016/s0735-1097(01)01485-1. [DOI] [PubMed] [Google Scholar]
- 17.Thombson CB, Eaton K, Princiotta SM, et al. Size dependent platelet subpopulations: relationship of platelet volume to ultrastructure, enzymatic activity, and function. Br J Haematol. 1982;50(3):509–19. doi: 10.1111/j.1365-2141.1982.tb01947.x. [DOI] [PubMed] [Google Scholar]
- 18.Tsiara S, Elisaf M, Jagroop IA, Mikhailidis DP. Platelets as predictors of vascular risk: is there a practical index of platelet activity? Clin Appl Thromb Hemost. 2003;9(3):177–90. doi: 10.1177/107602960300900301. [DOI] [PubMed] [Google Scholar]
- 19.Huczek Z, Kochman J, Filipiak KJ, et al. Mean platelet volume on admission predicts impaired reperfusion and long-term mortality in acute myocardial infarction treated with primary percutaneous coronary intervention. J Am Coll Cardiol. 2005;46(2):284–90. doi: 10.1016/j.jacc.2005.03.065. [DOI] [PubMed] [Google Scholar]
- 20.Yang A, Pizzulli L, Luderitz B. Mean platelet volume as marker of restenosis after percutaneous transluminal coronary angioplasty inpatients with stable and unstable angina pectoris. Thromb Res. 2006;117:371–77. doi: 10.1016/j.thromres.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 21.Siebers R, Maling T. Mean platelet volume in human essential hypertension. J Hum Hypertens. 1995;9:207. [PubMed] [Google Scholar]
- 22.Nadar SK, Blann AD, Kamath S, et al. Platelet indexes in relation to target organ damage in high-risk hypertensive patients: a substudy of the Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT) J Am Coll Cardiol. 2004;44(2):415–22. doi: 10.1016/j.jacc.2004.03.067. [DOI] [PubMed] [Google Scholar]
- 23.O’Brien E, Asmar R, Beilin L, et al. On behalf of the European Society of Hypertension Working Group on Blood Pressure Monitoring. Practice guidelines of the European Society of Hypertension for clinic, ambulatory and self-blood pressure measurement. J Hypertens. 2005;23:697–701. doi: 10.1097/01.hjh.0000163132.84890.c4. [DOI] [PubMed] [Google Scholar]
- 24.van der Loo B, Martin JF. A role for changes in platelet production in the cause of acute coronary syndromes. Arterioscler Thromb Vasc Biol. 1999;19(3):672–79. doi: 10.1161/01.atv.19.3.672. [DOI] [PubMed] [Google Scholar]
- 25.Elsenberg EH, van Werkum JW, van de Wal RM, et al. The influence of clinical characteristics, laboratory and inflammatory markers on high on-treatment platelet reactivity as measured with different platelet function tests. Thromb Haemost. 2009;102(4):719–27. doi: 10.1160/TH09-05-0285. [DOI] [PubMed] [Google Scholar]
- 26.Beyan C, Kaptan K, Ifran A. Platelet count, mean platelet volume, platelet distribution width, and plateletcrit do not correlate with optical platelet aggregation responses in healthy volunteers. J Thromb Thrombolysis. 2006;22(3):161–64. doi: 10.1007/s11239-006-9014-7. [DOI] [PubMed] [Google Scholar]
- 27.De Luca G, Verdoia M, Cassetti E, et al. Novara Atherosclerosis Study (NAS) group. Mean platelet volume is not associated with platelet reactivity and the extent of coronary artery disease in diabetic patients. Blood Coagul Fibrinolysis. 2013;24(6):619–24. doi: 10.1097/MBC.0b013e328360c75a. [DOI] [PubMed] [Google Scholar]
- 28.Spencer GG, Gurney D, Blann AD, et al. ASGOT Steering Committee, Anglo-Scandinavian Cardiac Outcomes Trial. VW factor, soluble P-selectin and target-organ damage in hypertension: A sub-study of the Anglo-Scandinavian Cardiac Outcomes trial (ASGOT) Hypertension. 2002;40(1):61–6. doi: 10.1161/01.hyp.0000022061.12297.2e. [DOI] [PubMed] [Google Scholar]
- 29.Gomi T, Ikeda T, Shibuya Y, Naqao R. Effects of anti-hyper-tensive treatment on platelet function in essential hypertension. Hypertens Res. 2000;23(6):567–72. doi: 10.1291/hypres.23.567. [DOI] [PubMed] [Google Scholar]
- 30.Siebers R, Maling T. Mean platelet volume in human essential hypertension. J Hum Hypertens. 1995;9(3):207. [PubMed] [Google Scholar]
- 31.Giles C, Inglis TC. Thrombocytopenia and macrothrombocytosis in gestational hypertension. Br J Obstet Gynaecol. 1981;88(11):1115–19. doi: 10.1111/j.1471-0528.1981.tb01764.x. [DOI] [PubMed] [Google Scholar]
- 32.Varol E, Akcay S, Icli A, et al. Mean platelet volume in patients with prehypertension and hypertension. Clin Hemorheol Microcirc. 2010;45(1):67–72. doi: 10.3233/CH-2010-1327. [DOI] [PubMed] [Google Scholar]
- 33.Kaya MG, Yarlioglues M, Gunebakmaz O, et al. Platelet activation and inflammatory response in patients with non-dipper hypertension. Atherosclerosis. 2010;209(1):278–82. doi: 10.1016/j.atherosclerosis.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 34.Guven A, Caliskan M, Ciftci O, Barutcu I. Increased platelet activation and inflammatory response in patients with masked hypertension. Blood Coagul Fibrinolysis. 2013;24:170–74. doi: 10.1097/MBC.0b013e32835aba36. [DOI] [PubMed] [Google Scholar]
- 35.Levy D, Garrison RJ, Savage DD, et al. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322(22):1561–66. doi: 10.1056/NEJM199005313222203. [DOI] [PubMed] [Google Scholar]
- 36.Mayet J, Shahi M, Hughes AD, et al. Left ventricular structure and function in previously untreated hypertensive patients: the importance of blood pressure, the nocturnal blood pressure dip and heart rate. J Cardiovasc Risk. 1995;2(3):255–61. [PubMed] [Google Scholar]
- 37.Inanc T, Kaya MG, Yarlioglues M, et al. The mean platelet volume in patients with non-dipper hypertension compared to dipper hypertension and normotensives. Blood Press. 2010;19(2):81–85. doi: 10.3109/08037050903516284. [DOI] [PubMed] [Google Scholar]
- 38.Ordu S, Ozhan H, Caglar O, et al. Mean platelet volume in patients with dipper and non-dipper hypertension. Blood Press. 2010;19(1):26–30. doi: 10.3109/08037050903416402. [DOI] [PubMed] [Google Scholar]
- 39.Roman MJ, Pickering TG, Schwartz JE, et al. Is the absence of a normal nocturnal fall in blood pressure (nondipping) associated with cardiovascular target organ damage? J Hypertens. 1997;15(9):969–78. doi: 10.1097/00004872-199715090-00007. [DOI] [PubMed] [Google Scholar]
- 40.Scuteri A, Cacciafesta M, dePropris AM, et al. Platelet size and left ventricular hypertrophy in hypertensive patients over 50 years of age. Eur J Clin Invest. 1995;25(11):874–76. doi: 10.1111/j.1365-2362.1995.tb01698.x. [DOI] [PubMed] [Google Scholar]
- 41.Możdżan M, Wierzbowska-Drabik K, Kurpesa M, et al. Echocardiographic indices of left ventricular hypertrophy and diastolic function in hypertensive patients with preserved LVEF classified as dippers and non-dippers. Arch Med Sci. 2013;20(9):268–75. doi: 10.5114/aoms.2013.34534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ajayi OE, Ajayi EA, Akintomide OA, et al. Ambulatory blood pressure profile and left ventricular geometry in Nigerian hypertensives. J Cardiovasc Dis Res. 2011;2:164–71. doi: 10.4103/0975-3583.85263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bulur S, Onder H, Aslantas Y, et al. Relation between indivces of end-organ damage and mean platelet volume in hypertensive patients. Blood Coagul Fibrinolysis. 2012;23:367–69. doi: 10.1097/MBC.0b013e32835291b1. [DOI] [PubMed] [Google Scholar]
- 44.Lip GY, Blann AD. Associations of platelet function in hypertensive patients with left ventricular hypertrophy. Platelets. 1999;10:71–72. doi: 10.1080/09537109976392. [DOI] [PubMed] [Google Scholar]
- 45.Bath PM, Carney C, Markandu ND, MacGregor GA. Platelet volume is not increased in essential hypertension. J Hum Hypertens. 1994;8(6):457–59. [PubMed] [Google Scholar]
- 46.Nadar S, Blann AD, Lip GY. Platelet morphology and plasma indices of platelet activation in essential hypertension: Lip GY: effects of amlodipine-based antihypertensive therapy. Ann Med. 2004;36(7):552–57. doi: 10.1080/07853890410017386. [DOI] [PubMed] [Google Scholar]
- 47.Gabbasov Z, Parfyonova Y, Popov E, et al. Association of platelet function in hypertensive patients with left ventricular hypertrophy, transient myocardial ischemia, and coronary artery disease. Platelets. 1998;9(3–4):191–95. doi: 10.1080/09537109876672. [DOI] [PubMed] [Google Scholar]
- 48.Thaulow E, Erikssen J, Sandvi KL, et al. Blood Platelet count are related to total and cardiovascular death in apparently healthy men. Circulation. 1991;84(2):613–17. doi: 10.1161/01.cir.84.2.613. [DOI] [PubMed] [Google Scholar]
- 49.Fager B, Sjogren A, Sjogren U. Platelet counts in myocardial infarction, angina pectoris and peripheral arterydisease. Acta Med Scand. 1985;217(1):21–26. doi: 10.1111/j.0954-6820.1985.tb01629.x. [DOI] [PubMed] [Google Scholar]
- 50.Glud T, Schmidt EB, Kristensen SD, Arnfred T. Platelet number and volume during myocardial infarction in relationto infarct size. Acta Med Scand. 1986;220(5):401–5. doi: 10.1111/j.0954-6820.1986.tb02787.x. [DOI] [PubMed] [Google Scholar]