Abstract
Background
Hereditary angioedema (HAE) is a rare disease caused by C1-esterase inhibitor (C1-INH) deficiency, characterized by periodic attacks of acute edema affecting subcutaneous (SC) tissues and mucous membranes. Human C1-INH concentrate given intravenously (IV) is effective and safe, but venous access may be difficult. We compared SC and IV administration of human pasteurized C1-INH concentrate with respect to pharmacokinetics, pharmacodynamics, and safety.
Study Design and Methods
This was a prospective, randomized, open-label, crossover study. Twenty-four subjects with mild or moderate HAE were randomly assigned during an attack-free interval to receive 1000 units of human pasteurized C1-INH concentrate IV or SC. Plasma levels of C1-INH activity and antigen, C4 antigen, cleaved high-molecular-weight kininogen (clHK), and C1-INH antibodies were measured.
Results
The mean relative bioavailability of functional C1-INH after SC administration was 39.7%. Maximum C1-INH activity after SC administration occurred within 48 hours and persisted longer than after IV administration. C4 antigen levels increased and clHK levels decreased after IV and SC administration, indicating the pharmacodynamic action of C1-INH. The mean half-life of functional C1-INH was 62 hours after IV administration and 120 hours after SC administration (p = 0.0595). C1-INH concentrate was safe and well tolerated when administered via both routes. As expected, SC administration resulted in a higher incidence of injection site reactions, all of which were mild.
Conclusion
With a relative bioavailability of 39.7%, SC administration of human pasteurized C1-INH yields potentially clinically relevant and sustained plasma levels of C1-INH and is safe and well tolerated.
Hereditary angioedema (HAE), caused by functional deficiency of C1-esterase inhibitor1 (C1-INH), is a rare disease characterized by recurrent, spontaneous, nonallergic edema in subcutaneous (SC) tissues and mucous membranes. In case of laryngeal edema, HAE is associated with high mortality rates when there is a delay in treating the attacks.2,3 HAE is a debilitating disease that can have a severe effect on quality of life.
C1-INH is a serine protease inhibitor that controls vascular permeability by acting on the initial activation phase of the complement, coagulation, contact, and fibrinolytic systems. The functional deficiency of C1-INH leads to increased activation of plasma kallikrein and Factor (F)XIIa with a subsequent release of bradykinin, which is a key mediator of vascular permeability.4 Additionally, C1-INH is the main inhibitor of FXIa, which plays an important role in the generation of thrombin, a positive modulator of vasopermeability.5-8
HAE Type I results from a quantitative deficiency in functional C1-INH, whereas the less common HAE Type II, affecting 15% of patients, results from a dysfunctional form of C1-INH circulating at normal or elevated plasma concentrations.4 Both defects are inherited as an autosomal dominant trait. HAE Type III is extremely rare, with mainly women being clinically affected; it is not associated with C1-INH deficiency and its pathophysiology is uncertain.9 Common anti-inflammatory treatments, such as corticosteroids, antihistamines, or epinephrine, are usually inappropriate for treating acute attacks caused by HAE.10 Clinical studies,11-13 as well as more than 30 years of clinical use,14,15 have shown that intravenous (IV) C1-INH replacement therapy with human C1-INH concentrate is an effective and safe treatment for acute edema attacks in patients with HAE. Therefore, C1-INH concentrate is recommended as first-line therapy in this indication.16
In patients with HAE requiring frequent IV treatment with C1-INH concentrate, either for acute edema attacks or for prophylaxis, venous access may become difficult over time. The SC administration of C1-INH concentrate is therefore being investigated as a potential alternative therapeutic approach, specifically for the prophylactic treatment of HAE. In support of this approach, a preclinical study with CSL Behring's human pasteurized C1-INH concentrate (Berinert, CSL Behring, Marburg, Germany) revealed a relative bioavailability of approximately 70% after SC administration in rabbits, compared with IV administration (Ingo Pragst, CSL Behring, May 2013). Building on this preclinical experience, the primary objective of our study was to compare the pharmacokinetics of the same preparation of C1-INH concentrate after IV and SC administration in subjects with mild or moderate HAE during an attack-free interval, evaluating the relative bioavailability of SC administration based on plasma levels of C1-INH activity. In addition to assessing the safety and tolerability of C1-INH concentrate when administered via both these routes, we also assessed plasma levels of C1-INH antigen and cleaved high-molecular-weight kininogen (clHK), serum levels of C4 antigen, and the presence of C1-INH antibodies after treatment. These additional endpoints were assessed to provide insight into the pharmacokinetic and pharmacodynamic effects of the C1-INH concentrate administered.
Materials and Methods
Study design and treatment
This was a single-center, prospective, randomized, open-label, crossover study in adults with mild or moderate HAE Type I or Type II. The study was conducted between September 22, 2008 (first subject first visit), and November 1, 2010 (last subject last visit). Subjects enrolled into the study had to present during an attack-free interval. In total, 24 subjects were each randomly assigned to one of two treatment sequences, AB or BA (A = IV; B = SC), at a ratio of 1:1. Blinded randomization envelopes were used. Blinding procedures were otherwise not needed because the study involved treatment with only one study drug. The sample size was calculated in accordance with the guideline on clinical investigation of recombinant or human plasma-derived F IX products (EMA/CHMP/BPWP/144552/2009).
According to the randomization sequence, human pasteurized C1-INH concentrate (Berinert, CSL Behring) was administered during an attack-free interval as either an IV infusion or an SC infusion, in each case as a single dose of 1000 U in 20 mL of solution, with a washout period of at least 7 days before study enrollment and between the two treatments. The IV infusion was administered over a time period of 3 minutes. The SC infusion was administered over a 15-minute period using two medical infusion pumps for continuous, simultaneous administration (MEDIS Infusa T1 portable syringe driver, OMT, Minden, Germany) of two doses of 500 U each at two different locations in the abdomen. A follow-up visit was performed 3 months after the second administration of C1-INH concentrate.
Plasma samples for pharmacokinetic and pharmacodynamic analyses were collected at 0, 0.25, 0.5, 0.75, 1, 2, 4, 6, 8, 12, 16, 20, 24, 36, 48, 60, 72, 120, and 168 hours after each treatment. In addition, after amending the protocol during the study, additional sampling times (at 336 ± 48 and 504 ± 48 hr after treatment) were introduced for the analysis of C1-INH activity and antigen levels, and C4 antigen levels, to investigate pharmacokinetic and pharmacodynamic trends over a longer period in six subjects. The analysis of clHK levels was also added, for which blood samples were collected at 0, 12, 36, 60, 168, and 504 hours after treatment in these six subjects.
Study population
The study was conducted at the University Hospital Frankfurt (Frankfurt am Main, Germany), after receiving approval from the applicable ethics committee. All potential study participants provided written informed consent before being screened for eligibility. Men and women could only be enrolled if they were at least 18 years of age and presented with mild or moderate HAE Type I (C1-INH activity < 50% and C1-INH antigen < 15.4 mg/dL) or Type II (C1-INH activity < 50% and C1-INH antigen in normal or elevated concentrations) during an attack-free interval. All but two of the enrolled subjects had mild HAE. Subjects were excluded from the study if HAE was not diagnosed or if their last treatment with C1-INH concentrate (i.e., their last attack) had occurred within 7 days before enrollment. Subjects with acquired angioedema and all other types of angioedema not associated with C1-INH deficiency were also excluded. Additionally, subjects who had received any investigational drug (excluding drugs appropriate for treating acute angioedema) during the 30 days before treatment in the current study, subjects who had received any other drug appropriate for the treatment of acute angioedema within 7 days before each administration of study drug in the current study, and subjects undergoing prophylaxis with danazol, antifibrinolytics, ε-aminocaproic acid, or tranexamic acid were also excluded. Further exclusion criteria included known hypersensitivity to the study drug, pregnancy (a rapid pregnancy test was required for women of childbearing potential), breast-feeding, malignant disease, immunodeficiency, and concurrent serious or acute illness or infection.
Pharmacokinetic and pharmacodynamic assessments
The primary endpoint was the bioavailability of functional C1-INH after SC administration relative to IV administration of human pasteurized C1-INH concentrate, calculated on the basis of plasma C1-INH activity. Secondary endpoints were analyses of plasma C1-INH activity and antigen levels, serum C4 antigen levels, and plasma clHK levels.
C1-INH activity was determined using the functional chromogenic assay (Berichrom C1-INH, commercially available from Siemens Healthcare Diagnostics, Eschborn, Germany). Standard human plasma served as the calibrator; the results are expressed as percentage of normal values. The assay was performed according to the manufacturer's instructions. The citrated plasma samples were analyzed undiluted.
Both C1-INH and C4 antigen levels were analyzed using nephelometric technology from Beckman Coulter (Brea, CA). The reagents for C1-INH antigen were obtained from Siemens Healthcare Diagnostics. The reagents for C4 antigen analysis were obtained from Beckman Coulter. The analysis of serum for C4 levels was conducted according to the manufacturer's instructions. Citrated plasma samples were analyzed undiluted.
ClHK was assessed according to the method of Berrettini and colleagues.17 Plasma samples underwent nonreducing sodium dodecyl sulfate–polyacrylamide gel electrophoresis (8% wt/vol). After electrophoretic transfer of the separated proteins from the gel onto a polyvinylidene difluoride membrane (Immobilon, Millipore, Bedford, MA), high-molecular-weight kininogen (HK) forms were identified with goat polyclonal anti-light chain-HK antibody that does not recognize the heavy chain (Nordic, Tiburg, the Netherlands) and visualized with a biotinylated rabbit anti-goat antibody (Sigma Chemical Company, St Louis, MO). The apparent molecular masses of proteins were derived by comparison with high-molecular-weight protein markers from Bio-Rad Laboratories (Richmond, CA). With this method, native HK appears as a band in the molecular range of 130 kDa and clHK is seen as two bands in the molecular ranges of 107 and 98 kDa.
Safety assessments
Safety endpoints included the incidence of treatment-emergent adverse events (AEs), analyses of standard laboratory safety variables, assessments of vital signs, and assessments of virus serology and the presence of C1-INH antibodies at 1 and 3 months after the last administration of C1-INH concentrate relative to baseline (before the first administration of C1-INH concentrate).
All AEs reported in this study occurred after administration of C1-INH concentrate and were considered treatment emergent. Standardized assessments were used for categorizing AEs according to intensity (mild, moderate, or severe) and causal relationship to administration of C1-INH concentrate (none, unlikely, possible, and probable). Missing values were not imputed.
Standard laboratory safety variables included hematology (hemoglobin [Hb], hematocrit, red blood cell count, total and differential white blood cell count, platelet count, mean corpuscular Hb, mean corpuscular volume), clinical chemistry (creatinine, uric acid, urea, total bilirubin, aspartate aminotransferase, alanine aminotransferase, gamma-glutamyl transferase, amylase, lipase), and coagulation (prothrombin time, activated partial thromboplastin time, fibrinogen).
Viral serology included analysis of hepatitis A virus antibodies, hepatitis B virus surface antigen, antibodies to hepatitis surface B antigen, antibodies to hepatitis B core antigen, hepatitis C virus (HCV) antibodies, human immunodeficiency virus Type 1 (HIV-1) and HIV-2 antibodies, and polymerase chain reaction (PCR) assays for HCV and HIV.
C1-INH antibodies were screened by a highly sensitive direct binding enzyme-linked immunosorbent assay (ELISA) in samples taken at the baseline, 1-month, and 3-month follow-up time points. C1-INH was coated to microplates and probed by citrated plasma samples. Antibodies were detected using a second peroxidase-conjugated antibody complex (Dako Cytomation, Glostrup, Denmark) that was not meant to be specific for single antibody isotypes. To achieve high sensitivity, the assay was designed to allow approximately 5% false-positive results. This cutoff was determined by testing of 140 samples from healthy subjects.
A result for the presence of C1-INH antibodies above cutoff was further characterized by antibody isotyping for relevant antibody isotypes, for example, immunoglobulin (Ig)M, IgG, and IgA (Sigma). In addition, C1-INH antibodies were described by obtaining relative antibody titers.
All samples with confirmed ELISA-based antibody responses above cutoff were analyzed further using a Bethesda-like assay to determine the antibody neutralization potential. For this analysis, citrated plasma samples were mixed with plasma from a normal plasma pool and incubated at 37°C for 2 hours. The residual C1-INH activity was measured using the functional chromogenic assay described above (Berichrom C1-INH).
Statistical analyses
Two data sets were analyzed. The safety set was used for the analysis of safety assessments and included all subjects who received any part of any administration of C1-INH concentrate (24 subjects). The pharmacokinetic per-protocol set was used for the analysis of pharmacokinetic and pharmacodynamic assessments and included all subjects who fulfilled the study inclusion criteria and completed the study without any prespecified major protocol deviation (23 subjects).
The bioavailability of human pasteurized C1-INH concentrate after SC administration relative to IV administration was determined by the geometric mean quotient of individual area under the curve (AUC) values for C1-INH activity using the most conservative approach possible (i.e., not extrapolated, dose adapted, with negative values set to 0, and no interpolation of values), including 90% confidence intervals (CIs).
Assessments of C1-INH activity, C1-INH antigen levels, and C4 antigen levels were analyzed by nonlinear regression, taking into account individual endogenous concentrations of C1-INH at baseline. Individual profiles were described by a one-compartment model. Standard formulas were used to obtain individual values for variables, including AUC for the dose of 1000 U per subject, time of maximum concentration (Tmax), maximum concentration (Cmax), terminal elimination half-life (t1/2), mean residence time (MRT), total clearance (CL), and volume of distribution at the terminal phase (VZ).
All variables were analyzed descriptively, including 95% CIs based on log-transformed data and graphs (boxplots). No imputation of missing data was applied for any variable. An exploratory analysis of variance and graphs were used to check for the influence of the factors “treatment” and “period” on the pharmacokinetic and pharmacodynamic variables as well as for carryover effects (“treatment × period” interaction).
The percentage of clHK compared to total HK and the safety assessments were analyzed using summary statistics. The differences over time for laboratory safety variables (comparing values between baseline, i.e., before first treatment and 3 months after baseline) were analyzed using an analysis of covariance model with “sequence” and “treatment” as fixed effects, the baseline value as a covariate, and “subject” as a random effect. The treatment difference for vital signs was compared using a two-sided, two-sample t test. All statistical analyses were performed using computer software (SAS, Version 9.1, SAS Institute, Cary, NC).
Results
Demographics and baseline characteristics
A total of 24 subjects with mild or moderate HAE Type I or Type II were enrolled, comprising the safety set (Table 1). One subject, in Sequence BA, was excluded from the pharmacokinetic per-protocol set because of unscheduled IV treatment with C1-INH concentrate less than 12 hours before the scheduled SC administration of C1-INH concentrate.
Table 1.
Demographic data (safety set)*
| Sequence AB (n = 12) | Sequence BA (n = 12) | |
|---|---|---|
| Sex† | ||
| Male | 7 (58.33) | 8 (66.67) |
| Female | 5 (41.67) | 4 (33.33) |
| Race† | ||
| Caucasian | 12 (100.00) | 11 (91.67) |
| Oriental | 0 (0.00) | 1 (8.33) |
| Age (years) | ||
| Mean (SD) | 37.7 (17.45) | 43.3 (18.68) |
| Median (range) | 38.0 (18-76) | 45.0 (17-71) |
| BMI (kg/m2) | ||
| Mean (SD) | 26.1 (5.62) | 26.1 (6.55) |
| Median (range) | 24.92 (21.1-40.8) | 24.68 (18.9-41.9) |
The sequences designate the order of administration in each treatment group: A = IV infusion; B = SC infusion.
Data are reported as number (%).
BMI = body mass index.
Bioavailability of C1-INH concentrate and assessments of C1-INH activity, C1-INH antigen levels, and C4 antigen levels
The mean relative bioavailability of functional C1-INH after SC administration of human pasteurized C1-INH concentrate was 39.7%, with the 90% CI ranging from 27.34% to 57.74% (Table 2). Overall, the mean plasma C1-INH activity was lower after SC administration of C1-INH concentrate than after IV administration (Fig. 1), as also reflected in the lower AUC values after SC administration (Table 2). After SC administration, the median Tmax was delayed relative to IV administration, as expected. Comparing the pharmacokinetic profile of C1-INH between the two different routes of administration, a slower onset of C1-INH activity and a lower Cmax were observed after SC treatment (Fig. 1). The mean t1/2 of C1-INH activity after IV administration was 62.0 hours. A significant effect of “treatment” was observed by analysis of variance for most variables, whereas “period” and “treatment × period” had no effect. For CL and t1/2, no factor had a significant effect.
Table 2.
Analysis of C1-INH activity levels after IV and SC administration of C1-INH concentrate (pharmacokinetic per-protocol set)*
| Variable | Mean (SD) | p value | |
|---|---|---|---|
| IV (n = 23) | SC (n = 23) | ||
| AUC (hr × %) | 1832.4 (927.10) | 881.7 (538.10) | 0.0001 |
| AUCst† (hr × %) | 220.5 (143.11) | 104.2 (66.37) | 0.0011 |
| Bioavailability (%)‡ | 39.7 (27.34-57.74) | ||
| Tmax (hr)§ | 0.5 (0-8) | 48.0 (1-456) | 0.0003 |
| Cmax (%) | 33.8 (7.513) | 9.8 (4.76) | <0.0001 |
| t1/2 (hr)|| | 62.0 (65.59) | 119.6 (126.13) | 0.0595 |
| MRT (hr) | 53.4 (38.25) | 87.0 (51.81) | 0.0149 |
| CL [U/(hr × %)] | 0.6 (0.39) | 1.2 (1.50) | 0.0982 |
| VZ (U/%) | 36.8 (13.74) | 133.3 (157.17) | 0.0038 |
p values are shown for the factor of “treatment” in an analysis of variance conducted for each pharmacokinetic variable including factors of “treatment,” “period,” and “treatment × period.” The factors of “period” and “treatment × period” had no effect on any of the pharmacokinetic variables.
Standardized to 15 U/kg body weight.
Mean bioavailability relative to IV administration based on AUCst (90% CI).
Median (range).
Calculation of t1/2 after SC infusion was only possible for 16 of 23 subjects due to an increase in C1-INH values toward the end of the observation period in seven subjects.
Figure 1.
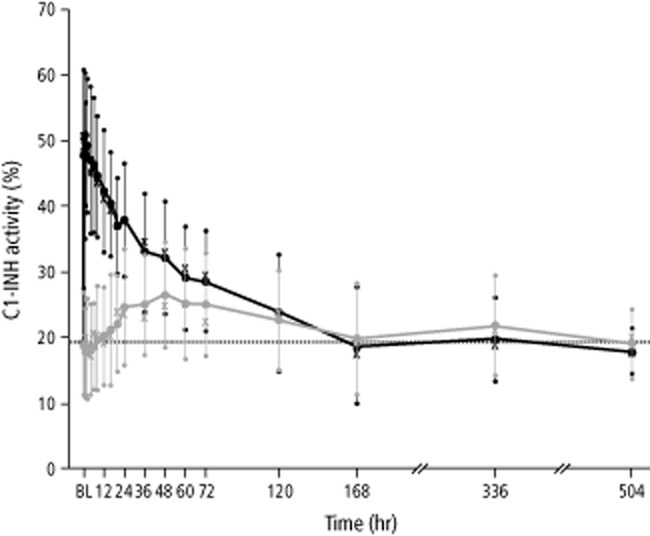
Mean C1-INH activity over time (pharmacokinetic per-protocol set, n = 23 subjects). Black and gray whiskers show SDs. Median values are indicated with “X.” Horizontal lines show baseline (BL) values (before first administration of study drug). Data for mean C1-INH activity at 336 and 504 hours were obtained in only six subjects. ( ) IV; (
) IV; ( ) SC.
) SC.
The results for C1-INH antigen and C4 antigen levels were comparable to C1-INH activity levels in terms of the relationship between the two modes of administration. The increases in mean C1-INH antigen and C4 antigen levels were delayed and less pronounced after SC administration than after IV administration (Figs. 2 and3). Similar to C1-INH activity, the median Tmax for C1-INH antigen and C4 antigen levels was markedly delayed after SC administration (Tables 3 and 4). Analysis of variance showed that only “treatment” had a significant influence (p < 0.05) on pharmacokinetic variables of C1-INH antigen and C4 antigen levels except with VZ for C4 antigen levels, where “period” had a significant effect (p = 0.0048).
Figure 2.
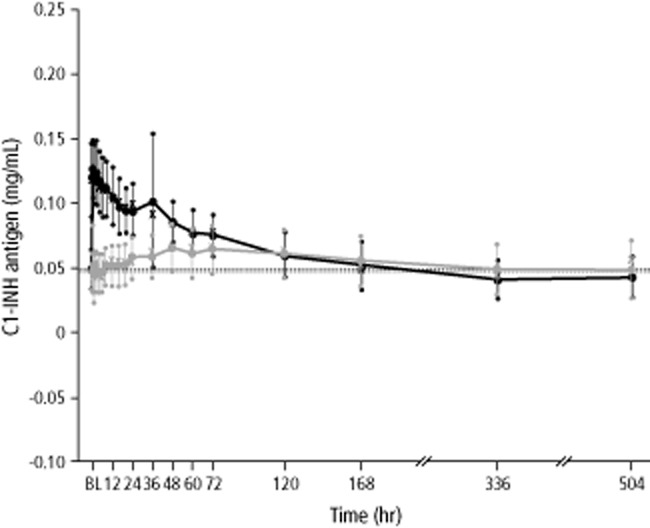
Mean concentrations of C1-INH antigen over time (pharmacokinetic per-protocol set, n = 16 subjects). Seven subjects with HAE Type II were excluded from the C1-INH antigen analyses. Black and gray whiskers show SDs. Median values are indicated with “X.” Horizontal lines show baseline (BL) values (before first administration of study drug). Data for mean concentrations of C1-INH antigen at 336 and 504 hours were obtained in only six subjects. ( ) IV; (
) IV; ( ) SC.
) SC.
Figure 3.
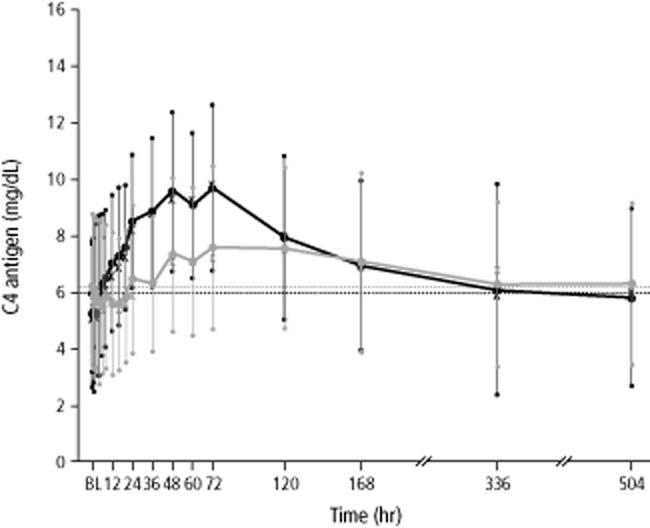
Mean concentrations of C4 antigen over time (pharmacokinetic per-protocol set, n = 23 subjects). Black and gray whiskers show SDs. Median values are indicated with “X.” Horizontal lines show baseline (BL) values (before first administration of study drug). Data for mean concentrations of C4 antigen at 336 and 504 hours were obtained in only six subjects. ( ) IV; (
) IV; ( ) SC.
) SC.
Table 3.
Analysis of C1-INH antigen levels after IV and SC administration of C1-INH concentrate (pharmacokinetic per-protocol set)*
| Variable | Mean (SD) | p value | |
|---|---|---|---|
| IV (n = 16)† | SC (n = 16)† | ||
| AUC (hr × mg/mL) | 4.7 (1.07) | 2.7 (1.84) | 0.0008 |
| AUCst‡ (hr × mg/mL) | 0.6 (0.14) | 0.3 (0.24) | 0.0030 |
| Tmax (hr)§ | 0.4 (0-36) | 48.1 (1-456) | 0.0052 |
| Cmax (mg/mL)|| | 0.09 (0.044) | 0.03 (0.021) | <0.0001 |
| t1/2 (hr) | 53.3 (28.19) | 94.4 (71.61) | 0.0643 |
| MRT (hr) | 54.9 (26.55) | 106.2 (58.99) | 0.0041 |
| CL [U/(hr × mg/mL)] | 206.8 (64.91) | 556.2 (777.85) | 0.0424 |
| VZ [U/(mg/mL)] | 14,842.7 (7,438.10) | 49,503.6 (51,319.02) | 0.0053 |
p values are shown for the factor of “treatment” in an analysis of variance conducted for each pharmacokinetic variable including factors of “treatment,” “period,” and “treatment × period.” The factors of “period” and “treatment × period” had no effect on any of the pharmacokinetic variables.
Seven subjects with HAE Type II were excluded from the C1-INH antigen analyses.
Standardized to 15 U/kg body weight.
Median (range).
Mean values were not rounded to one decimal place to avoid values of 0.0; decimal places of the corresponding SD were rounded accordingly.
Table 4.
Analysis of C4 antigen levels after IV and SC administration of C1-INH concentrate (pharmacokinetic per-protocol set)*
| Variable | Mean (SD) | p value | |
|---|---|---|---|
| IV (n = 23) | SC (n = 23) | ||
| AUC (hr × mg/dL) | 424.6 (177.07) | 227.1 (206.67) | 0.0013 |
| AUCst† (hr × mg/mL) | 50.5 (23.12) | 27.0 (25.01) | 0.0020 |
| Tmax (hr)‡ | 60.1 (24-168) | 120.0 (4-456) | 0.0121 |
| Cmax (mg/dL) | 4.1 (1.49) | 2.0 (1.06) | <0.0001 |
| t1/2 (hr) | 134.5 (216.66) | 242.1 (289.30) | 0.4741 |
| MRT (hr) | 83.0 (21.81) | 114.3 (55.05) | 0.0155 |
| CL [U/(hr × mg/dL)] | 1.9 (1.05) | 2.5 (1.77) | 0.3052 |
| VZ [U/(mg/dL)] | 207.4 (119.53) | 477.7 (293.73) | 0.0034 |
p values are shown for the factor of “treatment” in an analysis of variance conducted for each pharmacokinetic variable including factors of “treatment,” “period,” and “treatment × period.” The factors of “period” and “treatment × period” had no effect for any of the pharmacokinetic variables except VZ, where “period” had a significant effect (p = 0.0048).
Standardized to 15 U/kg body weight.
Median (range).
ClHK
After IV and SC administration of C1-INH concentrate, the percentage of clHK levels decreased for up to 36 hours (Fig. 4). This was more pronounced with IV administration, where the percentage of clHK decreased from a mean of 46.0% at baseline (0 hr) to 37.1% at 36 hours. In contrast, after SC administration, the lowest value was 42.0%, starting from a baseline value of 44.8%. After 36 hours, the clHK levels increased again to attain approximately baseline levels.
Figure 4.
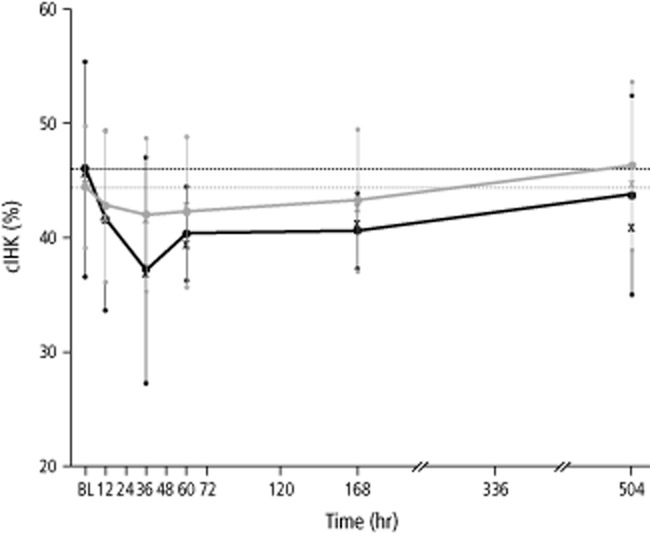
Mean clHK over time (pharmacokinetic per-protocol set, n = 6 subjects). Black and gray whiskers show SDs. Median values are indicated with “X.” Horizontal lines show baseline values (before first administration of study drug). ( ) IV; (
) IV; ( ) SC.
) SC.
Safety assessments
In total, 16 of the 24 subjects (66.7%) in the safety set experienced 46 treatment-emergent AEs (Table 5). There were no deaths during the study. One serious AE of pneumonia occurred after IV infusion of C1-INH concentrate. This event was moderate in intensity, not related to the study drug, and resolved without sequelae.
Table 5.
Treatment-emergent AEs after IV and SC administration of C1-INH concentrate (safety set)*
| IV (n = 24) | SC (n = 24) | |
|---|---|---|
| Subjects with AE | 7 (29.2) | 15 (62.5) |
| Number of AEs | 14 | 32 |
| AE causality | ||
| None | 10 (71.4) | 9 (28.1) |
| Unlikely | 4 (28.6) | 0 (0.0) |
| Possible | 0 (0.0) | 2 (6.3) |
| Probable | 0 (0.0) | 21 (65.6) |
| AE intensity | ||
| Mild | 10 (71.4) | 32 (100.0) |
| Moderate | 4 (28.6) | 0 (0.0) |
| Severe | 0 (0.0) | 0 (0.0) |
Data are reported as number (%).
The causality of 21 AEs occurring after SC administration was categorized as “probably related.” These events included application site irritation (n = 9), application site swelling (n = 6), application site hematoma (n = 4), application site pain (n = 1), and back disorder (n = 1). Two AEs were categorized as possibly related (both were application site irritation).
All AEs occurring after SC administration (n = 32) were mild in intensity. The only moderate AEs occurred after IV administration and were one case each of rhinitis, oropharyngeal pain, sinusitis, and the serious AE of pneumonia.
No significant changes in laboratory safety variables occurred over time, and there were no significant differences in vital signs between the two routes of administration. No virus transmissions due to the administration of study drug, or positive PCR results suggestive of virus transmission, were observed.
Only one subject, after SC administration of C1-INH concentrate, exhibited a change in C1-INH antibody titer. The C1-INH antibody titer of this subject was above cutoff at screening (1:150) and further increased at 1 (1:450) and 3 months (1:450) after the last administration of study drug. No inhibitory C1-INH antibodies were detected.
Discussion
IV administration of C1-INH concentrate is a well-established and effective therapy for the acute and prophylactic treatment of HAE.18 However, it can be an uncomfortable and often painful procedure for patients, especially when frequent administration is required for routine prophylaxis. SC administration may mitigate this limitation of IV therapy and thereby provide an attractive alternative therapeutic approach for HAE prophylaxis, especially for patients with limited venous access and for children.
Preclinical studies and clinical studies of SC treatment with two different preparations of C1-INH concentrate have indicated that this route of administration is safe and could achieve sustained and therapeutically potentially relevant systemic levels of C1-INH (Ingo Pragst, CSL Behring, May 2013).19-21 Against this background, our study investigated the bioavailability and the pharmacokinetics, pharmacodynamics, and safety of SC administration of a marketed human pasteurized C1-INH concentrate (Berinert) that is currently approved for IV treatment of acute angioedema in patients with HAE.
Compared with IV administration, the mean relative bioavailability of functional C1-INH after SC administration of 1000 U of human pasteurized C1-INH concentrate, as assessed by C1-INH activity, was 39.7%. With the same dose of a different preparation of C1-INH concentrate, the relative bioavailability after SC administration was 62% for 1000 U and 36% for 2000 U, based on C1-INH activity; C1-INH antigen levels were 44 and 28% for 1000 and 2000 U, respectively.20 The reason for this difference remains unclear. The relative bioavailability of 39.7% after SC administration of C1-INH concentrate in our study was sufficient to elicit pharmacodynamic responses, as indicated by recoveries in C4 antigen and clHK levels (increase and decrease, respectively), which was also seen after IV administration. Patients with HAE usually present with chronic reductions in C4 plasma levels, and a recovery in C4 levels may indirectly demonstrate efficacy of the administered C1-INH concentrate.4,22 In addition, clHK levels can be used to monitor bradykinin synthesis and therapeutic efficacy due to the involvement of the kinin-kallikrein system in acute HAE attacks and the presence of elevated clHK in the plasma of patients during HAE attacks.23-27
The lower activity levels of C1-INH when administered SC can likely be attributed to the gradual release of the C1-INH from the SC reservoir into the circulation. Due to its large molecular size, the C1-INH concentrate was most probably transferred from the SC reservoir to the vascular network via the lymphatic system.28 The bioavailability observed in our study in humans is lower compared with the relative bioavailability of approximately 70% observed previously in rabbits (Ingo Pragst, CSL Behring, May 2013). The reason for this finding is still unclear and warrants further investigations.
In our single-dose study, the mean maximum activity level of C1-INH in plasma was 33.8% after IV administration and 9.8% after SC administration. Various studies have indicated that C1-INH concentrations of at least 0.07 g/L, or approximately 30% of the functional activity level, are needed for effective long-term prophylaxis of angioedema attacks.29-33 However, the data from our study cannot provide insight into whether SC administration of C1-INH concentrate yields protective levels of C1-INH in plasma because the single-dose design, while appropriate for assessing relative bioavailability, was not suited to assessing trough concentrations or prophylactic efficacy of C1-INH, which would have required a multiple-dose design.
In addition to the practical advantages of administering C1-INH concentrate SC, the apparently lower fluctuation in plasma concentrations of C1-INH associated with SC administration, relative to IV administration, may provide an additional benefit in the prophylactic treatment of HAE. Sustained concentrations of C1-INH in the circulation could potentially enhance prophylactic efficacy over time and could also mean that the frequency with which C1-INH concentrate must be administered can be reduced. However, further research is needed to establish the extent to which these principles might apply in the light of the low relative bioavailability of C1-INH concentrate when administered SC, and the extent to which the efficacy of SC administered C1-INH can be influenced by varying the concentration and dosing regimen of the SC preparation.
No earlier data are available on the pharmacokinetics of C1-INH concentrate after SC administration that can be directly compared with the results from our study. However, with respect to IV administration, historical data are available from a number of studies with which we can compare our data. Of primary interest is that the mean t1/2 observed after IV administration of human pasteurized C1-INH concentrate in our study was notably longer than mean historical values obtained with the same preparation of C1-INH concentrate. The t1/2 of C1-INH appears to be influenced by the severity of HAE in the population analyzed and by whether the assessment was made in patients with symptomatic HAE (i.e., while experiencing an acute attack) or asymptomatic HAE (i.e., during an attack-free interval). In previous studies of the same preparation of C1-INH concentrate as used in our study, mean t1/2 values after IV administration were 32.7 hours (population estimate, 90% CI, 16.6-48.8 hr) in symptomatic patients,34 33.3 hours (standard deviation [SD], 19.8 hr) in asymptomatic patients with severe HAE,35 and 47.8 hours (SD, 21.2 hr) in asymptomatic patients with moderate HAE,35 compared with 62.0 hours (SD, 65.6 hr) in asymptomatic patients with mild or moderate HAE in our study. Similar to our result, mean t1/2 values of 56 (SD, 35) and 62 hours (SD, 38 hr) were reported for a different human plasma C1-INH concentrate in patients with asymptomatic HAE,36,37 and for a non-commercial C1-INH preparation the mean t1/2 value was 67.7 hours (SD, 4.9 hr) in patients with asymptomatic HAE and 64 hours (SD, 1.4 hr) in healthy individuals.38
Our study revealed no signals of concern regarding the tolerability and safety of SC administration of human pasteurized C1-INH concentrate. AEs were reported in 16 of the 24 subjects treated. Although more than twice as many events (32) occurred after SC administration than after IV administration (14), all of the events that occurred after SC administration were mild in intensity, and most events were associated with the injection site, such as hematoma, irritation, pain, and swelling. Relative to IV administration, such AEs are commonly associated with SC administration, as has been observed for another human plasma C1-INH concentrate20,21 and for SC immunoglobulin replacement therapy.39 As the C1-INH concentrate administered in our study is intended for IV administration, the volume administered was relatively high (10 mL per injection site), which may have exacerbated the injection-site reactions associated with SC administration. Increasing the concentration of a human C1-INH preparation intended for SC administration may provide a means of reducing the incidence of injection site reactions.
The development of inhibitory antibodies is often a concern with protein-based therapies, and low rates of noninhibitory antibody formation have been associated with the long-term administration of C1-INH concentrate.40 Furthermore, with respect to therapeutic-based proteins, SC administration is known to be more immunogenic than IV administration.41-43 In our study, only one subject exhibited an increase in C1-INH antibody titer, at 1 and 3 months after SC administration, but these antibodies were not inhibitory.
In conclusion, the relative bioavailability of functional C1-INH after SC administration of human pasteurized C1-INH concentrate was 39.7%. SC administration was safe and well tolerated. It was accompanied by pharmacodynamic responses expected of C1-INH therapy and indications that SC administration of C1-INH concentrate may achieve prolonged and less fluctuating systemic exposure to human pasteurized C1-INH concentrate than with IV administration. These observations, together with the additional convenience of SC administration, suggest that further investigation of the SC administration of C1-INH concentrate for prophylactic treatment of HAE may be warranted.
Acknowledgments
We acknowledge the assistance of the laboratory teams at the University Hospital in Frankfurt am Main and at CSL Behring in Marburg (Germany), and at the Department of Clinical Science “Luigi Sacco,” University of Milan (Italy). We are grateful for the viral serology performed at the Institute of Medical Virology at the University Hospital, Frankfurt am Main. In addition, we appreciate the support received from the Clinical Study Center Rhine-Main (KSRM) at the University Hospital, Frankfurt am Main. We acknowledge the statistical support provided by Heinz-Otto Keinecke from Accovion GmbH (Marburg office, Germany) and by Carmen Theek and Uwe Hehnke from Pierrel Research Europe GmbH (Essen, Germany). Medical writing support was provided by Dr Ioanna Bethani from Trilogy Writing & Consulting GmbH (Frankfurt am Main, Germany) and by Dr Ivanka Yaneva-Hoffmann from Pierrel Research Europe GmbH (Essen, Germany). Finally, we thank our patients for participating in our study.
Glossary
- AE(s)
adverse event(s)
- AUC
area under the curve
- C1-INH
C1-esterase inhibitor
- CL
total clearance
- clHK
cleaved high-molecular-weight kininogen
- Cmax
maximum concentration
- HAE
hereditary angioedema
- HK
high-molecular-weight kininogen
- MRT
mean residence time
- SC
subcutaneous
- t1/2
terminal elimination half-life
- Tmax
time of maximum concentration
- VZ
volume of distribution at the terminal phase
Conflict of Interest
IMS has received honoraria for lectures and funding for travel expenses from CSL Behring. She is a consultant for the following companies: Baxter, Bayer, CSL Behring, Octapharma, Shire, and Sobi. MC has been a speaker for Dyax/Sigma Tau, CSL Behring, ViroPharma, Pharming/SOBI, and Shire. He has attended advisory boards of the companies mentioned above, as well as of BioChrist, and he has been a consultant for Dyax/Sigma Tau, CSL Behring, and Pharming/SOBI. EAP has attended advisory boards, has received honoraria for lectures, and has participated in clinical trials, all sponsored by CSL Behring. She has also received sponsorship for educational purposes and has provided consultancy services or has participated in clinical trials sponsored by CSL Behring, Jerini AG/Shire, Sobi, and ViroPharma. HS is an employee of Siemens Healthcare Diagnostics, Germany. AF and UK are employees of CSL Behring, Germany. WK is a consultant for and has received honoraria for lectures and funding for travel expenses from the following companies: Baxter, Bayer, CSL Behring, Biotest, Grifols, Intersero, Novo Nordisk, Octapharma, Shire, Sobi, and ViroPharma. CS, ER, and TR declare no conflicts of interest relevant to this article.
References
- Donaldson VH, Evans RR. A biochemical abnormality in hereditary angioneurotic oedema: absence of serum inhibitor of C1-esterase. Am J Med. 1963;35:37–44. doi: 10.1016/0002-9343(63)90162-1. [DOI] [PubMed] [Google Scholar]
- Bork K, Siedlecki K, Bosch S, et al. Asphyxiation by laryngeal edema in patients with hereditary angioedema. Mayo Clin Proc. 2000;75:349–354. doi: 10.4065/75.4.349. [DOI] [PubMed] [Google Scholar]
- Frank MM, Gelfand JA, Atkinson JP. Hereditary angioedema: the clinical syndrome and its management. Ann Intern Med. 1976;84:580–593. doi: 10.7326/0003-4819-84-5-580. [DOI] [PubMed] [Google Scholar]
- Longhurst H, Cicardi M. Hereditary angio-edema. Lancet. 2012;379:474–481. doi: 10.1016/S0140-6736(11)60935-5. [DOI] [PubMed] [Google Scholar]
- Wuillemin WA, Hack CE, Bleeker WK, et al. Inactivation of factor XIa in vivo: studies in chimpanzees and in humans. Thromb Haemost. 1996;76:549–555. [PubMed] [Google Scholar]
- Garcia JG, Siflinger-Birnboim A, Bizios R, et al. Thrombin-induced increase in albumin permeability across the endothelium. J Cell Physiol. 1986;128:96–104. doi: 10.1002/jcp.1041280115. [DOI] [PubMed] [Google Scholar]
- Garcia JG, Pavalko FM, Patterson CE. Vascular endothelial cell activation and permeability responses to thrombin. Blood Coagul Fibrinolysis. 1995;6:609–626. doi: 10.1097/00001721-199510000-00001. [DOI] [PubMed] [Google Scholar]
- Cugno M, Cicardi M, Bottasso B, et al. Activation of the coagulation cascade in C1-inhibitor deficiencies. Blood. 1997;89:3213–3218. [PubMed] [Google Scholar]
- Bork K. Diagnosis and treatment of hereditary angioedema with normal C1 inhibitor. Allergy Asthma Clin Immunol. 2010;6:15. doi: 10.1186/1710-1492-6-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hack CE. 2004. Pathogenesis of angioedema attacks. In: Hereditary and acquired angioedema: problems and progress. Proceedings of the third C1 esterase inhibitor deficiency workshop and beyond. JACI 114:S75–9.
- Craig TJ, Levy RJ, Wasserman RL, et al. Efficacy of human C1 esterase inhibitor concentrate compared with placebo in acute hereditary angioedema attacks. J Allergy Clin Immunol. 2009;124:801–808. doi: 10.1016/j.jaci.2009.07.017. [DOI] [PubMed] [Google Scholar]
- Craig TJ, Wasserman RL, Levy RJ, et al. Prospective study of rapid relief provided by C1 esterase inhibitor in emergency treatment of acute laryngeal attacks in hereditary angioedema. J Clin Immunol. 2010;30:823–829. doi: 10.1007/s10875-010-9442-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman RL, Levy RJ, Bewtra AK, et al. Prospective study of C1 esterase inhibitor in the treatment of successive acute abdominal and facial hereditary angioedema attacks. Ann Allergy Asthma Immunol. 2011;106:62–68. doi: 10.1016/j.anai.2010.10.012. [DOI] [PubMed] [Google Scholar]
- Bork K. Human pasteurized C1-inhibitor concentrate for the treatment of hereditary angioedema due to C1-inhibitor deficiency. Expert Rev Clin Immunol. 2011;7:723–733. doi: 10.1586/eci.11.72. [DOI] [PubMed] [Google Scholar]
- Bork K, Korger G, Kreuz W. Review of the long-term safety of a human pasteurized C1 inhibitor concentrate. J Allergy Clin Immunol. 2012;129:AB222. Poster presented at the 2012 AAAAI Annual Meeting, Orlando, Florida. [Google Scholar]
- Bowen T, Cicardi M, Farkas H, et al. International consensus algorithm for the diagnosis, therapy and management of hereditary angioedema. Allergy Asthma Clin Immunol. 2010;6:24. doi: 10.1186/1710-1492-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berrettini M, Lämmle B, White T, et al. Detection of in vitro and in vivo cleavage of high molecular weight kininogen in human plasma by immunoblotting with monoclonal antibodies. Blood. 1986;68:455–462. [PubMed] [Google Scholar]
- Craig T, Pürsün EA, Bork K, et al. WAO Guideline for the management of hereditary angioedema. World Allergy Organ J. 2012;5:182–199. doi: 10.1097/WOX.0b013e318279affa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Zhang HM, Frank MM. Subcutaneous infusion of human C1 inhibitor in swine. Clin Immunol. 2010;136:323–328. doi: 10.1016/j.clim.2010.05.001. [DOI] [PubMed] [Google Scholar]
- Schranz J, Levy R, Lumry W, et al. Safety, pharmacokinetics (PK), and pharmacodynamics (PD) of subcutaneous (SC) Cinryze® C1 inhibitor (C1 INH) with recombinant human hyaluronidase (rHuPH20) in subjects with hereditary angioedema (HAE) J Allergy Clin Immunol. 2012;129:AB369. Poster presented at the 2012 AAAAI Annual Meeting, Orlando, Florida. [Google Scholar]
- ClinicalTrials.gov. 2012. A study to evaluate the safety, pharmacokinetics, and pharmacodynamics of subcutaneous CINRYZE administration. [cited 2013 May 13]. Accessed from: http://clinicaltrials.gov/ct2/show/NCT01095497.
- Zuraw BL, Sugimoto S, Curd JG. The value of rocket immunoelectrophoresis for C4 activation in the evaluation of patients with angioedema or C1-inhibitor deficiency. J Allergy Clin Immunol. 1986;78:1115–1120. doi: 10.1016/0091-6749(86)90259-9. [DOI] [PubMed] [Google Scholar]
- Davis AE., 3rd Hereditary angioedema: a current state-of-the-art review, III: mechanisms of hereditary angioedema. Ann Allergy Asthma Immunol. 2008;100:S7–12. doi: 10.1016/s1081-1206(10)60580-7. [DOI] [PubMed] [Google Scholar]
- Lämmle B, Zuraw BL, Heeb MJ, et al. Detection and quantitation of cleaved and uncleaved high molecular weight kininogen in plasma by ligand blotting with radiolabeled plasma prekallikrein or factor IX. Thromb Haemost. 1988;59:151–161. [PubMed] [Google Scholar]
- Nielsen EW, Johansen HT, Hogasen K, et al. Activation of the complement, coagulation, fibrinolytic and kallikrein-kinin systems during attacks of hereditary angioedema. Scand J Immunol. 1996;44:185–192. doi: 10.1046/j.1365-3083.1996.d01-298.x. [DOI] [PubMed] [Google Scholar]
- Cugno M, Cicardi M, Coppola R, et al. Activation of factor XII and cleavage of high molecular weight kininogen during acute attacks in hereditary and acquired C1-inhibitor deficiencies. Immunopharmacology. 1996;33:361–364. doi: 10.1016/0162-3109(96)00086-0. [DOI] [PubMed] [Google Scholar]
- Bühler R, Hovinga JK, Aebi-Huber I, et al. Improved detection of proteolytically cleaved high molecular weight kininogen by immunoblotting using an antiserum against its reduced 47 kDa light chain. Blood Coagul Fibrinolysis. 1995;6:223–232. doi: 10.1097/00001721-199505000-00005. [DOI] [PubMed] [Google Scholar]
- McDonald TC. Subcutaneous administration of biotherapeutics. Curr Opin Mol Ther. 2010;12:461–470. [PubMed] [Google Scholar]
- Späth PJ, Wüthrich B, Bütler R. Quantification of C1-inhibitor functional activities by immunodiffusion assay in plasma of patients with hereditary angioedema–evidence of a functionally critical level of C1-inhibitor concentration. Complement. 1984;1:147–159. doi: 10.1159/000467830. [DOI] [PubMed] [Google Scholar]
- Bork K, Witzke G. Long-term prophylaxis with C1-inhibitor (C1 INH) concentrate in patients with recurrent angioedema caused by hereditary and acquired C1-inhibitor deficiency. J Allergy Clin Immunol. 1989;83:677–682. doi: 10.1016/0091-6749(89)90082-1. [DOI] [PubMed] [Google Scholar]
- Farkas H, Harmat G, Füst G, et al. Clinical management of hereditary angio-oedema in children. Pediatr Allergy Immunol. 13:153–161. doi: 10.1034/j.1399-3038.2002.01014.x. [DOI] [PubMed] [Google Scholar]
- Levi M, Choi G, Picavet C, et al. Self-administration of C1-inhibitor concentrate in patients with hereditary or acquired angioedema caused by C1-inhibitor deficiency. J Allergy Clin Immunol. 2006;117:904–908. doi: 10.1016/j.jaci.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Kaplan AP, Joseph K. The bradykinin-forming cascade and its role in hereditary angioedema. Ann Allergy Asthma Immunol. 2010;104:193–204. doi: 10.1016/j.anai.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Bernstein JA, Ritchie B, Levy RJ, et al. Population pharmacokinetics of plasma-derived C1 esterase inhibitor concentrate used to treat acute hereditary angioedema attacks. Ann Allergy Asthma Immunol. 2010;105:149–154. doi: 10.1016/j.anai.2010.06.005. [DOI] [PubMed] [Google Scholar]
- Martinez-Saguer I, Rusicke E, Aygören-Pürsün E, et al. Pharmacokinetic analysis of human plasma-derived pasteurized factor concentrate in adults and children with hereditary angioedema: a prospective study. Transfusion. 2010;50:354–360. doi: 10.1111/j.1537-2995.2009.02394.x. [DOI] [PubMed] [Google Scholar]
- European Medicines Agency. Cinryze—assessment report. Procedure No. EMEA/H/C/001207. [cited 2013 Nov 13]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/001207/WC500108898.pdf.
- European Medicines Agency. Cinryze 500 Units powder and solvent for solution for injection. Summary of product characteristics. [cited 2013 Nov 13]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/001207/WC500108895.pdf.
- Brackertz D, Isler E, Kueppers F. Half-life of C1-INH in hereditary angioneurotic oedema (HAE) Clin Allergy. 1975;1:89–94. doi: 10.1111/j.1365-2222.1975.tb01839.x. [DOI] [PubMed] [Google Scholar]
- Skoda-Smith S, Torgerson TR, Ochs HD. Subcutaneous immunoglobulin replacement therapy in the treatment of patients with primary immunodeficiency disease. Ther Clin Risk Manag. 2010;6:1–10. doi: 10.1057/rm.2009.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig TJ, Bewtra AK, Bahna SL, et al. C1 esterase inhibitor concentrate in 1085 hereditary angioedema attacks—final results of the I.M.P.A.C.T.2 study. Allergy. 2011;66:1604–1611. doi: 10.1111/j.1398-9995.2011.02702.x. [DOI] [PubMed] [Google Scholar]
- Braun A, Kwee L, Labow MA, et al. Protein aggregates seem to play a key role among the parameters influencing the antigenicity of interferon alpha (IFN-alpha) in normal and transgenic mice. Pharm Res. 1997;14:1472–1478. doi: 10.1023/a:1012193326789. [DOI] [PubMed] [Google Scholar]
- Kromminga A, Schellekens H. Antibodies against erythropoietin and other protein-based therapeutics: an overview. Ann N Y Acad Sci. 2005;1050:257–265. doi: 10.1196/annals.1313.027. [DOI] [PubMed] [Google Scholar]
- Schellekens H, Jiskoot W. Erythropoietin-associated PRCA: still an unsolved mystery. J Immunotoxicol. 2006;3:123–130. doi: 10.1080/15476910600845567. [DOI] [PubMed] [Google Scholar]


