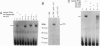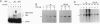Abstract
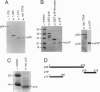
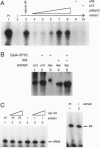
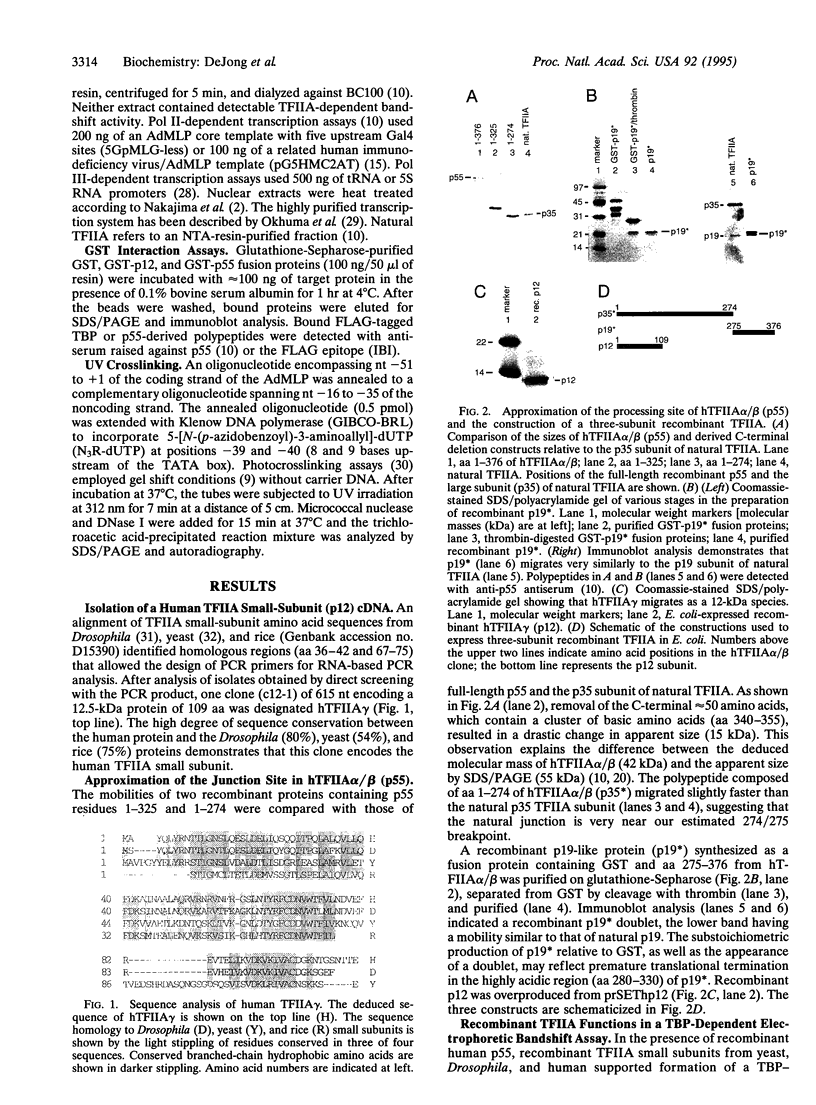
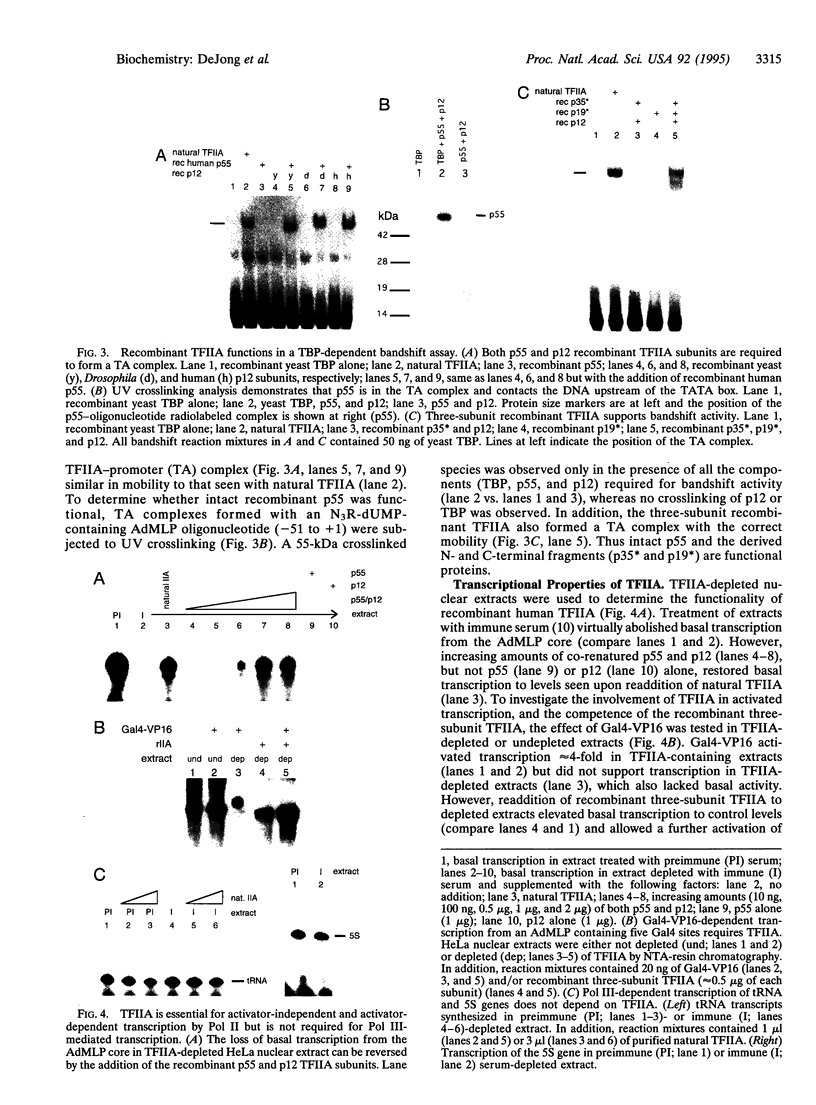
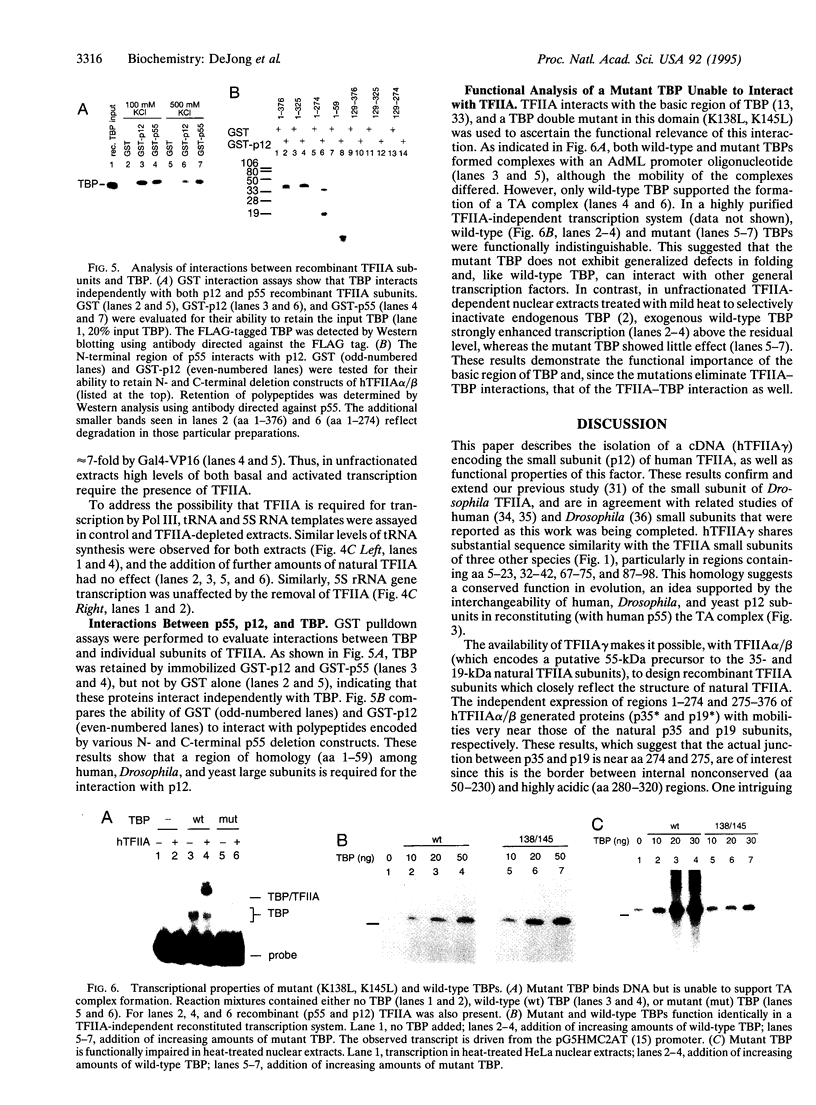
The human general transcription factor TFIIA is one of several factors involved in specific transcription by RNA polymerase II, possibly by regulating the activity of the TATA-binding subunit (TBP) of TFIID. TFIIA purified from HeLa extracts consists of 35-, 19-, and 12-kDa subunits. Here we describe the isolation of a cDNA clone (hTFIIA gamma) encoding the 12-kDa subunit. Using expression constructs derived from hTFIIA gamma and TFIIA alpha/beta (which encodes a 55-kDa precursor to the alpha and beta subunits of natural TFIIA), we have constructed a synthetic TFIIA with a polypeptide composition similar to that of natural TFIIA. The recombinant complex supports the formation of a DNA-TBP-TFIIA complex and mediates both basal and Gal4-VP16-activated transcription by RNA polymerase II in TFIIA-depleted nuclear extracts. In contrast, TFIIA has no effect on tRNA and 5S RNA transcription by RNA polymerase III in this system. We also present evidence that both the p55 and p12 recombinant subunits interact with TBP and that the basic region of TBP is critical for the TFIIA-dependent function of TBP in nuclear extracts.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aso T., Serizawa H., Conaway R. C., Conaway J. W. A TATA sequence-dependent transcriptional repressor activity associated with mammalian transcription factor IIA. EMBO J. 1994 Jan 15;13(2):435–445. doi: 10.1002/j.1460-2075.1994.tb06278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auble D. T., Hahn S. An ATP-dependent inhibitor of TBP binding to DNA. Genes Dev. 1993 May;7(5):844–856. doi: 10.1101/gad.7.5.844. [DOI] [PubMed] [Google Scholar]
- Bartholomew B., Kassavetis G. A., Braun B. R., Geiduschek E. P. The subunit structure of Saccharomyces cerevisiae transcription factor IIIC probed with a novel photocrosslinking reagent. EMBO J. 1990 Jul;9(7):2197–2205. doi: 10.1002/j.1460-2075.1990.tb07389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein R., DeJong J., Roeder R. G. Characterization of the highly conserved TFIIA small subunit from Drosophila melanogaster. J Biol Chem. 1994 Sep 30;269(39):24361–24366. [PubMed] [Google Scholar]
- Buratowski S., Hahn S., Guarente L., Sharp P. A. Five intermediate complexes in transcription initiation by RNA polymerase II. Cell. 1989 Feb 24;56(4):549–561. doi: 10.1016/0092-8674(89)90578-3. [DOI] [PubMed] [Google Scholar]
- Chi T., Carey M. The ZEBRA activation domain: modular organization and mechanism of action. Mol Cell Biol. 1993 Nov;13(11):7045–7055. doi: 10.1128/mcb.13.11.7045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang C. M., Roeder R. G. Expression and purification of general transcription factors by FLAG epitope-tagging and peptide elution. Pept Res. 1993 Mar-Apr;6(2):62–64. [PubMed] [Google Scholar]
- Conaway R. C., Conaway J. W. General initiation factors for RNA polymerase II. Annu Rev Biochem. 1993;62:161–190. doi: 10.1146/annurev.bi.62.070193.001113. [DOI] [PubMed] [Google Scholar]
- Cortes P., Flores O., Reinberg D. Factors involved in specific transcription by mammalian RNA polymerase II: purification and analysis of transcription factor IIA and identification of transcription factor IIJ. Mol Cell Biol. 1992 Jan;12(1):413–421. doi: 10.1128/mcb.12.1.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulombe B., Killeen M., Liljelund P., Honda B., Xiao H., Ingles C. J., Greenblatt J. Identification of three mammalian proteins that bind to the yeast TATA box protein TFIID. Gene Expr. 1992;2(2):99–110. [PMC free article] [PubMed] [Google Scholar]
- Coulombe B., Li J., Greenblatt J. Topological localization of the human transcription factors IIA, IIB, TATA box-binding protein, and RNA polymerase II-associated protein 30 on a class II promoter. J Biol Chem. 1994 Aug 5;269(31):19962–19967. [PubMed] [Google Scholar]
- DeJong J., Roeder R. G. A single cDNA, hTFIIA/alpha, encodes both the p35 and p19 subunits of human TFIIA. Genes Dev. 1993 Nov;7(11):2220–2234. doi: 10.1101/gad.7.11.2220. [DOI] [PubMed] [Google Scholar]
- Ge H., Roeder R. G. Purification, cloning, and characterization of a human coactivator, PC4, that mediates transcriptional activation of class II genes. Cell. 1994 Aug 12;78(3):513–523. doi: 10.1016/0092-8674(94)90428-6. [DOI] [PubMed] [Google Scholar]
- Ge H., Roeder R. G. The high mobility group protein HMG1 can reversibly inhibit class II gene transcription by interaction with the TATA-binding protein. J Biol Chem. 1994 Jun 24;269(25):17136–17140. [PubMed] [Google Scholar]
- Hoffmann A., Roeder R. G. Purification of his-tagged proteins in non-denaturing conditions suggests a convenient method for protein interaction studies. Nucleic Acids Res. 1991 Nov 25;19(22):6337–6338. doi: 10.1093/nar/19.22.6337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imbalzano A. N., Zaret K. S., Kingston R. E. Transcription factor (TF) IIB and TFIIA can independently increase the affinity of the TATA-binding protein for DNA. J Biol Chem. 1994 Mar 18;269(11):8280–8286. [PubMed] [Google Scholar]
- Inostroza J. A., Mermelstein F. H., Ha I., Lane W. S., Reinberg D. Dr1, a TATA-binding protein-associated phosphoprotein and inhibitor of class II gene transcription. Cell. 1992 Aug 7;70(3):477–489. doi: 10.1016/0092-8674(92)90172-9. [DOI] [PubMed] [Google Scholar]
- Kovelman R., Roeder R. G. Sarkosyl defines three intermediate steps in transcription initiation by RNA polymerase III: application to stimulation of transcription by E1A. Genes Dev. 1990 Apr;4(4):646–658. doi: 10.1101/gad.4.4.646. [DOI] [PubMed] [Google Scholar]
- Kretzschmar M., Kaiser K., Lottspeich F., Meisterernst M. A novel mediator of class II gene transcription with homology to viral immediate-early transcriptional regulators. Cell. 1994 Aug 12;78(3):525–534. doi: 10.1016/0092-8674(94)90429-4. [DOI] [PubMed] [Google Scholar]
- Lee D. K., DeJong J., Hashimoto S., Horikoshi M., Roeder R. G. TFIIA induces conformational changes in TFIID via interactions with the basic repeat. Mol Cell Biol. 1992 Nov;12(11):5189–5196. doi: 10.1128/mcb.12.11.5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman P. M., Berk A. J. A mechanism for TAFs in transcriptional activation: activation domain enhancement of TFIID-TFIIA--promoter DNA complex formation. Genes Dev. 1994 May 1;8(9):995–1006. doi: 10.1101/gad.8.9.995. [DOI] [PubMed] [Google Scholar]
- Ma D., Watanabe H., Mermelstein F., Admon A., Oguri K., Sun X., Wada T., Imai T., Shiroya T., Reinberg D. Isolation of a cDNA encoding the largest subunit of TFIIA reveals functions important for activated transcription. Genes Dev. 1993 Nov;7(11):2246–2257. doi: 10.1101/gad.7.11.2246. [DOI] [PubMed] [Google Scholar]
- Maldonado E., Ha I., Cortes P., Weis L., Reinberg D. Factors involved in specific transcription by mammalian RNA polymerase II: role of transcription factors IIA, IID, and IIB during formation of a transcription-competent complex. Mol Cell Biol. 1990 Dec;10(12):6335–6347. doi: 10.1128/mcb.10.12.6335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner W., Holland R., Waldschmidt R., Seifart K. H. Transcription factor IIA stimulates the expression of classical polIII-genes. Nucleic Acids Res. 1993 Feb 25;21(4):1013–1018. doi: 10.1093/nar/21.4.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisterernst M., Roeder R. G. Family of proteins that interact with TFIID and regulate promoter activity. Cell. 1991 Nov 1;67(3):557–567. doi: 10.1016/0092-8674(91)90530-c. [DOI] [PubMed] [Google Scholar]
- Meisterernst M., Roy A. L., Lieu H. M., Roeder R. G. Activation of class II gene transcription by regulatory factors is potentiated by a novel activity. Cell. 1991 Sep 6;66(5):981–993. doi: 10.1016/0092-8674(91)90443-3. [DOI] [PubMed] [Google Scholar]
- Nakajima N., Horikoshi M., Roeder R. G. Factors involved in specific transcription by mammalian RNA polymerase II: purification, genetic specificity, and TATA box-promoter interactions of TFIID. Mol Cell Biol. 1988 Oct;8(10):4028–4040. doi: 10.1128/mcb.8.10.4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohkuma Y., Sumimoto H., Hoffmann A., Shimasaki S., Horikoshi M., Roeder R. G. Structural motifs and potential sigma homologies in the large subunit of human general transcription factor TFIIE. Nature. 1991 Dec 5;354(6352):398–401. doi: 10.1038/354398a0. [DOI] [PubMed] [Google Scholar]
- Ozer J., Moore P. A., Bolden A. H., Lee A., Rosen C. A., Lieberman P. M. Molecular cloning of the small (gamma) subunit of human TFIIA reveals functions critical for activated transcription. Genes Dev. 1994 Oct 1;8(19):2324–2335. doi: 10.1101/gad.8.19.2324. [DOI] [PubMed] [Google Scholar]
- Ranish J. A., Hahn S. The yeast general transcription factor TFIIA is composed of two polypeptide subunits. J Biol Chem. 1991 Oct 15;266(29):19320–19327. [PubMed] [Google Scholar]
- Ranish J. A., Lane W. S., Hahn S. Isolation of two genes that encode subunits of the yeast transcription factor IIA. Science. 1992 Feb 28;255(5048):1127–1129. doi: 10.1126/science.1546313. [DOI] [PubMed] [Google Scholar]
- Reinberg D., Horikoshi M., Roeder R. G. Factors involved in specific transcription in mammalian RNA polymerase II. Functional analysis of initiation factors IIA and IID and identification of a new factor operating at sequences downstream of the initiation site. J Biol Chem. 1987 Mar 5;262(7):3322–3330. [PubMed] [Google Scholar]
- Sun X., Ma D., Sheldon M., Yeung K., Reinberg D. Reconstitution of human TFIIA activity from recombinant polypeptides: a role in TFIID-mediated transcription. Genes Dev. 1994 Oct 1;8(19):2336–2348. doi: 10.1101/gad.8.19.2336. [DOI] [PubMed] [Google Scholar]
- Usuda Y., Kubota A., Berk A. J., Handa H. Affinity purification of transcription factor IIA from HeLa cell nuclear extracts. EMBO J. 1991 Aug;10(8):2305–2310. doi: 10.1002/j.1460-2075.1991.tb07767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Gralla J. D., Carey M. The acidic activator GAL4-AH can stimulate polymerase II transcription by promoting assembly of a closed complex requiring TFIID and TFIIA. Genes Dev. 1992 Sep;6(9):1716–1727. doi: 10.1101/gad.6.9.1716. [DOI] [PubMed] [Google Scholar]
- Yokomori K., Admon A., Goodrich J. A., Chen J. L., Tjian R. Drosophila TFIIA-L is processed into two subunits that are associated with the TBP/TAF complex. Genes Dev. 1993 Nov;7(11):2235–2245. doi: 10.1101/gad.7.11.2235. [DOI] [PubMed] [Google Scholar]
- Yokomori K., Zeidler M. P., Chen J. L., Verrijzer C. P., Mlodzik M., Tjian R. Drosophila TFIIA directs cooperative DNA binding with TBP and mediates transcriptional activation. Genes Dev. 1994 Oct 1;8(19):2313–2323. doi: 10.1101/gad.8.19.2313. [DOI] [PubMed] [Google Scholar]