Abstract
Background
Granulocyte–colony-stimulating factor (G-CSF) is routinely used for mobilization of hematopoietic stem and progenitor cells preceding autologous transplantation after high-dose chemotherapy in hematologic malignancies. However, due to high mobilization failure rates, alternative mobilization strategies are required.
Study Design and Methods
Patients who poorly mobilized CD34+ hematopoietic cells (HCs) with G-CSF additionally received the CXCR4 antagonist plerixafor. The phenotype of CD34+ HCs collected after this plerixafor-induced “rescue” mobilization, in regard to adhesion molecule and CD133, CD34, and CD38 expression in comparison to CD34+ HCs collected after traditional G-CSF administration in good mobilizers, was analyzed flow cytometrically. To confirm previous studies in our patient cohort, the efficiency of mobilization and subsequent engraftment after this “on-demand” plerixafor mobilization were analyzed.
Results
Pronounced mobilization occurred after plerixafor administration in poor mobilizers, resulting in similar CD34+ cell yields as obtained by G-CSF in good mobilizers, whereby plerixafor increased the content of primitive CD133+/CD34+/CD38– cells. The surface expression profiles of the marrow homing and retention receptors CXCR4, VLA-4, LFA-1, and CD44 on mobilized CD34+ cells and hematopoietic recovery after transplantation were similar in patients receiving G-CSF plus plerixafor or G-CSF. Unexpectedly, the expression levels of respective adhesion receptors were not related to mobilization efficiency or engraftment.
Conclusion
The results show that CD34+ HCs collected by plerixafor-induced rescue mobilization are qualitatively equivalent to CD34+ HCs collected after traditional G-CSF mobilization in good mobilizers, in regard to their adhesive phenotype and engraftment potential. Thereby, plerixafor facilitates the treatment of poor mobilizers with autologous HC transplantation after high-dose chemotherapy.
Autologous hematopoietic cell (HC) transplantation is widely applied to reconstitute hematopoiesis after high-dose chemotherapy in patients with hematologic malignancies including multiple myeloma (MM), non-Hodgkin's lymphoma (NHL), and Hodgkin's lymphoma.1,2 Mobilized peripheral blood (PB) CD34+ hematopoietic stem and progenitor cells (HSPCs) are easy to collect, have a high engraftment potential, and are used as preferred source for transplantation. To mobilize HSPCs from the marrow (BM) into the PB, their adhesive interactions with accessory stromal cells, osteoclasts,3 osteoblasts, and extracellular matrix components in the BM need to be overcome. The chemokine CXCL12 and the α4β1 (VLA-4, CD49d/CD29) integrin ligand VCAM-1 are abundantly expressed by BM stromal cells and act as key mediators of HSPC retention in the BM.4 In line, disruption of CXCL12–CXCR4 and VCAM-1–VLA-4 interactions results in release of HSPCs into the PB.5-7 Additionally, the αLβ2 integrin LFA-1 (CD11a/CD18) and the adhesion receptor CD44 are involved in HSPC lodgment in the BM and CD44 is also implicated in mobilization of these cells.8-10 Importantly, the receptors CXCR4, VLA-4, LFA-1, and CD44 also mediate BM homing of HSPCs,9,11-14 wherefore their expression might influence the migration of these cells into the BM and engraftment after autologous transplantation.
Currently, the granulocyte–colony-stimulating factor (G-CSF) is used as standard mobilizing agent, alone or in combination with chemotherapy. G-CSF causes severe alterations of the BM microenvironment including osteoblast apoptosis,15 depletion of CD68+/CD169+ BM macrophages,16,17 inhibition of osteogenic mesenchymal stem cell differentiation,18 and bone formation.16,19 Collectively, these alterations cause a down regulation of molecules important for HSPC maintenance and retention including CXCL12, VCAM-1, SCF, and angiopoietin-116-20 in endosteal and vascular niches, which eventually facilitates HSPC mobilization. G-CSF has been suggested to further promote HSPC mobilization by inducing the expression of the proteases matrix metalloproteinase-9, cathepsin G, and neutrophil elastase, which together with mediators of the complement cascade21-23 and thrombolytic pathway24 cleave and inactivate the retention factors CXCL12, VCAM-1, and c-Kit.25-29 However, in 10% to 30% of all patients G-CSF fails to efficiently mobilize CD34+ HSPCs and does not support the collection of at least 2 × 106 CD34+ cells/kg body weight in one collection attempt, which are minimally required for transplantation and successful engraftment after high-dose chemotherapy.30-33 These patients require alternative mobilization regimens. Plerixafor (AMD3100), a bicyclam antagonist of CXCR4, which reversibly blocks CXCL12 binding and chemotactic signaling,34,35 has recently been identified as potent mobilizing agent. Broxmeyer and colleagues7 first showed that plerixafor and G-CSF act synergistically in the mobilization of functionally competent human stem cells. Subsequent studies demonstrated that plerixafor elevates CD34+ cell harvests in comparison to G-CSF alone in healthy volunteers36 and NHL and MM patients.37-39 Therefore, plerixafor was approved in combination with G-CSF for HSPC mobilization for autologous transplantation in lymphoma and MM patients.
In our study G-CSF was used as standard mobilizing agent. Patients identified as “poor mobilizers,” based on low circulating CD34+ cell counts during G-CSF administration, additionally received plerixafor (“on demand”). As plerixafor induces HSPC mobilization by mechanisms distinct from G-CSF, it may release HSPCs of different subsets and different adhesion and migration receptor expression profiles. We evaluated HSPC subset composition based on CD133, CD34, and CD38 expression and the surface expression of CXCR4, CD49d (α-subunit of VLA-4), CD11a (α-subunit of LFA-1), and CD44 on collected CD34+ cells. Additionally, we analyzed the mobilization efficiency of plerixafor “rescue” administration in comparison to standard G-CSF application, to confirm previous investigations demonstrating plerixafor efficiency37,39-44 in our patient cohort. To further compare the quality of mobilized HCs, we evaluated the time to engraftment after transplantation and the impact of CXCR4 and adhesion molecule expression on time to neutrophil and platelet (PLT) engraftment.
Materials and Methods
Patients and HSPC collection
Under approval from the local ethics committee (No. 415-E/1177/8-2010) and after written informed consent was obtained, 37 patients with hematologic malignancies (summarized in Table 1 and detailed in Table S1, available as supporting information in the online version of this paper), who underwent HSPC mobilization at the Third Medical Department of the Paracelsus Medical University Salzburg, were included in this study. Mobilization was induced by daily subcutaneous injections of 10 μg of G-CSF (Merckle Biotec, Ulm, Germany; or Novartis, Basel, Switzerland) per kg of body weight for 3 to 4 consecutive days before leukapheresis was started. A total of 75.7% of our patients additionally received chemotherapy (Table 1), which was followed by daily administration of 10 μg/kg G-CSF for 7 to 12 days starting from the day after chemotherapy. Leukapheresis was initiated at the discretion of the attaining physician and depended on circulating CD34+ cell numbers as monitored via a flow cytometer (FC-500, Beckman Coulter, Fullerton, CA). A total of 20 × 106 CD34+ cells/L PB were considered as minimum and at least 50 × 106 CD34+ cells/L PB as optimum to induce leukapheresis. If the minimum cell number was not attained during G-CSF administration, patients were considered as “poor mobilizers” and received a single subcutaneous injection of 240 μg/kg plerixafor followed by induction of leukapheresis after 10 to 12 hours. Cell collection was performed in one session with a commercially available apheresis system (COBE Spectra, Terumo BCT, Lakewood, CO). The number of collected CD34+ cells was determined by flow cytometer. Patients not responding to plerixafor were not subjected to leukapheresis and therefore not included in this study.
Table 1.
Patient characteristics
| Characteristics | Mobilization regimen | |
|---|---|---|
| G-CSF | G-CSF plus plerixafor | |
| Number of patients | 29 | 8 |
| Age (years) | 54 (18-73) | 62 (30-72) |
| Male sex | 20 (69.0) | 1 (12.5) |
| Chemotherapy previous to G-CSF | 24 (82.8) | 4 (50.0) |
| Chemotherapy regimen | ||
| Cyclophosphamide | 15 (51.7) | 3 (37.5) |
| R-DHAP | 3 (10.3) | 1 (12.5) |
| DHAP | 4 (13.8) | 0 |
| BEACOPP | 1 (3.4) | 0 |
| CHOEP | 1 (3.4) | 0 |
| Diagnosis | ||
| NHL | 10 (34.5) | 4 (50.0) |
| MM | 14 (48.3) | 3 (37.5) |
| Hodgkin's lymphoma | 3 (10.3) | 0 |
| Acute lymphocytic leukemia | 1 (3.4) | 0 |
| Acute myeloid leukemia | 1 (3.4) | 0 |
| Scleromyxedema | 0 | 1 (12.5) |
Data are reported as median (range) or number (%).
R-DHAP = rituximab–dexamethasone, cytarabine and cisplatin; DHAP = dexamethasone, cytarabine, and cisplatin; BEACOPP = bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone; CHOEP = cyclophosphamide, doxorubicin, vincristine, and prednisone plus etoposide.
Flow cytometry
Fresh leukapheresis products were stained with the following monoclonal antibodies (MoAbs): anti-CXCR4–PE, anti-CD49d–PE, anti-CD11a–PE, anti-CD34–FITC (BD Biosciences, Franklin Lakes, NJ) or corresponding isotype controls. Additionally, PB mononuclear cells (PBMNCs) were isolated by density gradient centrifugation, viably frozen, and stored in liquid nitrogen until use. Thawed PBMNCs were stained with anti-CD34–FITC (BD Biosciences), CD44–FITC, anti-CD38–PE, anti-CD34–PE (Beckman Coulter), and anti-CD133–APC MoAbs (Miltenyi Biotec, Bergisch-Gladbach, Germany). The percentage of cells expressing respective receptors and mean fluorescence intensity values in relation to isotype controls (MFIRs) were analyzed with a flow cytometer and its accompanying software (Gallios and Caluza, respectively, both Beckman Coulter).
Definition of engraftment
Neutrophil engraftment was defined as the first day after transplantation where neutrophil counts in the PB reached at least 500 × 106/L. PLT engraftment was defined as first (of 3 consecutive) day(s) where PLT counts reached at least 50 × 109/L PB based on guidelines of the European Group for Blood and Marrow Transplantation.45 In three cases (marked in Table S1), we observed lower but stable PLT counts in the absence of bleeding without further requirement of PLT transfusion at discharge from the hospital, which was defined as engraftment.
Statistical analyses
Statistical analyses were performed with computer software (GraphPad, San Diego, CA). The Kolmogorov-Smirnov test was used to evaluate normal distribution. Normally distributed data were analyzed by t test, nonnormally distributed by Mann-Whitney test. Correlation analyses were carried out using the Pearson's test for normally distributed or Spearman test for nonnormally distributed data. Two-tailed p values of less than 0.05 were considered significant.
Results
G-CSF and G-CSF plus plerixafor result in the mobilization of CD34+ cells with similar CXCR4 and adhesion molecule expression profiles
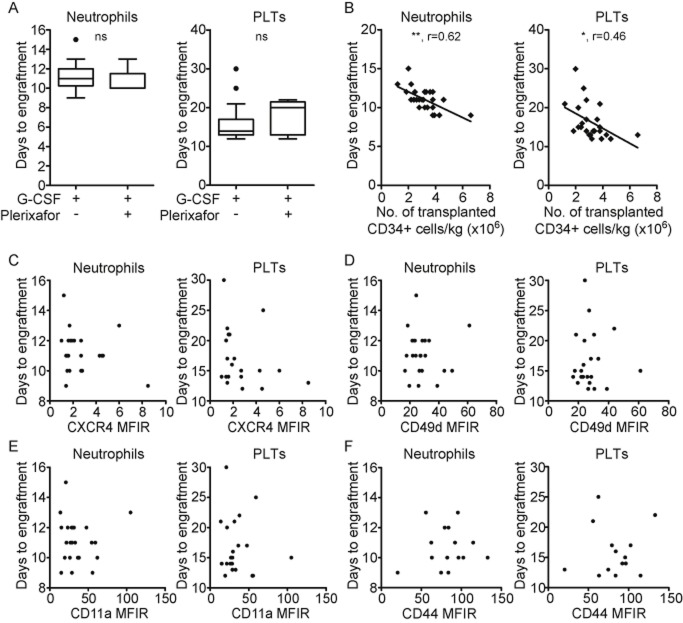
The surface expression of CXCR4, VLA-4, LFA-1, and CD44, which are involved in HSPC BM homing and retention, were measured on CD34+ cells in leukapheresis products collected after G-CSF plus plerixafor–induced rescue mobilization and standard G-CSF mobilization using multiparameter flow cytometry. HSPCs were identified by size and granularity (forward and side scatter) and CD34 expression (as exemplified in Fig. 1A). Representative fluorescence histograms of each receptor and the MFIRs are depicted on the left side of Figs. 1B through 1E. CXCR4 was weakly expressed on CD34+ cells of both treatment groups as shown by median MFIRs of 1.8 (G-CSF) and 2.0 (G-CSF plus plerixafor, Fig. 1B, middle) and median percentages of CXCR4-positive CD34+ cells of 4.3 (G-CSF) and 14.2 (G-CSF plus plerixafor, Fig. 1B, right). As CXCR4 levels were directly determined in fresh leukapheresis products the low levels were not caused from down regulation during density centrifugation or freezing and thawing. In contrast, the expression of VLA-4, as determined by flow cytometric analysis of CD49d (α-subunit of VLA-4), was high on the entire CD34+ population throughout all patients, independent of the mobilizing regimen (Fig. 1C). Similarly, LFA-1, as determined by analysis of CD11a (α-subunit of LFA-1) levels (Fig. 1D), and CD44 (Fig. 1E) were highly expressed on mobilized CD34+ cells of all patients and did not differ between G-CSF and G-CSF plus plerixafor–mobilized CD34+ cells.
Figure 1.
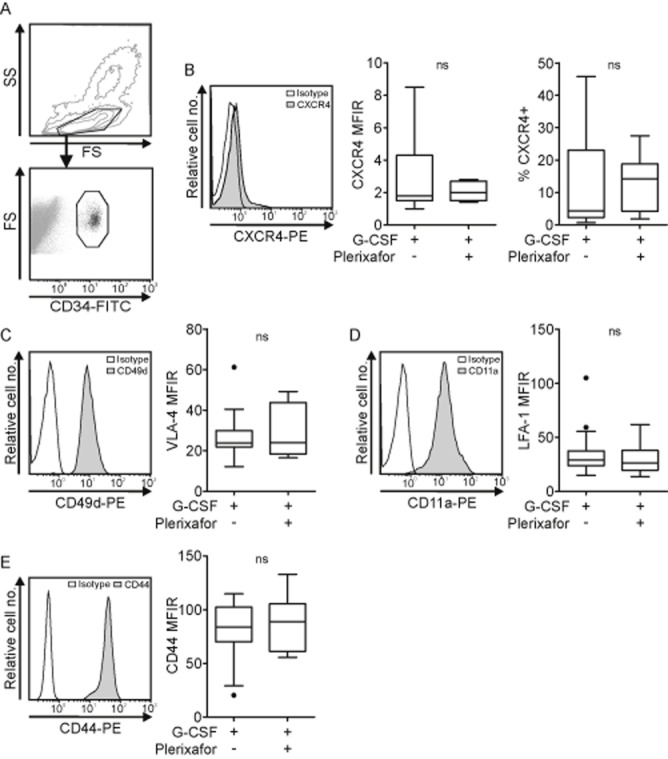
CXCR4 and adhesion molecule expression profiles in G-CSF and G-CSF plus plerixafor–mobilized HSPCs. (A) Representative density and dot plots illustrate the gating strategy for HSPCs based on forward and side scatter (FS and SS) and CD34 expression. (B) Representative fluorescence histograms (left), MFIRs of surface CXCR4 levels (middle), and percentages of CXCR4+ cells (right) of CD34+ cells collected after G-CSF– (n = 23) or G-CSF plus plerixafor–induced mobilization (n = 7). Representative fluorescence histograms of CD49d (C, left), CD11a (D, left), and CD44 (E, left) expression on CD34+ cells mobilized with G-CSF or G-CSF plus plerixafor. The corresponding MFIRs of CD49d (C, right, n(G) = 24, n(G+P) = 7), CD11a (D, right, n(G) = 24, n(G+P) = 7), and CD44 (E, right n(G) = 15, n(G+P) = 6) are shown in box plot format. G = G-CSF; G+P = G-CSF plus plerixafor. ns = not significant.
Collectively, our data show that CD34+ cells collected after G-CSF plus plerixafor–induced rescue mobilization or standard G-CSF administration exhibit similar CXCR4 and adhesion receptor expression profiles despite different mechanisms of release from the BM.
The addition of plerixafor to G-CSF causes increased mobilization of primitive CD133+/CD34+/CD38– HCs
To compare the HC subsets collected after either mobilization regimen, we characterized CD133, CD34, and CD38 expression in leukapheresis products via flow cytometry. Although CD34 is widely used as HSPC marker,46,47 CD34–/CD38–/Lin– HCs are capable of repopulating immunodeficient mice.48 Therefore, we additionally analyzed the alternative HSPC marker CD133,49 which is expressed on primitive CD34–/CD38–/Lin–50 as well as on CD34+ HCs, where it marks a subpopulation enriched for repopulating cells.51,52 Within the CD34+ population, CD38– cells represent a subset with extensive proliferation and repopulating capacity,53,54 while CD38+ cells express differentiation markers and have limited repopulating capacities.55
Figure 2A illustrates a typical CD34 and CD38 expression profile of CD133+ HCs in leukapheresis products collected after G-CSF (top) and G-CSF plus plerixafor administration (bottom). In all patients the main population of CD133+/CD34+ HCs also expressed CD38 (exemplified in Fig. 2A). While leukapheresis products collected after G-CSF treatment contained no or very small percentages of CD133+/CD34+/CD38– HCs, G-CSF plus plerixafor induced the collection of a significantly larger subpopulation of early CD133+/CD34+/CD38– HCs (median, 0.39% compared to 1.84%; p = 0.037, Fig. 2B).
Figure 2.
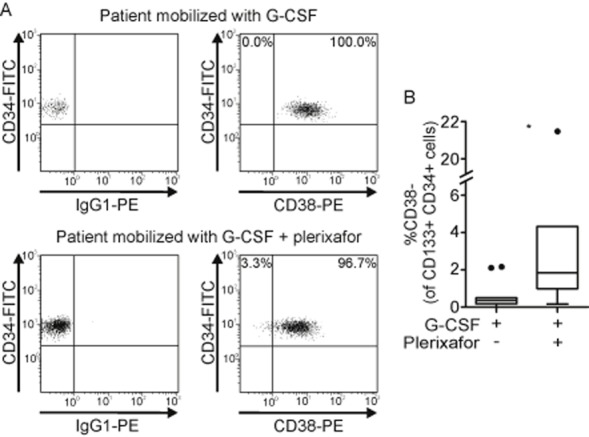
CD34 and CD38 expression on CD133+ HCs in leukapheresis products. (A) The dot plots show the surface expression of CD34 and CD38 on pregated CD133+ HCs and are representative for 11 leukapheresis products collected after G-CSF (top) and seven leukapheresis products collected after G-CSF plus plerixafor administration (bottom). (B) Percentages of CD38– cells within the CD133+/CD34+ cell population in G-CSF (n = 11) compared to G-CSF plus plerixafor (n = 7). *p < 0.05.
Plerixafor rescues HSPC mobilization in poor mobilizers
To determine how the addition of plerixafor to G-CSF in poor mobilizers affects mobilization efficiency, we first compared circulating CD34+ cell counts 1 day before and the next morning after plerixafor administration before leukapheresis. Figure 3A shows that plerixafor induced a 12-fold elevation of the median number of circulating CD34+ cells (×106/L PB) from 7.5 to 90.5. Further, we found that G-CSF plus plerixafor induced the mobilization of similar numbers of CD34+ cells (×106/L) to the PB (median, 96.5 × 106 cells/L) like G-CSF alone in good responders (median, 95.0 × 106 cells/L; Fig. 3B), as determined shortly before leukapheresis, and resulted in similar yields of CD34+ cells/kg by leukapheresis (median, 5.2 × 106 and 6.9 × 106 cells/kg; Fig. 3C). Of note, within G-CSF–treated patients, those who underwent chemotherapy before G-CSF treatment had significantly higher CD34+ cell counts (×106/L PB; median, 115.0 × 106 compared to 48.0 × 106 cells/L; Fig. 3D) and CD34+ cell yields/kg (median, 8.3 × 106 compared to 3.3 × 106 cells/kg; Fig. 3E). Preapheresis CD34+ cell numbers (×106/L) in the PB of the whole patient collective correlated with the number of CD34+ cells/kg yielded by subsequent leukapheresis (Fig. 3F), which confirms peripheral CD34+ counts to be helpful indicators to predict mobilization success.
Figure 3.
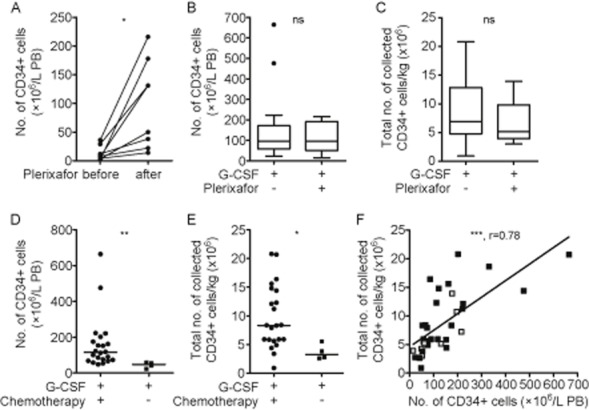
Plerixafor rescues HSPC mobilization in poor mobilizers. (A) Numbers of preapheresis CD34+ cells (×106/L PB) 1 day before and the next morning after plerixafor injection in poor mobilizers as determined by flow cytometry (n = 8). (B) Comparison of preapheresis CD34+ cell counts (×106/L PB) in good mobilizers receiving G-CSF (n = 28) and poor mobilizers receiving G-CSF plus plerixafor (n = 8). (C) Numbers of CD34+ cells/kg collected after G-CSF– (n = 26) or G-CSF plus plerixafor–induced mobilization (n = 8). Results are illustrated in box plot format, indicating the median. The 25th and 75th percentiles are marked by the edges of the box and the minimum and maximum observations (excluding outliers) are marked by the whiskers. Outliers are defined as cases that are more than 1.5-fold the interquartile range away from the edges of the box and are indicated as dots. (D) Preapheresis CD34+ cell numbers (×106/L PB) of good mobilizers who did (n = 22) or did not (n = 4) receive chemotherapy immediately before G-CSF. (E) Total CD34+ cell numbers/kg collected from good mobilizers, who received chemotherapy before G-CSF (n = 22) or not (n = 4). (F) Correlation of preapheresis CD34+ cell numbers (×106/L PB) with total CD34+ cells/kg collected via leukapheresis. (■) Patients treated with G-CSF (n = 27); ( ) patients treated with G-CSF plus plerixafor (n = 8). *p < 0.05; **p < 0.01; ***p < 0.001; ns = not significant.
) patients treated with G-CSF plus plerixafor (n = 8). *p < 0.05; **p < 0.01; ***p < 0.001; ns = not significant.
Together, our data show that the on-demand use of plerixafor potently induces mobilization of CD34+ cells in patients poorly responding to G-CSF (with or without chemotherapy) and facilitates mobilization rates similar to those of good mobilizers.
CXCR4, VLA-4, LFA-1, and CD44 expression on mobilized CD34+ cells is independent of mobilization efficiency
Next, we evaluated whether the surface expression of receptors involved in HSPC retention in the BM is related to mobilization efficiency. The relations of preapheresis CD34+ cell numbers (×106/L PB) with expression of respective receptors are shown in Fig. 4 and illustrate that the surface levels (MFIRs) of CXCR4 (Fig. 4A, left) and percentages of CXCR4 expressing CD34+ cells (Fig. 4A, right) and the surface levels of CD49d (VLA-4, Fig. 4B), CD11a (LFA-1, Fig. 4C), and CD44 (Fig. 4D) do not correlate with mobilization efficiency as analyzed in the total patient cohort. The separate analysis of both treatment groups similarly did not show any correlation (data not shown). However, it is worth mentioning that three patients displaying particularly high mobilization response (>300 × 106 CD34+ cells/L PB) exhibit a low expression of CXCR4, CD49d, and CD11a.
Figure 4.
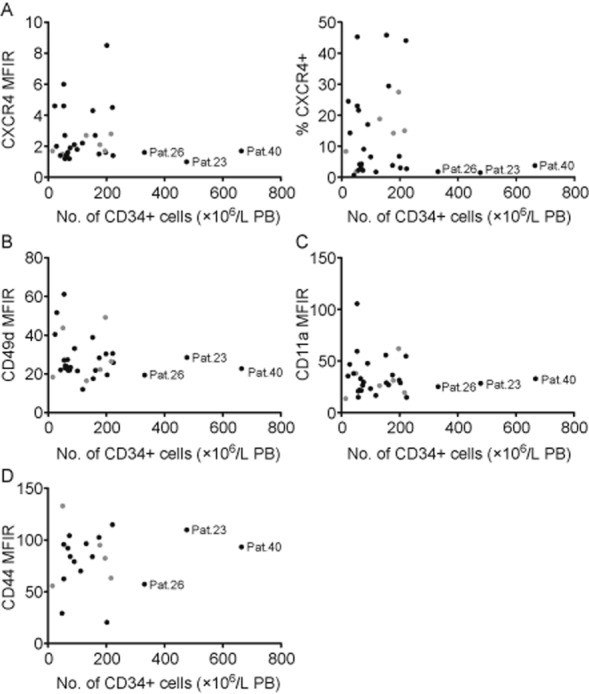
CXCR4, CD49d, CD11a, and CD44 expression on mobilized CD34+ cells are independent of mobilization efficiency. Preapheresis CD34+ cell numbers (×106/L PB) were correlated with MFIRs of CXCR4 (A, left, n = 31), percentages of CXCR4+ cells within the CD34+ population (A, right, n = 31) and MFIRs of CD49d (B, n = 32), CD11a (C, n = 32), and CD44 (D, n = 21) on CD34+ cells. (●) Patients receiving G-CSF; ( ) patients receiving G-CSF plus plerixafor.
) patients receiving G-CSF plus plerixafor.
Transplantation with HCs mobilized via G-CSF or G-CSF plus plerixafor results in similar time to engraftment
A rapid engraftment of transplanted HCs in patients after high-dose chemotherapy is essential in reconstituting the immune system and minimize the risk of severe infections. Therefore, we compared the time to of hematopoietic recovery (as defined by time to neutrophil and PLT engraftment) in 26 patients transplanted with autologous HCs collected after either mobilization regimen. Neutrophil engraftment after transplantation with HCs mobilized by G-CSF resulted in neutrophil engraftment after a median of 11 days, comparable to that of neutrophil engraftment after G-CSF plus plerixafor–induced mobilization (median, 10 days; Fig. 5A, left). PLT engraftment occurred after a median of 14 days in patients receiving G-CSF–mobilized HCs and after a median of 20 days in patients receiving G-CSF plus plerixafor-mobilized HCs (Fig. 5A, right). Next, we investigated which variables influenced time to engraftment. As reported previously for G-CSF–mobilized HCs, in our cohort of G-CSF and G-CSF plus plerixafor–treated patients, the number of transplanted CD34+ cells/kg inversely correlated with time to neutrophil engraftment (Fig. 5B, left) and weakly inversely correlated with time to PLT engraftment (Fig. 5B, right). To further assess the influence of CXCR4 and adhesion molecule expression on CD34+ cells in apheresis products on engraftment we related expression levels of each receptor to neutrophil and PLT engraftment time. As shown in Figs. 5C through 5F, none of the CXCR4, CD49d, CD11a, or CD44 surface expression levels were related to neutrophil or PLT engraftment. Our results show that G-CSF and G-CSF plus plerixafor mobilize HSPCs with similar engraftment kinetics and that the time to engraftment primarily depends on the number of transplanted CD34+ HCs but not on CXCR4 and adhesion molecule expression.
Figure 5.
Transplantation with HCs mobilized via G-CSF or G-CSF plus plerixafor results in similar time to engraftment. (A) The days to neutrophil (left, n(G) = 20, n(G+P) = 6) and PLT engraftment (right, n(G) = 19, n(G+P) = 5) after transplantation with G-CSF– or G-CSF plus plerixafor–mobilized HCs are shown in box plot format. (B) Correlation of transplanted CD34+ cell numbers/kg (mobilized by G-CSF or G-CSF plus plerixafor) with time to neutrophil (left, n = 26) and PLT engraftment (right, n = 24). Correlations of neutrophil (left) and PLT engraftment (right) with surface levels of CXCR4 (C, n(Neutrophils) = 23 and n(PLTs) = 21), CD49d (D, n(Neutrophils) = 24 and n(PLTs) = 22), CD11a (E, n(Neutrophils) = 24 and n(PLTs) = 22), and CD44 (F, n(Neutrophils) = 15 and n(PLTs) = 14). G = G-CSF; G+P = G-CSF plus plerixafor. *p < 0.05; **p < 0.01; ns = not significant.
Discussion
Limited data are currently available comparing the phenotype and engraftment properties of HSPCs collected after G-CSF plus plerixafor–induced rescue mobilization compared to traditional G-CSF administration. In our exploratory study on a total of 37 patients we found that while the on-demand addition of plerixafor to G-CSF in poor mobilizers altered the subset composition in leukapheresis products in favor of more primitive CD133+/CD34+/CD38– HCs, the CXCR4 and adhesion molecule expression profiles were similar on the bulk CD34+ population collected after both mobilization regimens. In line, plerixafor-induced rescue mobilization was similarly effective in mobilizing CD34+ cells and caused equal neutrophil and PLT engraftment after transplantation like G-CSF in good mobilizers.
Previous studies suggested that a modulation of adhesion receptor expression on BM HSPCs occurs during G-CSF administration, which might be functionally involved in the mobilization process. Specifically, CXCR4 surface expression was found to be dynamically modulated in response to G-CSF,25 and CXCR4, VLA-4, and LFA-1 surface levels were suggested to be down regulated during mobilization into the circulation.25,56,57 Although plerixafor is thought to elicit less pronounced effects on the BM microenvironment than G-CSF, HSPCs mobilization to the PB might be accompanied by alterations in their adhesion molecule expression. For example, plerixafor has recently been shown to induce release of CXCL12 from BM stromal cells into the PB,58 which in turn might affect CXCR4 expression on HSPCs, for example, by induction of CXCR4 internalization. Studies in an autologous transplantation rhesus macaque model demonstrated that plerixafor-mobilized CD34+ cells have higher VLA-4 and CXCR4 levels as G-CSF–mobilized CD34+ cells.59 In contrast, we did not detect any differences in the adhesion molecule expression on mobilized CD34+ cells, whether plerixafor was used or not. Importantly, our patients received G-CSF until the day before plerixafor injection and therefore might exhibit G-CSF–induced alterations on mobilized CD34+ cells. These receptor modulations alone might have not been sufficient to initiate pronounced mobilization but might facilitate release from the BM in addition to plerixafor-specific mechanisms. Our interpatient comparison of G-CSF plus plerixafor– versus G-CSF–mobilized subsets illustrated that the addition of plerixafor also induced the release of more primitive CD133+/CD34+/CD38– cells in poor mobilizers compared to G-CSF alone in good mobilizers. A previous study showed that the supplementation of plerixafor in a G-CSF–based regimen increased the percentage of CD38– cells in the circulating CD133+/CD34+ population within individual MM and NHL patients60 and another study reported elevated percentages of CD133+/CD38– within CD34+ cells if plerixafor was added to G-CSF instead of cyclophosphamide.61 Together these data show that the different mobilizing mechanisms of plerixafor and G-CSF cause the collection of different HC subset combinations.
A rapid hematopoietic recovery after HC transplantation is essential to reduce the risk of secondary infections. Recently, a minimal delay in engraftment independent of the CD34+ cell dose was associated with mobilization by G-CSF plus plerixafor in comparison to G-CSF.62 Our data (albeit determined in a small cohort) do not support this suggestion, as we have observed that transplantation with HCs mobilized by either regimen result in similar engraftment times. Although CD34+/CD38– cells possess extensive proliferation and repopulation potentials,53,54 greater percentages within CD34+ cells or total transplanted numbers were not related to faster engraftment (data not shown). However, effects on long-term hematopoiesis need to be further investigated. Furthermore, engraftment time after transplantation did not correlate with the expression of BM homing and retention receptors on CD34+ cells (that one could expect) but was strictly correlated with the number of transplanted CD34+ cells, supporting a previous study63 and underlining its dominant role in determining engraftment. A recent report shows that patients with prolonged time to neutrophil engraftment (>19 days) exhibited lower VLA-4 levels on collected CD34+ cells.64 Importantly, all our patients expressed high VLA-4 levels on collected CD34+ cells, which might exceed a certain threshold necessary to effectively mediate HSPC homing and retention within the BM. In line, neutrophil engraftment occurred faster in our entire patient cohort (≤17 days), which could further explain the lack of correlation. Although CXCR4 is essential for homing into the BM, we did not observe any association of CXCR4 levels and engraftment. Interestingly, previous studies have shown that CXCR4 expression on CD34+ cells is dynamically regulated and that CD34+ cells can release CXCR4 from intracellular stores to the cell surface upon cytokine stimulation.65 Therefore, we suggest that the low CXCR4 expression on collected CD34+ cells might be significantly increased upon injection into the recipient to facilitate migration into the BM.
Collectively, our results suggest that the use of plerixafor on-demand releases HSPCs that are qualitatively equivalent to HSPCs mobilized by G-CSF, in terms of BM homing and retention receptor expression and engraftment potential. Therefore, our study, albeit using a limited patient number, supports the use of plerixafor for poor mobilizers to rescue HSPC mobilization and facilitate their treatment with potentially curative high-dose therapy and HC transplantation.
Acknowledgments
The authors thank Jutta Rößlhuber for technical assistance.
Glossary
- BM
marrow
- HC(s)
hematopoietic cell(s)
- HSPC(s)
hematopoietic stem and progenitor cell(s)
- MFIR(s)
mean fluorescence intensity value(s) in relation to isotype control
- MM
multiple myeloma
- NHL
non-Hodgkin's lymphoma
- PB
peripheral blood
Conflict of Interest
The authors report no conflicts of interest or funding sources.
Supporting Information
Detailed patient characteristics
References
- Bensinger W, DiPersio JF, McCarty JM. Improving stem cell mobilization strategies: future directions. Bone Marrow Transplant. 2009;43:181–195. doi: 10.1038/bmt.2008.410. [DOI] [PubMed] [Google Scholar]
- Gratwohl A, Baldomero H, Schwendener A, et al. The EBMT activity survey 2008: impact of team size, team density and new trends. Bone Marrow Transplant. 2011;46:174–191. doi: 10.1038/bmt.2010.69. [DOI] [PubMed] [Google Scholar]
- Kollet O, Dar A, Shivtiel S, et al. Osteoclasts degrade endosteal components and promote mobilization of hematopoietic progenitor cells. Nat Med. 2006;12:657–664. doi: 10.1038/nm1417. [DOI] [PubMed] [Google Scholar]
- Motabi IH, DiPersio JF. Advances in stem cell mobilization. Blood Rev. 2012;26:267–278. doi: 10.1016/j.blre.2012.09.003. [DOI] [PubMed] [Google Scholar]
- Bonig H, Wundes A, Chang KH, et al. Increased numbers of circulating hematopoietic stem/progenitor cells are chronically maintained in patients treated with the CD49d blocking antibody natalizumab. Blood. 2008;111:3439–3441. doi: 10.1182/blood-2007-09-112052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez P, Rettig MP, Uy GL, et al. BIO5192, a small molecule inhibitor of VLA-4, mobilizes hematopoietic stem and progenitor cells. Blood. 2009;114:1340–1343. doi: 10.1182/blood-2008-10-184721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broxmeyer HE, Orschell CM, Clapp DW, et al. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J Exp Med. 2005;201:1307–1318. doi: 10.1084/jem.20041385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixido J, Hemler ME, Greenberger JS, et al. Role of beta 1 and beta 2 integrins in the adhesion of human CD34hi stem cells to bone marrow stroma. J Clin Invest. 1992;90:358–367. doi: 10.1172/JCI115870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen M, Le PF, Gagnerault MC, et al. Role of adhesion molecules in the homing and mobilization of murine hematopoietic stem and progenitor cells. Blood. 1998;92:894–900. [PubMed] [Google Scholar]
- Christ O, Kronenwett R, Haas R, et al. Combining G-CSF with a blockade of adhesion strongly improves the reconstitutive capacity of mobilized hematopoietic progenitor cells. Exp Hematol. 2001;29:380–390. doi: 10.1016/s0301-472x(00)00674-3. [DOI] [PubMed] [Google Scholar]
- Lapidot T, Dar A, Kollet O. How do stem cells find their way home? Blood. 2005;106:1901–1910. doi: 10.1182/blood-2005-04-1417. [DOI] [PubMed] [Google Scholar]
- Peled A, Kollet O, Ponomaryov T, et al. The chemokine SDF-1 activates the integrins LFA-1, VLA-4, and VLA-5 on immature human CD34(+) cells: role in transendothelial/stromal migration and engraftment of NOD/SCID mice. Blood. 2000;95:3289–3296. [PubMed] [Google Scholar]
- Kollet O, Spiegel A, Peled A, et al. Rapid and efficient homing of human CD34(+)CD38(-/low)CXCR4(+) stem and progenitor cells to the bone marrow and spleen of NOD/SCID and NOD/SCID/B2m(null) mice. Blood. 2001;97:3283–3291. doi: 10.1182/blood.v97.10.3283. [DOI] [PubMed] [Google Scholar]
- Avigdor A, Goichberg P, Shivtiel S, et al. CD44 and hyaluronic acid cooperate with SDF-1 in the trafficking of human CD34+ stem/progenitor cells to bone marrow. Blood. 2004;103:2981–2989. doi: 10.1182/blood-2003-10-3611. [DOI] [PubMed] [Google Scholar]
- Christopher MJ, Link DC. Granulocyte colony-stimulating factor induces osteoblast apoptosis and inhibits osteoblast differentiation. J Bone Miner Res. 2008;23:1765–1774. doi: 10.1359/JBMR.080612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler IG, Sims NA, Pettit AR, et al. Bone marrow macrophages maintain hematopoietic stem cell (HSC) niches and their depletion mobilizes HSCs. Blood. 2010;116:4815–4828. doi: 10.1182/blood-2009-11-253534. [DOI] [PubMed] [Google Scholar]
- Christopher MJ, Rao M, Liu F, et al. Expression of the G-CSF receptor in monocytic cells is sufficient to mediate hematopoietic progenitor mobilization by G-CSF in mice. J Exp Med. 2011;208:251–260. doi: 10.1084/jem.20101700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez-Ferrer S, Michurina TV, Ferraro F, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466(7308):829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levesque JP, Helwani FM, Winkler IG. The endosteal “osteoblastic” niche and its role in hematopoietic stem cell homing and mobilization. Leukemia. 2010;24:1979–1992. doi: 10.1038/leu.2010.214. [DOI] [PubMed] [Google Scholar]
- Chow A, Lucas D, Hidalgo A, et al. Bone marrow CD169+ macrophages promote the retention of hematopoietic stem and progenitor cells in the mesenchymal stem cell niche. J Exp Med. 2011;208:261–271. doi: 10.1084/jem.20101688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HM, Wu W, Wysoczynski M, et al. Impaired mobilization of hematopoietic stem/progenitor cells in C5-deficient mice supports the pivotal involvement of innate immunity in this process and reveals novel promobilization effects of granulocytes. Leukemia. 2009;23:2052–2062. doi: 10.1038/leu.2009.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratajczak J, Reca R, Kucia M, et al. Mobilization studies in mice deficient in either C3 or C3a receptor (C3aR) reveal a novel role for complement in retention of hematopoietic stem/progenitor cells in bone marrow. Blood. 2004;103:2071–2078. doi: 10.1182/blood-2003-06-2099. [DOI] [PubMed] [Google Scholar]
- Ratajczak MZ, Kim CH, Wojakowski W, et al. Innate immunity as orchestrator of stem cell mobilization. Leukemia. 2010;24:1667–1675. doi: 10.1038/leu.2010.162. [DOI] [PubMed] [Google Scholar]
- Tjwa M, Janssens S, Carmeliet P. Plasmin therapy enhances mobilization of HPCs after G-CSF. Blood. 2008;112:4048–4050. doi: 10.1182/blood-2008-07-166587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit I, Szyper-Kravitz M, Nagler A, et al. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat Immunol. 2002;3:687–694. doi: 10.1038/ni813. [DOI] [PubMed] [Google Scholar]
- Semerad CL, Christopher MJ, Liu F, et al. G-CSF potently inhibits osteoblast activity and CXCL12 mRNA expression in the bone marrow. Blood. 2005;106:3020–3027. doi: 10.1182/blood-2004-01-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levesque JP, Takamatsu Y, Nilsson SK, et al. Vascular cell adhesion molecule-1 (CD106) is cleaved by neutrophil proteases in the bone marrow following hematopoietic progenitor cell mobilization by granulocyte colony-stimulating factor. Blood. 2001;98:1289–1297. doi: 10.1182/blood.v98.5.1289. [DOI] [PubMed] [Google Scholar]
- Levesque JP, Hendy J, Takamatsu Y, et al. Mobilization by either cyclophosphamide or granulocyte colony-stimulating factor transforms the bone marrow into a highly proteolytic environment. Exp Hematol. 2002;30:440–449. doi: 10.1016/s0301-472x(02)00788-9. [DOI] [PubMed] [Google Scholar]
- Levesque JP, Hendy J, Winkler IG, et al. Granulocyte colony-stimulating factor induces the release in the bone marrow of proteases that cleave c-KIT receptor (CD117) from the surface of hematopoietic progenitor cells. Exp Hematol. 2003;31:109–117. doi: 10.1016/s0301-472x(02)01028-7. [DOI] [PubMed] [Google Scholar]
- Gordan LN, Sugrue MW, Lynch JW, et al. Poor mobilization of peripheral blood stem cells is a risk factor for worse outcome in lymphoma patients undergoing autologous stem cell transplantation. Leuk Lymphoma. 2003;44:815–820. doi: 10.1080/1042819031000067585. [DOI] [PubMed] [Google Scholar]
- Olivieri A, Brunori M, Capelli D, et al. Salvage therapy with an outpatient DHAP schedule followed by PBSC transplantation in 79 lymphoma patients: an intention to mobilize and transplant analysis. Eur J Haematol. 2004;72:10–17. doi: 10.1046/j.0902-4441.2004.00171.x. [DOI] [PubMed] [Google Scholar]
- Kuittinen T, Nousiainen T, Halonen P, et al. Prediction of mobilisation failure in patients with non-Hodgkin's lymphoma. Bone Marrow Transplant. 2004;33:907–912. doi: 10.1038/sj.bmt.1704466. [DOI] [PubMed] [Google Scholar]
- Akhtar S, Weshi AE, Rahal M, et al. Factors affecting autologous peripheral blood stem cell collection in patients with relapsed or refractory diffuse large cell lymphoma and Hodgkin lymphoma: a single institution result of 168 patients. Leuk Lymphoma. 2008;49:769–778. doi: 10.1080/10428190701843213. [DOI] [PubMed] [Google Scholar]
- Fricker SP, Anastassov V, Cox J, et al. Characterization of the molecular pharmacology of AMD3100: a specific antagonist of the G-protein coupled chemokine receptor, CXCR4. Biochem Pharmacol. 2006;72:588–596. doi: 10.1016/j.bcp.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Rosenkilde MM, Gerlach LO, Jakobsen JS, et al. Molecular mechanism of AMD3100 antagonism in the CXCR4 receptor: transfer of binding site to the CXCR3 receptor. J Biol Chem. 2004;279:3033–3041. doi: 10.1074/jbc.M309546200. [DOI] [PubMed] [Google Scholar]
- Liles WC, Rodger E, Broxmeyer HE, et al. Augmented mobilization and collection of CD34+ hematopoietic cells from normal human volunteers stimulated with granulocyte-colony-stimulating factor by single-dose administration of AMD3100, a CXCR4 antagonist. Transfusion. 2005;45:295–300. doi: 10.1111/j.1537-2995.2005.04222.x. [DOI] [PubMed] [Google Scholar]
- DiPersio JF, Micallef IN, Stiff PJ, et al. Phase III prospective randomized double-blind placebo-controlled trial of plerixafor plus granulocyte colony-stimulating factor compared with placebo plus granulocyte colony-stimulating factor for autologous stem-cell mobilization and transplantation for patients with non-Hodgkin's lymphoma. J Clin Oncol. 2009;27:4767–4773. doi: 10.1200/JCO.2008.20.7209. [DOI] [PubMed] [Google Scholar]
- Maziarz RT, Nademanee AP, Micallef IN, et al. Plerixafor plus granulocyte colony-stimulating factor improves the mobilization of hematopoietic stem cells in patients with non-Hodgkin lymphoma and low circulating peripheral blood CD34+ cells. Biol Blood Marrow Transplant. 2013;19:670–675. doi: 10.1016/j.bbmt.2013.01.005. [DOI] [PubMed] [Google Scholar]
- DiPersio JF, Stadtmauer EA, Nademanee A, et al. Plerixafor and G-CSF versus placebo and G-CSF to mobilize hematopoietic stem cells for autologous stem cell transplantation in patients with multiple myeloma. Blood. 2009;113:5720–5726. doi: 10.1182/blood-2008-08-174946. [DOI] [PubMed] [Google Scholar]
- Calandra G, McCarty J, McGuirk J, et al. AMD3100 plus G-CSF can successfully mobilize CD34+ cells from non-Hodgkin's lymphoma, Hodgkin's disease and multiple myeloma patients previously failing mobilization with chemotherapy and/or cytokine treatment: compassionate use data. Bone Marrow Transplant. 2008;41:331–338. doi: 10.1038/sj.bmt.1705908. [DOI] [PubMed] [Google Scholar]
- Fruehauf S, Ehninger G, Hubel K, et al. Mobilization of peripheral blood stem cells for autologous transplant in non-Hodgkin's lymphoma and multiple myeloma patients by plerixafor and G-CSF and detection of tumor cell mobilization by PCR in multiple myeloma patients. Bone Marrow Transplant. 2010;45:269–275. doi: 10.1038/bmt.2009.142. [DOI] [PubMed] [Google Scholar]
- Duarte RF, Shaw BE, Marin P, et al. Plerixafor plus granulocyte CSF can mobilize hematopoietic stem cells from multiple myeloma and lymphoma patients failing previous mobilization attempts: EU compassionate use data. Bone Marrow Transplant. 2011;46:52–58. doi: 10.1038/bmt.2010.54. [DOI] [PubMed] [Google Scholar]
- Worel N, Rosskopf K, Neumeister P, et al. Plerixafor and granulocyte-colony-stimulating factor (G-CSF) in patients with lymphoma and multiple myeloma previously failing mobilization with G-CSF with or without chemotherapy for autologous hematopoietic stem cell mobilization: the Austrian experience on a named patient program. Transfusion. 2011;51:968–975. doi: 10.1111/j.1537-2995.2010.02896.x. [DOI] [PubMed] [Google Scholar]
- Hubel K, Fresen MM, Salwender H, et al. Plerixafor with and without chemotherapy in poor mobilizers: results from the German compassionate use program. Bone Marrow Transplant. 2011;46:1045–1052. doi: 10.1038/bmt.2010.249. [DOI] [PubMed] [Google Scholar]
- Apperley J, Carreras E, Gluckman E, et al., editors. ESH-EBMT handbook on haematopoietic stem cell transplantation. 2008 edition. [cited 2014 Jan 21]. Available from: http://www.esh.org/esh-ebmt-handbook-on-haematopoietic-stem-cell-transplantation-2008/
- Civin CI, Strauss LC, Brovall C, et al. Antigenic analysis of hematopoiesis. III. A hematopoietic progenitor cell surface antigen defined by a monoclonal antibody raised against KG-1a cells. J Immunol. 1984;133:157–165. [PubMed] [Google Scholar]
- Bhatia M, Wang JC, Kapp U, et al. Purification of primitive human hematopoietic cells capable of repopulating immune-deficient mice. Proc Natl Acad Sci U S A. 1997;94:5320–5325. doi: 10.1073/pnas.94.10.5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia M, Bonnet D, Murdoch B, et al. A newly discovered class of human hematopoietic cells with SCID-repopulating activity. Nat Med. 1998;4:1038–1045. doi: 10.1038/2023. [DOI] [PubMed] [Google Scholar]
- Miraglia S, Godfrey W, Yin AH, et al. A novel five-transmembrane hematopoietic stem cell antigen: isolation, characterization, and molecular cloning. Blood. 1997;90:5013–5021. [PubMed] [Google Scholar]
- Gallacher L, Murdoch B, Wu DM, et al. Isolation and characterization of human CD34(-)Lin(-) and CD34(+)Lin(-) hematopoietic stem cells using cell surface markers AC133 and CD7. Blood. 2000;95:2813–2820. [PubMed] [Google Scholar]
- Yin AH, Miraglia S, Zanjani ED, et al. AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood. 1997;90:5002–5012. [PubMed] [Google Scholar]
- de Wynter EA, Buck D, Hart C, et al. CD34+AC133+ cells isolated from cord blood are highly enriched in long-term culture-initiating cells, NOD/SCID-repopulating cells and dendritic cell progenitors. Stem Cells. 1998;16:387–396. doi: 10.1002/stem.160387. [DOI] [PubMed] [Google Scholar]
- Larochelle A, Vormoor J, Hanenberg H, et al. Identification of primitive human hematopoietic cells capable of repopulating NOD/SCID mouse bone marrow: implications for gene therapy. Nat Med. 1996;2:1329–1337. doi: 10.1038/nm1296-1329. [DOI] [PubMed] [Google Scholar]
- Terstappen LW, Huang S, Safford M, et al. Sequential generations of hematopoietic colonies derived from single nonlineage-committed CD34+ Blood. 1991;77:1218–1227. [PubMed] [Google Scholar]
- Conneally E, Cashman J, Petzer A, et al. Expansion in vitro of transplantable human cord blood stem cells demonstrated using a quantitative assay of their lympho-myeloid repopulating activity in nonobese diabetic-scid/scid mice. Proc Natl Acad Sci U S A. 1997;94:9836–9841. doi: 10.1073/pnas.94.18.9836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohle R, Murea S, Kirsch M, et al. Differential expression of L-selectin, VLA-4, and LFA-1 on CD34+ progenitor cells from bone marrow and peripheral blood during G-CSF-enhanced recovery. Exp Hematol. 1995;23:1535–1542. [PubMed] [Google Scholar]
- Mohle R, Haas R, Hunstein W. Expression of adhesion molecules and c-kit on CD34+ hematopoietic progenitor cells: comparison of cytokine-mobilized blood stem cells with normal bone marrow and peripheral blood. J Hematother. 1993;2:483–489. doi: 10.1089/scd.1.1993.2.483. [DOI] [PubMed] [Google Scholar]
- Dar A, Schajnovitz A, Lapid K, et al. Rapid mobilization of hematopoietic progenitors by AMD3100 and catecholamines is mediated by CXCR4-dependent SDF-1 release from bone marrow stromal cells. Leukemia. 2011;25:1286–1296. doi: 10.1038/leu.2011.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larochelle A, Krouse A, Metzger M, et al. AMD3100 mobilizes hematopoietic stem cells with long-term repopulating capacity in nonhuman primates. Blood. 2006;107:3772–3778. doi: 10.1182/blood-2005-09-3592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fruehauf S, Veldwijk MR, Seeger T, et al. A combination of granulocyte-colony-stimulating factor (G-CSF) and plerixafor mobilizes more primitive peripheral blood progenitor cells than G-CSF alone: results of a European phase II study. Cytotherapy. 2009;11:992–1001. doi: 10.3109/14653240903121245. [DOI] [PubMed] [Google Scholar]
- Varmavuo V, Mantymaa P, Silvennoinen R, et al. CD34+ cell subclasses and lymphocyte subsets in blood grafts collected after various mobilization methods in myeloma patients. Transfusion. 2013;53:1024–1032. doi: 10.1111/j.1537-2995.2012.03848.x. [DOI] [PubMed] [Google Scholar]
- Alexander ET, Towery JA, Miller AN, et al. Beyond CD34+ cell dose: impact of method of peripheral blood hematopoietic stem cell mobilization (granulocyte-colony-stimulating factor [G-CSF], G-CSF plus plerixafor, or cyclophosphamide G-CSF/granulocyte-macrophage [GM]-CSF) on number of colony-forming unit-GM, engraftment, and Day +100 hematopoietic graft function. Transfusion. 2011;51:1995–2000. doi: 10.1111/j.1537-2995.2011.03085.x. [DOI] [PubMed] [Google Scholar]
- Weaver CH, Hazelton B, Birch R, et al. An analysis of engraftment kinetics as a function of the CD34 content of peripheral blood progenitor cell collections in 692 patients after the administration of myeloablative chemotherapy. Blood. 1995;86:3961–3969. [PubMed] [Google Scholar]
- Hartz B, Volkmann T, Irle S, et al. alpha4 integrin levels on mobilized peripheral blood stem cells predict rapidity of engraftment in patients receiving autologous stem cell transplantation. Blood. 2011;118:2362–2365. doi: 10.1182/blood-2011-02-331918. [DOI] [PubMed] [Google Scholar]
- Kollet O, Petit I, Kahn J, et al. Human CD34(+)CXCR4(-) sorted cells harbor intracellular CXCR4, which can be functionally expressed and provide NOD/SCID repopulation. Blood. 2002;100:2778–2786. doi: 10.1182/blood-2002-02-0564. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Detailed patient characteristics