Abstract
Surgical management of renal cell carcinoma is the most effective treatment for patients with localized disease. In patients with advanced renal cell carcinoma, immune modulation-based therapies are typically used to improve cancer specific survival via anti-angiogenic drugs. Similar to most cancers, tumor grade and stage are linked to the tumor’s biologic potential. Integrating these factors with patients’ performance status can help predict their long-term disease-free survival, the likelihood of tumor recurrence, and the median time to failure following surgery and immunotherapy. A novel integrated staging system and a postoperative renal cell carcinoma specific nomogram, along with standardized quality of life assessments have been shown to be useful clinical tools to aid in patient counseling, determining optimal follow-up imaging protocols, and identifying patients who might benefit from early enrollment in adjuvant therapy protocols. This article offers clinicians a review and summary of the most recent evidence-based research related to risk assessment among patients with newly diagnosed renal cell carcinoma.
Keywords: Renal Cancer, Risk Assessment, Outcomes, Nephrectomy, Quality of Life
1. INTRODUCTION
In the United States renal cell carcinoma (RCC) is expected to be responsible for 51,190 new cancer cases in 2007 causing approximately 12,890 deaths [1]. Renal cell carcinomas have proven to be nearly uniformly resistant to available chemotherapeutic agents [2]. Surgical management of renal cell carcinoma is the most effective treatment for patients with localized disease, and immune modulation based therapies are the standard of care for treating patients with advanced renal cell cancer (nodal or distant metastasis). Prior to 2006, the only available immune-mediated therapies for kidney cancer were Interferon (IFN-α) and Interleukin (IL)-2. Two recently developed oral tyrosine kinase inhibitors – Sunitinib (Sutent, Pfizer™) and Sorafenib (Nexavar, Bayer/Onyx™) are approved by the US Food and Drug Administration. Both of these agents are taken orally, better tolerated than IFN or IL-2, and have shown a benefit in terms of progression-free survival and response rates. Other agents which are actively being studied include vascular endothelial growth factor (VEGF) binding agents such as Bevacizumab (Avastin, Genentech™), other kinase inhibitors and mammalian on targets of rapamycin (mTOR) inhibitors, such as CCI-779 (Temsirolimus, Wyeth™) [3].
The discovery of a relationship for the Von Hippel-Lindau (VHL) tumor suppressor gene, hypoxia-inducible factor (HIF-1α), and VEGF in the growth of clear-cell renal cell carcinoma has identified a pathway for targeted therapy. In a study by Motzer, et al. the impact of these targeted agents on metastatic cancer was measured. “It was shown that the small molecule targeted inhibitors Sunitinib, temsirolimus, and Sorafinib, as well as the monoclonal antibody, Bevacizumab, demonstrated antitumor activity in randomized trials.” It was also suggested that one way to enhance the activity of the targeted approach to RCC therapy may be to combine agents that target different points in the VHL–hypoxia-inducible gene pathway; these trials are currently underway [4].
Accurate prediction of long-term disease-free survival immediately after surgical resection of clinically localized disease would be valuable for patient counseling, scheduling follow-up imaging and identifying poor risk group patients who might benefit from enrollment in adjuvant therapy protocols. During this discussion, pathologic stage refers specifically to the tumor stage only as outlined in the 2002 AJCC TNM pathologic staging system (T1–T4; Table I), updated from the 1997 AJCC TNM staging system to contain a more predictive ability for patient diagnosis [5]. The overall TNM stage [1997 AJCC TNM staging system (stages I – IV; Table II)] is a more global system taking into account the local regional disease status (tumor size, lymph node status), in addition to whether or not any distant disease is present (M-metastatic disease).
Table I.
TNM Classification of Renal Cell Carcinoma according to AJCC 2002 Pathologic Stagin
| TX | Primary tumor cannot be assessed |
| T0 | No evidence of primary tumor |
| T1 | Tumor 7.0cm or less in greatest dimension, limited to the kidney |
| T1a | Tumor 4.0 cm or less in greater dimension, limited to the kidney |
| T1b | Tumor more than 4.0 cm but not more than 7.0 cm in greatest dimension, limited to the kidney |
| T2 | Tumor greater than 7.0cm, limited to the kidney |
| T3 | Tumor extends into major veins, or invades adrenal or perinephric tissues But not beyond Gerota’s fascia |
| T3a | Tumor invades adrenal gland or perinephric tissues but not beyond Gerota’s fascia |
| T3b | Tumor grossly extends into renal vein(s) or vena cava below the Diaphragm |
| T4 | Tumor invades beyond Gerota’s fascia |
| N0 | No regional lymph node metastasis |
| N1 | Metastasis to a single regional lymph node |
| N2 | Metastasis in more than 1 regional lymph node |
| M0 | No distant metastasis |
Table II.
AJCC 1997 Overall TNM Staging system – Tumor Stage
| Stage I | T1N0M0 |
| Stage II | T2N0M0 |
| Stage III | T3N0M0, T1-3N1M0 |
| Stage IV | T4N0-2M0, any TN0-1M1 |
2. DETERMINANTS OF THE NATURAL HISTORY OF RENAL CELL CANCERS
The clinical behavior of a tumor is linked to its underlying genetic abnormalities. Changes at the molecular and cellular level lead to microscopic and eventually macroscopic pathology. A comprehensive understanding of the factors responsible for the biologic behavior of renal cell cancers facilitates our comprehension of the natural history of this disease in patients.
Tumor stage, tumor grade, and patient performance status remain the most useful and clinically available predictors of renal cell carcinoma patient outcome [5, 6]. Of these three principle clinical determinants, tumor stage is felt to be the most important prognostic factor for renal cell carcinoma [5, 7–11].
In 1997, the TNM system underwent revisions. T1 stage was expanded to include tumors up to 7cm (previously < 2.5cm). The staging system for cases with inferior vena cava tumor thrombus was also changed. Tumor thrombus above or below the diaphragm was changed from T4 to T3c and T3c to T3b with renal vein involvement respectively [12]. Further revisions were made in 2002 to the TNM staging system. ‘The T1 stage was expanded into T1, T1a, and T1b. T1 was limited to tumors up to 7cm or less, limited to the kidney. T1a stage was limited to a tumor no more than 4cm or less, limited to the kidney. Stage T1b was to include tumors more than 4cm but not greater than 7cm, limited to the kidney. Mean 5-year cancer-specific survival from contemporary cohort studies for the revised 1997 TNM staging system was 95.4%, 87.6%, 63% and 23% for stages I to IV lesions [6, 12–17]. The estimated 5-year cancer-specific survival rates by the 2002 tumor classification were 97%, 87%, 71%, 53%, 44%, 37%, and 20% in patients with pT1a, pT1b, pT2, pT3a, pT3b, pT3c, and pT4’ [5].
The second most important predictor of renal cell carcinoma behavior is tumor grade. The Fuhrman [18] pathologic grading system which is based on nuclear size, shape and content remains the most commonly used system in North America. This system is a 4-tier grading scheme with grade 3 and 4 tumors being more poorly differentiated than grade 1 and 2 tumors. On average, 10% of tumors are grade 1, 35% grade 2, 35% grade 3 and 20% grade 4 tumors [19]. In a recent study, it was determined that the Fuhrman grading system was not appropriate for diagnosis of chromophobe RCC. The weakness in the Fuhrman system is that it was developed before current RCC classification by histologic, genetic, and clinical factors. Additionally, chromophobe RCC accounts for 2.1–11% of RCC in large series [20]. At a recent UICC (Union Internationale Contre le Cancer)/ AJCC (American Joint Committee on Cancer) workgroup meeting [21] a 3-tier grading system has been proposed which would combine grades 1 and 2 in the Fuhrman grading system. This system has not yet been implemented. Recently, Dall’Oglio et al, determined that microvascular tumor invasion (MVI) is an independent prognostic predictor of RCC. Patients with tumors lacking MVI, 91% were found to have a median survival of 5 years, compared to those with tumors containing MVI (39%), with a relative risk of death of 5.16 [22].
The Karnovsky or Eastern Cooperative Oncology Group (ECOG) patient performance status scales have provided a useful single scale to evaluate the impact of multiple patient signs and symptoms (Table III). The scores range from 0 to 4; a score of 0 indicates normal activity and a score of 4 identifies someone who is completely bedridden. While initially used to help determine which patients should be eligible for immunotherapy regimens, recent studies [6] indicate that the ECOG scale is a good prognostic factor that can be extended to all stages of renal cell carcinoma. In 661 patients who underwent nephrectomy for metastatic and non-metastatic disease at the University of California, Los Angeles, the ECOG performance status at presentation was prognostic of the overall 5-year survival rate (51% - ECOG ≥ 1 vs. 81% - ECOG of 0).
Table III.
ECOG Performance Status Scale
| 0 | Normal activity |
| 1 | Symptomatic but ambulatory |
| 2 | Bedridden less than 50% of the time |
| 3 | Bedridden more than 50% of the time |
| Completely bedridden | |
Several clinical characteristics, such as time from diagnosis to metastasis, tumor location, weight loss and total number of metastatic sites have been used to predict clinical outcomes in advanced renal cell cancer. Certain tumor histologic types such as sarcomatoid renal cell carcinoma, renal medullary carcinoma, and collecting duct carcinoma are aggressive cancers that progress rapidly and have a different natural history than clear cell renal cell carcinomas [23–26].
3. BIOMEDICAL RISK ASSESSMENT FOR NEWLY DIAGNOSED RCC PATIENTS
Accurately predicting the natural history of renal cell carcinoma and the long-term disease-free survival in patients diagnosed with renal cell cancer can help physicians, patients, and families cope better with the new diagnosis of RCC. Investigators have evaluated four paradigms: 1) improve or modify the current renal cell cancer staging system; 2) stratify renal cell cancer patients into risk groups based on established clinical predictors of survival, similar to prostate cancer patients – low-risk, intermediate-risk and high-risk group patients; 3) develop a post-operative nomogram based on known determinants of renal cell cancer disease progression; and 4) identify specific genes at the molecular level in an attempt to identify a genetic expression profile of the different types of renal cell carcinoma.
3.1. Improvement of the Renal Cell Carcinoma Staging System
Typically patients are given cancer-specific survival rates based primarily on the overall TNM stage (I–IV) of the disease noted at the time of surgical removal of the cancerous kidney. The Mayo Clinic group developed the stage, size, grade and necrosis (SSIGN) scoring algorithm for patients with clear cell RCC undergoing radical nephrectomy [27]. Additionally, the SSIGN score (Table IV) is now the most efficacious instrument for RCC follow up planning and design of adjuvant therapy clinical studies, allowing the clinician a more dynamic outcome prediction model [28]. Independent predictors of survival were based on an analysis of 1,801 patients’ TNM stage, a tumor size of ≥ 5 cm, nuclear grade, and histological tumor necrosis [29]. This showed decreased survival correlating with an increased SSIGN score for scores of 0–1 and ≥ 10 and with 5-year cancer survival rates of 99.4% and 7.4%, respectively. This SSIGN system has been externally validated, finding that patients with SSIGN scores of 0–2 and 3–4 had 5-year cancer-specific survival rates of 100% and 91%, respectively. In contrast, patients with scores of 5–6 and 7–9 had 5-year cancer-specific survival rates of 64% and 47%, respectively. All patients with a score of 10 or more died of disease within 2 years of surgery. Additionally, all pathological features in the SSIGN score algorithm except tumor size were associated with patient outcome in a multivariate setting. The measure of predictive ability of the SSIGN score was 0.88, which was considerably higher than that achieved using any single feature [30].
Table IV.
SSIGN score algorithm
| Feature: | Score: |
|---|---|
|
| |
| Primary Tumor classification: | |
| pT1 | 0 |
| pT2 | 1 |
| pT3a | 2 |
| pT3b | 2 |
| pT3c | 2 |
| pT4 | 0 |
|
| |
| Regional lymph node involvement: | |
| pNx | 0 |
| pN0 | 0 |
| pN1 | 2 |
| pN2 | 2 |
|
| |
| Distant metastases: | |
| pM0 | 0 |
| pM1 | 4 |
|
| |
| Primary tumor size (cm): | |
| Less than 5 | 0 |
| 5 or Greater | 2 |
|
| |
| Nuclear grade: | |
| 1 | 0 |
| 2 | 0 |
| 3 | 1 |
| 4 | 3 |
|
| |
| Coagulative tumor necrosis: | |
| Absent | 0 |
| Present | 2 |
The University of California Los Angeles integrated staging system (UISS) combines the TNM stage with additional prognostic variables to better stratify patients into prognostic categories. Tsui et al. [16] retrospectively reviewed the records of 643 patients who underwent partial or radical nephrectomy for renal cell carcinoma between 1987 and 1998 (mean follow-up 47 ± 40 months). The 1997 AJCC TNM stage, ECOG performance status, tumor grade, and 1997 pathological tumor stage (T1 – T4) were all statistically significant at predicting survival (p <0.001). In a multivariate model, the most significant predictors of patient survival were 1997 AJCC TNM stage (p<0.001) and tumor grade (p<0.001). ECOG performance status was also a significant predictor (p=0.031) and the 1997 pathological tumor stage was not a significant independent prognostic indicator (p=0.138). For patients with T1 lesions, the 5-year cancer-specific survival rate was 91% for grade 1, 83% for grade 2, 60% for grade 3 and 0% for grade 4 disease. In patients with T4 lesions, the 5-year cancer-specific survival rate was 46% for grade 2, 38% for grade 3 and 0% for grade 4 disease.
Belldegrun and colleagues [6] proposed a single integrated cancer staging system (UISS) for renal cell carcinoma that would accurately predict cancer-specific survival and be simple to use for both nonmetastatic and metastatic patients. The integrated staging system (UISS) takes into account the most significant prognostic variables for patients without metastatic disease (M−) and those with metastatic disease (M+). For patients with non-metastatic disease, the variables that were found to be significant on multivariate analysis were Fuhrman grade, histologic type (sarcomatoid vs. others), number of symptoms, and whether immunotherapy had been given.
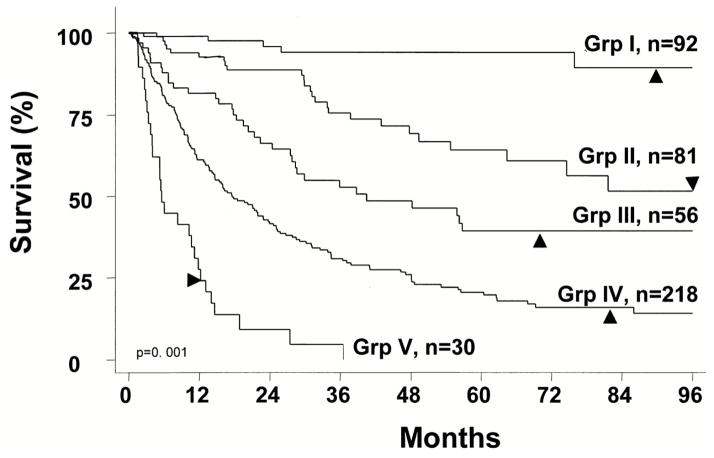
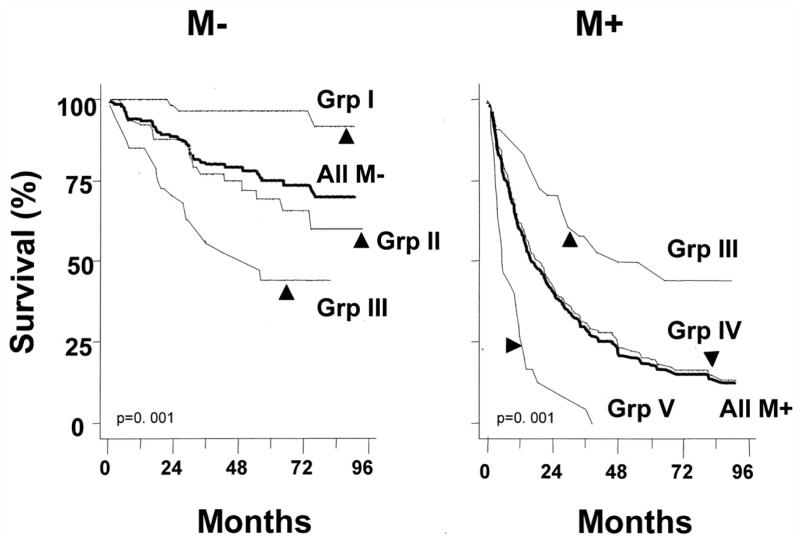
To determine the prognostic UISS group (I–V) for a given patient, three numbers should be plugged into the UISS categorization: 1) AJCC 1997 TNM stage (I to IV); 2) Grade 1 to 4; and 3) whether the patient has ECOG performance status that equals 0 (fully active) or higher (restricted physical activity or worse) (Table IV). The UISS can discriminate between favorable disease (UISS I, II), unfavorable disease (UISS IV, V), and intermediate disease (UISS III) (Figures 1,2). The UISS can also detect survival differences between different histologic types of renal cell carcinoma. Papillary tumors had the best prognosis while sarcomatoid and collecting duct tumors had the worst prognosis. Clear-cell and chromophobe had similar prognosis and were considered together. Papillary tumors were categorized as favorable (UISS I, II) most of the time (29 of 42; 69%). Sarcomatoid tumors were categorized as unfavorable (UISS IV, V) the majority of the time (28 of 30; 93%), and no patient with a sarcomatoid tumor was categorized as favorable (UISS I, II).
Fig 1.
Kaplan-Meier survival analysis of the study population according to the UISS cat tegories
Fig 2.
Kaplan-Meier survival analysis of the study population according to the formulated UISS categories separately for metastatic (M+) and nonmetastatic (M−) pa atients
The UISS staging system has been validated as a renal cell cancer survival predictor in patients treated for non-metastatic and metastatic renal cell cancer at the M.D. Anderson Cancer Center [31]. The UISS system has also been externally validated through a large multicenter study. The UISS stratified both localized and metastatic RCC into three different risk groups (low, intermediate, and high) determining the localized RCC 5-year survival rates to be 92%, 67%, and 44%. For metastatic RCC, the 3-year survival rates were 37%, 23%, and 12%. A trend toward a higher risk of death was observed in all centers for increasing UISS risk category, for both 3 and 5-year survival rates. A greater variability in survival rates among centers was observed for high-risk patients [32].
3.2. Risk Group Stratification for Patients with Surgically Resected Renal Cell Carcinoma
Frank et al, has determined that while the while the original SSIGN score is an efficacious post-operative tool for counseling patients, determining the need for adjuvant therapy and stratifying patients for clinical trials, it is static and it only estimated outcome via date of surgery only. The D-SSIGN score was developed to account for the time that patients remain free of disease during surveillance and allows clinicians to continually adjust this surveillance based on more accurate risk assessment of patient prognosis. The D-SSIGN score uses the cause specific survival (CSS rates) and SSIGN score to create a more dynamic algorithm. It was concluded that within each SSIGN score CSS rates increase as the disease-free interval following surgery increases [28].
Similar to prostate cancer risk group stratification, the UISS integrative staging system was used to stratify patients with renal cell carcinoma into low-, intermediate- and high-risk groups [11]. Likewise, from this risk group stratification a comprehensive clinical algorithm was proposed to predict relevant end points for patients with and without metastasis at the time of diagnosis. A retrospective review was done of patients who underwent nephrectomy for unilateral disease at the UCLA Medical Center between 1989 and 2000 [11]. The final cohort for this study consisted of 814 patients. Patients were divided into those with no metastasis at diagnosis (NM) and those with lymph node involvement and/or metastasis at diagnosis (M). For patients with no metastasis (NM) the following UISS groups were designated as low-risk group (UISS I), intermediate-risk group (UISS II), and high-risk group (UISS III–V). For patients with metastasis (M), by definition there had to be lymph node involvement (N+) or systemic disease (M+). Low-risk group patients had a UISS II–III category, intermediate-risk group had UISS IV category and high-risk group patients had a UISS V category.
Clinical endpoints were overall-survival, disease-specific survival, freedom from recurrence (local, systemic or both), time from nephrectomy to immunotherapy (IMT), time from recurrence to IMT, percentage of patients undergoing IMT, percentage of patients with recurrence that undergo IMT, survival after IMT, freedom from progression after IMT, survival after IMT for early recurrence (≤ 6 months after nephrectomy) and survival after IMT for late recurrence (> 6 months after nephrectomy).
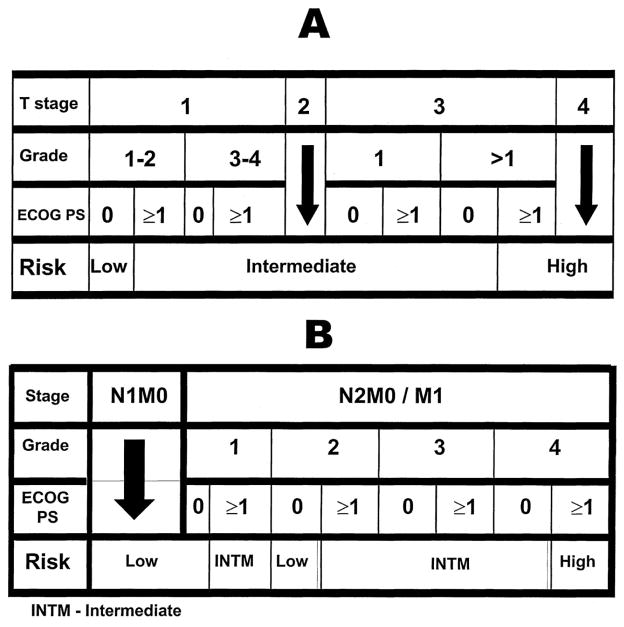
A decision box was developed (nonmetastatic and metastatic) to guide the user to the patient’s UISS risk group (Figure 3). To predict the outcome of a patient with renal cell carcinoma, three steps must be completed. First, select the decision box that corresponds to the patient’s status at diagnosis (no metastasis – N0M0 or metastasis – N1/N2/M1). Second, begin at the top of the decision box and progress downward by identifying the patient’s pathological tumor stage, Fuhrman tumor grade, and ECOG performance status. This guides the user to the patient’s risk group (low, intermediate, or high). Third, now that the risk group has been determined, the patient’s prognosis can be predicted in terms of the aforementioned clinical endpoints. Tables V–VIII show the predicted clinical outcomes for (M) and (NM) patients: overall and disease-specific survival (Table V); local and systemic failure (Table VI); and the effect of immunotherapy (Table VII, VIII).
Fig 3.
Decision box A assigns N0M0 nephrectomized patients into risk groups
Table V.
UISS Categorization Table with 2- and 5-year projected survivorship
| UISS | 1997 TNM | Grade | ECOG | No pts. | 2Yr. | 5Yr. |
|---|---|---|---|---|---|---|
|
| ||||||
| Stage | ||||||
| I | I | 1,2 | 0 | 92 | 96% | 94% |
| II | I | 1,2 | 1 or more | 28 | 89% | 67% |
| I | 3,4 | Any | 16 | |||
| II | Any | Any | 19 | |||
| III | Any | 0 | 14 | |||
| III | 1 | 1 or more | 4 | |||
| III | III | 2–4 | 1 or more | 28 | 66% | 39% |
| IV | 1,2 | 0 | 20 | |||
| IV | IV | 3,4 | 0 | 20 | 42% | 23% |
| IV | 1–3 | 1 or more | 198 | |||
| V | IV | 4 | 1 or more | 30 | 9% | 0% |
TNM Stages: Stage I=T1N0M0, Stage II=T2N0M0, Stage III=T3N0-1M0, T1-3N1M0 and Stage IV= T4N0-2M0, any TN0-1M1
Table VIII.
Impact of Immunotherapy (IMT) on Early and Late Failure in NM Patients by UISS Risk Groups
| NM at Diagnosis | |||
|---|---|---|---|
|
| |||
| LR | IR | HR | |
| IMT delivered for any recurrence, (%) | 43 | 77 | 76 |
| Early failure (≤ 6 months after Nx) (%) | 0.8 | 3.2 | 8 |
| Late failure (> 6 months after Nx) (%) | 1.6 | 19.5 | 30 |
| Early failure | |||
| Median time from failure to IMT (months) | 3.3 | 1.1 | |
| Any early failure treated with IMT (%) | 100 | 75 | 71 |
| Survival from early failure to death with IMT (%) | |||
| 1 year | 100 | 37 | |
| 2 year | 67 | 28 | |
| 5 year | 0 | 0 | |
| Late failure | |||
| Median time from failure to IMT (months) | 3.3 | 5.1 | 2.7 |
| Any early failure treated with IMT (%) | 33 | 77 | 78 |
| Survival from late failure to death with IMT (%) | |||
| 1 year | 79 | 86 | |
| 2 year | 70 | 58 | |
| 5 year | 36 | 15 | |
| Progression after immunotherapy (%) | 66 | 81.4 | 86 |
| Progression-free after IMT in early failures (%) | |||
| 1 year | 33.3 | 8.3 | |
| 2 year | 0 | 0 | |
| 5 year | 0 | 0 | |
| Progression-free after IMT in late failures (%) | |||
| 1 year | 37.8 | 35.9 | |
| 2 year | 18.9 | 15.1 | |
| 5 year | 0 | 0 | |
NM – non-metastasis; LR – low risk, IR – intermediate risk group and HR – high risk group; Nx- Nephrectomy
Table VI.
Overall and Disease-Specific Survival for LR, IR and HR Patients with and without Metastasis
| NM at Diagnosis | M at Diagnosis | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| LR | IR | HR | LR | IR | HR | |
| Overall survival % | ||||||
| 1 year | 97.5 | 95.4 | 84.4 | 85.3 | 62.8 | 20.2 |
| 2 year | 96.5 | 87.2 | 70.9 | 63.0 | 40.5 | 10.1 |
| 5 year | 83.8 | 71.9 | 44.0 | 30.3 | 19.3 | 0.0 |
| Disease-specific survival % | ||||||
| 1 year | 100 | 97.2 | 89.0 | 86.7 | 63.1 | 21.0 |
| 2 year | 98.8 | 90.6 | 77.7 | 65.0 | 40.9 | 10.5 |
| 5 year | 91.1 | 80.4 | 54.7 | 32.0 | 19.5 | 0.0 |
NM – non-metastasis; M – Metastasis; LR – low risk, IR – intermediate risk group and HR – high risk group
Table VII.
Local and Systemic Failure in NM Patients by UISS Risk Groups
| NM at Diagnosis | |||
|---|---|---|---|
|
| |||
| LR | IR | HR | |
| Failed locally, (%) | 0 | 3.7 | 8.6 |
| Freedom from local recurrence after nephrectomy % | |||
| 1 year | 100 | 98.8 | 93.5 |
| 2 year | 100 | 98.8 | 88.8 |
| 5 year | 100 | 94.7 | 85.4 |
| Failed systemically, (%) | 5.5 | 29 | 46.7 |
| Freedom from systemic failure after nephrectomy % | |||
| 1 year | 97.4 | 88.5 | 76.0 |
| 2 year | 96.3 | 80.1 | 59.5 |
| 5 year | 91.4 | 70.6 | 40.1 |
| Any failure (Local and/or Systemic) % | 5.5 | 29.5 | 50 |
| Freedom from any failure after nephrectomy % | |||
| 1 year | 97.4 | 88.5 | 74.3 |
| 2 year | 96.3 | 80.1 | 57.5 |
| 5 year | 91.4 | 64.0 | |
| 37.3 | |||
NM – non-metastasis; LR – low risk, IR – intermediate risk group and HR – high risk group
3.3. Postoperative Prognostic Nomogram for Renal Cell Carcinoma
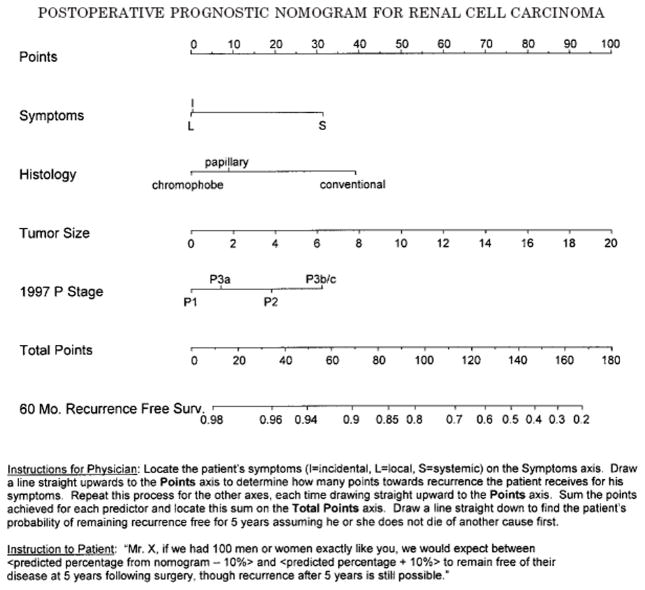
Kattan et al. [33] developed a postoperative nomogram for patients with renal cell carcinoma who underwent radical or partial nephrectomy. The nomogram was based upon a cohort of 601 patients with unilateral renal carcinoma who underwent surgery at Memorial Sloan-Kettering Cancer Center from July 1989 to December 1998. Patients with bilateral disease, lymph node positive disease before or at surgery, oncocytoma histological subtype and those with known metastatic disease who underwent a cytoreductive nephrectomy were excluded form the study. The Kattan model was compared to UISS model, Yaycioglu model, and Cindolo model, which concluded that postoperative models discriminate substantially better than preoperative ones. The Kattan model was consistently found to be the most accurate, with the UISS model only slightly less accurate. It was also concluded that the Kattan model can be useful in the UISS intermediate-risk patients for a more complete prognosis [34]. A Cox proportional hazards model without restricted cubic splines was used as the basis for designing the nomogram (Figure 4). The nomogram uses a continuous point system to predict the probability that the patient will not encounter renal cell carcinoma (recurrent or new bilateral asynchronous disease) within the first five years following surgery.
Figure 4.
Nomogram for recurrence of renal cell carcinoma based on 601 patients treated with nephrectomy at our institution
Potential advantages of the nomogram include the ability to: 1) identify patients who are at low vs. high risk for recurrence; 2) help tailor follow-up visits based on patient risk category; and 3) identify patients at high risk of recurrence who would benefit from adjuvant therapy protocols. Lastly, the design of this nomogram simultaneously considers all prognostic variables, as opposed to other approaches that compare single prognostic factors one at a time.
Limitations of this nomogram are the following: 1) it is not perfectly accurate; in fact, the nomogram predictions are within 10% of actual probabilities; 2) the nomogram only predicts disease recurrence within a maximum of five years following surgery; and 3) the nomogram relies upon postoperative variables, therefore making it an inadequate preoperative counseling tool. Lastly, the nomogram does not differentiate between local vs. distant disease recurrence and Fuhrman grade was excluded from the nomogram.
Kattan and colleagues [35] published an updated nomogram in 2005 (N= 833 patients who underwent a radical or partial nephrectomy from January 1989 to August 2002), focusing on predicting recurrence for patients with conventional clear cell renal cell carcinoma. The nomogram model was based on 701 patients where disease recurrence was noted in 72 of 701 patients (10.3%) and the 5-year probability of freedom from recurrence for the entire patient cohort was 80.9%.
In a 2007 retrospective analysis by Lane et. al, a preoperative prognostic nomogram for solid enhancing renal tumors treated with partial nephrectomy was found to be accurate, discriminating (bootstrap corrected concordance index .0644), and calibrated. Age, gender, tumor size, and smoking history were clinically associated with predicting benign pathology [36].
3.4. Gene Expression Profiling of Clear Cell Renal Cell Carcinomas: Gene Identification and Prognostic Classification
The ability to identify which genes are associated with more or less aggressive cancers can lead to a more accurate prognosis of an individual renal tumor and its likely response to treatment. Takahashi et al. [37] collected normal and cancerous tissue from 29 patients with clear cell renal cell carcinoma (ccRCC) who underwent radical nephrectomy at Tokushima University Hospital in Japan. Median patient follow- up was 83.7 months (range: 3.2 months – 137.2 months). Gene expression profiles of 29 ccRCC were compared to patient-matched normal tissue and samples were hybridized to 21,632 cDNA microarrays. Genes that were at least 3-fold up- (n=32 genes) or down-regulated (n=77 genes) in at least 75% of the tumors are listed (Table IX). Lysyl oxidase (an extracellular enzyme involved in the connective tissue maturation pathway) was upregulated in ccRCC. Recently Kininogen was shown to be an inhibitor of angiogenesis.
Table IX.
Impact of Immunotherapy (IMT) in M Patients by UISS Risk Groups
| M at Diagnosis | |||
|---|---|---|---|
|
| |||
| LR | IR | HR | |
| IMT delivered (%) | 69 | 65 | 58 |
| Median time from Nx to IMT (months) | 1.4 | 1.5 | 1.5 |
| Survival from IMT to death % | |||
| 1 year | 85 | 62 | 25 |
| 2 year | 55 | 42 | 17 |
| 5 year | 26 | 23 | 0 |
| Progression-after immunotherapy % | 64.7 | 79 | 93.3 |
| Progression-free after IMT % | |||
| 1 year | 45 | 30 | 0 |
| 2 year | 33 | 21 | 0 |
| 5 year | 25 | 12 | 0 |
M – metastasis; LR – low risk, IR – intermediate risk group and HR – high risk group; Nx- Nephrectomy; IMT- Immunotherapy
In five patients, prognostic classification by expression profiling had a better prediction than staging. For example, one patient had a stage III, grade 3 tumor invading into the renal vein at operation (high risk by staging) but had low-risk gene expression profile and the patient has no evidence of disease 7.5 years following the operation. Likewise, a patient with a stage II, grade 2 tumor (low risk by staging) had a high-risk gene expression profile and died of ccRCC 4.6 years after the operation. Molecular profiling of an individual cancer has the potential to improve our ability to predict clinical outcomes and to identify patients who are at a higher risk of disease recurrence. Expression of gene markers allows clinicians to choose the most appropriate therapies for RCC patients that will maximize patient response and minimize noxious treatment exposure. The proposed functionality of molecular markers as prognostic indicators ranges from marking tumor aggressiveness and metastasis to an inherent ability to be recognized by the host immune system and to predict treatment responses of RCC patients [38].
Current efforts to incorporate molecular information have led to a great deal of enthusiasm to carbonic anhydrase (CA) IX. CA IX is a member of the carbonic anhydrase family thought to have a role in the regulation of intracellular and extracellular pH during periods of hypoxia in tumor cells [29]. Bui and colleagues noted that 94% of conventional clear cell renal cell carcinoma stained positive for CA IX [39]. The key cutoff point is a CA IX of < or > 85%. Low CA IX staining has been associated with poorer survival in patients with metastatic RCC and lower response rates to IL-2 immunotherapy [40]. CA IX and Ki67 (a monoclonal antibody) have been shown to correlate with RCC prognosis. The univariate statistical analysis showed that high Ki67 staining and low CA IX staining correlated significantly with poor median survival and the multivariate analysis with the combined parameter consisting of Ki67 and CA IX was a significant predictor of survival, even displacing the histological grade [41]. Recently, epidermal growth factor receptor (EGFR) was thought to be one of the positive regulators involved in the invasive progression of RCC. With the recent discovery of inhibitor of differentiation or DNA binding (Id-1), Li et al. [42] correlated the over-expression of Id-1 with EGFR expression as a novel marker for advanced RCC. It was suggested that Id-1 may play an important role in the development of RCC and indicate that Id-1 is a potential marker of patients with a poor prognosis [42].
4. QUALITY OF LIFE ASSESSMENT AS A PROGNOSTIC AND FOLLOW-UP TOOL
4.1. QOL-Associated Negative Outcomes
QOL scores offer important insights that can guide clinicians as they search for optimal ways of helping their patients cope with their cancer diagnosis and its consequences. Moreover, there is a growing body of evidence showing that quality of life is also correlated with quantity of life for a number of cancers [43]. However, unlike other prognostic indicators that are irreversible, QOL assessments can help clinicians identify ways to improve patients’ quality of life, their psychosocial well being, and potentially the duration of their survival.
Several studies have shown the connection between increased quality of life (in particular psychosocial aspects) and positive cancer-related outcomes. Von Essen et al.’s 2002 study showed that heightened quality of life and psychosocial function were associated with less anxiety and subsequently greater satisfaction with psychosocial care in patients diagnosed with endocrine gastrointestinal tumors [44]. In another study, while depression induced by the diagnosis of the cancer has not been found to correlate with decreased survival rates, depression prior to cancer diagnosis has been found to correlate with decreased survival rates [45]. Since depression often goes undiagnosed, screening all patients with a cancer diagnosis for depression makes it possible to explore psychosocial treatment options designed to improve the patient’s overall QOL.
Brief, standardized surveys are available for making quality of life assessments to help clinicians quickly anticipate when an intervention or referral for psychosocial issues is appropriate and to help providers individualize patient education and coaching to improve pain control [46]. These instruments are sufficiently simple that they can easily be incorporated into a busy practice. To limit patient and staff burden, clinicians can select the shortest version of the appropriate instruments and still derive a meaningful clinical assessment.
4.2. Background on QOL Tools
Among the most commonly used instruments for assessing cancer patients’ quality of life are: Functional Assessment of Cancer Therapy-General (FACT-G), FACT-Kidney Symptom Index (FKSI-15/FKSI-10), Impact of Events Scale (IES/IES-Revised), Medical Outcomes Study Short-Form Health Surveys (SF-36/SF-12), Quality of Well Being Instrument (QWB), European Organization for Research and Treatment of Cancer Core Quality of Life Questionaire (EORTC QLQ-C30), Symptom Checklist-90-R (SCL-90-R), Brief Symptom Inventory (BSI-53/BSI-18), and Distress Thermometer (DT) (Table X) [47–49].
Table X.
Commonly up-regulated genes in clear cell renal cell carcinoma
| Gene name | Average fold | % of RCC |
|---|---|---|
| Ceruloplasmin | 16.9 | 96.2 |
| Nicotinamide N-methyltransferase | 13.5 | 96.6 |
| Fatty acid-binding protein 7, brain | 13.2 | 87.5 |
| Lysyl oxidase | 11.2 | 95.8 |
| Tumor necrosis factor, alpha-induced protein 6 | 10.5 | 100 |
| Caveolin 1, caveolae protein 22kD | 5.4 | 92.9 |
| Vascular endothelial growth factor | 5.1 | 96.4 |
| Commonly down-regulated genes in clear cell renal cell carcinoma
| ||
|---|---|---|
| Gene name | Average fold | % of RCC |
| Kininogen | 27.2 | 100 |
| Fatty acid-binding protein 1, liver | 22.8 | 95.8 |
| Phenylalanine hydroxylase | 20.4 | 96 |
| Plasminogen | 12 | 100 |
| Metallothionein 1G | 10 | 100 |
| Metallothionein 1 H | 8.4 | 96.6 |
| RNA helicase-related protein | 6.9 | 96.6 |
The FACT-G is a cancer-specific QOL assessment tool that has been further modified into the FKSI-15 and FKSI-10 for kidney cancer (Table X) [50–52]. Both the FKSI-15 and FKSI-10 are shortened versions of the FACT-G that assess kidney cancer symptoms using 15 and 10 items, respectfully.
Broader, cross-disease QOL assessment tools can also be used to assess renal cell carcinoma. The IES, SF-36, and EORTC QLQ-C30have all been used to assess patients’ QOL after surgical treatment for renal cell carcinoma (Table X) [47, 53]. The IES assesses human response to a stressful life event while the SF-36 assesses general physical and mental health [54–56]. The IES and SF-36 have been revised into more commonly used versions: the IES-R and the SF-12 [46, 57]. The EORTC QLQ-C30 has 30 items that assess 5 functional scales (physical, role, emotional, cognitive, and social) and 3 symptom scales (fatigue, pain, and nausea/vomiting) [58].
Additional cross-disease QOL assessment tools have been used for various cancer types and may also be applied to renal cell carcinoma patients. The QWB is a common QOL assessment tool that has been used to measure change in QOL associated with breast cancer treatment [59, 60]. The BSI-18 is a shortened version of the BSI-53, the latter which was streamlined from the SCL-90-R (Table X) [61–64]. All three have been used to assess QOL in cancer patients, the BS-18 being used most commonly [65, 66]. A 2004 study conducted by Palapattu et al. used the BSI-18 to show that preoperative somatization was a predictor of local bladder carcinoma recurrence or distant metastasis. Albeit such indicators are beneficial, constraints on physicians’ time have led to the development of an even less time-consuming screening instrument, the DT [48].
Clinicians may select a combination of these instruments to give the most comprehensive psychosocial assessment of their patients’ well-being. Since psychosocial well-being is a dynamic trait, periodic reassessments are appropriate.
4.3. Selecting QOL Tools for Renal Cell Carcinoma Patients
While longer forms of QOL assessment are useful for research purposes, shortened forms can sufficiently measure patient QOL in the clinical setting. For example, the SF-12 was found to capture 80% to 85% of the SF-36 results [67]. There are many options for administration and scoring of patients’ QOL assessments prior to physician contact. Paper and pencil versions are the simplest means of data collection. To expedite the data analysis, staff can then enter the patients’ responses into software scoring programs so the interpreted results can be immediately available during the patient visit [68, 69]. Alternatively, the same questions can be uploaded into a computer tablet where imbedded scoring software will instantaneously interpret the data for the clinical staff [70–72].
Post-operative patient perceptions of renal cell carcinoma surgical outcomes have been shown to be associated with patient anxiety and overall health [47]. Therefore, while the short QOL assessments (SF-12, FKSI-10, BSI-18, and DT) can be used on a routine basis, the event-specific IES-Revised should be considered as a supplement to these assessments during the vulnerable post-operative period.
5. CONCLUSION
Risk assessment tools, such as the integrated staging system, stage, size, grade and necrosis (SSIGN) scoring algorithm and the postoperative renal cell cancer nomogram, have the potential to allow better risk stratification of patients into low-, intermediate-, and high-risk groups using familiar clinical determinants such as performance status (activity level) and pathologic tumor grade and stage. As investigators continue to identify specific genes at the molecular level in patients with renal cell carcinoma, this information has the potential to further delineate the expected behavior of select cancers, as well as the identification of novel mechanisms to improve patient survival and quality of life. Quality of life assessments are recommended for all patients and on an on-going basis to enable the clinical staff to deliver optimal patient care and reduce psychosocial distress that can negatively influence both quality and quantity of survival.
Table XI.
Quality of Life (QOL) Assessment Tools
| Screening focus | Admin. Time | # of Questions | Response Type | Use To-Date | |
|---|---|---|---|---|---|
| FACT-G | Physical, social/family, emotional, and functional well-being | 5–10 min | 27 | scale | Various other cancers |
| FKSI-15 | Thirty-four symptoms related to kidney cancer | 2–3 min | 15 | scale | Renal cancer |
| FKSI-10 | Thirty-four symptoms related to kidney cancer | <2 min | 10 | scale | Renal cancer |
| IES | Response to a stressful life event: intrusion and avoidance | 1–3 min | 20 | scale | Renal cell carcinoma, various other cancers |
| SF-36 | Physical & mental health (8 health concepts) | 5–10 min | 36 | both (scale; yes/no) | Renal cell carcinoma, various other cancers |
| SF-12 | Physical & mental health | < 2 min | 12 | both | Various other cancers |
| QWB | Mobility, physical activity, and social activity | 14 min (average) | 60 | scale | Various other cancers |
| EORTC- QLQ-C30 | Physical, role, emotional, cognitive, and social functioning; fatigue, pain, and nausea/vomiting | 11 min (average) | 30 | scale | Renal cell carcinoma, various other cancers |
| SCL-90-R | Psychological health | 12–20 min | 90 | scale | Various other cancers |
| BSI-53 | Psychological health (9 symptom dimensions*) | 8–15 min | 53 | scale | Various other cancers |
| BSI-18 | Psychological health (3 symptom dimensions**) | 1–3 min | 18 | scale | Bladder cancer, various other cancers |
| DT | Distress in cancer patients | <BSI-18 | 34 | yes/no*** | Various other cancers |
somatization, obsessive-compulsive, interpersonal sensitivity, depression, anxiety, hostility, phobic anxiety, paranoid ideation, psychoticism
somatization, depression, anxiety
one 11-pt scale included
Biographies
Tracy M. Downs, M.D. is a urologic oncologist and an assistant professor of surgery at Veterans Administration Medical Center at the University of California, San Diego, La Jolla, California.
Matthew Schultzel is a second year osteopathic medical student at Touro College of Medicine, New York, New York.
Helen Shi is a second year medical student at the University of California, San Diego, La Jolla, California.
Catherine Sanders is a second year medical student at The Ohio State University, Columbus, Ohio.
Zunera Tahir is an undergraduate student at the University of California, San Diego, La Jolla, California.
Georgia Robins Sadler, M.B.A., Ph.D. is the associate director for community outreach at Moores UCSD Cancer Center and a professor of surgery at the University of California, San Diego, La Jolla, California.
References
- 1.American Cancer Society. Cancer Facts & Figures 2007. 2007. [Google Scholar]
- 2.Rini BI. New approaches in advanced renal cell carcinoma. Urol Oncol. 2005;23(1):65–6. doi: 10.1016/j.urolonc.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 3.Stadler W. New therapeutic options for renal cell carcinoma. Clin Adv Hematol Oncol. 2006;4(6):429–30. [PubMed] [Google Scholar]
- 4.Motzer RJ, Bukowski RM. Targeted therapy for metastatic renal cell carcinoma. J Clin Oncol. 2006;24(35):5601–8. doi: 10.1200/JCO.2006.08.5415. [DOI] [PubMed] [Google Scholar]
- 5.Frank I, et al. Independent validation of the 2002 American Joint Committee on cancer primary tumor classification for renal cell carcinoma using a large, single institution cohort. J Urol. 2005;173(6):1889–92. doi: 10.1097/01.ju.0000158043.94525.d6. [DOI] [PubMed] [Google Scholar]
- 6.Zisman A, et al. Improved prognostication of renal cell carcinoma using an integrated staging system. J Clin Oncol. 2001;19(6):1649–57. doi: 10.1200/JCO.2001.19.6.1649. [DOI] [PubMed] [Google Scholar]
- 7.Skinner DG, et al. Diagnosis and management of renal cell carcinoma. A clinical and pathologic study of 309 cases. Cancer. 1971;28(5):1165–77. doi: 10.1002/1097-0142(1971)28:5<1165::aid-cncr2820280513>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 8.Nurmi MJ. Prognostic factors in renal carcinoma. An evaluation of operative findings. Br J Urol. 1984;56(3):270–5. doi: 10.1111/j.1464-410x.1984.tb05385.x. [DOI] [PubMed] [Google Scholar]
- 9.Maldazys JD, deKernion JB. Prognostic factors in metastatic renal carcinoma. J Urol. 1986;136(2):376–9. doi: 10.1016/s0022-5347(17)44873-7. [DOI] [PubMed] [Google Scholar]
- 10.Thrasher JB, Paulson DF. Prognostic factors in renal cancer. Urol Clin North Am. 1993;20(2):247–62. [PubMed] [Google Scholar]
- 11.Zisman A, et al. Risk group assessment and clinical outcome algorithm to predict the natural history of patients with surgically resected renal cell carcinoma. J Clin Oncol. 2002;20(23):4559–66. doi: 10.1200/JCO.2002.05.111. [DOI] [PubMed] [Google Scholar]
- 12.Guinan P, et al. TNM staging of renal cell carcinoma: Workgroup No. 3. Union International Contre le Cancer (UICC) and the American Joint Committee on Cancer (AJCC) Cancer. 1997;80(5):992–3. doi: 10.1002/(sici)1097-0142(19970901)80:5<992::aid-cncr26>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 13.Stein J, et al. The surgical management for renal cell carcinoma: Long-term results in a large group of patients. Journal of Urology; 93rd Annual Meeting of the American Urological Association, Inc; May 30–June 4, 1998; San Diego, California, USA. 1998. p. 192. [Google Scholar]
- 14.Javidan J, et al. Prognostic significance of the 1997 TNM classification of renal cell carcinoma. J Urol. 1999;162(4):1277–81. [PubMed] [Google Scholar]
- 15.Kinouchi T, et al. Impact of tumor size on the clinical outcomes of patients with Robson State I renal cell carcinoma. Cancer. 1999;85(3):689–95. doi: 10.1002/(sici)1097-0142(19990201)85:3<689::aid-cncr19>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 16.Tsui KH, et al. Prognostic indicators for renal cell carcinoma: a multivariate analysis of 643 patients using the revised 1997 TNM staging criteria. J Urol. 2000;163(4):1090–5. doi: 10.1016/s0022-5347(05)67699-9. quiz 1295. [DOI] [PubMed] [Google Scholar]
- 17.Pantuck AJ, Zisman A, Belldegrun AS. The changing natural history of renal cell carcinoma. J Urol. 2001;166(5):1611–23. [PubMed] [Google Scholar]
- 18.Fuhrman SA, Lasky LC, Limas C. Prognostic significance of morphologic parameters in renal cell carcinoma. Am J Surg Pathol. 1982;6(7):655–63. doi: 10.1097/00000478-198210000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Green LK, et al. Role of nuclear grading in stage I renal cell carcinoma. Urology. 1989;34(5):310–5. doi: 10.1016/0090-4295(89)90333-6. [DOI] [PubMed] [Google Scholar]
- 20.Delahunt B, et al. Fuhrman grading is not appropriate for chromophobe renal cell carcinoma. Am J Surg Pathol. 2007;31(6):957–60. doi: 10.1097/01.pas.0000249446.28713.53. [DOI] [PubMed] [Google Scholar]
- 21.Medeiros LJ, et al. Grading of renal cell carcinoma: Workgroup No. 2. Union Internationale Contre le Cancer and the American Joint Committee on Cancer (AJCC) Cancer. 1997;80(5):990–1. doi: 10.1002/(sici)1097-0142(19970901)80:5<990::aid-cncr25>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 22.Dall’oglio MF, et al. Microvascular tumour invasion in renal cell carcinoma: the most important prognostic factor. BJU Int. 2007 doi: 10.1111/j.1464-410X.2007.07015.x. [DOI] [PubMed] [Google Scholar]
- 23.Motzer RJ, Mazumdar M. Predicting survival of patients with metastatic renal cell carcinoma. Urologe A. 2004;43(Suppl 3):135–6. doi: 10.1007/s00120-004-0602-x. [DOI] [PubMed] [Google Scholar]
- 24.Motzer RJ, et al. Treatment outcome and survival associated with metastatic renal cell carcinoma of non-clear-cell histology. J Clin Oncol. 2002;20(9):2376–81. doi: 10.1200/JCO.2002.11.123. [DOI] [PubMed] [Google Scholar]
- 25.Kim HL, et al. Paraneoplastic signs and symptoms of renal cell carcinoma: implications for prognosis. J Urol. 2003;170(5):1742–6. doi: 10.1097/01.ju.0000092764.81308.6a. [DOI] [PubMed] [Google Scholar]
- 26.Elson PJ, Witte RS, Trump DL. Prognostic factors for survival in patients with recurrent or metastatic renal cell carcinoma. Cancer Res. 1988;48(24 Pt 1):7310–3. [PubMed] [Google Scholar]
- 27.Frank I, et al. An outcome prediction model for patients with clear cell renal cell carcinoma treated with radical nephrectomy based on tumor stage, size, grade and necrosis: the SSIGN score. J Urol. 2002;168(6):2395–400. doi: 10.1016/S0022-5347(05)64153-5. [DOI] [PubMed] [Google Scholar]
- 28.Thompson RH, et al. Dynamic outcome prediction in patients with clear cell renal cell carcinoma treated with radical nephrectomy: the D-SSIGN score. J Urol. 2007;177(2):477–80. doi: 10.1016/j.juro.2006.09.057. [DOI] [PubMed] [Google Scholar]
- 29.Lam JS, et al. Renal cell carcinoma 2005: new frontiers in staging, prognostication and targeted molecular therapy. J Urol. 2005;173(6):1853–62. doi: 10.1097/01.ju.0000165693.68449.c3. [DOI] [PubMed] [Google Scholar]
- 30.Ficarra V, et al. External validation of the Mayo Clinic Stage, Size, Grade and Necrosis (SSIGN) score to predict cancer specific survival using a European series of conventional renal cell carcinoma. J Urol. 2006;175(4):1235–9. doi: 10.1016/S0022-5347(05)00684-1. [DOI] [PubMed] [Google Scholar]
- 31.Han KR, et al. Validation of an integrated staging system toward improved prognostication of patients with localized renal cell carcinoma in an international population. J Urol. 2003;170(6 Pt 1):2221–4. doi: 10.1097/01.ju.0000096049.64863.a1. [DOI] [PubMed] [Google Scholar]
- 32.Patard JJ, et al. Use of the University of California Los Angeles integrated staging system to predict survival in renal cell carcinoma: an international multicenter study. J Clin Oncol. 2004;22(16):3316–22. doi: 10.1200/JCO.2004.09.104. [DOI] [PubMed] [Google Scholar]
- 33.Kattan MW, et al. A postoperative prognostic nomogram for renal cell carcinoma. J Urol. 2001;166(1):63–7. [PubMed] [Google Scholar]
- 34.Cindolo L, et al. Comparison of predictive accuracy of four prognostic models for nonmetastatic renal cell carcinoma after nephrectomy: a multicenter European study. Cancer. 2005;104(7):1362–71. doi: 10.1002/cncr.21331. [DOI] [PubMed] [Google Scholar]
- 35.Sorbellini M, et al. A postoperative prognostic nomogram predicting recurrence for patients with conventional clear cell renal cell carcinoma. J Urol. 2005;173(1):48–51. doi: 10.1097/01.ju.0000148261.19532.2c. [DOI] [PubMed] [Google Scholar]
- 36.Lane BR, et al. A Preoperative Prognostic Nomogram for Solid Enhancing Renal Tumors 7 cm or Less Amenable to Partial Nephrectomy. J Urol. 2007 doi: 10.1016/j.juro.2007.03.106. [DOI] [PubMed] [Google Scholar]
- 37.Takahashi M, et al. Gene expression profiling of clear cell renal cell carcinoma: gene identification and prognostic classification. Proc Natl Acad Sci U S A. 2001;98(17):9754–9. doi: 10.1073/pnas.171209998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leppert JT, et al. The role of molecular markers in the staging of renal cell carcinoma. BJU Int. 2007;99(5 Pt B):1208–11. doi: 10.1111/j.1464-410X.2007.06812.x. [DOI] [PubMed] [Google Scholar]
- 39.Bui MH, et al. Carbonic anhydrase IX is an independent predictor of survival in advanced renal clear cell carcinoma: implications for prognosis and therapy. Clin Cancer Res. 2003;9(2):802–11. [PubMed] [Google Scholar]
- 40.Atkins M, McDermott D, Regan M, Stanbridge E, Upton M, Youmans P, Febbo M, Lechpammer M, Signoretti S. Carbonic Anhydrase IX (CAIX) expression predicts for renal cell cancer (RCC) patient response and survival to IL-2 therapy. Journal of Clinical Oncology. 2004;22(14s):4512. [Google Scholar]
- 41.Bui MH, et al. Prognostic value of carbonic anhydrase IX and KI67 as predictors of survival for renal clear cell carcinoma. J Urol. 2004;171(6 Pt 1):2461–6. doi: 10.1097/01.ju.0000116444.08690.e2. [DOI] [PubMed] [Google Scholar]
- 42.Li X, et al. Prognostic significance of Id-1 and its association with EGFR in renal cell cancer. Histopathology. 2007;50(4):484–90. doi: 10.1111/j.1365-2559.2007.02637.x. [DOI] [PubMed] [Google Scholar]
- 43.Coates A, Porzsolt F, Osoba D. Quality of life in oncology practice: prognostic value of EORTC QLQ-C30 scores in patients with advanced malignancy. Eur J Cancer. 1997;33(7):1025–30. doi: 10.1016/s0959-8049(97)00049-x. [DOI] [PubMed] [Google Scholar]
- 44.Von Essen L, et al. ‘Satisfaction with care’: associations with health-related quality of life and psychosocial function among Swedish patients with endocrine gastrointestinal tumours. Eur J Cancer Care (Engl) 2002;11(2):91–9. doi: 10.1046/j.1365-2354.2002.00293.x. [DOI] [PubMed] [Google Scholar]
- 45.Stommel M, Given BA, Given CW. Depression and functional status as predictors of death among cancer patients. Cancer. 2002;94(10):2719–27. doi: 10.1002/cncr.10533. [DOI] [PubMed] [Google Scholar]
- 46.Oliver JW, et al. Individualized patient education and coaching to improve pain control among cancer outpatients. J Clin Oncol. 2001;19(8):2206–12. doi: 10.1200/JCO.2001.19.8.2206. [DOI] [PubMed] [Google Scholar]
- 47.Clark PE, et al. Quality of life and psychological adaptation after surgical treatment for localized renal cell carcinoma: impact of the amount of remaining renal tissue. Urology. 2001;57(2):252–6. doi: 10.1016/s0090-4295(00)00927-4. [DOI] [PubMed] [Google Scholar]
- 48.Jacobsen PB, et al. Screening for psychologic distress in ambulatory cancer patients. Cancer. 2005;103(7):1494–502. doi: 10.1002/cncr.20940. [DOI] [PubMed] [Google Scholar]
- 49.Palapattu GS, et al. Preoperative somatic symptoms are associated with disease progression in patients with bladder carcinoma after cystectomy. Cancer. 2004;101(10):2209–13. doi: 10.1002/cncr.20639. [DOI] [PubMed] [Google Scholar]
- 50.Brucker PS, et al. General population and cancer patient norms for the Functional Assessment of Cancer Therapy-General (FACT-G) Eval Health Prof. 2005;28(2):192–211. doi: 10.1177/0163278705275341. [DOI] [PubMed] [Google Scholar]
- 51.Functional Assessment of Chronic Illness Therapy (FACIT) 2007 Jul 23; < http://www.facit.org>.
- 52.Cella D, et al. Development and validation of the Functional Assessment of Cancer Therapy-Kidney Symptom Index (FKSI) J Support Oncol. 2006;4(4):191–9. [PubMed] [Google Scholar]
- 53.Poulakis V, et al. Quality of life after surgery for localized renal cell carcinoma: comparison between radical nephrectomy and nephron-sparing surgery. Urology. 2003;62 (5):814–20. doi: 10.1016/s0090-4295(03)00687-3. [DOI] [PubMed] [Google Scholar]
- 54.Horowitz M, Wilner N, Alvarez W. Impact of Event Scale: a measure of subjective stress. Psychosom Med. 1979;41(3):209–18. doi: 10.1097/00006842-197905000-00004. [DOI] [PubMed] [Google Scholar]
- 55.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–83. [PubMed] [Google Scholar]
- 56.Ware JE, Jr, Snow KK, Kosinski M, Gandek B. SF-36 Health Survey. Manual and Interpretation Guide. Boston: The Health Institute, New England Medical Center; 1993. [Google Scholar]
- 57.Bisson JI, et al. The prevalence and predictors of psychological distress in patients with early localized prostate cancer. BJU Int. 2002;90(1):56–61. doi: 10.1046/j.1464-410x.2002.02806.x. [DOI] [PubMed] [Google Scholar]
- 58.Aaronson NK, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–76. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 59.Vacek PM, et al. Factors influencing quality of life in breast cancer survivors. Qual Life Res. 2003;12(5):527–37. doi: 10.1023/a:1025098108717. [DOI] [PubMed] [Google Scholar]
- 60.Andresen EM, Rothenberg BM, Kaplan RM. Performance of a self-administered mailed version of the Quality of Well-Being (QWB-SA) questionnaire among older adults. Med Care. 1998;36(9):1349–60. doi: 10.1097/00005650-199809000-00007. [DOI] [PubMed] [Google Scholar]
- 61.Carlson LE, et al. High levels of untreated distress and fatigue in cancer patients. Br J Cancer. 2004;90(12):2297–304. doi: 10.1038/sj.bjc.6601887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Derogatis L. The SCL-90R Manual II: Administrative, Scoring, and Procedures. Johns Hopkins; Baltimore, MD: 1977. [Google Scholar]
- 63.Derogatis L. Brief Symptom Inventory (BSI) Manual: Administration, Scoring, and Procedures Manual. 3. Minneapolis, MN: NCS Pearson, Inc; 1993. [Google Scholar]
- 64.Derogatis L. The Brief Symptom Inventory (BSI) 18: Administration, Scoring, and Procedures Manual. Minneapolis, MN: NCS Pearson, Inc; 2001. [Google Scholar]
- 65.Compas BE, et al. Adjustment to breast cancer: age-related differences in coping and emotional distress. Breast Cancer Res Treat. 1999;54(3):195–203. doi: 10.1023/a:1006164928474. [DOI] [PubMed] [Google Scholar]
- 66.Zabora J, et al. The prevalence of psychological distress by cancer site. Psychooncology. 2001;10(1):19–28. doi: 10.1002/1099-1611(200101/02)10:1<19::aid-pon501>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 67.Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34 (3):220–33. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 68.Pearson A. Scoring Options by Assessment. 2007 Jun 15; < http://www.pearsonassessments.com/scoring/assess_scoring_option.htm>.
- 69.QualityMetric. QualityMetric Health Outcomes™ Scoring Software 2.0. 2007 Jun 15; < http://www.qualitymetric.com/products/sfscoring/scoringsoftwarev2.aspx>.
- 70.Velikova G, et al. Automated collection of quality-of-life data: a comparison of paper and computer touch-screen questionnaires. J Clin Oncol. 1999;17(3):998–1007. doi: 10.1200/JCO.1999.17.3.998. [DOI] [PubMed] [Google Scholar]
- 71.Taenzer P, et al. Impact of computerized quality of life screening on physician behaviour and patient satisfaction in lung cancer outpatients. Psychooncology. 2000;9(3):203–13. doi: 10.1002/1099-1611(200005/06)9:3<203::aid-pon453>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 72.Pearson A. Patient Assessment Device (PAD) 2007 Jun 15; < http://www.pearsonassessments.com/catalog/fpadfl.pdf>.