Abstract
Background
Both increases and decreases in ambient temperature have been associated with increased cardiovascular mortality and morbidity. However, the mechanism(s) remain unclear.
Objectives
We examined associations between biomarkers of pathways thought to, in part, explain these associations and changes in ambient temperature in a panel of predominantly post-myocardial infarction or post-stent patients.
Methods
We studied 76 subjects who had a recent coronary event and were participating in a cardiac rehabilitation program. In these patients, we measured heart rate variability, repolarization, and baroreflex sensitivity parameters using Holter ECG recordings before and during supervised, graded, twice weekly, exercise sessions. Hourly temperature measurements were made at a monitoring site near the rehabilitation center.
Results
Using linear mixed models, we observed decreases in rMSSD (square root of the mean of the sum of the squared differences between adjacent NN intervals) and deceleration capacity, associated with increases in ambient temperature in the previous four days. Additionally, decreased rMSSD was associated with both increasing temperature (mean in previous 6 hours) in the summer and decreasing temperature (mean in the previous 3 weeks) in the winter.
Conclusions
In a panel of cardiac rehabilitation patients, changes in ambient temperature were associated with decreases in markers of heart rate variability and baroreflex sensitivity, which may lead to increased risk of arrhythmic events and sudden death in post-infarction patients.
Keywords: ambient temperature, cardiac rehabilitation, heart rate variability, repolarization
INTRODUCTION
Multiple studies have demonstrated acute increases in cardiovascular mortality and morbidity associated with both increasing and decreasing ambient temperature (Bhaskaran et al., 2009; Rocklov, Ebi, & Forsberg, 2011; Ye et al., 2011). Due to climate change, there is the potential for more extreme temperatures, both hot and cold, which may increase cardiovascular morbidity and mortality. (Huang et al., 2011; IPCC, 2007) However, the mechanisms through which these acute changes in temperature may act are unclear. Knowledge of these mechanisms may provide an avenue for prevention of cardiovascular morbidity associated, in part, with these more severe temperatures. Autonomic nervous system dysfunction is one proposed mechanism (Hampel et al., 2010; Ren et al., 2011). Changes in heart rate variability have been associated with both acute air temperature decreases and increases in experimental studies among healthy young adult subjects (Bruce-Low, Cotterrell, & Jones, 2006; Liu, Lian, & Liu, 2008; Yamamoto, Iwamoto, Inoue, & Harada, 2007; Yao et al., 2009), observational studies of healthy older males (Ren et al., 2011), and myocardial infarction survivors (Hampel et al., 2010). However, individuals with underlying cardiovascular disease, such as cardiac rehabilitation patients and older adults, may be more susceptible to changes in temperature due to existing underlying disease (Abrignani et al., 2009; Guo, Barnett, Pan, Yu, & Tong, 2011; Muggeo & Hajat, 2009; Ye et al., 2011).
Previously, we conducted a study of cardiovascular biomarker responses to increases in ambient particulate matter concentrations in a panel of cardiac rehabilitation patients with a recent coronary event (myocardial infarction [MI] or unstable angina). We reported decreased heart rate variability (HRV) and baroreflex sensitivity, and delayed repolarization associated with increased ultrafine particle (particles <100nm in diameter), accumulation mode particle (100–500nm) and fine particle (<2.5 μm) concentrations in the previous few hours and days (Rich et al., 2012). Using these same data, we now examine whether acute ambient temperature changes impact these same markers. Based upon these previously observed biomarker responses to pollutants and knowledge that these responses are markers of pathways thought to underlie previous reports of cardiorespiratory mortality and morbidity, we hypothesized that increases in ambient temperature would be associated with increased heart rate, decreased heart rate variability and baroreflex sensitivity, and delayed repolarization. Several studies have discerned a U- or J-shaped relationship where the lowest mortality is found at moderate temperatures, with higher mortality with warmer temperatures (response time within a few days) and colder temperatures (response time within a few weeks) (Anderson & Bell, 2009; Baccini et al., 2008; Bhaskaran et al., 2009; Braga, Zanobetti, & Schwartz, 2002; Chang, Shipley, Marmot, & Poulter, 2004; Goodman, Dockery, & Clancy, 2004; Guo et al., 2011; Kovats, Hajat, & Wilkinson, 2004; Liang, Liu, Chou, & Kuo, 2008; Linares & Diaz, 2008; McMichael et al., 2008; Ye et al., 2011) Therefore, we also hypothesized that 1) in the winter months, decreases in ambient temperature would be associated with increased heart rate, decreased heart rate variability and baroreflex sensitivity, and delayed repolarization, especially over longer time periods (weeks versus days or hours), while 2) in the summer, increases in ambient temperature would be associated with these same changes, especially over shorter time periods (hours or days versus weeks).
METHODS
Study population
The study population has been described in detail elsewhere.(Rich et al., 2012) We recruited 76 subjects who had a recent coronary event (MI or unstable angina) and were referred to the University of Rochester Cardiac Rehabilitation Center (Center) by their cardiologist. We excluded subjects if they had cardiomyopathy in the absence of coronary disease, coronary bypass grafting within the last three months, type I diabetes, chronic atrial fibrillation, anemia, left bundle branch block, presence of a prosthetic heart valve or pacemaker, used amiodarone regularly, were active smokers or living with an active smoker, or resided greater than 10 miles (16.1 km) from the air pollution monitoring site at the Center. The study was approved by the Research Subjects Review Board of the University of Rochester, and informed written consent was obtained from all subjects.
Study protocol
Subjects participated in the standard cardiac rehabilitation program. For the study protocol, they participated in a maximum of 20 supervised exercise sessions during a 10-week program between June 2006 and November 2009. At these visits, study participants came to the Center 30–60 minutes prior to exercise. During this pre-exercise time, we recorded ECG and made blood pressure measurements before they began exercise. After these measurements, subjects exercised for 45–60 minutes on a bicycle, treadmill, or rowing machine, including warm-up (2–5 minutes of gentle stretching) and cool-down periods (10 minutes resting). We again recorded ECG measurements during and after the exercise session. We used these 1,359 subject-visits and their ECG recordings in the statistical analyses described below.
During the visits, we measured subjects’ heart rate variability and baroreflex sensitivity using 3-lead (modified V2, V5, and AVF) Holter ECG recordings (Burdick Altair-Disc holter recorder; Cardiac Science, Bothell, WA), which were analyzed using the Vision Premier Burdick Holter System (Cardiac Science, Bothell, WA) and custom-made programs at the University of Rochester Medical Center (Bauer et al., 2008; Cygankiewicz et al., 2008). The Holters were first automatically annotated by the commercial Holter scanning algorithm (Vision Premier Burdick Holter System) and then annotated by a trained technician.
Our methods for measuring HRV parameters have been described previously (Rich et al., 2012). RR intervals were exported to a custom made HRV program that produced a set of HRV parameters. Short-term, ‘pre-exercise,’ resting recordings provided information regarding HRV parameters unaffected by sympathetic stimuli during exercise, whereas the ‘whole session’ recording (including the exercise session) reflected the overall behavior of heart rate and autonomic responses to daily conditions, including exercise. Based in part on Bigger et al (Bigger et al., 1992), filtering criteria eliminated two RR intervals after atrial beats or premature ventricular beats. We did not apply pre-processing filtering to eliminate extreme values. We examined 5-minute segments during the resting period to standardize conditions for all HRV and repolarization parameters, requiring at least 200 beats for HRV analyses. As a post-processing approach, we evaluated outliers and determined whether the values were valid or not based on intra-lab ranges developed during a prior study (Schneider et al., 2010).
We measured time domain HRV parameters, including the mean NN interval time between successive normal to normal beats (MeanNN) as a measure of heart rate, the standard deviation of all normal to normal beat intervals (SDNN; marker of overall variability in heart rate), and the square root of the mean of the sum of squared differences between adjacent NN intervals (rMSSD; marker of parasympathetic modulation of heart rate) during the entire recording (‘whole session’). We also measured heart rate turbulence slope (HRT) and deceleration capacity (DC) across the ‘whole session’ using programs adopted from Bauer et al (Bauer et al., 2006) and from Schmidt et al. (Schmidt et al., 1999) HRT is a measure of baroreflex sensitivity (Bauer et al., 2008; Cygankiewicz, Wranicz, Bolinska, Zaslonka, & Zareba, 2004) and is characterized by a brief acceleration and subsequent deceleration of heart rate following a spontaneous premature ventricular contraction, with decreased HRT associated with increased risk of cardiac death (Bauer et al., 2008; Stein & Deedwania, 2009; Stein & Barzilay, 2011). DC is an additional measure of heart rate dynamics, reflecting the variability in heart rate during periods when the heart is slowing down, complementing information based on the other HRV and HRT parameters (Bauer et al., 2008). It describes the capacity of the sinus rhythm to slow down, without necessarily being linked to one particular physiological regulation process (e.g. respiratory, baroreflex mediated, or circadian). DC seems to correlate well with low frequency power, which is believed to reflect baroreflex responses (Goldstein, Bentho, Park, & Sharabi, 2011; Lewek et al., 2009).
Repolarization is a critical mechanism of the electrophysiology of cardiac cells, and plays an important role in arrhythmogenesis and the risk of sudden death. To measure repolarization duration, we used the ‘pre-exercise’ recordings only and manually measured the QT interval duration in lead II, correcting it for heart rate (QTc) using Bazett’s formula (Bazett, 1920). We also measured the difference between the peak and end of the T-wave (TpTe) as a measure of late repolarization duration.
Ambient Temperature and Pollution Measurements
Hourly temperature, relative humidity, and barometric pressure measurements were taken at the New York State Department of Environmental Conservation (NYS DEC) site in Rochester (~ 5.5 km from the Rehabilitation Center). We then used these hourly values to calculate the mean temperature, relative humidity (RH), and barometric pressure levels in the 24 hours before each clinic visit. In the same manner we calculated longer (e.g. mean temperature from lag hours 0–47, 0–71, 0–95, 0–119, 0–143, 0–167, and 0–304) and shorter mean weather values (lag hours 0 to 5). These mean temperatures, RH, and barometric pressure levels were then used in the statistical analyses described below.
Particle size distributions for ultrafine particles (UFP; 10–100 nm diameter) and for accumulation mode particles (AMP; 100–500 nm diameter) were measured using a wide range particle spectrometer (model 1000XP; MSP Corporation, Shoreview, MN) at the Cardiac Rehabilitation Center. Concentrations of PM2.5 were measured using a tapered element oscillating microbalance (ThermoFisher, Franklin, MA) at the New York State Department of Environmental Conservation site in Rochester (~ 5.5 km from the Cardiac Rehabilitation Center).
Statistical Analysis
We used linear mixed models, with a compound symmetry covariance structure, to estimate the change in outcome (QTc, TpTe, MeanNN, SDNN, rMSSD, HRT, and DC) associated with each interquartile range increase in the mean temperature in the previous 24 hours, controlling for each subject’s visit number, days since study inception, calendar month, weekday, and time of day. Barometric pressure, relative humidity, and ozone concentrations were not consistently associated with outcomes, and therefore not included in analyses. We also estimated changes in each outcome associated with interquartile range (IQR) increases in the mean temperature for shorter (mean of lag hours 0 to 5) and longer lag times (mean temperature from lag hours 0–47, 0–71, 0–95, 0–119, 0–143, 0–167 [1 week], and 0–304 [3 weeks]). From these models, we present the change in each outcome (and its 95% confidence interval) associated with each interquartile range increase in temperature in the specified time period.
Next, to examine whether there were different outcome responses to temperature changes in the winter (e.g. increased heart rate, decreased heart rate variability, decreased baroreflex sensitivity, and delayed repolarization associated with decreased temperature) versus the summer (increased heart rate, decreased heart rate variability, decreased baroreflex sensitivity, and delayed repolarization associated with increased temperature), we conducted separate analyses restricted to only winter months (December–February), and then to only summer months (June–August). To determine whether the effect of temperature within the summer was dependent upon the actual level of temperature, we re-ran the summer analysis replacing the continuous temperature variable with indicator variables for temperature quartile. We did the same analysis within the winter.
To evaluate whether any changes in these HRV, repolarization, and heart rate turbulence parameters associated with temperature changes were independent of increases in ambient pollutant concentrations in the previous few hours/days, we re-ran the models described above controlling for any pollutant previously found to be associated with each specific outcome.(Rich et al., 2012) Second, we evaluated whether our estimated changes in each marker associated with increased temperature were independent of heart rate, by including MeanNN in the same models described above. All data management and statistical analyses were done using SAS version 9.2 (SAS Institute Inc., Cary, NC).
RESULTS
Study subject characteristics are shown in Table 1. Of the 76 subjects, 63 (83%) completed all 20 rehabilitation visits, with six subjects (8%) completing less than 10 visits. Most subjects were older, white males, with a majority having a history of myocardial infarction, stent, or hypertension. Nearly all subjects were taking statins, with most also taking beta-blockers and angiotensin-converting enzyme inhibitors. Descriptive statistics of the HRV, repolarization, and heart rate turbulence parameters are shown in Table 2. The distribution of temperature, relative humidity, barometric pressure, and several air pollutants are shown in Table 3. The mean temperature throughout the study period was 11.8°C, with a mean in the winter and summer of −1.1°C and 22.2°C, respectively (Table 3).
Table 1.
Characteristics of study population at baseline (N=76).
| Characteristic | N | % |
|---|---|---|
| Age | ||
| <50 years | 13 | 17% |
| 50–54 years | 11 | 14% |
| 55–59 years | 10 | 13% |
| 60–64 years | 14 | 18% |
| 65–69 years | 12 | 16% |
| 70–74 years | 9 | 12% |
| 75–79 years | 5 | 7% |
| ≥ 75 years | 2 | 3% |
| Male | 51 | 67% |
| White | 68 | 88% |
| Body mass index category | ||
| Normal Weight (18.5 kg/m2 to <25 kg/m2) | 10 | 13% |
| Overweight (25 kg/m2 to <30 kg/m2) | 31 | 41% |
| Obese (≥30 kg/m2) | 35 | 46% |
| History of: | ||
| Myocardial infarction | 45 | 59% |
| Coronary bypass surgery | 4 | 5% |
| Stent | 65 | 86% |
| Chronic obstructive pulmonary disease | 13 | 17% |
| Type 2 diabetes mellitus | 17 | 22% |
| Hypertension | 45 | 59% |
| Smoking | ||
| Never | 40 | 53% |
| Former | 36 | 47% |
| Daily Medication use at 1st Visit: | ||
| Angiotensin Receptor Blocker | 10 | 13% |
| Beta-blocker | 66 | 87% |
| Angiotensin Converting Enzyme Inhibitor | 50 | 66% |
| Calcium channel blocker | 7 | 9% |
| Digitalis | 1 | 1% |
| Diuretic | 20 | 26% |
| Statin | 73 | 96% |
Table 2.
Mean and standard deviation of each outcome, at the first cardiac rehabilitation visit.
| Biomarker levels at baseline | Mean | Standard Deviation | Minimum | 25th %tile | Median | 75th %tile | Maximum |
|---|---|---|---|---|---|---|---|
| Pre-exercise resting period | |||||||
| QTC (ms) | 419.91 | 32.67 | 354.57 | 399.12 | 412.66 | 432.67 | 593.50 |
| TpTe (ms) | 89.06 | 12.05 | 55.89 | 81.64 | 87.04 | 93.51 | 149.25 |
| Whole Session | |||||||
| MeanNN (ms) | 733.32 | 110.4 | 475.88 | 653.96 | 728.02 | 817.29 | 1102.68 |
| SDNN (ms) | 132.07 | 43.32 | 27.76 | 101.10 | 126.38 | 157.04 | 296.99 |
| rMSSD (ms) | 77.70 | 38.15 | 11.47 | 50.97 | 72.56 | 96.82 | 274.62 |
| Heart Rate Turbulence Slope (ms/RR) | 6.08 | 4.31 | 0 | 2.89 | 4.83 | 8.06 | 19.50 |
| Deceleration Capacity (ms) | 3.84 | 1.40 | 0.0005 | 2.93 | 3.84 | 4.72 | 9.79 |
Table 3.
Descriptive statistics of daily air pollution concentrations and weather characteristics during the study period (June 26, 2006 to November 25, 2009. N=1249 possible days of measurement)
| Weather Characteristic | Mean | Standard Deviation | Minimum | 25th %tile | Median | 75th %tile | Maximum |
|---|---|---|---|---|---|---|---|
| Whole Year | |||||||
| Temperature (°C) | 11.8 | 10.1 | −13.8 | 3.6 | 13.0 | 20.1 | 31.3 |
| Relative Humidity (%) | 64.7 | 12.7 | 12.5 | 56.6 | 65.6 | 73.6 | 93.2 |
| Barometric Pressure (inches Hg) | 29.4 | 0.27 | 27.4 | 29.2 | 29.4 | 29.6 | 30.1 |
| Winter (December–February) | |||||||
| Temperature (°C) | −1.1 | 5.8 | −13.8 | −5.1 | −1.2 | 2.1 | 16.4 |
| Relative Humidity (%) | 68.9 | 10.8 | 41.0 | 61.3 | 70.0 | 77.5 | 92.3 |
| Barometric Pressure (inches Hg) | 29.3 | 0.22 | 27.4 | 29.2 | 29.3 | 29.5 | 29.7 |
| Summer (June–August) | |||||||
| Temperature (°C) | 22.2 | 3.5 | 10.7 | 19.8 | 22.1 | 24.3 | 31.3 |
| Relative Humidity (%) | 63.4 | 9.7 | 42.8 | 56.3 | 62.8 | 70.6 | 86.5 |
| Barometric Pressure (inches Hg) | 29.4 | 0.27 | 28.5 | 29.2 | 29.4 | 29.6 | 30.0 |
Changes in each outcome associated with each IQR increase in the mean temperature in the previous 6, 24, 48, 72 and 96 hours, as well as the previous 1 week (168 hours) and 3 weeks (304 hours) are presented in Table 4. Consistent with our a priori hypotheses, IQR increases in temperature were associated with increased QTc, increased TpTe, and decreased MeanNN at all moving average times. IQR increases in temperature were also associated with increased SDNN at all moving average times (inconsistent with our a priori hypothesis). However, all of these estimates were not statistically significant, with 95% confidence intervals including both negative and positive values. Although increases in temperature at all lags were associated with decreased rMSSD, the largest rMSSD change was associated with each IQR increase in temperature in the previous 24 hours (12.81 ms; 95% confidence interval = −18.76, −6.86). Consistently, decreases in DC were associated with increasing temperatures as well, with the largest DC decrease observed at 72 hours (0.35 ms; 95% CI = −0.57, −0.13). IQR increases in temperature were also associated with decreased HRT at all lags. The largest HRT decrease was associated with each IQR increase in temperature in the previous 96 hours (1.60 ms/RR; 95% CI = −3.16, −0.05; Table 4).
Table 4.
Change in each outcome associated with each IQR increase in ambient temperature, by moving average of time before outcome measurement was made.
| Biomarkers and moving averages evaluated | N Subjects (Subject-Visits) | IQR (°C) | Change in Biomarker | 95% CI | |
|---|---|---|---|---|---|
| PRE-EXERCISE RESTING PERIOD | QTc (ms) | ||||
| 0–5 | 76 (1263) | 17.4 | 2.10 | −1.69, 5.89 | |
| 0–23 | 17.4 | 2.23 | −1.86, 6.32 | ||
| 0–47 | 17.3 | 1.62 | −2.76, 6.00 | ||
| 0–71 | 17.4 | 2.36 | −2.38, 7.10 | ||
| 0–95 | 17.6 | 3.06 | −2.03, 8.15 | ||
| 0–167 (1 week) | 17.6 | 3.40 | −2.48, 9.29 | ||
| 0–503 (3 weeks) | 17.6 | 6.80 | −1.6, 15.21 | ||
|
| |||||
| TpTe (ms) | |||||
| 0–5 | 76 (1253) | 17.4 | 0.89 | −0.74, 2.52 | |
| 0–23 | 17.4 | 1.12 | −0.65, 2.88 | ||
| 0–47 | 17.3 | 0.95 | −0.94, 2.84 | ||
| 0–71 | 17.4 | 0.48 | −1.56, 2.53 | ||
| 0–95 | 17.6 | 0.43 | −1.77, 2.63 | ||
| 0–167 (1 week) | 17.6 | 1.70 | −0.83, 4.23 | ||
| 0–503 (3 weeks) | 17.6 | 1.79 | −1.82, 5.41 | ||
|
| |||||
| WOHLE SESSION | MeanNN(ms) | ||||
| 0–5 | 76 (1359) | 17.4 | −6.83 | −17.74, 4.08 | |
| 0–23 | 17.4 | −8.56 | −20.37, 3.24 | ||
| 0–47 | 17.3 | −4.53 | −17.18, 8.13 | ||
| 0–71 | 17.4 | −4.54 | −18.21, 9.14 | ||
| 0–95 | 17.6 | −4.01 | −18.77, 10.74 | ||
| 0–167 (1 week) | 17.6 | −4.26 | −21.39, 12.86 | ||
| 0–503 (3 weeks) | 17.6 | −4.43 | −28.86, 20.00 | ||
|
| |||||
| SDNN(ms) | |||||
| 0–5 | 76 (1357) | 17.4 | 0.28 | −4.62, 5.18 | |
| 0–23 | 17.4 | 0.79 | −4.52, 6.09 | ||
| 0–47 | 17.3 | 2.58 | −3.10, 8.26 | ||
| 0–71 | 17.4 | 2.65 | −3.49, 8.78 | ||
| 0–95 | 17.6 | 3.15 | −3.47, 9.77 | ||
| 0–167 (1 week) | 17.6 | 6.06 | −1.61, 13.74 | ||
| 0–503 (3 weeks) | 17.6 | 7.50 | −3.46, 18.45 | ||
|
| |||||
| HRT(ms/RR)a | |||||
| 0–5 | 76 (504) | 17.4 | −0.41 | −1.59, 0.77 | |
| 0–23 | 17.4 | −0.63 | −1.90, 0.64 | ||
| 0–47 | 17.3 | −0.60 | −1.94, 0.74 | ||
| 0–71 | 17.4 | −1.23† | −2.68, 0.23 | ||
| 0–95 | 17.6 | −1.60* | −3.16, −0.05 | ||
| 0–167 (1 week) | 17.6 | −1.45 | −3.29, 0.39 | ||
| 0–503 (3 weeks) | 17.6 | −0.73 | −3.39, 1.94 | ||
|
| |||||
| DC(ms) | |||||
| 0–5 | 76 (1314) | 17.4 | −0.29** | −0.46, −0.11 | |
| 0–23 | 17.4 | −0.32** | −0.51, −0.13 | ||
| 0–47 | 17.3 | −0.34** | −0.54, −0.14 | ||
| 0–71 | 17.4 | −0.35** | −0.57, −0.13 | ||
| 0–95 | 17.6 | −0.32** | −0.56, −0.08 | ||
| 0–167 (1 week) | 17.6 | −0.25† | −0.52, 0.03 | ||
| 0–503 (3 weeks) | 17.6 | −0.40* | −0.79, −0.01 | ||
|
| |||||
| rMSSD (ms) | |||||
| 0–5 | 76 (1353) | 17.4 | −11.48** | −16.98, −5.98 | |
| 0–23 | 17.4 | −12.81** | −18.76, −6.86 | ||
| 0–47 | 17.3 | −9.99** | −16.39, −3.59 | ||
| 0–71 | 17.4 | −7.78* | −14.7, −0.86 | ||
| 0–95 | 17.6 | −6.78† | −14.25, 0.69 | ||
| 0–167 (1 week) | 17.6 | −3.48 | −12.15, 5.19 | ||
| 0–503 (3 weeks) | 17.6 | −0.03 | −12.41, 12.35 | ||
Note: Since HRT is measured only when premature ventricular contractions (PVC) occur, these analyses include only those subject-visits with 1 or more PVCs.
p<0.10
p<0.05
p<0.01
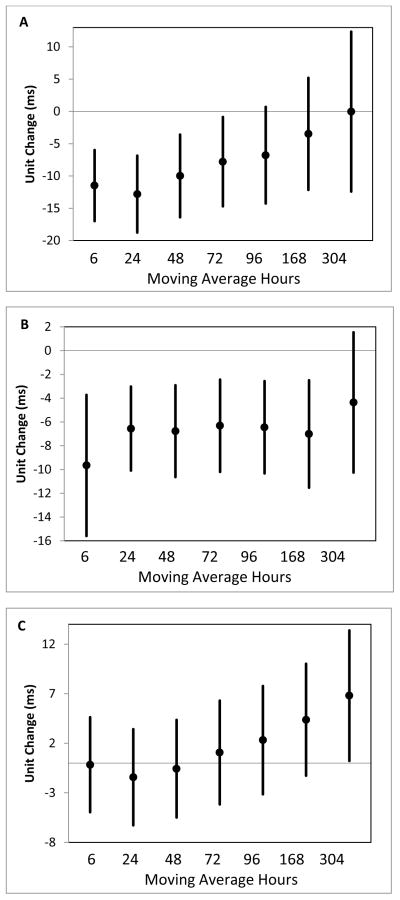
In the winter months (December–February), we observed the largest increase in rMSSD associated with each IQR increase in temperature in the previous 3 weeks (6.93 ms; 95% CI = 0.17, 13.69; Table 5; Figure 1), but little change associated with increased temperature in previous 48 hours. There was an increasing effect size as the moving average of time increased from 72 hours to 3 weeks. Whereas in the summer, we observed the largest change in rMSSD associated with each IQR increase in temperature in the previous 6 hours (−9.66ms; 95% CI = −15.60, −3.72; Table 5; Figure 1). In the winter, each IQR increase in the average temperature over the previous 96 hours was associated with a significant decrease in HRT (−1.21 ms/RR; −2.41, −0.01; Table 5), while in the summer, IQR temperature increases were associated with consistent, but smaller and not statistically significant decreases in HRT. In the winter and summer with fewer subjects per group, IQR increases in temperature at all lags were associated with small non-significant decreases in DC (Table 5).
Table 5.
Change in each outcome associated with each IQR increase or decrease in ambient temperature, by moving average of time before outcome measurement was made, by season.
| Biomarkers and moving averages evaluated | Winter (December–February) | Summer (June–August) | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| N Subjects (N Subject-Visits) | IQR (°C) | Change in Biomarker per IQR increase | 95% CI | N Subjects (N Subject-Visits) | IQR (°C) | Change in Biomarker per IQR increase | 95% CI | ||
| WHOLE SESSION | HRT(ms/RR)a | ||||||||
| 0–5 | 30 (118) | 7.9 | −0.47 | −1.71, 0.77 | 41 (121) | 7.2 | −0.10 | −1.83, 1.63 | |
| 0–23 | 7.3 | −0.86 | −2.08, 0.35 | 4.3 | −0.32 | −1.29, 0.65 | |||
| 0–47 | 6.9 | −0.53 | −1.74, 0.69 | 4.2 | −0.66 | −1.74, 0.42 | |||
| 0–71 | 6.5 | −0.82 | −2.06, 0.41 | 3.8 | −0.61 | −1.71, 0.50 | |||
| 0–95 | 6.1 | −1.21* | −2.41, −0.01 | 3.5 | −0.60 | −1.75, 0.55 | |||
| 0–167 (1 week) | 5.5 | −1.02 | −2.27, 0.24 | 3.1 | −0.86 | −2.30, 0.58 | |||
| 0–503 (3 weeks) | 4.6 | −0.34 | −1.94, 1.25 | 2.5 | −0.07 | −1.76, 1.62 | |||
|
| |||||||||
| DC(ms) | |||||||||
| 0–5 | 30 (276) | 7.9 | −0.14 | −0.32, 0.04 | 41 (379) | 7.2 | −0.10 | −0.29, 0.09 | |
| 0–23 | 7.3 | −0.11 | −0.30, 0.07 | 4.3 | −0.06 | −0.18, 0.05 | |||
| 0–47 | 6.9 | −0.12 | −0.30, 0.07 | 4.2 | −0.09 | −0.21, 0.04 | |||
| 0–71 | 6.5 | −0.12 | −0.32, 0.08 | 3.8 | −0.08 | −0.20, 0.05 | |||
| 0–95 | 6.1 | −0.12 | −0.33, 0.09 | 3.5 | −0.06 | −0.19, 0.06 | |||
| 0–167 (1 week) | 5.5 | −0.09 | −0.31, 0.12 | 3.1 | −0.05 | −0.20, 0.09 | |||
| 0–503 (3 weeks) | 4.6 | −0.09 | −0.34, 0.16 | 2.5 | −0.01 | −0.20, 0.18 | |||
|
| |||||||||
| rMSSD (ms) | |||||||||
| 0–5 | 30 (283) | 7.9 | −0.17 | −4.95, 4.62 | 41 (390) | 7.2 | −9.66** | −15.60, −3.72 | |
| 0–23 | 7.3 | −1.43 | −6.28, 3.42 | 4.3 | −6.56** | −10.09, −3.03 | |||
| 0–47 | 6.9 | −0.57 | −5.49, 4.35 | 4.2 | −6.77** | −10.63, −2.91 | |||
| 0–71 | 6.5 | 1.07 | −4.17, 6.30 | 3.8 | −6.32** | −10.19, −2.44 | |||
| 0–95 | 6.1 | 2.32 | −3.14, 7.77 | 3.5 | −6.45** | −10.33, −2.56 | |||
| 0–167 (1 week) | 5.5 | 4.37 | −1.28, 10.01 | 3.1 | −7.01** | −11.52, −2.49 | |||
| 0–503 (3 weeks) | 4.6 | 6.80* | 0.22, 13.39 | 2.5 | −4.36 | −10.26, 1.53 | |||
Note: Since HRT is measured only when premature ventricular contractions (PVC) occur, these analyses include only those subject-visits with 1 or more PVCs.
p<0.10
p<0.05
p<0.01
Figure 1.
Unit change and 95% confidence interval in rMSSD measured across the whole rehabilitation session associated with an IQR in ambient temperature for the whole year (A), summer months (B), and winter months (C).
Next, we evaluated whether the effect of temperature within the summer or within the winter was dependent upon the actual level of temperature. Within the summer, with each increasing quartile of temperature, we saw increasingly greater decreases in rMSSD (test for trend p=0.0002) (Table 6). In the winter, with each increasing quartile of mean temperature, we observed larger increases in rMSSD, as hypothesized, though the test for trend p-value was not significant (p=0.14) (Table 6). This suggests that our reported temperature effects on rMSSD were not limited to just the extreme temperatures.
Table 6.
Change in rMSSD associated with each quartile of mean temperature in the previous 6 hours (summer) or previous 3 weeks (winter).
| Quartile | Minimum (°C) | Median (°C) | Maximum (°C) | Change in rMSSD (ms) | 95% CI | Test for trend p-value | |
|---|---|---|---|---|---|---|---|
| SUMMER (6 hours) | 1st Quartile | 8.00 | 17.04 | 18.42 | 0.00 | -- | 0.0002 |
| 2nd Quartile | 18.43 | 20.20 | 21.88 | −5.10 | −12.91, 2.70 | ||
| 3rd Quartile | 21.96 | 23.45 | 25.26 | −13.70 | −22.22, −5.17 | ||
| 4th Quartile | 25.34 | 27.54 | 34.75 | −17.52 | −27.81, −7.23 | ||
|
|
|||||||
| WINTER (3 weeks) | 1st Quartile | −7.52 | −6.09 | −2.43 | 0.00 | -- | 0.14 |
| 2nd Quartile | −2.39 | −1.50 | −0.63 | 3.51 | −6.91, 13.93 | ||
| 3rd Quartile | −0.62 | 0.17 | 1.97 | 8.32 | −3.99, 20.64 | ||
| 4th Quartile | 1.98 | 4.81 | 7.41 | 9.46 | −4.94, 23.86 | ||
Last, when we included air pollutant concentrations for these same lag times in our models, or when we included heart rate in our models, our effect estimates were not substantially different from our main analysis (Online Resources 1 and 2).
DISCUSSION
In a panel of post-infarction cardiac rehabilitation patients, increased temperature was associated with decreases in rMSSD, DC, and HRT in the next few days, suggesting parasympathetic modulation of heart rate and baroreflex sensitivity are impacted by acute temperature changes. In the summer, decreases in rMSSD were associated with increased temperature in the previous 6 hours, while in the winter, decreases in rMSSD were associated with decreased temperature in the previous 3 weeks. We saw no such season-specific associations between increased temperature and changes in HRT or DC. These relationships were independent of long term time trends, day of the week, hour of the day, particulate and gaseous pollutant concentrations, duration of participation in the rehabilitation program, and heart rate. These associations also were not limited to just the extreme temperatures in the summer or winter. These small changes in parasympathetic modulation of heart rate and baroreflex sensitivity may make patients with decreased cardiovascular health more susceptible to future cardiac events.
Similar to previous studies, we found a decrease in heart rate variability (here rMSSD) associated with increases in temperature (Bruce-Low et al., 2006; Ren et al., 2011; Yamamoto et al., 2007). We found similarly sized decreases in rMSSD associated with each lagged temperature increase, with the largest in the previous 6 hours and 24 hours. This rapid rMSSD HRV response is similar to what we and others have observed with pollution (Brook et al., 2010; Rich et al., 2012; U.S. EPA, 2009). We also found that cold winter-time temperature and warmer summer temperatures both resulted in decreased rMSSD. However, this parasympathetic response to temperature was much more rapid in the summer (within 6 hours) than in the winter (within 3 weeks). Ren et al (Ren et al., 2011) reported only decreased HRV associated with increasing temperature in the warm season, but no effect of decreased temperature in the winter. However, other studies have reported adverse changes in CV biomarkers associated with decreased temperature (Okamoto-Mizuno, Tsuzuki, Mizuno, & Ohshiro, 2009; Schneider et al., 2008). Although these winter effects (i.e. decreased rMSSD associated with a decrease in the 3 week mean temperature) need to be replicated in further studies, this seasonal difference in temperature response is similar to the U-shaped relationship between temperature and mortality observed previously (Baccini et al., 2008; Braga et al., 2002; Kovats et al., 2004; Liang et al., 2008; Lin et al., 2009; Linares & Diaz, 2008; McMichael et al., 2008). To our knowledge, this is the first study to examine the relationship between heart rate turbulence or deceleration capacity and ambient temperature.
The HRV, repolarization, and HRT variables included in our analysis were intended to allow us to assess whether acute temperature changes impacted the autonomic regulation of the heart and baroreflex sensitivity of the cardiovascular system. Our findings suggest that temperature increases, especially in the warm season, decrease parasympathetic modulation of heart rate and modify baroreflex sensitivity in as little time as 6 hours. The short timing of these HRT and rMSSD responses to temperature increases (HRT: 96 hours; rMSSD 6 and 24 hours) is consistent with the HRT and rMSSD responses to ambient air pollution we observed in this panel previously (HRT: 96 hours; rMSSD: 6 and 24 hours) (Rich et al., 2012). However, previously we reported prolongation of late repolarization duration (reduced TpTe) associated with increased AMP in the previous 24–47 hours (Rich et al., 2012), but we found no such repolarization parameter response to temperature increases. Further work is needed to replicate our season specific findings of decreased rMSSD HRV associated with increased temperature in the summer and decreased temperature in the winter.
Although this study had several strengths, including a large sample size, multiple observations per participant, and a wide range of temperatures throughout the study period, it had a few limitations. First, the temperature measure used was an outdoor temperature, but given the recent health event(s) of these subjects, they may have spent the majority of their time indoors. Therefore, they may not have been exposed to the outdoor temperature but for short periods of time each day (e.g. when traveling to the rehabilitation center), depending on the season. However, this error is likely non-differential with respect to rMSSD level (e.g. high versus low), resulting in a bias toward the null and underestimates of effect. Second, the secondary analyses examining temperature/outcome responses within the winter and summer seasons separately had limited sample sizes, decreasing the precision of our estimates. However, these were exploratory analyses examining the possible U-shaped response between temperature and outcomes. Future studies should be adequately powered to estimate and replicate these season specific effects. Third, we estimated a large number of temperature-outcome associations, increasing the potential for type one error. Our primary focus, however, was toward overall patterns of biomarker changes associated with multiple moving average temperature changes, not whether each individual effect was statistically significant. These patterns were consistent across moving averages of temperature and also consistent with our a priori hypotheses.
Last, most of the subjects included in the analysis for the winter season are different from those included in the analysis for the summer season. Four of the subjects had visits in both the winter and summer seasons (e.g. 14–19 visits in 1 season and 1–6 visits in the other season), with 26 subjects only in the winter analyses and 37 different subjects only in the summer analyses. Therefore, any seasonal difference in biomarker response to increased temperature by season could also be due to differences between the people studied each season. However, “winter” and “summer” subjects had similar study characteristics (data not shown), suggesting this was not the case.
In this panel of patients participating in a cardiovascular rehabilitation program, increases in outdoor temperature were associated with decreases in rMSSD, HRT, and DC. However, changes in rMSSD were in opposite directions in winter (decreased rMSSD associated with decreased temperature in the previous 3 weeks) and summer (decreased rMSSD associated with increased temperature in the previous 6 hours), providing some mechanistic explanation for previous reports of a U-shape relationship between ambient temperature and cardiovascular mortality. These sub-clinical changes in cardiac physiology (i.e. parasympathetic modulation of heart rate and baroreflex sensitivity) may be risk factors for future cardiovascular events. However, further work is needed to confirm these findings.
Supplementary Material
Acknowledgments
This work was supported by the New York State Energy Research and Development Authority (contract 8650), the U.S. Environmental Protection Agency (EPA) through a grant from the Science to Achieve Results (RD83241501) a Syracuse Center of Excellence CARTI (Collaborative Activities for Research and Technology Innovation) project award which was supported by a grant from the U.S. EPA (X-83232501-0), the Electric Power Research Institute (EPRI agreement W06325), and the National Institute of Environmental Health Sciences Center (P30 ES01247). EPRI is primarily supported by the electric industry in the United States and abroad. EPRI is an independent nonprofit 501(c)(3) organization that funds external research at a number of universities and institutes worldwide. Although the research described in this article has been funded in part by the U.S. EPA, it has not been subjected to the Agency’s required peer and policy review. Therefore, it does not necessarily reflect the views of the U.S. EPA and no official endorsement should be inferred. We thank the study participants for their enthusiastic participation and L. Kopin for her coordination of the study.
References
- Abrignani MG, Corrao S, Biondo GB, et al. Influence of climatic variables on acute myocardial infarction hospital admissions. Int J Cardiol. 2009;137(2):123–129. doi: 10.1016/j.ijcard.2008.06.036. [DOI] [PubMed] [Google Scholar]
- Anderson BG, Bell ML. Weather-related mortality: How heat, cold, and heat waves affect mortality in the united states. Epidemiology. 2009;20(2):205–213. doi: 10.1097/EDE.0b013e318190ee08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccini M, Biggeri A, Accetta G, et al. Heat effects on mortality in 15 european cities. Epidemiology. 2008;19(5):711–719. doi: 10.1097/EDE.0b013e318176bfcd. [DOI] [PubMed] [Google Scholar]
- Bauer A, Kantelhardt JW, Barthel P, et al. Deceleration capacity of heart rate as a predictor of mortality after myocardial infarction: Cohort study. Lancet. 2006;367(9523):1674–1681. doi: 10.1016/S0140-6736(06)68735-7. [DOI] [PubMed] [Google Scholar]
- Bauer A, Malik M, Schmidt G, et al. Heart rate turbulence: Standards of measurement, physiological interpretation, and clinical use: International society for holter and noninvasive electrophysiology consensus. J Am Coll Cardiol. 2008;52(17):1353–1365. doi: 10.1016/j.jacc.2008.07.041. [DOI] [PubMed] [Google Scholar]
- Bazett HC. An analysis of the time-relations of electrocardiograms. Heart. 1920;7:353–370. [Google Scholar]
- Bhaskaran K, Hajat S, Haines A, Herrett E, Wilkinson P, Smeeth L. Effects of ambient temperature on the incidence of myocardial infarction. Heart. 2009;95(21):1760–1769. doi: 10.1136/hrt.2009.175000. [DOI] [PubMed] [Google Scholar]
- Bigger JT, Jr, Fleiss JL, Steinman RC, Rolnitzky LM, Kleiger RE, Rottman JN. Correlations among time and frequency domain measures of heart period variability two weeks after acute myocardial infarction. Am J Cardiol. 1992;69(9):891–898. doi: 10.1016/0002-9149(92)90788-z. [DOI] [PubMed] [Google Scholar]
- Braga AL, Zanobetti A, Schwartz J. The effect of weather on respiratory and cardiovascular deaths in 12 U.S. cities. Environ Health Perspect. 2002;110(9):859–863. doi: 10.1289/ehp.02110859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook RD, Rajagopalan S, Pope CA, 3rd, et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the american heart association. Circulation. 2010;121(21):2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- Bruce-Low SS, Cotterrell D, Jones GE. Heart rate variability during high ambient heat exposure. Aviat Space Environ Med. 2006;77(9):915–920. [PubMed] [Google Scholar]
- Chang CL, Shipley M, Marmot M, Poulter N. Lower ambient temperature was associated with an increased risk of hospitalization for stroke and acute myocardial infarction in young women. J Clin Epidemiol. 2004;57(7):749–757. doi: 10.1016/j.jclinepi.2003.10.016. [DOI] [PubMed] [Google Scholar]
- Cygankiewicz I, Wranicz JK, Bolinska H, Zaslonka J, Zareba W. Relationship between heart rate turbulence and heart rate, heart rate variability, and number of ventricular premature beats in coronary patients. J Cardiovasc Electrophysiol. 2004;15(7):731–737. doi: 10.1046/j.1540-8167.2004.03613.x. [DOI] [PubMed] [Google Scholar]
- Cygankiewicz I, Zareba W, Vazquez R, et al. Heart rate turbulence predicts all-cause mortality and sudden death in congestive heart failure patients. Heart Rhythm. 2008;5(8):1095–1102. doi: 10.1016/j.hrthm.2008.04.017. [DOI] [PubMed] [Google Scholar]
- Goldstein DS, Bentho O, Park MY, Sharabi Y. Low-frequency power of heart rate variability is not a measure of cardiac sympathetic tone but may be a measure of modulation of cardiac autonomic outflows by baroreflexes. Exp Physiol. 2011;96(12):1255–1261. doi: 10.1113/expphysiol.2010.056259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman PG, Dockery DW, Clancy L. Cause-specific mortality and the extended effects of particulate pollution and temperature exposure. Environ Health Perspect. 2004;112(2):179–185. doi: 10.1289/ehp.6451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Y, Barnett AG, Pan X, Yu W, Tong S. The impact of temperature on mortality in tianjin, china: A case-crossover design with A distributed lag non-linear model. Environ Health Perspect. 2011 doi: 10.1289/ehp.1103598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampel R, Schneider A, Bruske I, et al. Altered cardiac repolarization in association with air pollution and air temperature among myocardial infarction survivors. Environ Health Perspect. 2010;118(12):1755–1761. doi: 10.1289/ehp.1001995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Barnett AG, Wang X, Vaneckova P, FitzGerald G, Tong S. Projecting future heat-related mortality under climate change scenarios: A systematic review. Environ Health Perspect. 2011;119(12):1681–1690. doi: 10.1289/ehp.1103456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IPCC. Contribution of working group II to the fourth assessment report of the intergovernmental panel on climate change. Cambridge: Cambridge University Press; 2007. Climate change 2007: Impacts, adaptation and vulnerability. [Google Scholar]
- Kovats RS, Hajat S, Wilkinson P. Contrasting patterns of mortality and hospital admissions during hot weather and heat waves in greater london, UK. Occup Environ Med. 2004;61(11):893–898. doi: 10.1136/oem.2003.012047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewek J, Wranicz JK, Guzik P, Chudzik M, Ruta J, Cygankiewicz I. Clinical and electrocardiographic covariates of deceleration capacity in patients with ST-segment elevation myocardial infarction. Cardiol J. 2009;16(6):528–534. [PubMed] [Google Scholar]
- Liang WM, Liu WP, Chou SY, Kuo HW. Ambient temperature and emergency room admissions for acute coronary syndrome in taiwan. Int J Biometeorol. 2008;52(3):223–229. doi: 10.1007/s00484-007-0116-5. [DOI] [PubMed] [Google Scholar]
- Lin S, Luo M, Walker RJ, Liu X, Hwang SA, Chinery R. Extreme high temperatures and hospital admissions for respiratory and cardiovascular diseases. Epidemiology. 2009;20(5):738–746. doi: 10.1097/EDE.0b013e3181ad5522. [DOI] [PubMed] [Google Scholar]
- Linares C, Diaz J. Impact of high temperatures on hospital admissions: Comparative analysis with previous studies about mortality (madrid) Eur J Public Health. 2008;18(3):317–322. doi: 10.1093/eurpub/ckm108. [DOI] [PubMed] [Google Scholar]
- Liu W, Lian Z, Liu Y. Heart rate variability at different thermal comfort levels. Eur J Appl Physiol. 2008;103(3):361–366. doi: 10.1007/s00421-008-0718-6. [DOI] [PubMed] [Google Scholar]
- McMichael AJ, Wilkinson P, Kovats RS, et al. International study of temperature, heat and urban mortality: The ‘ISOTHURM’ project. Int J Epidemiol. 2008;37(5):1121–1131. doi: 10.1093/ije/dyn086. [DOI] [PubMed] [Google Scholar]
- Muggeo VM, Hajat S. Modelling the non-linear multiple-lag effects of ambient temperature on mortality in santiago and palermo: A constrained segmented distributed lag approach. Occup Environ Med. 2009;66(9):584–591. doi: 10.1136/oem.2007.038653. [DOI] [PubMed] [Google Scholar]
- Okamoto-Mizuno K, Tsuzuki K, Mizuno K, Ohshiro Y. Effects of low ambient temperature on heart rate variability during sleep in humans. Eur J Appl Physiol. 2009;105(2):191–197. doi: 10.1007/s00421-008-0889-1. [DOI] [PubMed] [Google Scholar]
- Ren C, O’Neill MS, Park SK, Sparrow D, Vokonas P, Schwartz J. Ambient temperature, air pollution, and heart rate variability in an aging population. Am J Epidemiol. 2011;173(9):1013–1021. doi: 10.1093/aje/kwq477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich DQ, Zareba W, Beckett W, et al. Are ambient ultrafine, accumulation mode, and fine particles associated with adverse cardiac responses in patients undergoing cardiac rehabilitation? Environ Health Perspect. 2012;120(8):1162–1169. doi: 10.1289/ehp.1104262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocklov J, Ebi K, Forsberg B. Mortality related to temperature and persistent extreme temperatures: A study of cause-specific and age-stratified mortality. Occup Environ Med. 2011;68(7):531–536. doi: 10.1136/oem.2010.058818. [DOI] [PubMed] [Google Scholar]
- Schmidt G, Malik M, Barthel P, et al. Heart-rate turbulence after ventricular premature beats as a predictor of mortality after acute myocardial infarction. Lancet. 1999;353(9162):1390–1396. doi: 10.1016/S0140-6736(98)08428-1. [DOI] [PubMed] [Google Scholar]
- Schneider A, Hampel R, Ibald-Mulli A, et al. Changes in deceleration capacity of heart rate and heart rate variability induced by ambient air pollution in individuals with coronary artery disease. Part Fibre Toxicol. 2010;7:29-8977-7-29. doi: 10.1186/1743-8977-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider A, Schuh A, Maetzel FK, Ruckerl R, Breitner S, Peters A. Weather-induced ischemia and arrhythmia in patients undergoing cardiac rehabilitation: Another difference between men and women. Int J Biometeorol. 2008;52(6):535–547. doi: 10.1007/s00484-008-0144-9. [DOI] [PubMed] [Google Scholar]
- Stein PK, Barzilay JI. Relationship of abnormal heart rate turbulence and elevated CRP to cardiac mortality in low, intermediate, and high-risk older adults. J Cardiovasc Electrophysiol. 2011;22(2):122–127. doi: 10.1111/j.1540-8167.2010.01967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein PK, Deedwania P. Usefulness of abnormal heart rate turbulence to predict cardiovascular mortality in high-risk patients with acute myocardial infarction and left ventricular dysfunction (from the EPHESUS study) Am J Cardiol. 2009;103(11):1495–1499. doi: 10.1016/j.amjcard.2009.01.362. [DOI] [PubMed] [Google Scholar]
- U.S. EPA. Integrated science assessment for particulate matter. Research Triangle Park, NC: National Center for Environmental Assessment, Office of Research and Development; 2009. [Google Scholar]
- Yamamoto S, Iwamoto M, Inoue M, Harada N. Evaluation of the effect of heat exposure on the autonomic nervous system by heart rate variability and urinary catecholamines. J Occup Health. 2007;49(3):199–204. doi: 10.1539/joh.49.199. [DOI] [PubMed] [Google Scholar]
- Yao Y, Lian Z, Liu W, Jiang C, Liu Y, Lu H. Heart rate variation and electroencephalograph--the potential physiological factors for thermal comfort study. Indoor Air. 2009;19(2):93–101. doi: 10.1111/j.1600-0668.2008.00565.x. [DOI] [PubMed] [Google Scholar]
- Ye X, Wolff R, Yu W, Vaneckova P, Pan X, Tong S. Ambient temperature and morbidity: A review of epidemiological evidence. Environ Health Perspect. 2011 doi: 10.1289/ehp.1003198. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.