Abstract
Various methods are currently under investigation to preserve fertility in males treated with high-dose chemotherapy and radiation for malignant and nonmalignant disorders. Human umbilical cord mesenchymal stem cells (HUC-MSCs), which possess potent immunosuppressive function and secrete various cytokines and growth factors, have the potential clinical applications. As a potential alternative, we investigate whether injection of HUC-MSCs into the interstitial compartment of the testes to promote spermatogenic regeneration efficiently. HUC-MSCs were isolated from different sources of umbilical cords and injected into the interstitial space of one testis from 10 busulfan-treated mice (saline and HEK293 cells injections were performed in a separate set of mice) and the other testis remained uninjected. Three weeks after MSCs injection, Relative quantitative reverse transcription polymerase chain reaction was used to identify the expression of 10 of germ cell associated, which are all related to meiosis, demonstrated higher levels of spermatogenic gene expression (2–8 fold) in HUC-MSCs injected testes compared to the contralateral uninjected testes (five mice). Protein levels for germ cell-specific genes, miwi, vasa and synaptonemal complex protein (Scp3) were also higher in MSC-treated testes compared to injected controls 3 weeks after treatment. However, no different expression was detected in saline water and HEK293 cells injection control group. We have demonstrated HUC-MSCs could affect mouse germ cell-specific genes expression. The results also provide a possibility that the transplanted HUC-MSCs may promote the recovery of spermatogenesis. This study provides further evidence for preclinical therapeutic effects of HUC-MSCs, and explores a new approach to the treatment of azoospermia.
Keywords: azoospermia, germ cell specific genes, human umbilical cord-derived mesenchymal stem cells, infertility
INTRODUCTION
Infertility affects 7% of men worldwide. Azoospermia is one of the most common diseases of male infertility, with 20% of infertile men diagnosed with azoospermia.1,2 Currently, the most effective procedures for treatment of human infertility is assisted reproductive technique (ART) combined with drug therapy and surgical treatment. With recent advancements and well-establishment in ART in vitro fertilisation/intracytoplasmic sperm injection (ICSI), its application is becoming increasingly prevalent,3 particularly ICSI for treatment of severe male factor infertility including oligo-and azoospermia. However, this approach has its limitation if no spermatozoa can be retrieved. Hence, ART does not solve the reproductive health problems of male infertility patients in an absolutely satisfactory manner. To close this gap, stem cell research is the new hope for novel and effective therapy methods with a higher degree of safety and lower cost.
With the rapid development of stem cell research, stem cell-based clinical applications are becoming much more widespread. Specifically, mesenchymal stem cells (MSCs) are increasingly being investigated for applications in the growing field of regenerative medicine. Recent clinical trials have suggested the use of MSCs for therapeutic purposes can achieve highly successful results, including in autoimmune diseases,4,5 diseases of the nervous system,6,7 diabetes8 and hematopoietic disease.9 Among the types of MSCs, human umbilical cord-derived (HUC-MSCs), derived from Wharton's jelly (WJ), have been shown to be a valuable source of MSCs and can be used for cell therapy.10,11,12 HUC-MSCs, in addition to their prominent advantages of abundant supply, painless collection, and fast self-renewal, to the most important, have proved less immunologically alloreactive and been used for treating graft versus host disease in vivo.13 HUC-MSCs express MSC marker CD73, CD90, and do not express human leukocyte antigen in alloreactive transplantation. The immune suppression properties are great useful in transplantation cell therapy. Furthermore, HUC-MSCs can synthesize and secret endocrine factors, such as cytokines, to support the function of other cells (including embryonic stem cells, hematopoietic stem cells, immunological cells, and neuronal cells), and to migrate toward pathological areas.
Due to these advantages mentioned above, especially their low immunological alloreactivity,13 several studies carried out have achieved to improve spermatogenesis via the administration of MSCs, which achieve their therapeutic effect by differentiating into germ cells.14,15 Yue et al.16 showed that bone marrow stem cells could differentiate into putative germ cells after transplantation in mouse testis, but do not undergo the postmeiotic phase to produce mature sperm. It is hard to determine MSCs undergo germ cell differentiation or whether they colonized interstitial compartments and favored mouse spermatogonia to restart spermatogenesis. However, whether injection of HUC-MSCs directly into the interstitial compartment of the testis can affect or promote spermatogenic regeneration have not yet been determined.
We described that HUC-MSCs can affect mouse germ-cell-specific genes expression. To observe its curative effect, we injected HUC-MSCs into the testis of mice suffering from azoospermia caused by busulfan treatment. Our results demonstrate that the transplanted HUC-MSCs can increase expression of the germ cell-specific genes, and potentially probably promote recovery of spermatogenesis. This study is to provide the evidence necessary for further therapeutic effects of preclinical research to be carried out and explore a new means of treating azoospermia.
MATERIALS AND METHODS
Ethics statement and animals
All the involving animals were approved by the Animal Care and Use Committee of Huazhong University of Science and Technology. All the experimental procedures involving animals as described here were approved by the Animal Care and Use Committee of Huazhong University of Science and Technology. Specific pathogen free grade male BALB/c mice (8 weeks old, average weight 23.5 g, No. 4200601018) were obtained from Center for Diseases Control and Prevention of Hubei Province, China. They were kept under standard conditions, at 22°C–25°C with a 12-h light/dark cycle, five mice/cage, with food and water freely available throughout the study.
Azoospermia model and hematoxylin and eosin (HE) staining
Busulfan (Sigma, St. Louis, USA) was dissolved in dimethyl sulfoxide (DMSO) (Cryoserv, Bad Soden, Germany) to a concentration of 10 mg ml−1.17 Immediately prior to dosing, 0.1–0.2 ml of isotonic saline water was added to approximately 0.1 ml BU–DMSO solution for further dilution. A total of 45 male BALB/c mice aged 8 weeks (22–25 g) were weighed and given a single injection. All the mice were given a single dose of 35 mg kg−1 busulfan as previously described.18 At 5 weeks after the single injection, the mice survived were prepared for transplantation. The testes of 5 mice were selected for histology examination by HE staining. HE staining was performed as previously described.19
Cell culture
Parent's written consent for tissue donation was required prior to the collection of umbilical cords from full-term birth babies according to the regulation of Wuhan Shangzhi Maternity Hospital and Institutional Review Board (IRB) of Huazhong University of Science and Technology. Moreover, we got a formal written waiver for ethics approval from IRB of Huazhong University of Science and Technology because no human subject in this study involved. HUC was obtained from Wuhan Shangzhi Maternity Hospital. The method was as previously described.20 In brief, the procedure was based on the plastic adhesion capacities of MSCs alone. The umbilical cords from three different individuals were separately processed within 6 h of vaginal delivery. HUC-derived MSCs were isolated from the WJ without enzyme digestion or dissection. After washing in phosphate buffered saline (PBS) to remove blood on the surface, the vessels were removed and the cord was cut into small pieces (0.5–1 cm). The cells were cultured in Dulbecco's modified Eagle's Medium (DMEM) supplemented with antibiotics (penicillin 100 mg ml−1, streptomycin 10 mg ml−1,) and 10% fetal bovine serum (all from Gibco, NY, USA) in 5% CO2 in a 37°C incubator. The medium was changed every 3–4 days until the plastic adherent cells reached confluence. At this point, the cells were harvested with 0.05% trypsin/ethylenediaminetetraacetic acid (EDTA) and subcultured at a density of 3 × 103 cells cm−2. The third passage cultured cells were selected for use. HEK293 cells were cultured in DMEM medium containing 10% fetal bovine serum under standard condition of 5% CO2 at 37°C. Cells were harvested at a density of 6 × 105 cells ml−1.
Characterizing human umbilical cord-mesenchymal stem cells by flow cytometry
Human umbilical cord-MSCs from WJ after harvesting at the third passage were immediately treated with 0.05% trypsin-EDTA and incubated with 1 μg 10−6 cells fluorescein isothiocynate-conjugated or phycoerythryne-conjugated antibodies at 4°C for 40 min in the dark. Anti-CD31, anti-CD105, anti-CD73 antibodies (Biolegend, San Diego, USA) were used. After washing, 1 × 106 stained cells were analyzed using a FACScan flow cytometer (Beckman, Fullerton, USA).
Karyotype analysis
Human umbilical cord-MSCs were cultured in medium supplemented with 10μl ml−1 of colcemid (Sigma, St. Louis, USA) for up to 4 h. Conventional cytogenetics was performed by the standard procedures and G banding.21 The karyotypes were described according to the International System for Human Cytogenetic Nomenclature (2009). Briefly, 50 μl KaryoMAX™ Colcemid Solution (Gibco, NY, USA) was added to 50%–75% confluent cells in a 6 cm dish. The cells were incubated for an additional 30 min. The cells were then rinsed with PBS and trypsinized for 3 min, transferred to a centrifuge tube and centrifuged for 10 min at 1000 rpm. Then the supernatant was removed and the cells were resuspended in 10 ml of pre-warmed hypotonic solution (0.075 mol l−1 KCl), incubated at 37°C for 30 min and centrifuged for 10 min at 1000 rpm. The supernatant was removed, the cellular sediment agitated and 10 ml of fresh, ice-cold fixative made up of one part acetic acid to three parts methanol was added dropwise. This cell suspension was chilled at 4°C for 30 min. This stage was repeated. At this stage, the preparation was stained with Giemsa.
Adipogenic and osteogenic differentiation
To induce adipogenic differentiation, cells were stained with Oil Red O as previously described.22 1 × 104 cells cm−2 were cultured in 24-well plates in DMEM high glucose (Sigma, St. Louis, USA) supplemented with 10% FBS, 0.5 mmol l−1 isobutyl-methylxantine, 1 μmol l−1 dexame thasone, 5 μg ml−1 insulin and 150 μmol l−1 indomethacin (Sigma, St. Louis, USA). The cells were cultured, replacing the medium every 3 days. After 21 days of culture, the cells contained lipid droplets; they were fixed in a 10% solution of formaldehyde in aqueous phosphate buffer for about 1 h. Cells were stained with Oil red O solution.
To induce osteogenic differentiation, 1 × 104 cells cm−2 were cultured in 24-well plates in DMEM (Sigma, St. Louis, USA) supplemented with 10% FBS, 10 mmol l−1 β-glycerophosphate (Sigma, St. Louis, USA), 0.2 mmol l−1 ascorbic acid (Sigma, St. Louis, USA), and 100 nmol l−1 dexamethasone (Sigma, St. Louis, USA). Cells were cultured for 25 days, replacing the medium every 3 days. Then the cells were fixed with 10% formalin (Sigma, St. Louis, USA) for 15 min and assessed by Von Kossa staining.23
Cell transplantation
The third passage of HUC-MSCs was prepared for transplantation. After harvesting, the cells were pelleted by centrifugation at 600 g for 5 min, after which a single-cell suspension was obtained by gently digesting in calcium-and magnesium-free Hanks’ balanced salt solution. The cells were then counted, pelleted by centrifugation at 600 g for 5 min, and resuspended in physiological saline at a concentration of 1 × 107 cells ml−1. All 40 busulfan-treated mice were randomly divided into four groups (each group included 10 mice): group 1 was model mice without injection as a blank control. Group 2 was injected with physiological saline as a negative control in the left side testis. Group 3 was injected with HEK293 cells also in the left side testis. Group 4 was injected with HUC-MSCs in the left side testis. The mice were positioned supine decubitus on the operation table. Microinjection needles were constructed from 20 μl glass micropipettes. A small incision was made and 10 μl of the cell suspension (approximately 1 × 105 cells) was directly injected under the tunica albuginea.
Relative quantitative reverse transcription-polymerase chain reaction
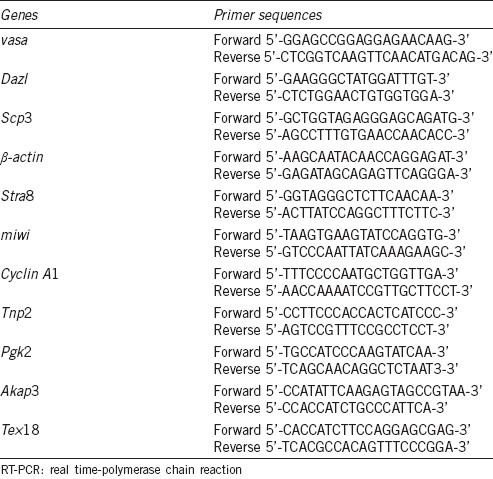
The expression level of meiosis related genes (Table 1) was analyzed by relative quantitative real time PCR. Three weeks after injection, five mice of each group were selected and total ribonucleic acid (RNA) was extracted from testis using Tri-Reagent (Sigma, St. Louis, USA), according to the manufacturer's recommendations. Subsequently, RNA isolated from the tissues was reverse transcribed with Mmlv reverse transcriptase (Fermentas, Glen Burnie, USA) for 1 h at 42°C in the presence of an oligoT primer. PCR primer sequences are listed in Table 1 (Shenggong, Shanghai, China).
Table 1.
Primer sequences for RT-PCR
The amplification reaction was performed using SYBR Green I fluorescence with M × 3000P thermocycler (Stratagene, Santa Clara, USA) under the following conditions: complementary DNA was initially denaturated at 95°C for 10 min, then amplified for 35 cycles, 95°C for 30 s, annealing at 60°C for 30 s, and extension 72°C for 30 s, and 8 s at 85°C (fluorescence data was acquired). A melting curve was generated at the end of every run. The expression of target genes was measured relative to that of β-actin as an internal gene. All samples were performed in triplicate. After amplification, the PCR products were examined on a 1.5% agarose gel and photographed under ultraviolet light.
Western blot analysis
Three weeks after injection, five mice of each group were selected and western blots were performed as described previously.24 Total testis tissue protein was rinsed twice with PBS and homogenized using a lysis buffer (Roche Applied Science, Indianapolis, USA). The protein concentration of the tissue homogenates was determined with a bicinchoninic acid protein assay kit (Pierce Biotechnology, Rockford, USA). Equal amounts of proteins were loaded onto a 10% acrylamide gel. Proteins were separated by electrophoresis and transferred to membranes. The membranes were blocked and incubated with mouse monoclonal antibodies against Piwi-like protein 1 (miwi), DEAD (Asp-Glu-Ala-Asp) box polypeptide 4 (DDX4, vasa) and synaptonemal complex protein 3 (Scp3) (1:2000; Santa Cruz, CA, USA) overnight at 4°C. After washing, the membranes were incubated for 60 min with horseradish peroxidase-linked goat anti-mouse IgG (1:1000; Santa Cruz, CA, USA). Mouse monoclonal antibodies against β-actin (1:2000) were used as a control.
Statistical analysis
Each of the experiments, from different HUC-MSCs transplantation to gene expression detecting, was performed in triplicate. Data are from the experiment presented as a mean ± standard deviation. Statistical analyses using SPSS 14 software (SPSS, Chicago, USA) were performed using the Student's t-test. All P values ≤ 0.05 were considered as statistically significant.
RESULTS
Histological examination of testis
After busulfan administration (35 mg kg−1), the testes of five mice were selected for the HE staining. Testicular sections show most of the seminiferous tubules walls became thinner at the end of week 5, the wall consisted of seminiferous epithelial cells and the spermatogonium is in the outer layer. Spermatogenic cells, including spermatocytes, spermatids, and spermatozoa were severely depleted (Figure 1).
Figure 1.
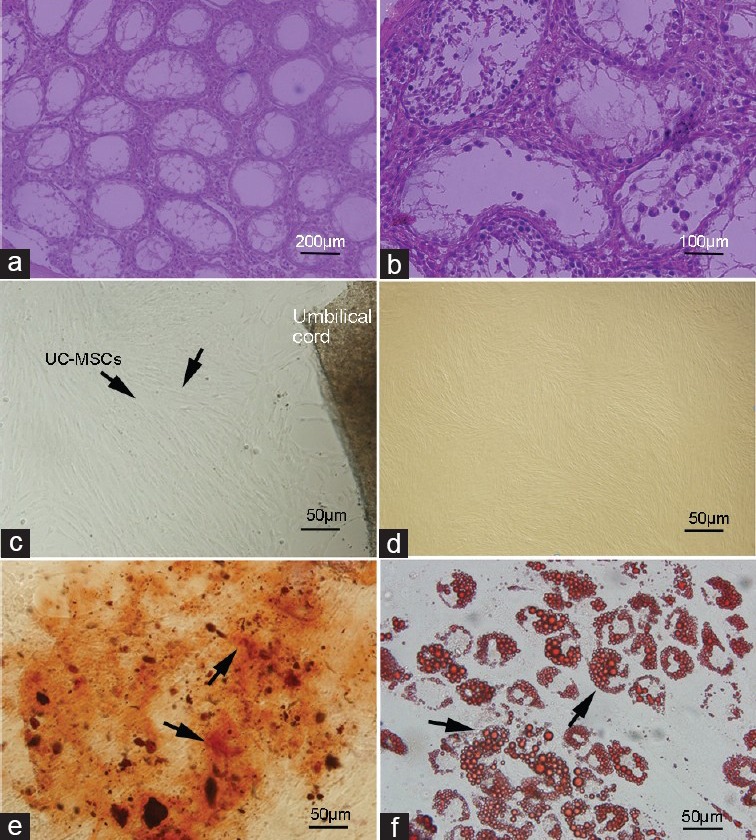
Histological examination of testis, cell culture and differentiation potential detection. Histological examination of testis sections from 5-week-old busulfan treated mice. Paraffin sections were stained with hematoxylin and eosin. 5 weeks after busulfan treatment, endogenous spermatogenesis was destroyed, and the testes of most are depleted in germ cells although they contain somatic cells and spermatogonia (a) ×200 (b) ×400. The umbilical cord samples were cut into small pieces, after 2 weeks, the cells migrate out of the tissue, and reach confluence. (c) Most of the cells were spindle-shaped and fibroblast-like. (d) At the third passage, adherent cells had the mesenchymal stem cell (MSC)-like phenotype. Differentiation capacity of human umbilical cord (HUC)-MSCs after expansion was detected. HUC-MSCs were induced to osteogenic (Von Kossa staining) (e) at days 21) lines and differentiate along adipogenic (Oil Red O staining) (f) at days 25). Multi-potent differentiation of HUC-MSCs was demonstrated.
Cell culture and characterizing of cells
The umbilical cord tissues were cut into small pieces, after 2 weeks, the cells migrate out of the tissue and reach confluence. Most of the cells were spindle-shaped and fibroblast-like (Figure 1c). At the third passage, adherent cells had the MSC-like phenotype (Figure 1b). After 21 days of osteogenic differentiation induction, mineralized deposits were observed. The presence of mineralized bone matrix was shown by Von Kossa staining (Figure 1e). After induction, adipogenic differentiation of HUC-MSCs was observable between 15 and 25 days. The cells contained a large amount of small lipid vacuoles stained using Oil Red O solution at 25 days (Figure 1f).
Immunostaining of HUC-MSCs showed strong positive signals against CD73 and CD 105. Cells were not immunostained by CD31 which is a marker of endothelial cells. These results indicate that cells which are isolated from WJ of HUC are not hematopoietic origin (Figure 2a). Normal karyotype observed with HUC-MSCs at passages 3 (Figure 2b).
Figure 2.
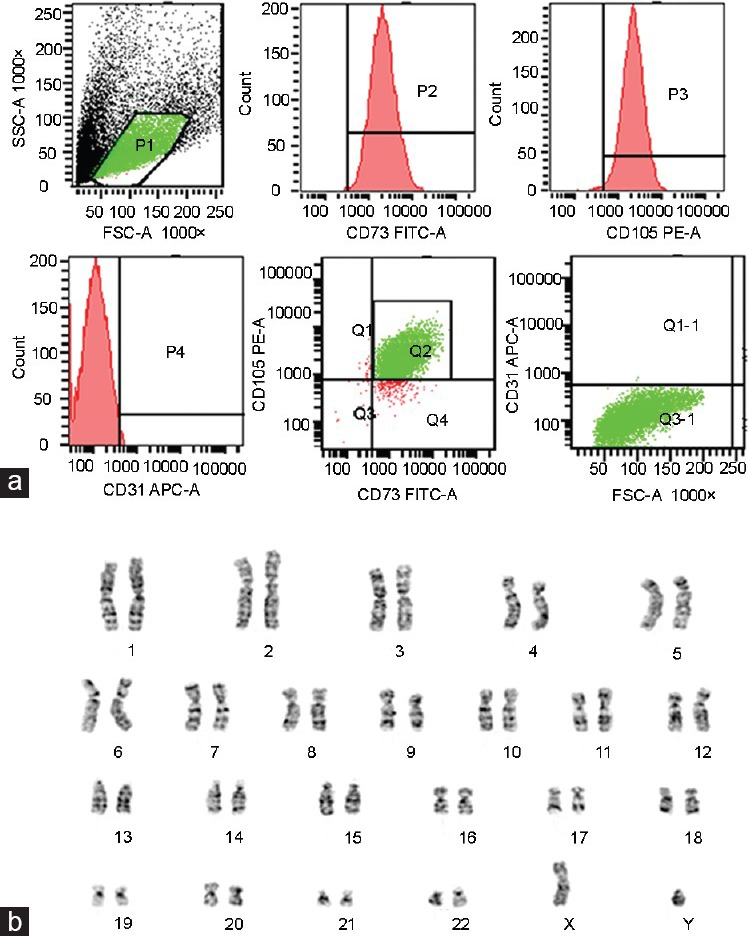
Immunophenotype and karyotype of human umbilical cord mesenchymal stem cells (HUC-MSCs). (a) The immunophenotype of HUC-MSCs as analyzed by flow cytometry assay. Representative flow cytometry analysis of HUC-MSCs after expansion to the third passage when labeled with typical HUC-MSCs antibodies against human antigens CD73, CD105 and CD31. (b) Normal karyotype observed with HUC-MSCs at passages 3.
Relative quantitative reverse transcription-polymerase chain reaction analysis of germ cell specific genes
As mentioned in the introduction, the main task of this study is to determine whether the HUC-MSCs which were injected into testis affects the spermatogenesis. Therefore, we focused on the different testis spermatogenic genes expression level between the HUC-MSCs injected and the control side. At week 3 after azoospermia model were prepare, five mice were chosen randomly and were evaluated separately. The expression of genes involved in germ cell development, which expressed especially during meiosis, was examined by relative quantitative reverse transcriptase-PCR. The genes which expressed stage-specific during meiotic we selected, including Dazl, DDX4 (vasa), Stra8, Scp3, Cyclin A1, Tnp2, Pgk2, miwi, Tex 18, and Akap3, as shown in Figure 3, the expression of meiosis associated genes in injected testis was higher than the other side which as a control. Meanwhile, when the saline water and HEK293 cells were separately injected into the testis as a negative group, the expression of all the genes we detected were unchanged compared to control side testis (Figure 3).
Figure 3.
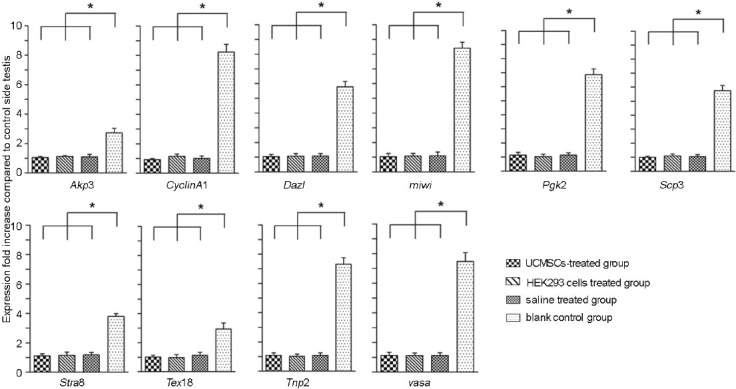
Relative quantitative reverse transcription polymerase chain reaction (RT-PCR) analysis of germ cell specific genes. Relative messenger ribonucleic acid expression levels were analyzed by relative quantitative RT-PCR. Each group contains five mice. One side of the testis (left) was injected with saline, HEK293 cells and HUC-MSCs separately. The other side (right) was as control without injection. After 3 weeks,10 of germ cell associated genes were detected. β-actin was used as an internal gene control. The value of each mouse was calculated by the testis which injected compared to the control side. Five mice were calculated for each gene. Compared to the saline-treated group and HEK293 cells group, the expression of germ-cell-specific-genes in UC-MSCs-injected group was higher than the control sides. Results are expressed as the mean ± s.d. for experiments * P ≤ 0.05.
Western blot analysis
Western blot analysis was performed to further confirm the protein expression of three germ cell-specific genes in the testis of injecting HUC-MSCs comparing to control side (Figure 4). Five mice were chosen randomly and different animals were evaluated separately. The testes of five mice were selected and the expression of miwi, vasa, Scp3 were detected at a higher level in injected testis than control side on week three after injecting HUC-MSCs. The expression of β-actin was used as an internal gene (P ≤ 0.05). However, no difference can be detected between the testes which treated with saline and without treatment. The expression level of these three genes in saline-treated group was low. Meanwhile, when injecting HEK293 cells into the testes, as negative control group, no difference can be detected between two sides, and the level of expression was also very low. Totally, the level of protein expression which was detected by Western blot was very similar to mRNA expression by relative quantitative RT-PCR analysis.
Figure 4.
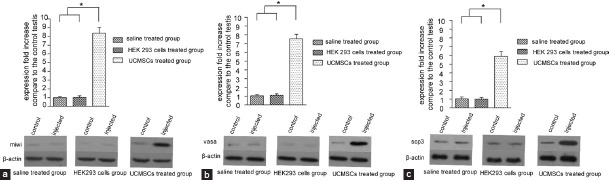
Western blot analysis of germ cell specific genes. (a) Detection of miwi, (b) vasa, (c) Scp3 protein expression by western blot analysis. The expression of β-Actin was used as a control gene. The statistics method is similar to messenger ribonucleic acid expression. In each group five mice were selected. Expression of miwi, vasa, Scp3 was detected at a higher level in injected testis than control side on week three after injecting HUC-MSCs compared to another group. Results are expressed as the mean ± standard deviation for experiments * P ≤ 0.05.
DISCUSSION
Our study shows that transplantation of HUC-derived MSCs is an effective approach to increase the expression of germ cell specific genes in busulfan-treated mice and it is the survived mouse germ cells after busulfan treatment that proliferates to yield meiotic germ cells. We evaluated two side testes of each mouse by separate not by pooled, to avoid the variant between individuals. Specifically, we choose three different individual sources of HUC-MSCs in this study. And the consistent results indicate that HUC-MSCs from three different individual sources have the similar cellular property and function. Independently-derived preparations of HUC-MSCs would help to account for variation introduced by the biological source of injected materials. Thus, the repeatability of these data are further confirmed. We use busulfan to treat the BLAB/c mice to get azoospermia model. Busulfan is a kind of anticancer drug and myeloablative alkylating agent, which can affect the immunologic function of the animals seriously. When using busulfan to induce the azoospermia model, immunity of the animals is suppressed severely. It is reported that, to determine the highest busulfan dose that immunocompetent mice can tolerate, mice were treated with increasing doses and survival was evaluated. At a dose of 35 mg kg−1, all mice lived up to 6 weeks, while at doses of 50 and 75 mg kg−1, all mice died within a few hours following treatment.25 In our experiment, we use BALB/c mouse and the injection of dose of 35 mg kg−1 busulfan is the optimal dose for inducing azoospermia model.
Stem cell-based therapies represent one of the major challenges of translational medicine. HUC-MSCs in the WJ of umbilical cord have many properties that can be easily obtained and processed, and expand rapidly compared to other stem cells. Thus, HUC-MSCs may have high therapeutic potential in the field of stem cell therapies. Work from other laboratories suggests HUC-MSCs transplanted into brain striatum are viable for 4 months without immunological suppression.26 Moreover, transplantation with HUC-MSCs in rat spinal cord and liver also promotes the regeneration of corticospinal fibers and controls the type 1 diabetes, respectively.27 Together these studies suggest that HUC-MSCs may be an important source for allogeneic transplantation therapy in the future.
Several researches indicate that stem cell therapy promises to be a potential in infertility. Many stem cells, such as ESCs,28,29 bone marrow stem cells,30 human fetal lung-MSCs14 were the candidate for the germ cell formation. However, whether MSCs can undergo germ cell differentiation and form spermatogonia in vivo is controversial and the literature in support of this concept that MSCs can colonized interstitial compartments and contribute directly to spermatogenesis is limited. Nayernia et al.30 showed that murine BM-MSCs could differentiate into male germ cells for the first time. Lue et al.16 showed that BM-MSCs, transplanted into testis of a busulfan-treated infertility mouse model, appeared to differentiate into germ cells. Tournaye's group in 2009 demonstrated that stem cells from bone marrow do not contribute to spermatogenesis upon transplantation.31 In our study, we injected HUC-MSCs into the interstitium of experimentally germ cell deficient mouse testes, and detecting the spermatogenesis genes expression. In this experimental setting, due to the change of the cellular microenvironment (niche), the possibility that HUC-MSCs differentiate into germ cells may decrease when injecting HUC-MSCs into the interstitium of the testis. HUC-MSCs may provide signals that might activate appropriate metabolic pathways.
Spermatogenesis occurs in successive mitosis, meiosis and spermiogenesis in the testis and the whole process of male germ cell differentiation from spermatogonia to sperm is under the complex regulation of many factors.32 A large number of stage-specific genes during spermatogenesis encoded proteins have been identified, and these genes play pivotal and specific roles in different steps of germ cell developmental regulation. Here, we select different genes expressed during meiosis which are stage-specific. Interestingly, there was no genes expression change when control cells injected into testis during meiosis. However, after 3 weeks of HUC-MSCs injection, mRNA levels of 10 genes related to meiosis were increased. We detected the protein expression of the germ cell specific gene miwi, vasa, and Scp3. There were distinctly differences between the control side and the injected testis. These genes were suggested to be important in spermatocytes, and they also might play pivotal role in meiosis. Prior studies from other laboratories state that bone marrow stem cells differentiate into germ cells in mice after transplantation into the testis. And this cell type did not pass through meiosis to develop into mature sperm. The differential potential of bone marrow stem cells in vitro reprogramming should be further investigated. Our thoughts focus on the endocrinological functions of the HUC-MSCs to explain the rationale why injection of MSCs into the interstitium of the testis should have an influence on the testicular stem cells in the seminiferous tubules. The differentiation of germ cells occurs in the tubular seminiferous epithelium depending on the supporting of sertoli cells and leydig cells and secondary mediators such as hormones and cytokines. That is, HUC-MSCs injection can alter the expression of genes through several indirect interactions and the release of more cytokines or growth factors from the HUC-MSCs which may be necessary for maintenance of stem cells and affect secretion function by supporting cells in seminiferous epithelium. Further studies were required to understand the exact mechanisms of HUC-MSCs functions in gene expression regulation during spermatogenesis and the promotion of recovery of spermatogenesis after the transplantation of HUC-MSCs in the testis. Our data also provide new information for further studies of using HUC-MSCs as clinical cellular repair potential and is crucial to raise the possibility that HUC-MSCs as nearer match clinical method to treat azoospermia.
The major concern is the possibility that the effects observed are the result of an inflammatory response in the injected testes resulting from injection of human cells. HUC-MSCs13 just like other MSCs, in addition to the regenerative properties, possess an immunosuppression and immunoregulatory capacity, and elicit immunosuppressive effects in many cases. HUC-MSCs are immunoprivileged cells, due to the low expression of class II major histocompatibility complex and co-stimulatory molecules in their cell surface, making them avoidable to the immune system.10 They are implicated in immune regulation resulting from suppressing initial immune responses and clean up inflammatory factors. Such immense plasticity makes them extremely valuable for stem cell-based therapy in the treatment of some illnesses. Actually, in our experiment we adopt the third passage HUC-MSCs, which is the lower passage MSCs can be used in transplantation. The regulation effects are most likely due to soluble factors secreted by MSCs.10 Previous clinical trials showed that the immunosuppressive properties of MSCs are the basis of their use in treating human graft-versus-host disease.33 Moreover, HUC-MSCs unlike ESCs, do not induce tumors after transplantation.34 So the xenotransplantation of HUC-MSCs into mouse testis was tolerated without immunological rejection or resulting in death.35 However, the main mechanism of the HUC-MSCs paracrine function to the testis is still elusive.
To control for the potential role inflammation, we choose HEK293 cells as human cells control to inject into the testes of model mice. The result indicates that HEK293 cells could not result in the increased expression of spermatogenic genes or stimulate spermatogenesis compared to the opposite testis without injection. This result demonstrates that, injecting human cells into the testis of busulfan treated mouse could not affect the expression of germ cell specific genes in the testis. So we can infer that, the reason of increased expression of germ cell specific genes when injecting HUC-MSCs into the testis of the busulfan-treated mouse, do not caused by the human cells injection or inflammation. Similarly, from the group of saline-treated results, indicates that compared to untreated testes, the expression level of germ cell associated genes do not change and the progress of spermatogenesis recovery did not appear.
CONCLUSIONS
Current data do not support the possibility that HUC-MSCs transdifferentiate into spermatogonia in vivo directly. Instead, indirect paracrine mechanisms are likely to promote spermatogenesis which requires further investigation. Our study provides experimental evidence in preclinical models of infertility suggesting the possible clinical benefits of HUC-MSCs. It is a promising candidate, at least partly, for promoting spermatogenesis. Before HUC-MSCs can be translated into human trials, the efficacy and safety of HUC-MSC-based treatment must require in depth evaluation for use in the field of regenerative medicine for male fertility treatment.
AUTHOR CONTRIBUTIONS
RFY carried out HUC-MSCs cultures, azoospermia model establishment, HE staining, adipogenic and osteogenic differentiation, cell transplantation, Relative quantitative RT-PCR and western blot, participated in the design of the study, performed the statistical analysis and drafted the manuscript. THL carried out flow cytometry, participated in HEK293 cell cultures, cell transplantation, and the statistical analysis. KZ carried out karyotype analysis, participated in cell transplantation, Relative quantitative RT-PCR. CLX conceived of the study, and participated in its design and coordination and helped to draft the manuscript. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
ACKNOWLEDGMENTS
This work is supported by Projects in the National Science and Technology Pillar Program during the twelfth five-year plan period (No. 2012BAI32B03) and a grant from Science and Technology Department of Hubei Province, China (Research and development project: No. 2008BCC007).
REFERENCES
- 1.Harton GL, Tempest HG. Chromosomal disorders and male infertility. Asian J Androl. 2012;14:32–9. doi: 10.1038/aja.2011.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.De Kretser DM. Male infertility. Lancet. 1997;349:787–90. doi: 10.1016/s0140-6736(96)08341-9. [DOI] [PubMed] [Google Scholar]
- 3.Silber SJ, Barbey N. Scientific molecular basis for treatment of reproductive failure in the human: An insight into the future. Biochim Biophys Acta. 2012;1822:1981–96. doi: 10.1016/j.bbadis.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Figueroa FE, Carrión F, Villanueva S, Khoury M. Mesenchymal stem cell treatment for autoimmune diseases: a critical review. Biol Res. 2012;45:269–77. doi: 10.4067/S0716-97602012000300008. [DOI] [PubMed] [Google Scholar]
- 5.Bernardo ME, Fibbe WE. Safety and efficacy of mesenchymal stromal cell therapy in autoimmune disorders. Ann N Y Acad Sci. 2012;1266:107–17. doi: 10.1111/j.1749-6632.2012.06667.x. [DOI] [PubMed] [Google Scholar]
- 6.Joyce N, Annett G, Wirthlin L, Olson S, Bauer G, et al. Mesenchymal stem cells for the treatment of neurodegenerative disease. Regen Med. 2010;5:933–46. doi: 10.2217/rme.10.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martino G, Franklin RJ, Baron Van Evercooren A, Kerr DA Stem Cells in Multiple Sclerosis (STEMS) Consensus Group. Stem cell transplantation in multiple sclerosis: current status and future prospects. Nat Rev Neurol. 2010;6:247–55. doi: 10.1038/nrneurol.2010.35. [DOI] [PubMed] [Google Scholar]
- 8.Domínguez-Bendala J, Lanzoni G, Inverardi L, Ricordi C. Concise review: mesenchymal stem cells for diabetes. Stem Cells Transl Med. 2012;1:59–63. doi: 10.5966/sctm.2011-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pelosi E, Castelli G, Testa U. Human umbilical cord is a unique and safe source of various types of stem cells suitable for treatment of hematological diseases and for regenerative medicine. Blood Cells Mol Dis. 2012;49:20–8. doi: 10.1016/j.bcmd.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 10.Weiss ML, Anderson C, Medicetty S, Seshareddy KB, Weiss RJ, et al. Immune properties of human umbilical cord Wharton's jelly-derived cells. Stem Cells. 2008;26:2865–74. doi: 10.1634/stemcells.2007-1028. [DOI] [PubMed] [Google Scholar]
- 11.Fan CG, Zhang QJ, Zhou JR. Therapeutic potentials of mesenchymal stem cells derived from human umbilical cord. Stem Cell Rev. 2011;7:195–207. doi: 10.1007/s12015-010-9168-8. [DOI] [PubMed] [Google Scholar]
- 12.Friedman R, Betancur M, Boissel L, Tuncer H, Cetrulo C, et al. Umbilical cord mesenchymal stem cells: adjuvants for human cell transplantation. Biol Blood Marrow Transplant. 2007;13:1477–86. doi: 10.1016/j.bbmt.2007.08.048. [DOI] [PubMed] [Google Scholar]
- 13.Cho PS, Messina DJ, Hirsh EL, Chi N, Goldman SN, et al. Immunogenicity of umbilical cord tissue derived cells. Blood. 2008;111:430–8. doi: 10.1182/blood-2007-03-078774. [DOI] [PubMed] [Google Scholar]
- 14.Hua J, Yu H, Dong W, Yang C, Gao Z, et al. Characterization of mesenchymal stem cells (MSCs) from human fetal lung: potential differentiation of germ cells. Tissue Cell. 2009;41:448–55. doi: 10.1016/j.tice.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 15.Huang P, Lin LM, Wu XY, Tang QL, Feng XY, et al. Differentiation of human umbilical cord Wharton's jelly-derived mesenchymal stem cells into germ-like cells in vitro. J Cell Biochem. 2010;109:747–54. doi: 10.1002/jcb.22453. [DOI] [PubMed] [Google Scholar]
- 16.Lue Y, Erkkila K, Liu PY, Ma K, Wang C, et al. Fate of bone marrow stem cells transplanted into the testis: potential implication for men with testicular failure. Am J Pathol. 2007;170:899–908. doi: 10.2353/ajpath.2007.060543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schuler US, Renner UD, Kroschinsky F, Johne C, Jenke A, et al. Intravenous busulphan for conditioning before autologous or allogeneic human blood stem cell transplantation. Br J Haematol. 2001;114:944–50. doi: 10.1046/j.1365-2141.2001.03044.x. [DOI] [PubMed] [Google Scholar]
- 18.Brinster RL, Zimmermann JW. Spermatogenesis following male germ-cell transplantation. Proc Natl Acad Sci U S A. 1994;91:11298–302. doi: 10.1073/pnas.91.24.11298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagaosa K, Kishimoto A, Kizu R, Nakagawa A, Shiratsuchi A, et al. Perturbation of spermatogenesis by androgen antagonists directly injected into seminiferous tubules of live mice. Reproduction. 2007;133:21–7. doi: 10.1530/REP-06-0236. [DOI] [PubMed] [Google Scholar]
- 20.Fang TC, Pang CY, Chiu SC, Ding DC, Tsai RK. Renoprotective effect of human umbilical cord-derived mesenchymal stem cells in immunodeficient mice suffering from acute kidney injury. PLoS One. 2012;7:e46504. doi: 10.1371/journal.pone.0046504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qiao C, Xu W, Zhu W, Hu J, Qian H, et al. Human mesenchymal stem cells isolated from the umbilical cord. Cell Biol Int. 2008;32:8–15. doi: 10.1016/j.cellbi.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 22.Peng KW, Liou YM. Differential role of actin-binding proteins in controlling the adipogenic differentiation of human CD105-positive Wharton's Jelly cells. Biochim Biophys Acta. 2012;1820:469–81. doi: 10.1016/j.bbagen.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 23.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–7. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 24.Li H, Ding X, Guo C, Guan H, Xiong C. Immunization of male mice with B-cell epitopes in transmembrane domains of CatSper1 inhibits fertility. Fertil Steril. 2012;97:445–52. doi: 10.1016/j.fertnstert.2011.11.043. [DOI] [PubMed] [Google Scholar]
- 25.Robert-Richard E, Ged C, Ortet J, Santarelli X, Lamrissi-Garcia I, et al. Human cell engraftment after busulfan or irradiation conditioning of NOD/SCID mice. Haematologica. 2006;91:1384. [PubMed] [Google Scholar]
- 26.Yang CC, Shih YH, Ko MH, Hsu SY, Cheng H, et al. Transplantation of human umbilical mesenchymal stem cells from Wharton's jelly after complete transection of the rat spinal cord. PLoS One. 2008;3:e3336. doi: 10.1371/journal.pone.0003336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsai PJ, Wang HS, Shyr YM, Weng ZC, Tai LC, et al. Transplantation of insulin-producing cells from umbilical cord mesenchymal stem cells for the treatment of streptozotocin-induced diabetic rats. J Biomed Sci. 2012;19:47. doi: 10.1186/1423-0127-19-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Toyooka Y, Tsunekawa N, Akasu R, Noce T. Embryonic stem cells can form germ cells in vitro. Proc Natl Acad Sci U S A. 2003;100:11457–62. doi: 10.1073/pnas.1932826100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Geijsen N, Horoschak M, Kim K, Gribnau J, Eggan K, et al. Derivation of embryonic germ cells and male gametes from embryonic stem cells. Nature. 2004;427:148–54. doi: 10.1038/nature02247. [DOI] [PubMed] [Google Scholar]
- 30.Nayernia K, Lee JH, Drusenheimer N, Nolte J, Wulf G, et al. Derivation of male germ cells from bone marrow stem cells. Lab Invest. 2006;86:654–63. doi: 10.1038/labinvest.3700429. [DOI] [PubMed] [Google Scholar]
- 31.Van Saen D, Goossens E, De Block G, Tournaye H. Bone marrow stem cells transplanted to the testis of sterile mice do not differentiate into spermatogonial stem cells and have no protective effect on fertility. Fertil Steril. 2009;91:1549–52. doi: 10.1016/j.fertnstert.2008.09.036. [DOI] [PubMed] [Google Scholar]
- 32.Guo R, Yu Z, Guan J, Ge Y, Ma J, et al. Stage-specific and tissue-specific expression characteristics of differentially expressed genes during mouse spermatogenesis. Mol Reprod Dev. 2004;67:264–72. doi: 10.1002/mrd.20026. [DOI] [PubMed] [Google Scholar]
- 33.McGuirk JP, Weiss ML. Promising cellular therapeutics for prevention or management of graft-versus-host disease (a review) Placenta. 2011;32(Suppl 4):S304–10. doi: 10.1016/j.placenta.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ma L, Zhou Z, Zhang D, Yang S, Wang J, et al. Immunosuppressive function of mesenchymal stem cells from human umbilical cord matrix in immune thrombocytopenia patients. Thromb Haemost. 2012;107:937–50. doi: 10.1160/TH11-08-0596. [DOI] [PubMed] [Google Scholar]
- 35.Li J, Ezzelarab MB, Cooper DK. Do mesenchymal stem cells function across species barriers? Relevance for xenotransplantation. Xenotransplantation. 2012;19:273–85. doi: 10.1111/xen.12000. [DOI] [PMC free article] [PubMed] [Google Scholar]


