Abstract
Male accessory gland inflammation or infection (MAGI) is a potentially underdiagnosed complication of type 2 diabetes (DM2); specifically, we reported in a recent study that the frequency of MAGI was 43% among DM2 patients. In previous studies, we have demonstrated that diabetic autonomic neuropathy (DAN) is associated with a peculiar ultrasound characterization of the seminal vesicles (SVs) in DM2 patients. The aim of the present study was to evaluate the frequency of MAGI in two different categories of DM2 patients (i.e. patients with and without symptoms that possibly reflect DAN) and the respective ultrasound characterizations. Sixty DM2 patients with a mean (± s.e.m.) age of 42.0 ± 6.0 years (range: 34–47 years) were classified according to the presence or the absence of symptoms that could possibly reflect DAN (group A: DM2 with symptoms possibly reflecting DAN, n = 28 patients and group B: DM2 without symptoms possibly reflecting DAN, n = 32 patients). The patients in Group A exhibited a significantly higher frequency of MAGI compared with those in group B patients (P < 0.05); moreover, the Group A patients exhibited a significantly higher frequency of ultrasound signs suggestive of vesiculitis (P < 0.05). Finally, the concentrations of lymphocytes but not the concentrations of the leukocytes in the semen were significantly higher (P < 0.05) in group A compared with group B.
Keywords: diabetic neuropathy, male accessory gland inflammation or infection, male infertility
INTRODUCTION
Male accessory gland inflammation or infection (MAGI) has been identified as a diagnostic category with a negative impact on male reproductive function and fertility. They share the following common characteristics: they are common diseases, they primarily have a chronic course, they rarely cause obstruction of the seminal pathways and they can exhibit unpredictable intracanalicular spread to one or more sexual accessory glands of the reproductive tract on one or both sides.
We recently reported an increased frequency of MAGI among patients with type 2 diabetes (DM2) and assumed that MAGI may represent a possible undiagnosed complication of this condition.1 We also reported differential ultrasound characterizations of the seminal vesicles (SVs) in these patients,2 particularly among those with diabetic autonomic neuropathy (DAN).3
Conventionally, MAGIs are classified into uncomplicated (prostatitis) and complicated forms (prostatovesiculitis and prostato-vesciculo-epididymitis).4 In our clinical experience, ultrasound characterization represents a valid tool for the evaluation of the real anatomical extent of the inflammatory process.5
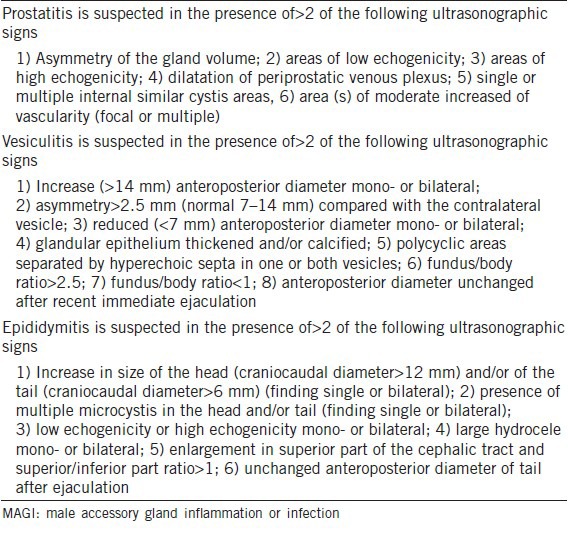
According to World Health Organization (WHO; 1993),6 MAGI is diagnosed when abnormal sperm parameters are found and associated with at least one factor A plus one factor B, one factor A plus one factor C, one factor B plus one factor C or two factors C (Table 1).
Table 1.
The clinical criteria adopted for the diagnosis of MAGI (WHO, 1993)6
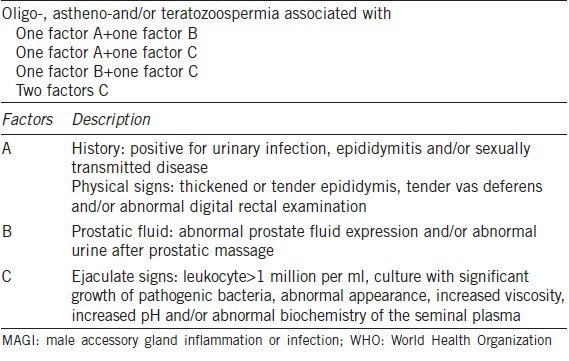
Based on these criteria, the aim of the present study was to evaluate the frequencies of MAGI in infertile DM2 patients, to compare patients with and without symptoms that possibly reflect DAN and to evaluate the ultrasonic characteristics of these patients.
PATIENTS AND METHODS
A total of 60 consecutive infertile DM2 patients were recruited over the last 2 years (June 2011–2013) from the Andrological Center of the University of Catania (Italy) during medical counseling for couples’ infertility and divided into the following two groups: group A, 28 DM2 patients with symptoms possibly reflecting DAN and group B, 32 DM2 patients without any symptoms that could possibly reflect DAN. Table 2 shows the main clinical characteristics of the two examined groups.
Table 2.
Main clinical characteristics of the two groups examined
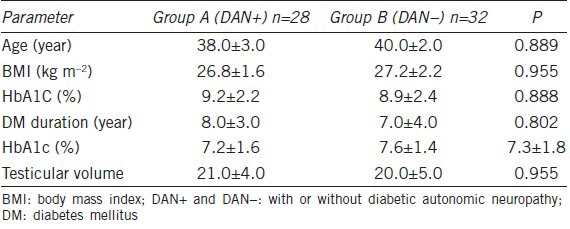
The enrolled patients had a mean (± standard error of the mean (s.e.m.)) age of 42.0 (±6.0) years (range: 34–47 years), a mean (± s.e.m.) body mass index (BMI) of 27.5 (±2.0) kg m−2 (range: 25–30 kg m−2), a mean (± s.e.m.) glycosylated hemoglobin level of 9.1 (±2.6%) (range 7.6%–10.6%) and a mean (± s.e.m.) duration of diabetes of 7.0 (±5.0) years (range: 2.0–12.0 years).
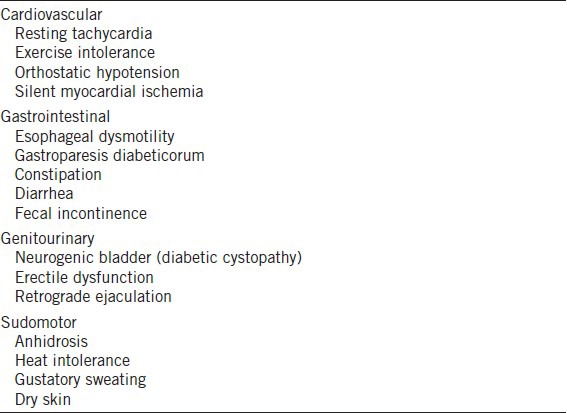
Diagnoses of diabetic neuropathy according to the guidelines of 19th Annual Diabetic Neuropathy Study Group of the European Association for the Study of Diabetes (NEURODIAB) and the 8th International Symposium on Diabetic Neuropathy in Toronto, Canada, 13–18 October 20097 had been made for all examined patients. According to these criteria, we arbitrarily selected only patients with symptoms that could possibly reflect complete autonomic neuropathy (two or more clinical cardiovascular and gastrointestinal and genitourinary and pseudomotor signs of DAN) (Table 3).
Table 3.
Symptoms possibly reflecting autonomic neuropathy detected in group A patients (at least two by type)
Each enrolled patient underwent a complete andrological diagnostic work-up that included the following: (i) a physical examination, (ii) a sperm analysis, (iii) a hormonal evaluation and (iv) an ultrasound evaluation.
Exclusion criteria
The exclusion criteria were as follows:
-
1
Ultrasound signs of proximal (epididymal) and/or distal (ampullo-prostato-vesicular) obstruction
-
2
Hormonal disorders (i.e. hypogonadism, hyperestrogenism, hyperprolactinemia and hypothyroidism). Specifically, endocrine factors can affect sexual function and cause long pauses during ejaculation in men with reduced sexual frequencies. Therefore, we arbitrarily chose to exclude these patients to minimize factors that promote stasis in the SV
-
3
Idiopathic orthostatic hypotension, Shy-Drager syndrome, Addison's disease, hypopituitarism, pheochromocytoma, hypovolemia, medications with anticholinergic or sympatholytic effects, amyloid neuropathy and other peripheral autonomic neuropathies.
Moreover, the patients were excluded if they exhibited any of the following:
Cigarette smoking, alcohol consumption, occupational chemical exposure, fever, drugs taken within the 3 months prior to enrollment in this study, azoospermia, testicular volume <15 ml (testicular volume is significantly correlated with testicular function; specifically, sperm parameters are subnormal in patients with total testicular volumes (right plus left testicular volumes) below 30 ml as measured by orchidometry,8 past or present cryptorchidism or varicocele.
Measurement of glycated hemoglobin
HbA1c was determined by high-pressure liquid chromatography. The equipment, calibration and controls were from the Bio-Rad company (USA) for the model Variant II. The reference ranges were 3.7%–6.2% and up to 48 nmol mol−1.
Measurement of serum hormone concentrations
The hormone assays were performed by electrochemiluminescence with a Hitachi-Roche (Cobas 6000) from Roche Diagnostics (Indianapolis, USA). The references interval were as follows: luteinizing hormone, 1.6–9.0 mIU ml−1; follicle-stimulating hormone, 2–12 mIU ml−1; estradiol, 8–43 pg ml−1; total testosterone, 2.8–8 ng ml−1 and prolactin, 4–15 ng ml−1. Blood samples were drawn from all examined patients between 08:00 and 10:00.
Sperm analysis
Semen samples were collected by masturbation into sterile containers following 2–7 days of sexual abstinence and were transported to the laboratory within 30 min of ejaculation. According to the 2010 WHO guidelines, each sample was evaluated for seminal volume, pH, sperm count, progressive motility, morphology and round cell concentration.9
Fructose levels in the semen were measured using a spectrophotometric method and were assessed one hour after ejaculation using the Mann resorcinol and HCl methods.10
The characterizations of the leukocytes in the semen were made with the traditional methods and flow cytometry for the correct identification of neutrophils and lymphocytes.
Seminal leukocytes (neutrophils)
The protocol used was adapted from that of Endtz.11 The working solution used for the test was obtained by adding 1 μl of H2O2 to 20 μl of a 0.09% 3,3‘- diaminobenzidine tetrahydrochloride stock solution (DAB, ISOPAC, Sigma, Milan, Italy) in 40% ethanol. In each assay, 20 μl of semen were incubated with 20 μl of working solution in an Eppendorf tube for 5 min at room temperature. Before setting up the slide, 40 μl of PBS was added. Peroxidase-positive cells were marked by yellow-brown-red staining, while peroxidase-negative cells remained colorless. At least 100 round cells were counted using an optical microscope at ×400 magnification, and the percentages of the peroxidase-positive and negative cells were evaluated. The total leukocyte counts are expressed in 106 ml−1 of semen.
Leukocytes flow cytometric analysis (lymphocytes)
The analyses were conducted with an EPICS XL Flow Cytometer (Coulter Electronics, IL, Italy), equipped with an argon laser at 488 nm and the following three fluorescence detectors: green (FL-1 at 525 nm), orange (FL-2 to 575 nm) and red (FL-3 at 620 nm). For each sample, 100 000 events were measured at low flow velocities and analyzed using Sistem II™, version 3.0.
To obtain the absolute leukocytes counts, 100 μl of each liquefied semen sample was incubated with a mixture containing Syto-16 green fluorescent nucleic acid stain to identify the spermatozoa and exclude debris (the final concentration was 200 nmol l−1, Molecular Probes, Eugene, Oregon, USA), 7-amino-actinomycin D (7-AAD Via-Probe, BD Pharmingen, San Diego, CA, USA) to assess viability, anti-CD45-APC (pan-leukocyte antigen) to recognize white blood cells and anti-CD16-PE for PMN recognition. The addition of 100 μl of Flow-Count™ Fluorospheres (Beckmann-Coulter, Fullerton, CA, USA) at 1034 beads per ml allowed for the determination of the absolute leukocyte count by flow cytometry. After incubation in the dark for 20 min at room temperature, 1 ml of PBS was added and the sample was analyzed by flow cytometry (FACSCalibur, Becton Dickinson, San Jose, CA, USA). For each test, 100 000 events were acquired.
Ultrasound evaluation
All patients underwent scrotal and transrectal ultrasound evaluation after one day of sexual abstinence before and one hour after ejaculation, using a transrectal 7.5 MHz biplan biconvex transducer (Esaote GPX Megas, Genova, Italy). We arbitrarily chose to examine patients after a single day of sexual abstinence to minimize factors that promote stasis in the SV.
The scrotal ultrasound evaluation was performed in two phases. In the first phase, the patient was in a supine position (with the penis resting on the suprapubic region), and in the second phase, the patient was in an upright position to allow for evaluations of reflux along the pampiniform plexus, testicular pain, testicular malposition and the extent of any fluid collections. The examination was performed with a GX Megas Esaote (Esaote SpA - Genova (Italy)) device, which was equipped with linear, high-resolution and high-frequency (7.5–14 MHz) probes dedicated to the study of soft body areas, a color Doppler for the detection of slow flow and scanning surface of at least 5 cm. Testicular volumes were calculated automatically by the ultrasound machine using the ellipsoid formula (length × width × thickness × 0.52). The testes were considered normal in size (volumes between 15 and 25 cm3), low-normal (volumes between 10 and 12 cm3) and hypotrophic (volumes less than 10 cm3).8 The parenchymal echo structures were considered normal when thin, densely packed and homogeneously deployed echoes were present. The presence of a finely inhomogeneous echo pattern and weakly hypo- or hyperechogenic areas was considered indicative of primary testicular disease. During the Doppler evaluation, flows were detected at the level of the spermatic artery and at the level of the testicular artery and its branches. The velocity analyses were considered normal when low resistance, a prolonged systolic phase, flow maintenance during diastole and a low resistance index were present (IR: 0.62). Systolic flow speed along the centripetal arteries was considered normal if it was lower than 15 cm s–1 and/or between 4 and 12 cm s–1.8,12,13
In the transrectal ultrasound evaluations of the prostates and the SVs, the following prostate ultrasound parameters were recorded: volume, parenchymal echogenicity and vascularization, the presence of parenchymal and/or ductal cystis, and/or calculi, and/or calcifications and/or area(s) of acinar ectasia. Moreover, the symmetry of the lobes and the characteristics of the venous periprostatic plexus were recorded.
The following direct SV ultrasound parameters were recorded: (i) body anteroposterior diameter (DAP); (ii) fundus DAP; (iii) parietal thickness of the right and left SVs and (iv) the numbers of polycyclic areas within both SVs. These values were used to calculate the following derived parameters: (i) fundus/body ratios; (ii) differences of the parietal thicknesses between the right and the left SVs; (iii) differences in interparietal diameter between the right and the left SVs and (iv) pre- and postejaculatory DAP differences (Figure 1).
Figure 1.
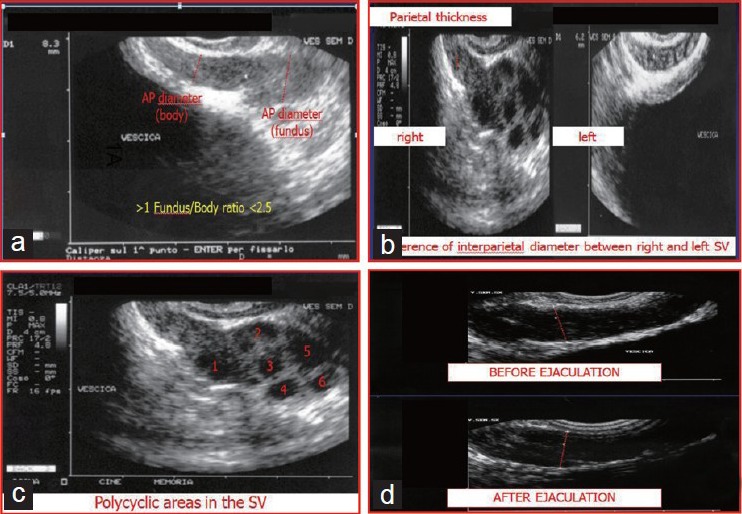
Ultrasound parameters of the seminal vesicles (SVs) examined in the present study. (a) Body anteroposterior diameter (DAP), fundus DAP and fundus/body ratio; (b) Parietal thicknesses of the right and left SVs, difference in the parietal thicknesses between the right and the left SV and difference between the interparietal diameters of the right and the left SVs; (c) Number of polycyclic areas within both SVs; (d) Pre- and postejaculatory DAP differences.
The operator (Single Laboratory Validation) repeated the measurements of these parameters twice and these parameters are expressed as the mean in the final report. For measurements below 1 mm, the calculations were performed on ultrasound paper with a millimetric measurement system.
Table 4 shows the ultrasound criteria (published by our group) that were adopted by our clinical practice for the confirmation of clinical diagnoses of MAGI.5
Table 4.
Ultrasound criteria for MAGI
The protocol was approved by the Institutional Review Board and an informed written consent was obtained from each patient and all controls.
Statistical analyses
The results are reported as the means ± s.e.m. throughout the study. The data were analyzed with one-way analysis of variance followed by Duncan's multiple range tests and Student's t-tests for direct comparison of the two groups. Statistical analyses were performed using Statistical Package for Social Sciences 9.0 for Windows. P < 0.05 was accepted as statistically significant.
RESULTS
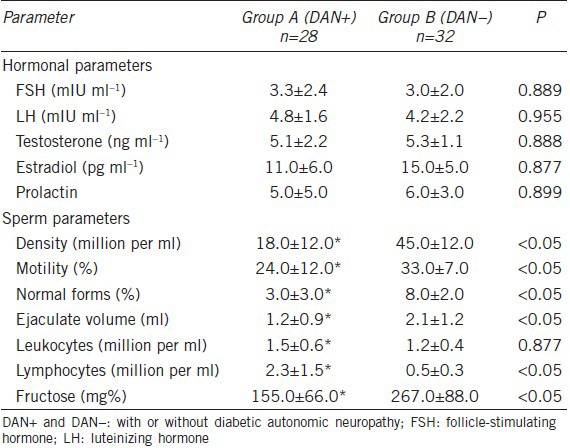
No patients exhibited alterations in the examined hormonal parameters (Table 5). In group A, the mean values of the following sperm parameters were significantly lower than those of group B: density, progressive motility, normal forms, ejaculate volume, concentration of lymphocytes and fructose levels (P < 0.05, Table 5). Specifically, isolated oligospermia was observed in 43% of the group A patients and in 23% of the group B patients. Isolated teratozoospermia was observed in 18% of group A patients and in 10% of group B patients. Isolated asthenozoospermia was observed in 17% of group A and 10% of group B patients. Oligoteratozoospermia was detected in 36% of group A and in 20% of group B patients. Oligoasthenozoospermia was detected in 28% of group A and 18% of group B patients. Oligoasthenoteratozoospermia was detected in 46% of group A and 32% of group B patients. The condition of leukocytospermia was detected in 45% of the patients in group A and in 39% of the patients in group B (this difference was not significant). The group A patients exhibited a significant increase in the concentration of seminal lymphocytes compared with the group B patients (2.3 ± 1.5 vs 0.5 ± 0.3 million per ml; P < 0.05).
Table 5.
Hormonal and sperm parameters of the two examined groups
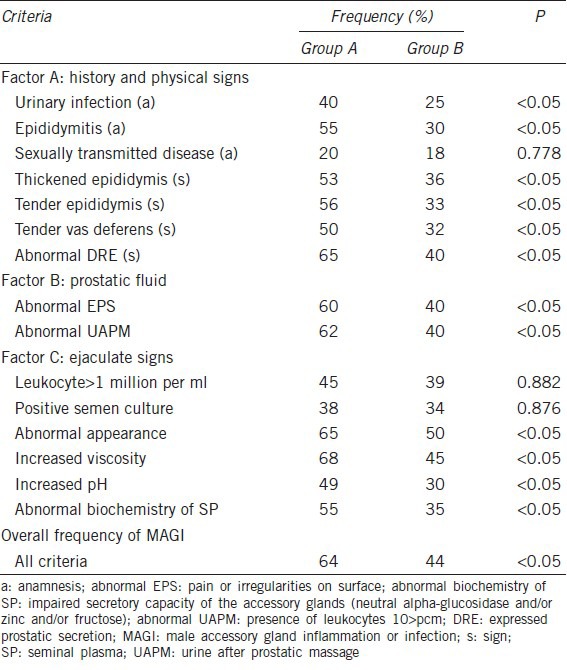
All of the diagnostic criteria that are used in clinical practice for the diagnosis of MAGI were found to be higher in Group A, with the exceptions of the presence in the anamnesis of reported sexually transmitted infections and the criteria of the C factor for the frequency of leukocytospermia and positive semen cultures (Table 6). Finally, total of 18 patients in group A and 14 patients in group B exhibited diagnoses of MAGI.
Table 6.
Detection of the diagnostic criteria for MAGI in the two groups examined
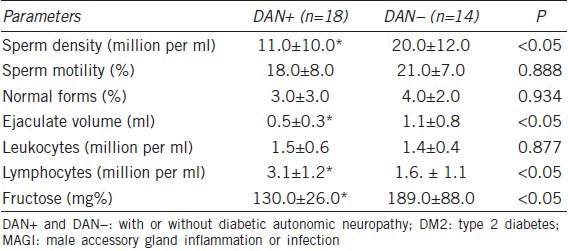
The ultrasound evaluations of the patients with DM2 and associated MAGI comparing patients with (n = 18) and without (n = 14) symptoms possibly reflecting DAN revealed significantly increased frequencies of all ultrasound criteria suggestive of vesiculitis in the patients with symptoms possibly reflecting DAN (P < 0.05) (Table 7). A total of nine patients with symptoms possibly reflecting DAN (50%) and three (21%) patients without symptoms possibly reflecting DAN exhibited ultrasound findings of prostato-vesciculo-epididymitis (i.e. more extensive anatomical involvement). Finally, the DM2 patients with MAGI and symptoms possibly reflecting DAN were significantly different from the DM2 patients with MAGI without symptoms possibly reflecting DAN symptoms in the following semen parameters: sperm density, concentration of lymphocytes, ejaculate volume and fructose levels (Table 8).
Table 7.
Ultrasound evaluations of the DM2 patients with MAGI (DAN+ vs DAN–)
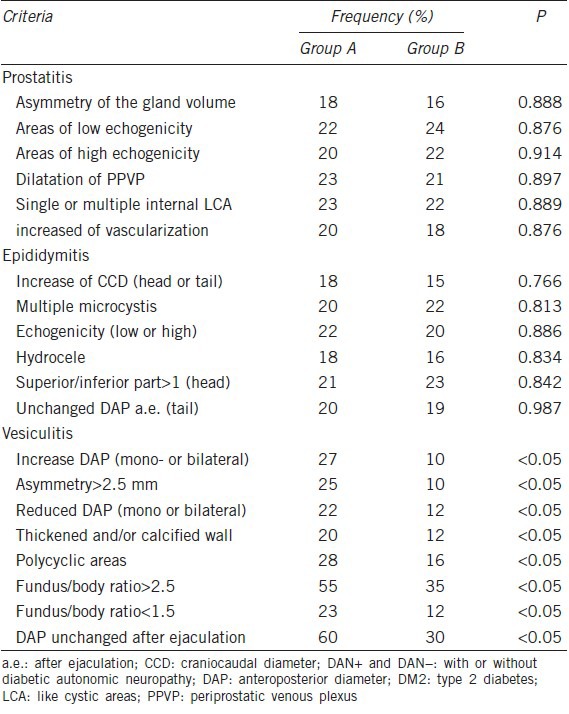
Table 8.
Sperm parameters of the DM2 patients with MAGI (DAN+ vs DAN–)
DISCUSSION
In a recent study, we showed that the prevalence of MAGI in DM2 patients is approximately 43%.1 The present study confirms the previous data and supports this hypothesis through the evaluations of two different groups of patients with DM2 (i.e. those with and without symptoms possibly reflecting DAN). Specifically, the patients with symptoms of DAN exhibited higher frequencies of MAGI compared with the DM2 patients without symptoms of DAN, and the ultrasound characterizations of the patients with DM2 and MAGI showed that the frequencies of meeting the criteria suggestive of vesiculitis were significantly higher in the group with symptoms possibly reflecting DAN compared with the group without symptoms possibly reflecting DAN. Furthermore, the frequencies of ultrasound findings of prostato-vesciculo-epididymitis (i.e. more extensive anatomical involvement) were higher in the group with symptoms possibly reflecting DAN than in the group without symptoms possibly reflecting DAN. Moreover, the semen qualities of the DM2 patients with symptoms possibly reflecting DAN were lower than those of the DM2 patients without symptoms of DAN; specifically, the concentrations of leukocytes in the ejaculates did not aid differential diagnoses, but the concentrations of seminal lymphocytes were significantly higher in the patients with symptoms possibly reflecting DAN. Finally, the sperm densities, semen lymphocytes concentrations, ejaculate volumes and fructose levels were significantly different between the DM2 patients with MAGI that had or did not have symptoms possibly reflecting DAN.
In our opinion, the most relevant aspects of the present study are as follows:
-
1
Greater inflammatory involvement of the SVs in patients with symptoms possibly reflecting DAN was demonstrated by the higher frequency of abnormal ultrasound findings and the reduced concentrations of fructose in the semen of these patients
-
2
Differential diagnoses were improved by the characterization of the lymphocytes in the semen compared with only characterizing the leucocytes, which is the normally recommended clinical practice8 (WHO, 2010).
We demonstrated that DAN may cause alterations in the structures and functions of the SVs. Specifically, the innervations of the SVs arise from the pelvic nerve and the hypogastric plexus, which supplies adrenergic and cholinergic fibers.14 The cholinergic fibers are found in the mucosa and stimulate secretory activity and these fibers are not found at the level of the muscularis.15 Dopamine D1 and D2 receptors have been identified at the level of the vesicular smooth muscle.16 Neuronal nitric oxide synthase activity and heme oxygenase 2 activity, which synthesizes carbon monoxide, have been demonstrated at the level of the tunica adventitia.17 Regarding the role of the vasoactive intestinal polypeptide (VIP), the data are conflicting; the initial evidence showed that VIP has no effect on resting tension or nerve-mediated responses;18 however, another study demonstrated a decrease in the sensitivity of the VIP receptor/effector system in the SV membrane of an animal model, which suggests a physiopathological role for VIP in seminal neuropathy diabetes.19 Other neurotransmitters involved in the possible functional alterations of the SV are the following: neuropeptide Y, calcitonin gene-related peptide and noradrenaline.20 Moreover, nerve growth factor and muscarinic M3 receptor contents are alerted in the SV.21
In our opinion, the ultrasound SV abnormalities that we found in patients with DM could be the consequence of alterations that are similar to those that occur after inflammation of the SV; however, autonomic neuropathy per se in these patients may be the reason for their abnormal inflammatory responses;22 i.e. parietal inflammatory lesions caused by vesicular stasis, the reparative lesions of the muscular and fibroelastic fibers and the epithelial cell alterations. Moreover, the functional alteration of the cilia in the main columnar cells or their metaplasia favors stasis and the production of proteins involved in clot formation. Another possible mechanism is that the ratio of elastic and collagen fibers is altered, which results in the consequent alteration of the ampullo-deferento-vesicular voiding mechanism (muscular wall). The other main hypothesis involves alterations of vesicular neurotransmission, particularly the ineffective purinergic transmission or ATP clearance.23,24 Finally, another important hypothesis is that parasympathetic dysfunction precedes sympathetic dysfunction in DAN;25 specifically, the lack of NO from nNOS-positive cholinergic autonomic nerve fibers26 may explain some of the structural changes.
In our experience, unchanged SV DAPs after ejaculation are a pathological characteristic of other categories of neuropathic patients; specifically, those with alcoholism or dysvitaminosis neuropathy. We reported that this finding is associated with increased leukocyte concentrations in the semen, which favors the onset of chronic inflammatory responses of the prostate-ampullo-vesicular tract.27
The other key aspect of the present study is the importance of the diagnostic characterization of lymphocytes in the semen. We demonstrated that the biological role of leukocytes in semen is not only antimicrobial;28,29,30 rather, leukocytes have other important biological actions. For example, another significant effect of their presence in the semen is the elimination of immature germ cells via an endogenous anti-inflammatory response.31,32,33 Routine practice includes only a peroxidase test for leukocyte measurement as recommended by the WHO manual, but this method is only able to detect granulocytes. Flow cytometry allows for the identification of all leukocyte subpopulations present in the semen.31,33 Specifically, granulocytes are the predominant component (50%–60%), followed by macrophages (20%–30%), T lymphocytes (2%–5%) and B lymphocytes.1,33 Leukocytospermia (>1 million per ml peroxidase-positive leukocytes in the semen) is present in approximately 10%–20% of infertile patients,34 and a large percentage of semen samples with leukocytospermia produce negative results in microbiological tests.34,35 Finally, bacteriospermia and elevated seminal leukocytes are prevalent, but are not statistically associated.34
Another important aspect is that the true origin of leukocytes in the semen is difficult to understand. The origin of leukocytes from the epididymis has been confirmed by studies performed using the vasectomy model,36,37 and these models produce evidence of local inflammatory responses following the procedure. However, in patients with leukocytospermia, seminal citric acid concentrations (a marker of prostate function) appear to be lower, which suggests that prostatitis is the main cause of the presence of leukocytes in the semen. However, there is no clear evidence regarding the potential role of altered contractile and/or secretory functions of the VS in terms of the presence of leukocytes and/or lymphocytes in the semen.
In conclusion, this study confirmed the elevated frequency of MAGI in DM2 patients with symptoms that possibly reflect autonomic neuropathy and suggests that DAN is a probable risk factor for the development of MAGI. An important role may be attributed to the greater frequencies of ultrasonic signs of vesiculitis, and further studies should clarify the role of seminal lymphocytes in these patients and should specifically clarify the origin of seminal lymphocytes.
AUTHOR CONTRIBUTIONS
SLV and RAC are the principal investigators. AEC and EV have performed the statistical analysis and the final revision of the manuscript.
COMPETING INTERESTS
The authors declare that they have no competing interests.
REFERENCES
- 1.Condorelli RA, Calogero AE, Vicari E, Duca Y, Favilla V, et al. Prevalence of MAGI in patients with type 2 diabetes mellitus. J Endocrinol Invest. 2013;36:770–4. doi: 10.3275/8950. [DOI] [PubMed] [Google Scholar]
- 2.La Vignera S, Condorelli RA, Di Mauro M, D’Agata R, Vicari E, et al. Seminal vesicles and diabetic neuropathy: ultrasound evaluation. J Androl. 2011;32:478–83. doi: 10.2164/jandrol.110.011676. [DOI] [PubMed] [Google Scholar]
- 3.La Vignera S, Vicari E, Condorelli R, D’Agata R, Calogero AE. Ultrasound characterization of the seminal vesicles in infertile patients with type 2 diabetes mellitus. Eur J Radiol. 2011;80:e64–7. doi: 10.1016/j.ejrad.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 4.La Vignera S, Vicari E, Condorelli RA, D’Agata R, Calogero AE. Male accessory gland infection and sperm parameters (review) Int J Androl. 2011;34:e330–47. doi: 10.1111/j.1365-2605.2011.01200.x. [DOI] [PubMed] [Google Scholar]
- 5.La Vignera S, Calogero AE, Condorelli RA, Vicari LO, Catanuso M, et al. Ultrasonographic evaluation of patients with male accessory gland infection. Andrologia. 2012;44:26–31. doi: 10.1111/j.1439-0272.2010.01132.x. [DOI] [PubMed] [Google Scholar]
- 6.Rowe P, Comhaire F, Hargreave TB, Mellows HJ, editors. Cambridge: Cambridge University Press; 1993. World Health Organization manual for the standardised investigation and diagnosis of the infertile couple. [Google Scholar]
- 7.Tesfaye S, Boulton AJ, Dyck PJ, Freeman R, Horowitz M, et al. Toronto Diabetic Neuropathy Expert Group. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33:2285–93. doi: 10.2337/dc10-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sakamoto H, Yajima T, Nagata M, Okumura T, Suzuki K, et al. Relationship between testicular size by ultrasonography and testicular function: measurement of testicular length, width, and depth in patients with infertility. Int J Urol. 2008;15:529–33. doi: 10.1111/j.1442-2042.2008.02071.x. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization. 5th ed. Cambridge: Cambridge University Press; 2010. WHO Laboratory Manual for the Examination and processing of human semen. [Google Scholar]
- 10.Mann T, Lutwak-Mann C. Evaluation of the functional state of male accessory glands by the analysis of seminal plasma. Andrologia. 1976;8:237–42. doi: 10.1111/j.1439-0272.1976.tb02139.x. [DOI] [PubMed] [Google Scholar]
- 11.Endtz AW. A rapid staining method for differentiating granulocytes from “germinal cells” in Papanicolaou-stained semen. Acta Cytol. 1974;18:2–7. [PubMed] [Google Scholar]
- 12.Pilatz A, Rusz A, Wagenlehner F, Weidner W, Altinkilic B. Reference values for testicular volume, epididymal head size and peak systolic velocity of the testicular artery in adult males measured by ultrasonography. Ultraschall Med. 2013;34:349–54. doi: 10.1055/s-0032-1313077. [DOI] [PubMed] [Google Scholar]
- 13.Pinggera GM, Mitterberger M, Bartsch G, Strasser H, Gradl J, et al. Assessment of the intratesticular resistive index by colour Doppler ultrasonography measurements as a predictor of spermatogenesis. BJU Int. 2008;101:722–6. doi: 10.1111/j.1464-410X.2007.07343.x. [DOI] [PubMed] [Google Scholar]
- 14.Mariotti A, Durham J, Mawhinney M. Protein kinases and the androgen-induced proliferation of accessory sex organ smooth muscle. Biol Reprod. 1992;46:551–60. doi: 10.1095/biolreprod46.4.551. [DOI] [PubMed] [Google Scholar]
- 15.Dixon JS, Jen PY, Gosling JA. The distribution of vesicular acetylcholine transporter in the human male genitourinary organs and its co-localization with neuropeptide Y and nitric oxide synthase. Neurourol Urodyn. 2000;19:185–94. doi: 10.1002/(sici)1520-6777(2000)19:2<185::aid-nau9>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 16.Hyun JS, Baig MR, Yang DY, Leungwattanakij S, Kim KD, et al. Localization of peripheral dopamine D1 and D2 receptors in rat and human seminal vesicles. J Androl. 2002;23:114–20. doi: 10.1002/j.1939-4640.2002.tb02604.x. [DOI] [PubMed] [Google Scholar]
- 17.Grozdanovic Z, Goessl C. Comparative localization of heme oxygenase-2 and nitric oxide synthase in the autonomic innervation to the human ductus deferens and seminal vesicle. J Urol. 1999;162:2156–61. doi: 10.1016/S0022-5347(05)68151-7. [DOI] [PubMed] [Google Scholar]
- 18.Moss HE, Crowe R, Burnstock G. The seminal vesicle in eight and 16 week streptozotocin-induced diabetic rats: adrenergic, cholinergic and peptidergic innervation. J Urol. 1987;138:1273–8. doi: 10.1016/s0022-5347(17)43583-x. [DOI] [PubMed] [Google Scholar]
- 19.Rodriguez-Pena MS, Guijarro LG, Juarranz MG, Rodriguez-Henche N, Bajo AM, et al. Analysis of vasoactive intestinal peptide receptors and the G protein regulation of adenylyl cyclase in seminal vesicle membranes from streptozotocin-diabetic rats. Cell Signal. 1994;6:147–56. doi: 10.1016/0898-6568(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 20.Morrison JF, Dhanasekaran S, Howarth FC. Neuropeptides in the rat corpus cavernosum and seminal vesicle: effects of age and two types of diabetes. Auton Neurosci. 2009;146:76–80. doi: 10.1016/j.autneu.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 21.Chen HE, Xiao DZ. Changes of nerve growth factor and M3 subtype muscarinic receptor in the seminal vesicle of diabetic rats. Zhonghua Nan Ke Xue. 2011;17:1002–6. [PubMed] [Google Scholar]
- 22.Vinik AI, Erbas T, Casellini CM. Diabetic cardiac autonomic neuropathy, inflammation and cardiovascular disease. J Diabetes Investig. 2013;4:4–18. doi: 10.1111/jdi.12042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Westfall DP, Todorov LD, Mihaylova-Todorova ST. ATP as a cotransmitter in sympathetic nerves and its inactivation by releasable enzymes. J Pharmacol Exp Ther. 2002;303:439–44. doi: 10.1124/jpet.102.035113. [DOI] [PubMed] [Google Scholar]
- 24.Burnstock G. Purines and sensory nerves. Handb Exp Pharmacol. 2009;194:333–92. doi: 10.1007/978-3-540-79090-7_10. [DOI] [PubMed] [Google Scholar]
- 25.Cellek S. Point of NO return for nitrergic nerves in diabetes: a new insight into diabetic complications. Curr Pharm Des. 2004;10:3683–95. doi: 10.2174/1381612043382792. [DOI] [PubMed] [Google Scholar]
- 26.Uckert S, Stanarius A, Stief CG, Wolf G, Jonas U, et al. Immunocytochemical distribution of nitric oxide synthase in the human seminal vesicle: a light and electron microscopical study. Urol Res. 2003;31:262–6. doi: 10.1007/s00240-003-0322-5. [DOI] [PubMed] [Google Scholar]
- 27.Vicari E, Calogero AE, Valenti D, Condorelli R, La Vignera S. Relationship between degree of neuropathy and post-ejaculatory seminal vesicles voiding and seminal leukocyte concentrations in diabetic patients. J Endocrinol Invest. 2008;31:17. [Google Scholar]
- 28.Seshadri S, Flanagan B, Vince G, Lewis-Jones DJ. Detection of subpopulations of leucocytes in different subgroups of semen sample qualities. Andrologia. 2012;44:354–61. doi: 10.1111/j.1439-0272.2011.01189.x. [DOI] [PubMed] [Google Scholar]
- 29.Olivier AJ, Liebenberg LJ, Coetzee D, Williamson AL, Passmore JA, et al. Isolation and characterization of T cells from semen. J Immunol Methods. 2012;375:223–31. doi: 10.1016/j.jim.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 30.Batstone GR, Doble A, Gaston JS. Autoimmune T cell responses to seminal plasma in chronic pelvic pain syndrome (CPPS) Clin Exp Immunol. 2002;128:302–7. doi: 10.1046/j.1365-2249.2002.01853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seshadri S, Flanagan B, Vince G, Lewis Jones DI. Leucocyte subpopulations in the seminal plasma and their effects on fertilisation rates in an IVF cycle. Andrologia. 2012;44:396–400. doi: 10.1111/j.1439-0272.2012.01293.x. [DOI] [PubMed] [Google Scholar]
- 32.Filippini A, Riccioli A, Padula F, Lauretti P, D’Alessio A, et al. Control and impairment of immune privilege in the testis and in semen. Hum Reprod Update. 2001;7:444–9. doi: 10.1093/humupd/7.5.444. [DOI] [PubMed] [Google Scholar]
- 33.Wolff H. The biologic significance of white blood cells in semen. Fertil Steril. 1995;63:1143–57. doi: 10.1016/s0015-0282(16)57588-8. [DOI] [PubMed] [Google Scholar]
- 34.Domes T, Lo KC, Grober ED, Mullen JB, Mazzulli T, et al. The incidence and effect of bacteriospermia and elevated seminal leukocytes on semen parameters. Fertil Steril. 2012;97:1050–5. doi: 10.1016/j.fertnstert.2012.01.124. [DOI] [PubMed] [Google Scholar]
- 35.Ostaszewska-Puchalska I, Zdrodowska-Stefanow B, Badyda J, Galewska Z. Antichlamydial antibodies and citric acid in patients with chronic prostatitis. Arch Immunol Ther Exp (Warsz) 2007;55:57–60. doi: 10.1007/s00005-007-0006-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marchlewicz M. Localization of immunocompetent cells in the human epididymis. Folia Histochem Cytobiol. 2001;39:173–4. [PubMed] [Google Scholar]
- 37.Nashan D, Cooper TG, Knuth UA, Schubeus P, Sorg C, et al. Presence and distribution of leucocyte subsets in the murine epididymis after vasectomy. Int J Androl. 1990;13:39–49. doi: 10.1111/j.1365-2605.1990.tb00958.x. [DOI] [PubMed] [Google Scholar]


