Abstract
Reactive stromal changes in prostate cancer (PCa) are likely involved in the emergence of castration-resistant PCa (CRPC). This study was designed to investigate stromal changes in patients with clinically advanced PCa and analyze their prognostic significance. Prostate needle biopsies obtained from 148 patients before castration therapy were analyzed by Masson trichrome staining and immunohistochemical analysis of vimentin and desmin. Reactive stroma grading was inversely correlated with Gleason score. Stroma grade (Masson stain 82.8% vs 45.6%, P < 0.001) and vimentin expression (P = 0.005) were significantly higher, and desmin expression (P = 0.004) significantly lower, in reactive stroma of tumors with a Gleason score of 6–7 than in adjacent peritumoral tissue. Kaplan-Meier analysis showed a significant association between reactive stroma grade in tumors and the occurrence of CRPC in patients with a Gleason score of 6–7 (P = 0.009). Furthermore, patients with higher vimentin or lower desmin expression had a shorter disease-free period. In multivariate analysis, only vimentin expression was a significant predictor of tumor relapse (hazard ratio 1.78, 95% confidence interval 1.12–10.26, P = 0.012). These findings indicate that the intensity of reactive stroma is associated with castration responsiveness, especially in patients with a lower Gleason score where the abundant stroma component is most frequently found. High expression of vimentin in tumor stroma was independently associated with poor outcomes in patients with Gleason scores of 6–7, and may serve as a new prognostic marker in daily practice.
Keywords: cancer-associated fibroblasts, castration resistance, prostate cancer, reactive stroma, vimentin
INTRODUCTION
Prostate cancer (PCa) is the most common malignant tumor in older men. Castration by drugs or surgery is the first-line treatment for clinically advanced PCa, but it cannot completely prevent disease progression. PCa that progresses after castration therapy is called castration-resistant PCa (CRPC).1 Once PCa progresses to CRPC, it is difficult to treat and patient prognosis is very poor.
Stromal-epithelial interactions play a key role in PCa carcinogenesis, as well as in response to castration therapy.2,3 Most studies assessing the mechanisms of CRPC have focused on cancerous epithelial cells,4,5,6 with less known about stromal cells, especially under anti-androgen conditions. Reactive stromal changes in PCa include changes in extracellular matrix components, increased vascular density and infiltration of activated myofibroblasts.7,8,9 These activated myofibroblasts, often termed cancer-associated fibroblasts (CAFs), are thought to play a central role in PCa growth and metastasis.
The prognostic significance of reactive stroma has been evaluated in patients with clinically localized PCa.10,11,12 Quantitation of reactive stroma has been shown useful in identifying patients at increased risk of biochemical recurrence after radical prostatectomy. In patients with clinically advanced PCa, however, the association between reactive stroma changes and the occurrence of CRPC has not been determined.
Castration-induced involution of normal prostate glands was found to be caused by primary changes in the prostate stroma.13 Moreover, the stroma in androgen-sensitive Dunning tumors was found to be androgen-insensitive after surgical castration in an experimental animal model, with the effects of castration enhanced by therapy that targets this unresponsive stroma.14 We hypothesized that reactive stromal cells, especially CAFs, could contribute to tumor growth after initially successful castration therapy. We tested this hypothesis by assessing changes in reactive stroma from prostate needle biopsies obtained from patients before castration therapy, using Masson trichrome staining and immunohistochemical analysis.
MATERIALS AND METHODS
Tissue samples
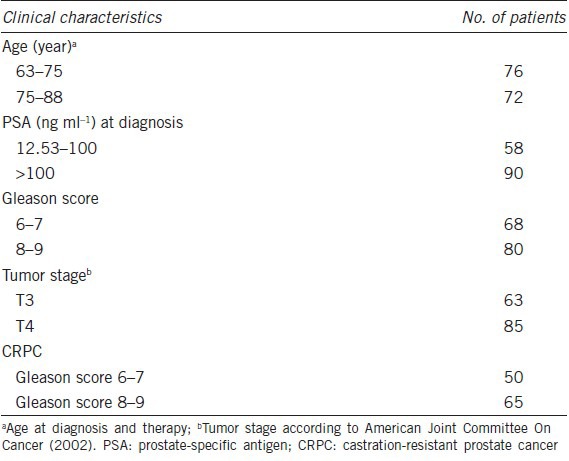
Slides were obtained from 148 patients who underwent diagnostic prostatic needle biopsies at Nanjing Hospital Affiliated to Nanjing Medical University between 2005 and 2008. All enrolled patients had been diagnosed with clinically advanced PCa (T3–T4), according to the criteria of the American Joint Committee on Cancer, 2002). Median patient age was 71 years (range, 63–88 years), with prostate-specific antigen (PSA) concentrations ranging from 12.53 to >100 ng l−1 and Gleason scores ranging from 6 to 9. Patient characteristics are shown in Table 1. All of the patients were treated with drugs or surgery, with none undergoing radiotherapy or strontium-89 particle treatment for bone metastasis.
Table 1.
Demographic and clinical characteristics of patients with prostate cancer
Integrated clinical follow-up data were available for all 148 patients. Serum PSA concentrations were measured, and digital rectal examinations and imaging modalities performed, every 3 months for the first 2 years, and every 6 months thereafter, with 48 months defined as the end point of this study. Adverse events, such as the occurrence of CRPC and disease-specific death, were recorded. CRPC was defined as three consecutive increases in PSA above the nadir with a castration level of serum testosterone, continued increase in PSA after anti-androgen withdrawal, or clear clinical or radiological evidence of progression. This study was approved by the Ethics Committee of Nanjing Medical University (Nanjing, China).
Of the 148 patients, 68 had Gleason scores of 6–7; of these, 50 patients (74%) were diagnosed with CRPC during the follow-up period, 5 (7%) died and 13 (19%) remained sensitive to castration therapy, with no tumor relapse and a low PSA concentration (<4 ng l−1); the latter were defined as having androgen-dependent PCa. The remaining 80 patients had Gleason scores of 8–9; of these, 65 (81%) were diagnosed with CRPC, 11 (14%) died, and 4 (5%) had androgen-dependent PCa.
Masson trichrome staining
Specimens were fixed in 10% buffered formalin, embedded in paraffin, cut at 4 μm thickness, and routinely stained with hematoxylin and eosin. The paraffin sections were stained with 1% hydrochloric acid for differentiation, and Masson composite staining solution (Fuzhou Maxim Biotech Co., Ltd., Fuzhou, China) was added dropwise for 5–10 min. The sections were subsequently washed with distilled water, treated with 1% phosphotungstic acid, and incubated with brilliant green staining solution for 5–10 min. After treatment with 1% glacial acetic acid for 1 min and dehydration with 95% alcohol several times, the sections were cemented using neutral gum for observation.
Using this procedure, prostatic stroma smooth muscle cells were stained red, and CAFs and collagen fibers were stained green. In each sample, we analyzed five areas with the most intense green staining under high magnification (×200), both in prostatic carcinoma and adjacent peritumoral tissues. Reactive stroma grade scored as grade 0 (tumors with < 5% tumor stromal area relative to the total tumor area), grade 1 (6%–15%), grade 2 (16%–50%), and grade 3 (>51%, stroma/epithelium ratio ≥ 1).11
Immunohistochemistry
Paraffin sections 5 μm thick were treated with EDTA for antigen repair, and endogenous enzyme activity was blocked by incubation in 3% hydrogen peroxide for 10 min at room temperature. Antigen retrieval was performed using 0.01 mol l−1 sodium citrate buffer (pH 6.0) at 95°C for 45 min. After incubation in 5% bovine serum albumin in phosphate-buffered saline for 10 min, the tissue sections were incubated with the primary polyclonal antibodies to vimentin and desmin (Nanjing Ningao Biotechnology Co., Ltd., Shanghai, China) overnight at 4°C. After washing, the sections were incubated with secondary antibody for 20 min at room temperature, followed by incubation with an Envision Dual Labeled Polymer kit (BioGenex, San Ramon, CA, USA) according to the manufacturer's instructions. All sections were counterstained with hematoxylin. Immunohistochemical analysis was performed in areas previously selected during Masson staining.
Immunohistochemically, CAFs were characterized by expression of vimentin and non-expression of desmin. Cells that expressed desmin but did not express vimentin were characteristic of a smooth muscle cell phenotype. The percentages of stromal cells positive for vimentin and desmin were quantified in five fields under low magnification (×100). Staining intensity was graded on a scale of 0–3, indicating 0%, ≤33%, 33%–66%, and > 66% positive stromal cells, respectively.12
All slides were examined independently by two trained pathologists blinded to survival data, and any differences were recorded and resolved by a joint review.
Statistical analysis
The χ2 test and Fisher's exact test were used to compare Masson trichrome staining in tumor stroma and adjacent peritumoral tissues and the expression of vimentin and desmin in samples with stroma grades 0/1 and 2/3. Spearman rank correlation coefficients, the Kaplan-Meier method and multivariate Cox proportional hazards regression analyses were performed to test the prognostic significance of various factors on patient survival. SAS version 9.12 (SAS Inc., Cary, USA) software was used and P < 0.05 was considered statistically significant.
RESULTS
Distribution patterns of reactive stroma in advanced prostate cancer tissues
Masson trichrome staining showed green- and red-stained stromal cells with uneven signal density and heterogeneity in the PCa tissues (Figure 1a). The green-stained stromal cells were vimentin-positive with decreased or negative desmin expression, consistent with the characteristics of CAFs (Figure 1b and 1c).
Figure 1.
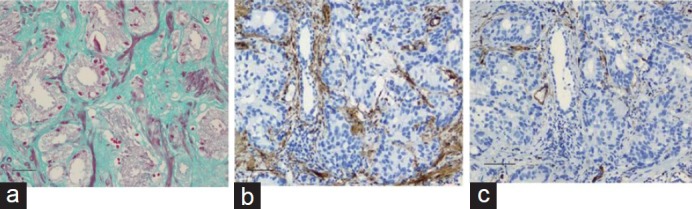
Stromal cell phenotype in prostate cancer detected by (a) Masson trichrome staining (×200), (b) vimentin (×100), and (c) desmin (×100). Scale bars = 20 μm.
Reactive tumor stroma grade was inversely correlated with Gleason score. Stroma grade 2 and 3 by Masson staining was observed in 82.8% of tumors with a Gleason score of 6–7, and in 15.0% of tumors with a Gleason score of 8–9 (P < 0.001). Tumors with a Gleason score of 6–7 also showed significantly higher expression of vimentin (87.5% vs 18.7%, P < 0.001) and significantly lower expression of desmin (44.1% vs 76.8%, P < 0.001) than tumors with a Gleason score of 8–9 (Table 2).
Table 2.
Intensity of reactive stroma and immunohistochemical expression of stroma markers in advanced prostate cancer and adjacent peritumoral tissue
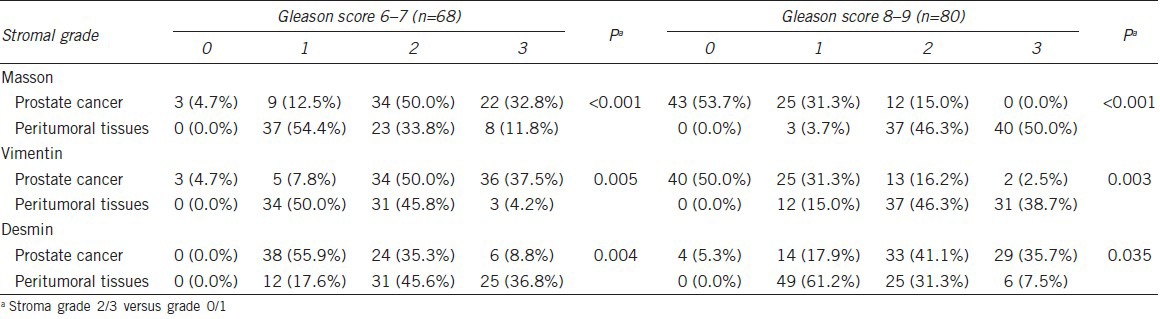
Assessments of tumors with a Gleason score of 6–7 showed that the intensity of green-stained stromal cells (stroma grades 2 and 3) was significantly higher in tumor stroma than in adjacent peritumoral tissues (82.8% vs 45.6%, P < 0.001). Furthermore, vimentin expression was significantly higher (P = 0.005) and desmin expression significantly lower (P = 0.004) in the tumor stroma than in adjacent peritumoral tissue (Table 2 and Figure 2). Pearson correlation analysis showed that Masson staining was positively correlated with vimentin (r = 0.796, P < 0.001) and negatively correlated with desmin (r = −0.122, P = 0.602) expression.
Figure 2.
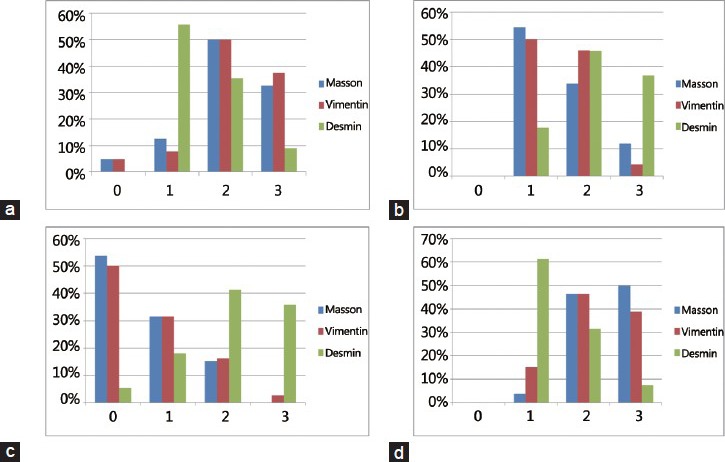
Distribution of reactive stroma in tumors with different Gleason score. (a) Stroma grade in the tumors with Gleason score of 6–7, (b) Stroma grade in peritumoral tissue with Gleason score of 6–7, (c) Stroma grade in the tumors with Gleason score of 8-9, (d) Stroma grade in peritumoral tissue with Gleason score of 8–9.
In tumors with a Gleason score of 8–9, however, high stroma grade was significantly less frequent in tumors than in adjacent peritumoral tissues (15.0% vs 96.3%, P < 0.001). When comparing samples with stromal grades 2/3 and grade 0/1, we found that stroma in adjacent peritumoral tissues showed decreased expression of vimentin (P = 0.003) and increased expression of desmin (P = 0.035) than in tumor stroma (Table 2 and Figure 2). Pearson correlation analysis showed that Masson staining was positively correlated with both vimentin (r = 0.833, P < 0.001) and desmin (r = 0.452, P = 0.007) expression.
Relationship between reactive tumor stroma and castration-resistant prostate cancer
Due to the abundant stromal component in tumors with Gleason scores of 6–7, and the significantly higher level of vimentin expression in the tumor stroma than in adjacent peritumoral tissues in this subset of tumors, we further explored the prognostic significance of reactive stromal changes in these patients treated with castration therapy. We did not further analyze patients with Gleason scores of 8–9 because these tumors had little or no stromal component and are regarded as being functionally stroma-independent.
Kaplan-Meier analysis showed a significant association between reactive tumor stromal and CRPC in patients with Gleason scores of 6–7 (P = 0.009) (Figure 3a). Patients with higher vimentin expression (P = 0.044) or lower desmin expression (P = 0.045) had a significantly shorter disease-free period and displayed a poorer response to castration therapy (Figure 3b and 3c).
Figure 3.
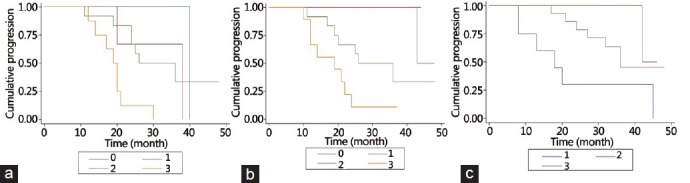
Kaplan-Meier analysis showed a significant association between intensity of reactive stroma and the occurrence of castration-resistant prostate cancer in tumors with Gleason score of 6–7 (log-rank P = 0.009, (a). Patients with higher vimentin expression (log-rank P = 0.044, (b) or lower desmin expression (log-rank P = 0.045, (c) had a shorter disease-free period.
Multivariate analysis using a Cox proportional hazard regression model indicated that only vimentin expression in the tumor stroma was an independent predictor of disease relapse (hazard ratio [HR] 1.78, 95% confidence interval [CI] 1.12–10.26, P = 0.012). Age (HR 1.08, 95% CI 0.96–1.23, P = 0.207), serum PSA (HR 1.01, 95% CI 0.99–1.03, P = 0.339), Gleason score (HR 1.64, 95% CI 0.28–9.57, P = 0.53), Masson staining (HR 8.56, 95% CI 0.95–6.36, P = 0.447), and desmin expression (HR 1.06, 95% CI 0.26–4.31, P = 0.938) were not significantly associated with disease recurrence after castration therapy.
DISCUSSION
Although castration results in a marked involution of normal prostate glands, this response is less prominent in patients with PCa.15 Although the reasons for this are largely unknown, a recent study indicated that this reduced response may be due to a limited or insensitive stromal response.14 In an experimental animal model, transforming growth factor beta (TGF-β) signaling from stromal fibroblasts was found to play a key role in mediating the prostatic response to androgen ablation.16 Furthermore, knockout from stromal fibroblasts of the TGF-β type II receptor gene did not markedly reduce the apoptosis of epithelial cells after surgical castration.17 Moreover, low androgen receptor level in the tumor stroma has been associated with a poor response to castration therapy.18,19 These findings, taken together, emphasize the important role of tumor stroma in mediating epithelial androgen responsiveness.
The stroma surrounding PCa tumors is altered, differing significantly from normal prostate stroma.20 Reactive stroma cells, especially CAFs, shield cancer cells from the host immune response and produce larger quantities of growth factor to promote tumor progression. The prognostic significance of reactive stroma has been well documented in organ-confined PCa,10,11,12 but, to our knowledge, was not previously assessed in patients with clinically advanced disease.
Since prostatic biopsies have been shown as useful for defining and scoring reactive stroma in PCa tissues,11,12,21 we used a prostate scoring system11 on prostate needle biopsies to quantify reactive stromal changes in clinically advanced PCa. We found that tumor stromal grade was significantly higher in tumors with a Gleason score of 6–7 than in tumors with a Gleason score of 8–9. This was similar to previous findings, which showed that most tumors with Gleason scores ≥ 8 had stromal grade 0, whereas those with Gleason scores ≤ 7 or less had stromal grade 2.8,10 Most poorly differentiated PCa tumors have little or no stromal component. This absence of stroma is usually accompanied by a higher epithelial grade and a poorer prognosis than in patients containing some degree of tumor stroma, a finding in agreement with clinical observations in patients with Gleason scores ≥ 8 who receive castration therapy. Tumors that lose stromal components can become stroma-independent, suggesting that the histological grading of epithelial tumors may be predictive of patient survival.
Serum PSA response is the most frequently used prognostic marker in patients having tumors with a Gleason score of 6–7.22 However, it is often difficult to correctly evaluate patients who have a better or poorer response to castration therapy. Because of the abundant stromal component in this tumor subset, we further analyzed the predictive value of reactive stroma changes in these patients. We found that reactive stromal grade was significantly higher in tumor stroma than in adjacent peritumoral tissue. Furthermore, a higher level of vimentin expression in the tumor stroma was associated with a shorter disease-free period in both univariate and multivariate analyses. In addition, loss of smooth muscle cells, immunohistochemically assessed by desmin expression, was also associated with a poorer outcome, although only in univariate analysis. These findings indicate that vimentin expression in the tumor stroma could serve as a useful marker to identify patients who have a better or worse response to castration therapy.
A previous study reported that desmin was the best predictor of biochemical recurrence in patients who underwent radical prostatectomy, whereas vimentin was not associated with disease recurrence.10 In contrast, we and Tomas and Kruslin8 found that vimentin was a better predictor than desmin. These findings indicate that the appearance of CAFs in reactive stroma has a greater influence on tumor recurrence than the loss of smooth muscle cells. Although, we confirmed that reactive stroma can be clearly shown by Masson trichrome staining, we found that Masson staining was not useful in predicting survival in patients who undergo castration therapy. These findings, therefore, suggest that Masson staining is a less specific or reliable marker than vimentin.
This study had several limitations. First, larger number of subjects is required to confirm our results. Second, verification of green-stained stromal cells and immunohistochemical quantification of vimentin expression depended on interpretation by individual pathologists. Third, we did not analyze the prognostic significance of stromal cells in patients with Gleason scores of 8–9, although the stromal component was more abundant in adjacent peritumoral tissues than in the tumor. Theoretically, these stromal cells can shield cancer cells from the host immune response and this subset of tumors is regarded as functionally stroma-independent.
Despite these limitations, our results demonstrate that changes in the reactive stroma in patients with advanced PCa were associated with responsiveness to castration therapy. Quantitation of reactive stroma can be used to identify patients who are prone to castration resistance, especially in patients with lower Gleason grades, in which CAFs occur most frequently. The level of vimentin expression in the tumor stroma could serve as a novel marker for patients with Gleason scores of 6–7 treated with castration therapy. Our study also confirmed the concept that prostate tumors are not purely epithelial, and that reactive stroma must be considered a biologically relevant part of the tumor.10 Reactive stromal changes that occur in PCa may be a new target for early intervention.
AUTHOR CONTRIBUTIONS
JPW and HBS designed the study, analyzed the data and drafted the manuscript. WBH, JHZ, JPW, and HZ performed Masson staining and immunohistochemical analyses. WBH also participated in the design of the study and analyzed the data. LWX, JGZ, and JHS participated in the collection of clinical data. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
ACKNOWLEDGMENTS
We would like to thank Dr. Mei-Lin Wang (School of Public Health, Nanjing Medical University, China) for statistical analysis and critical comments. This work was supported by a grant from the Nature Science Research Project of JiangSu Province (No. BK2008073), a grant from the Nanjing Science and Technology Committee (No. 201303006) and Nanjing Medical Science and technique Development Foundation (No. QRX11030).
REFERENCES
- 1.Donkena KV, Yuan H, Young CY. Recent advances in understanding hormonal therapy resistant prostate cancer. Curr Cancer Drug Targets. 2010;10:402–10. doi: 10.2174/156800910791208544. [DOI] [PubMed] [Google Scholar]
- 2.Thalmann GN, Rhee H, Sikes RA, Pathak S, Multani A, et al. Human prostate fibroblasts induce growth and confer castration resistance and metastatic potential in LNCaP Cells. Eur Urol. 2010;58:162–71. doi: 10.1016/j.eururo.2009.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Niu Y, Altuwaijri S, Yeh S, Lai KP, Yu S, et al. Targeting the stromal androgen receptor in primary prostate tumors at earlier stages. Proc Natl Acad Sci USA. 2008;105:12188–93. doi: 10.1073/pnas.0804701105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burnstein KL. Regulation of androgen receptor levels: implications for prostate cancer progression and therapy. J Cell Biochem. 2005;95:657–69. doi: 10.1002/jcb.20460. [DOI] [PubMed] [Google Scholar]
- 5.Scher HI, Sawyers CL. Biology of progressive, castration-resistant prostate cancer: directed therapies targeting the androgen-receptor signaling axis. J Clin Oncol. 2005;23:8253–61. doi: 10.1200/JCO.2005.03.4777. [DOI] [PubMed] [Google Scholar]
- 6.Wang Q, Li W, Zhang Y, Yuan X, Xu K, et al. Androgen receptor regulates a distinct transcription program in androgen-independent prostate cancer. Cell. 2009;138:245–56. doi: 10.1016/j.cell.2009.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tuxhorn JA, Ayala GE, Smith MJ, Smith VC, Dang TD, et al. Reactive stroma in human prostate cancer: induction of myofibroblast phenotype and extracellular matrix remodeling. Clin Cancer Res. 2002;8:2912–23. [PubMed] [Google Scholar]
- 8.Tomas D, Kruslin B. The potential value of (Myo) fibroblastic stromal reaction in the diagnosis of prostatic adenocarcinoma. Prostate. 2004;61:324–31. doi: 10.1002/pros.20109. [DOI] [PubMed] [Google Scholar]
- 9.Taylor RA, Risbridger GP. Prostatic tumor stroma: a key player in cancer progression. Curr Cancer Drug Targets. 2008;8:490–7. doi: 10.2174/156800908785699351. [DOI] [PubMed] [Google Scholar]
- 10.Ayala G, Tuxhorn JA, Wheeler TM, Frolov A, Scardino PT, et al. Reactive stroma as a predictor of biochemical-free recurrence in prostate cancer. Clin Cancer Res. 2003;9:4792–801. [PubMed] [Google Scholar]
- 11.Yanagisawa N, Li R, Rowley D, Liu H, Kadmon D, et al. Stromogenic prostatic carcinoma pattern (carcinomas with reactive stromal grade 3) in needle biopsies predicts biochemical recurrence-free survival in patients after radical prostatectomy. Hum Pathol. 2007;38:1611–20. doi: 10.1016/j.humpath.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 12.Tomas D, Spajić B, Milosević M, Demirović A, Marusić Z, et al. Intensity of stromal changes predicts biochemical recurrence-free survival in prostatic carcinoma. Scand J Urol Nephrol. 2010;44:284–90. doi: 10.3109/00365599.2010.485578. [DOI] [PubMed] [Google Scholar]
- 13.Ohlson N, Bergh A, Stattin P, Wikström P. Castration-induced epithelial cell death in human prostate tissue is related to locally reduced IGF-1 levels. Prostate. 2007;67:32–40. doi: 10.1002/pros.20480. [DOI] [PubMed] [Google Scholar]
- 14.Johansson A, Jones J, Pietras K, Kilter S, Skytt A, et al. A stroma targeted therapy enhances castration effects in a transplantable rat prostate cancer model. Prostate. 2007;67:1664–76. doi: 10.1002/pros.20657. [DOI] [PubMed] [Google Scholar]
- 15.Wikström P, Ohlson N, Stattin P, Bergh A. Nuclear androgen receptors recur in the epithelial and stromal compartments of malignant and non-malignant human prostate tissue several months after castration therapy. Prostate. 2007;67:1277–84. doi: 10.1002/pros.20569. [DOI] [PubMed] [Google Scholar]
- 16.Placencio VR, Sharif-Afshar AR, Li X, Huang H, Uwamariya C, et al. Stromal transforming growth factor-beta signaling mediates prostatic response to androgen ablation by paracrine Wnt activity. Cancer Res. 2008;68:4709–18. doi: 10.1158/0008-5472.CAN-07-6289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li X, Martinez-Ferrer M, Botta V, Uwamariya C, Banerjee J, et al. Epithelial Hic-5/ARA55 expression contributes to prostate tumorigenesis and castrate responsiveness. Oncogene. 2011;30:167–77. doi: 10.1038/onc.2010.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Y, Li CX, Ye H, Chen F, Melamed J, et al. Decrease in stromal androgen receptor associates with androgen-independent disease and promotes prostate cancer cell proliferation and invasion. J Cell Mol Med. 2008;12:2790–8. doi: 10.1111/j.1582-4934.2008.00279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wikström P, Marusic J, Stattin P, Bergh A. Low stroma androgen receptor level in normal and tumor prostate tissue is related to poor outcome in prostate cancer patients. Prostate. 2009;69:799–809. doi: 10.1002/pros.20927. [DOI] [PubMed] [Google Scholar]
- 20.Zhou H, Sun HB, Huang WB, Xu Z, Su JH, et al. Phenotypic differences of stroma cells in benign and malignant human prostate tissues. Zhonghua Yi Xue Za Zhi. 2012;92:516–9. [PubMed] [Google Scholar]
- 21.Billis A, Meirelles L, Freitas LL, Polidoro AS, Fernandes HA, et al. Adenocarcinoma on needle prostatic biopsies: does reactive stroma predicts biochemical recurrence in patients following radical prostatectomy? Int Braz J Urol. 2013;39:320–7. doi: 10.1590/S1677-5538.IBJU.2013.03.04. [DOI] [PubMed] [Google Scholar]
- 22.Crawford ED, Bennett CL, Andriole GL, Garnick MB, Petrylak DP. The utility of prostate-specific antigen in the management of advanced prostate cancer. BJU Int. 2013;112:548–60. doi: 10.1111/bju.12061. [DOI] [PubMed] [Google Scholar]


