Abstract
This case-controlled study was designed to evaluate the association between various baseline parental factors and the risk of hypospadias in China. Patients were selected from tertiary referral hospitals in Anhui, a province in mid-eastern China. A questionnaire was given to the parents of each patient. The final database included 193 cases and 835 controls. The incidence of additional coexistent anomalies was 13.0%, primarily cryptorchidism (9.8%). Ten patients (5.1%) were from families with genital anomaly, including five families (2.6%) with hypospadias. The risks of hypospadias was higher for children of mothers > 35 (odds ratio [OR] =1.47) and < 18 (OR = 2.95) years of age, and in mothers who had consumed alcohol (OR = 2.67), used drugs (OR = 1.53) and had an infection (OR = 1.87) during pregnancy. The risk of hypospadias was also higher when mothers (OR = 1.68) and fathers (OR = 1.74) were engaged in agriculture. Other factors assessed were not associated with the risk of hypospadias.
Keywords: genetics, hypospadias, maternal exposures, paternal exposures, pregnancy, risk factors
INTRODUCTION
Hypospadias is a birth defect in boys, in which the urinary tract opening is not at the tip of the penis. It is the second most common genital anomaly in children1 and can lead to severe mental and physical consequences.
Many studies have shown that hypospadias is associated with exposure to endocrine-disrupting agents. For example, placental insufficiency, resulting in reduced supplies of nutrients and gonadotropins to the fetus, may result in fetus hypospadias.2 In addition, genetic factors are thought to play a pivotal role in the development of hypospadias. Although, many candidate genes have been identified, few have shown a clear correlation with hypospadias.3 Environmental factors may also be involved in the etiology of hypospadias. Exogenous chemicals, for example, may induce hypospadias, but the adverse effects of these agents remain unclear. Other environmental risk factors also have been associated with hypospadias, but most have shown inconsistent results.4
Although the etiology of hypospadias remains unclear for most patients, epidemiologic evidence can provide some clues. Familial clustering, maternal age, parental smoking and drinking, drug use during pregnancy, exposure to viruses, and other infections have been proposed as risk factors for hypospadias.5,6,7
Large geographic studies have shown that the incidence of hypospadias ranges from 2 to 8 in 1000, and from 2 to 39 in 10 000, live-births.8,9 Hypospadias is therefore one of the most frequent birth defects known; although, its incidence varies widely among different ethnic populations. Although reports from Europe, the USA, and Japan have shown that the rate of hypospadias has increased over the past 30 years,9,10 few studies have evaluated the rates of hypospadias and the risk factors for this condition in China. This large case-controlled study was therefore designed to assess maternal and paternal risk factors for hypospadias in Chinese boys.
PATIENTS AND METHODS
Recruitment of cases and controls
For this retrospective investigation, cases and controls were recruited from six tertiary referral hospitals in the cities of Hefei, Lu‘an and Bengbu, all of which are located in Anhui Province, China, a province with more than 60 million residents and with a population demographically representative of the population throughout China. The total number of eligible cases and controls were 193 and 835, respectively. All of the cases were boys aged 5 to 19 years who were inpatients in the six hospitals between January 2003 and December 2012 and who did or did not undergo surgery for hypospadias. The controls consisted of 835 randomly selected boys without any congenital anomalies who were hospitalized in the Pediatric Departments of the same hospitals over the same time period.
Data collection
A questionnaire designed by the research group included questions about: (1) each patient's demographic and clinical characteristics; (2) information about hypospadias, including phenotypes, with or without other congenital anomalies such as cryptorchidism, horseshoe kidney, or polycystic kidney disease; (3) familial clustering of genital anomalies; (4) paternal information, including occupation, habitation, drug use, smoking and drinking; (5) maternal lifestyle habits throughout the pregnancy, including maternal age, parity, multiple births, smoking, drinking, drug use, cosmetic use and infection. All lifestyle information was related to the period before and during each pregnancy.
Each questionnaire was completed by the attending urologist based on information supplied by the parents of each patient. All parents provided written informed consent, including consent regarding the questionnaires. All the questionnaires were collected by the urology department of the First Affiliated Hospital of Anhui Medical University.
Statistical analysis
All statistical analyses were performed using the Statistical Package for Social Sciences, version 17.0 (SPSS Inc., Chicago, IL, USA). The chi-squared test and Fisher's exact test were used to evaluate qualitative data. Odds ratios (OR) and 95% confidence intervals (95% CI) were estimated using conditional logistic regression, taking into account the matched case-control design of the study. Statistical significance was set at P < 0.05.
RESULTS
Parental exposure
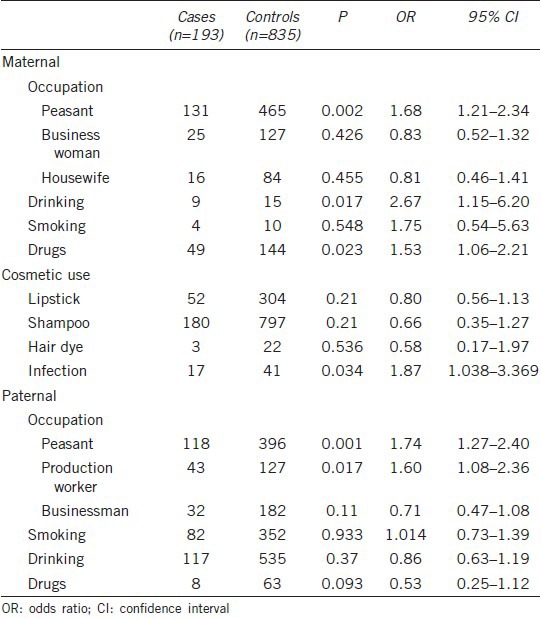
Table 1 shows the maternal and paternal factors assessed for risk of hypospadias. The most risk of hypospadias was markedly associated with participation in agriculture by both mothers (OR = 1.68, 95% CI = 1.21–2.34) and fathers (OR = 1.74, 95% CI = 1.27–2.40). Similarly, fathers engaged in factory work were more likely to have an affected child (OR = 1.60, 95% CI = 1.08–2.36). Infection by a virus or bacteria during pregnancy also enhanced the risk of hypospadias (OR = 1.87, 95% CI = 1.04–3.37). Of the mothers of children with hypospadias, 47 had taken drugs during pregnancy, including antiemetic, antibiotic, and antipsychotic agents, with drug taking showing a correlation with hypospadias (OR = 1.53, 95% CI = 1.06–2.21). Additionally, maternal alcohol consumption increased risk of hypospadias (OR = 2.67, 95% CI = 1.15–6.2), whereas maternal use of cosmetics, including lipstick, shampoo and hair dye, was not associated with hypospadias. Neither paternal drinking nor smoking was associated with hypospadias.
Table 1.
Parental risk factors for hypospadias
Pregnancy characteristics
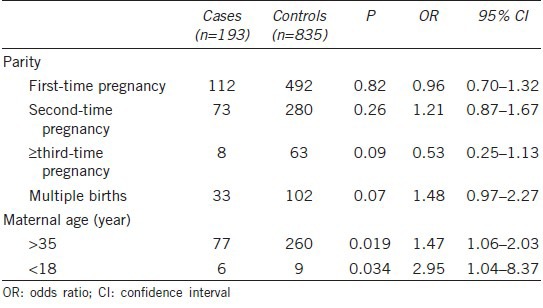
Table 2 shows the characteristics of each mother's pregnancy. Hypospadias was more likely to occur in infants of mothers aged > 35 (P = 0.019, OR = 1.47) and < 18 (P = 0.034, OR = 2.95) years. In addition, multiple births was a potential risk factor for hypospadias (P = 0.07, OR = 1.48), whereas parity was not (OR = 0.96, 95% CI = 0.70–1.32).
Table 2.
Pregnancy characteristics
Family clustering
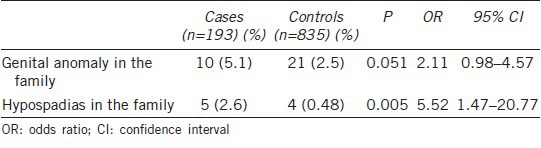
Table 3 lists the occurrence of hypospadias in family clusters. Hypospadias occurred more frequently in infants whose family members had hypospadias (2.6%, P = 0.005) or other genital anomalies (5.1%, P = 0.051).
Table 3.
Family clustering, n (%)
Coexistent anomaly
Interestingly, we found that coexistent anomalies were common in boys with hypospadias, being present in 25 of the 193 patients (13.0%), including 19 (9.8%) with cryptorchidism. Of the other six patients, one had an obstruction of the ureteropelvic junction, and five had nonurological anomalies, such as inguinal hernia. None of the hypospadiac boys had a horseshoe kidney or polycystic kidney disease.
DISCUSSION
Hypospadias has been correlated with fetal growth retardation. The latter is likely due to a problem early in gestation, as shown by the proportional retardation of weight, length, and brain growth (head circumference).11 These anomalies may be caused by placental insufficiency, which may result from many factors, including maternal age, parity, and multiple births.12 Advanced maternal age was found to be associated with an increased incidence of structural birth defects, including urethral abnormalities.13 Similarly, we observed significant relationships between hypospadias and maternal age extremes. Although parity and multiple births have been reported as being associated with increased risks of hypospadias, in agreement with results showing an association between hypospadias and growth restriction,14 we did not observe any association between hypospadias and either parity or multiple births.
The worldwide increase in the incidence of hypospadias has been regarded as caused by environmental chemicals, especially endocrine disruptors.3,15 Several endocrine disruptors, including the pesticides di-(2-ethylhexyl) phthalate, di-n-butyl-phthalate, mono-(2-ethylhexyl) phthalate, and dioxins/furans have been implicated in male birth defects, including hypospadias.15 Individuals engaged in agricultural work have increased exposure to pesticides, both directly and indirectly. Entering a field after it has been treated with pesticides can be a major source of chemical exposure, because residual hazardous materials may remain on foliage and soil for some time after spraying.11 Other chemicals may be present in pesticides, herbicides, and fertilizers.15 Indeed, we found that mothers involved in farming or other agricultural activities, or those who used insect repellents, were more likely to give birth to an infant with hypospadias. Interestingly, we also observed found an association between hypospadias and fathers whose occupation involved painting. The father's work may increase maternal exposure to these chemicals during the perinatal period. Similarly, the incidence of cryptorchidism was significantly higher in the children of mothers exposed to petrochemicals than in the general population.16 That study, however, could not compare the rates of hypospadias in industrialized and nonindustrialized areas owing to the small number of boys with this condition.16
In contrast to previous studies, which described maternal factors associated with the risk of hypospadias, we found that paternal factors were also involved. However, paternal smoking and drinking were not associated with hypospadias, in agreement with previous findings.17,18 Our results also suggested that, in contrast to previous findings,17,18 maternal alcohol consumption during the perinatal period may be associated with hypospadias.
Viral infection during pregnancy has also been associated with hypospadias,11,19 including in carriers of hepatitis B antigen11 and in women who had experienced a viral infection or influenza during the first trimester of pregnancy.19 Similarly, we found that the incidence of infection differed significantly in our case and control groups.
Most therapeutic drugs taken by Chinese women during pregnancy, including corticosteroids, antibiotics, antipsychotics, antifungal and antiasthmatic drugs, have not been associated with hypospadias.20 Nausea is a common symptom during early pregnancy, with some mothers taking antiemetics. We observed an association between antiemetic use and hypospadias, although this finding was based on relatively few subjects. Little is known about the mechanisms by which maternal antiemetic use may result in an infant with hypospadias.
The familial clustering of hypospadias, with 7% of cases having affected first, second or third degree relatives,21 suggests an important genetic element in its etiology. The heritability of hypospadias has been estimated to range from 55% to 77%, indicating that phenotypic variability can be attributed to genetic variability.22 We also observed an association between hypospadias and familial clustering. Of the 193 cases, five were from two families. Although many candidate genes have been identified, few have been found to contribute to the specific susceptibility of Chinese ethnic populations.
An almost linear relationship has been observed between the severity of hypospadias and the frequency of an associated anomaly.23 The most common associated anomalies in boys with hypospadias are cryptorchidism and inguinal hernia, present in about 8%–10% and 9%–15% of these patients, respectively.24 In addition, 17.6% of hypospadiac boys in Istanbul were found to have malformations of the genital or inguinal region.25 Our results were in good agreement with these previous findings.
Other risk factors reportedly associated with hypospadias include obesity; maternal drinking; and maternal exposure to lipstick, shampoo, hair dye, perfume and oral hormonal contraceptives.26 A lack of meat and fish in maternal diets has also been associated with hypospadias.8 However, because eggs, chicken, and fish are traditional meals for Chinese mothers during pregnancy, this factor was excluded from our analysis.
Relatively little is known about rates of genital anomalies, including hypospadias, in China. However, one limitation of our study was that all possible parental and environmental risk factors were not included in the analysis. Therefore, further prospective research through multiple approaches on a larger scale is still urgently necessary.
CONCLUSIONS
We found that the prevalence at birth of hypospadias was higher in infants whose mothers were at extremes of maternal age (<18 and > 35 years). We also found that hypospadias was frequently accompanied by additional anomalies. Hypospadias was also associated with parents whose occupation was farming, as well as with maternal use of drugs, alcohol consumption and infection during pregnancy. These risk factors were consistent with the hypothesis that genetic predisposition, placental insufficiency, and environmental chemicals may interfere with hormones before conception or during pregnancy.
AUTHOR CONTRIBUTIONS
LFX contributed to the conception and design of the study and to data acquisition and analysis, as well as drafting the manuscript. CZL, XGC, and SF assisted in the study conception and design. JL helped draft and revise the manuscript. XGC, SF, JZ, LZ, ST, and CQJ contributed to data acquisition. All authors approved the final version of this manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
ACKNOWLEDGMENTS
This work was supported by the First Affiliated Hospital of Bengbu Medical College and Lu‘an People's Hospital.
REFERENCES
- 1.Baskin LS. Can we prevent hypospadias? J Pediatr Urol. 2007;3:420–5. doi: 10.1016/j.jpurol.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 2.Yinon Y, Kingdom JC, Proctor LK, Kelly EN, Salle JL, et al. Hypospadias in males with intrauterine growth restriction due to placental insufficiency: the placental role in the embryogenesis of male external genitalia. Am J Med Genet A. 2010;152A:75–83. doi: 10.1002/ajmg.a.33140. [DOI] [PubMed] [Google Scholar]
- 3.Carmichael SL, Shaw GM, Lammer EJ. Environmental and genetic contributors to hypospadias: a review of the epidemiologic evidence. Birth Defects Res A Clin Mol Teratol. 2012;94:499–510. doi: 10.1002/bdra.23021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van der Zanden LF, van Rooij IA, Feitz WF, Franke B, Knoers NV, et al. Aetiology of hypospadias: a systematic review of genes and environment. Hum Reprod Update. 2012;18:260–83. doi: 10.1093/humupd/dms002. [DOI] [PubMed] [Google Scholar]
- 5.Gill SK, Broussard C, Devine O, Green RF, Rasmussen SA, et al. Association between maternal age and birth defects of unknown etiology: United States, 1997-2007. Birth Defects Res A Clin Mol Teratol. 2012;94:1010–8. doi: 10.1002/bdra.23049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hackshaw A, Rodeck C, Boniface S. Maternal smoking in pregnancy and birth defects: a systematic review based on 173 687 malformed cases and 11.7 million controls. Hum Reprod Update. 2011;17:589–604. doi: 10.1093/humupd/dmr022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pierik FH, Burdorf A, Deddens JA, Juttmann RE, Weber RF. Maternal and paternal risk factors for cryptorchidism and hypospadias: a case-control study in newborn boys. Environ Health Perspect. 2004;112:1570–6. doi: 10.1289/ehp.7243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akre O, Boyd HA, Ahlgren M, Wilbrand K, Westergaard T, et al. Maternal and gestational risk factors for hypospadias. Environ Health Perspect. 2008;116:1071–6. doi: 10.1289/ehp.10791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manson JM, Carr MC. Molecular epidemiology of hypospadias: review of genetic and environmental risk factors. Birth Defects Res A Clin Mol Teratol. 2003;67:825–36. doi: 10.1002/bdra.10084. [DOI] [PubMed] [Google Scholar]
- 10.Toppari J, Kaleva M, Virtanen HE. Trends in the incidence of cryptorchidism and hypospadias, and methodological limitations of registry-based data. Hum Reprod Update. 2001;7:282–6. doi: 10.1093/humupd/7.3.282. [DOI] [PubMed] [Google Scholar]
- 11.Sun G, Tang D, Liang J, Wu M. Increasing prevalence of hypospadias associated with various perinatal risk factors in Chinese newborns. Urology. 2009;73:1241–5. doi: 10.1016/j.urology.2008.12.081. [DOI] [PubMed] [Google Scholar]
- 12.Kalfa N, Philibert P, Baskin LS, Sultan C. Hypospadias: interactions between environment and genetics. Mol Cell Endocrinol. 2011;335:89–95. doi: 10.1016/j.mce.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 13.Fisch H, Golden RJ, Libersen GL, Hyun GS, Madsen P, et al. Maternal age as a risk factor for hypospadias. J Urol. 2001;165:934–6. [PubMed] [Google Scholar]
- 14.Carmichael SL, Shaw GM, Laurent C, Olney RS, Lammer EJ, et al. Maternal reproductive and demographic characteristics as risk factors for hypospadias. Paediatr Perinat Epidemiol. 2007;21:210–8. doi: 10.1111/j.1365-3016.2007.00809.x. [DOI] [PubMed] [Google Scholar]
- 15.Choi H, Kim J, Im Y, Lee S, Kim Y. The association between some endocrine disruptors and hypospadias in biological samples. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2012;47:2173–9. doi: 10.1080/10934529.2012.680387. [DOI] [PubMed] [Google Scholar]
- 16.Chul Kim S, Kyoung Kwon S, Pyo Hong Y. Trends in the incidence of cryptorchidism and hypospadias of registry-based data in Korea: a comparison between industrialized areas of petrochemical estates and a non-industrialized area. Asian J Androl. 2011;13:715–8. doi: 10.1038/aja.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brouwers MM, van der Zanden LF, de Gier RP, Barten EJ, Zielhuis GA, et al. Hypospadias: risk factor patterns and different phenotypes. BJU Int. 2010;105:254–62. doi: 10.1111/j.1464-410X.2009.08772.x. [DOI] [PubMed] [Google Scholar]
- 18.Brouwers MM, Feitz WF, Roelofs LA, Kiemeney LA, de Gier RP, et al. Risk factors for hypospadias. Eur J Pediatr. 2007;166:671–8. doi: 10.1007/s00431-006-0304-z. [DOI] [PubMed] [Google Scholar]
- 19.Morera AM, Valmalle AF, Asensio MJ, Chossegros L, Chauvin MA, et al. A study of risk factors for hypospadias in the Rhône-Alpes region (France) J Pediatr Urol. 2006;2:169–77. doi: 10.1016/j.jpurol.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 20.Carmichael SL, Ma C, Werler MM, Olney RS, Shaw GM, et al. Maternal corticosteroid use and hypospadias. J Pediatr. 2009;155:39–44. doi: 10.1016/j.jpeds.2009.01.039. 44.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fredell L, Iselius L, Collins A, Hansson E, Holmner S, et al. Complex segregation analysis of hypospadias. Hum Genet. 2002;111:231–4. doi: 10.1007/s00439-002-0799-y. [DOI] [PubMed] [Google Scholar]
- 22.Schnack TH, Zdravkovic S, Myrup C, Westergaard T, Christensen K, et al. Familial aggregation of hypospadias: a cohort study. Am J Epidemiol. 2008;167:251–6. doi: 10.1093/aje/kwm317. [DOI] [PubMed] [Google Scholar]
- 23.Bouvattier C. How and when to evaluate hypospadias? Arch Pediatr. 2013;20(Suppl 1):S5–10. doi: 10.1016/S0929-693X(13)71403-9. [DOI] [PubMed] [Google Scholar]
- 24.Leung AK, Robson WL. Current status of cryptorchidism. Adv Pediatr. 2004;51:351–77. [PubMed] [Google Scholar]
- 25.Akin Y, Ercan O, Telatar B, Tarhan F, Comert S. Hypospadias in Istanbul: incidence and risk factors. Pediatr Int. 2011;53:754–60. doi: 10.1111/j.1442-200X.2011.03340.x. [DOI] [PubMed] [Google Scholar]
- 26.Nordenvall AS, Frisén L, Nordenström A, Lichtenstein P, Nordenskjöld A. Population based nationwide study of hypospadias in Sweden, 1973 to 2009: incidence and risk factors. J Urol. 2014;191:783–9. doi: 10.1016/j.juro.2013.09.058. [DOI] [PubMed] [Google Scholar]


