Abstract
Monocarboxylic acid transporter 2 (MCT2) transports pyruvate and lactate outside and inside of sperms, mainly as energy sources and plays roles in the regulation of spermatogenesis. We investigated the association among genetic variations in the MCT2 gene, male infertility and MCT2 expression levels in sperm. The functional and genetic significance of the intron 2 (+28201A > G, rs10506398) and 3’ untranslated region (UTR) single nucleotide polymorphism (SNP) (+2626G > A, rs10506399) of MCT2 variants were investigated. Two MCT2 polymorphisms were associated with male infertility (n = 471, P < 0.05). In particular, the MCT2-3’ UTR SNP (+2626 G > A) had a strong association with the oligoasthenoteratozoospermia (OAT) group. The +2626GG type had an almost 2.4-fold higher sperm count than that of the +2626AA type (+2626GG; 66 × 106 vs +2626AA; 27 × 106, P < 0.0001). The MCT2-3’ UTR SNP may be important for expression, as it is located at the MCT2 3’ UTR. The average MCT2 protein amount in sperm of the +2626GG type was about two times higher than that of the +2626AA type. The results suggest that genetic variation in MCT2 has functional and clinical relevance with male infertility.
Keywords: 3’ UTR, male infertility, monocarboxylic acid transporter 2, single nucleotide polymorphism, sperm
INTRODUCTION
Monocarboxylic acid transporters (MCTs) facilitate the influx and efflux of pyruvate (CH3COCOO-) and lactate (CH3CH (OH) COO-) across the plasma membrane in various organs.1 The MCT family comprises of 14 members, of which four MCTs (1–4) carry metabolic compounds.
Lactate is an important energy substrate for spermatogenic cells provided by sertoli cells.2 MCT1, 2 and 4 are present in the testis.3 In particular, ubiquitously expressed MCT1 is present in the sperm head, but disappears in the distal epididymis where it is important for sperm maturation and movement. MCT2 is present in the sperm tail throughout the epididymis, and has a 10-fold higher affinity for monocarboxylates than that of MCT1.1,4
Sperm energy metabolism has been investigated as it relates to survival and motility, which is important for male fertility.5,6 Motility is essential for spermatozoa function and sperms require a continuous ATP source. Pyruvate and lactate are metabolized to produce ATP and thus, are good energy substrates for sperm survival and movement. Therefore, they may represent ideal candidates as risk factors for human disease.7
Because MCT2 transports pyruvate and lactate outside and inside of sperm, we investigated the association among MCT2 single nucleotide polymorphisms (SNP) with male infertility and selected sperm parameters. We analyzed intron 2 (+28201A > G, rs10506398) and the 3’ untranslated region (UTR) SNP (+2626G > A, rs10506399) in the MCT2 gene for their association with male infertility. We selected two polymorphisms based on minor allele frequency data from the database of National Genome Research Institute, National Institutes of Health, Republic of Korea. We also investigated the MCT2-3’ UTR SNP for its clinical relevance, particularly in sperm parameters from infertile patients. In addition to the clinical association study, we show MCT2 expression levels in sperm based on the MCT2 3’ UTR polymorphism to investigate the direct role of MCT2.
MATERIALS AND METHODS
Patients and controls
Patients and age-matched controls were used in this case-control association study, which has been explained in detail in previous studies.8,9,10 Briefly, nonobstructive infertile men (n = 471) were identified by physical examinations, Y chromosome deletion analysis and hormone assays from January 2002 to August 2007. They were classified into two subtypes of azoospermia (no spermatozoa in the ejaculate; n = 181) and oligoasthenoteratozoospermia (OAT; n = 290). The OAT group included men with at least one abnormality in sperm concentration (<20 × 106), motility (<50%) or morphology (<14%). The experiments were performed at the CHA General Hospital, Seoul, Korea. Informed consent was required to participate in this study. The institutional review board of CHA Hospital approved this study.
In total, 265 fertile men who had at least one child and who lacked any history of requiring assisted reproductive technology were included as the control group.8,10 The control group was consecutively enrolled from the Division of Genome Resources, National Genome Research Institute, National Institutes of Health, Republic of Korea.
Polymorphism genotyping
Genotyping for the two MCT2 SNPs (+28201A > G and +2626 G > A) was performed by pyrosequencing. The pyrosequencing assay was designed with pyrosequencing assay design software (Biotage AB, Uppsala, Sweden). The sequences for the pyrosequencing primers were: +28201A > G (rs10506398); forward primer: 5‘- biotin-AAGTACCTCTGTTGTGGCAAAAAT-3‘, reverse primer; 5‘- GCAGAGGTTATGTTGCCTTTG AA-3‘, sequence primer; 5‘- AAAACATATCATGTTCGATT-3‘. +2626 G > A (rs10506399); forward primer: 5‘- biotin-GGCATCAATCCAGATTCACTGT-3‘, reverse primer; 5‘- ATTAAAACCACCCCTCACTCTCTT-3‘, sequence primer and 5‘- GCAGGATTGTAAGTAGACC-3‘.
Sperm preparation and protein extraction
Sperm cells were extracted from a modified two-gradient (50%–80%) Percoll centrifugation method from ejaculated human semen obtained from nine genotyped men.11 The sperm pellet was washed three times and resuspended in phosphate buffered saline, and then reanalyzed with a Sperm Class analyzer system (Microptic S.L., Barcelona, Spain). The sperm pellet (99% purity) was resuspended in denaturing lysis buffer (20 mmol l–1 Tris, pH 7.4, 2% sodium dodecyl sulfate and 1 mM phenylmethanesulfonyl fluoride). The supernatant was collected after centrifugation and protein was quantified by the bicinchoninic acid assay. Total protein quantity was dependent on sperm number because of purity (99%). Twenty micrograms of protein obtained from each patient was loaded into each lane for 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The proteins were transferred to a nitrocellulose membrane using an electrophoretic method (Bio-Rad Laboratories, Inc., Hercules, CA, USA). The human MCT2 antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Construction of reporter plasmids
The pEGFP-3’ UTR-G and A reporter vectors were constructed with the MCT2 3› UTR fragment into the pEGFP-C1 vector (Invitrogen, Paisley, UK) at the XhoI and BamHI sites for the green fluorescent protein (GFP) reporter assays. A 1.2-kb fragment containing the 3’ UTR of the human MCT2 gene was prepared by polymerase chain reaction amplification from the full-length cDNA clone. The MCT2 3’ UTR fragment was amplified from full-length MCT2 cDNA (KRIBB, Daejon, Republic of Korea) using the following primers: forward 5‘-CCGCTCGAGCGTCAAAGTTTCAAATGCACA-3’ and reverse 5‘- CGGGATCCCAAGAATCAAGATCGGGTGAA-3‘. Reporter plasmids were transfected in HEK293 and HEK293T (human embryonic kidney cells) using DharmaFECT® Duo (Thermo Fisher Scientific, Waltham, MA, USA), according to the manufacturer's protocol. The transfected 293T cells were grown in DMEM supplemented with 10% fetal bovine serum.
Statistical analysis
Multiple logistic regression models were used to calculate odds ratios (OR), 95% confidence intervals (CIs) and corresponding P. All analyses were performed using Statistical Analysis Software (SAS Institute, Cary, NC, USA).12 Student's t-test and analysis of variance were used to compare the expression levels of the MCT2 genotypes (GG vs AA). A P < 0.05 was considered significant.
RESULTS
We investigated two sequence variations in the MCT2 gene by pyrosequencing in a case-control study using peripheral blood DNA from infertile and fertile Korean men. We selected two SNPs from intron 2 (+28201A > G, rs10506398) and the 3’ UTR SNP (+2626G > A, rs10506399) in the MCT2 gene. We found that both SNPs had a significant association with male infertility (n = 363, P < 0.05). The variant type (+28201GG) was 4.71% in fertile males (n = 265) for MCT2 +28201A > G SNP, but 11.97% in infertile males (n = 471, P = 0.035). In particular, the variant type (+2626AA) for the MCT2-3’ UTR SNP (+2626G > A) was 3.39% in fertile males (n = 265) and 13.8% in infertile males (n = 471, P < 0.0001) (Table 1). We analyzed whether the frequency of the +2626G > A genotype followed the Hardy-Weinberg equilibrium law. No deviation from Hardy-Weinberg equilibrium was observed in the infertile and fertile samples (P = 0.544; degrees of freedom = 1). We classified infertile men into two subgroups of OAT (spermatozoa in the ejaculate; n = 290) and azoospermia (no spermatozoa in the ejaculate; n = 181), and analyzed the associations in each group. Interestingly, the MCT2-3’ UTR SNP had a strong association with OAT (n = 290, OR, 6.13; P = 0.0001), but a weak association with azoospermia (n = 181, OR, 2.28; P = 0.06).
Table 1.
Distribution of the MCT2 +2626G>A genotype
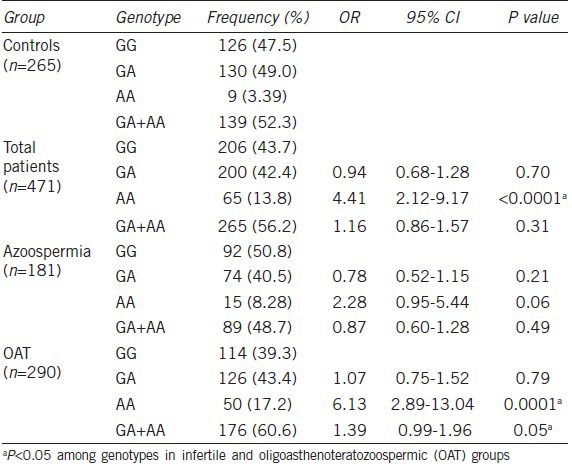
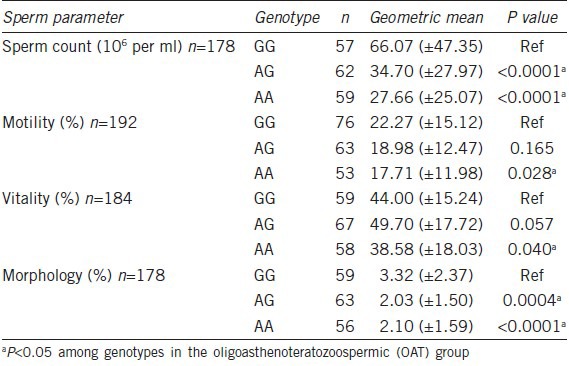
We further investigated sperm cell pathophysiology in this +2626GG genotype and analyzed its association with sperm count, vitality, motility and morphology from OAT subjects. As a result, the +2626GG genotype had a significant association with all four sperm parameters (n = 181, P < 0.05). In particular, the +2626GG type had an almost 2.4-fold higher sperm count than the +2626AA type (+2626GG; 66 × 106 vs +2626AA; 27 × 106, P < 0.0001) (Table 2). We also analyzed the association between the MCT2 SNP and luteinizing hormone, follicle-stimulating hormone, prolactin and testosterone levels, but found no significant associations (n = 181, P > 0. 05).
Table 2.
Sperm counts, motility, vitality and morphology according to +2626G>A genotype of oligoasthenoteratozoospermic males
Both MCT2 SNPs had a significant association with male infertility. Furthermore, the +2626AA types had a significant correlation with all sperm parameters. The +2626G > A polymorphism may be important for regulating gene expression, as it was located at the MCT2 3’ UTR. Therefore, we verified whether MCT2 expression was dependent on the MCT2 3’ UTR genotype directly from sperm of genotyped patients undergoing in vitro fertilization.
We assessed MCT2 protein expression (about 40 kDa) by Western blot using total sperm protein from nine genotyped individuals. The MCT2 expression from five +2626GG (G1–G5) and four +2626AA (A1–A4) types is shown in Figure 1a. Strong MCT2 expression was observed in the 2626GG types, but overall weak expression was found in the 2626AA types. Of the four +2626AA types, A3 showed strong MCT2 expression. The expression level was measured by relative optical density (ROD) and graphed (Figure 1b). Average MCT2 expression in sperm of the +2626GG types (ROD = 0.94) was about two times higher than that of the +2626AA types (ROD = 0.44). MCT2 3’ UTR SNP was important for gene expression in infertile males.
Figure 1.
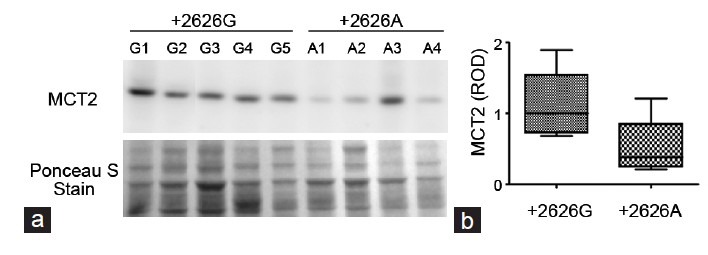
MCT2 expression in sperm is dependent on the +2626G>A polymorphism. (a) MCT2 protein expression in human sperm was determined by Western blot. The genotype of each sample is indicated in each lane; +2626 GG types (G1–G5) and +2626 AA types (A1–A4). Ponceau S staining of the same blot is shown after protein transfer. (b) The intensity of each band was determined and graphed in a box and whisker diagram. ROD, relative optical density, and the median value are indicated inside the bar (1 for the +2626GG type and 0.47 for the +2626AA type).
DISCUSSION
Previous genetic association studies have shown that single-gene or polygenic variations are associated with male infertility.9,13,14 Here, we investigated the MCT2 SNP for its clinical relevance in infertile patients.
We further determined whether the MCT2 3’ UTR SNP affected MCT2 gene expression in an in vitro GFP reporter assay. We constructed two reporters expressing GFP along with the 3’ UTR sequences (G or A at +2626 position). We constructed two pEGFP-3’ UTR-G (+2626G, G type) and pEGFP-3’ UTR-A (+2626A, A type) reporters to directly compare the polymorphic effects. The G type had almost twofold more fluorescence activity relative to that of the A type (data not shown).
Overall weak expression from the 2626AA sperm types may explain their association with the sperm parameters. A low amount of MCT2 in 2626AA types of sperm may affect energy metabolism compared to that in 2626GG types of sperm. However, further mechanistic studies are needed to support the results of this association study.
Sperm energy metabolism may be closely related with survival and motility. The principal sources of ATP production are not only glycolysis but also mitochondrial oxidative phosphorylation. Human sperm obtain a high proportion of their ATP from glycolysis,15 and respond to changes in energy demand by adjusting glycolytic flux rather than by mitochondrial respiration.16 Pyruvate and lactate are metabolized to produce ATP and thus, are good energy substrates for sperm survival and movement. Mammalian spermatozoa use lactate as an anaerobic energy source. MCT2 supports germ cell metabolic processes, but inhibiting MCT2 may affect sperm parameters.17
3’ UTR polymorphisms may have an effect on the regulation of gene expression during spermatogenesis and may affect mRNA stability and translational efficacy during spermatogenesis.18 Some 3’ UTR polymorphisms are associated with differential gene expression, but the underlying mechanisms are not fully understood.19 Posttranscriptional control of gene expression in these phases can be mediated by 3’ UTR sequences of messenger RNAs where they may be regulated by RNA-binding proteins or miRNAs, indicating that the 3’ UTR may play important roles in spermatogenesis.18,20 Our findings suggest a new paradigm and support the pursuit of 3’ UTR MCT2 SNPs in all reproductive diseases to better understand its gene regulatory role in genetic risk.
CONCLUSIONS
MCT2 genetic variations were associated with infertility in Korean males. We identified the clinical meaning of the 3’ UTR MCT2 SNP for male infertility. We determined that the 3’ UTR MCT2 SNP alters MCT2 expression during spermatogenesis.
AUTHOR CONTRIBUTIONS
JL and SL designed the experiments. JL, DRL and SL performed the experiments. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
ACKNOWLEDGMENTS
This study was supported by a grant (A120520) from the Korea Healthcare Technology R and D Project, Ministry of Health and Welfare, Korea.
REFERENCES
- 1.Garcia CK, Goldstein JL, Pathak RK, Anderson RG, Brown MS. Molecular characterization of a membrane transporter for lactate, pyruvate, and other monocarboxylates: implications for the Cori cycle. Cell. 1994;76:865–73. doi: 10.1016/0092-8674(94)90361-1. [DOI] [PubMed] [Google Scholar]
- 2.Mannowetz N, Wandernoth P, Wennemuth G. Basigin interacts with both MCT1 and MCT2 in murine spermatozoa. J Cell Physiol. 2011;227:2154–62. doi: 10.1002/jcp.22949. [DOI] [PubMed] [Google Scholar]
- 3.Brauchi S, Rauch MC, Alfaro IE, Cea C, Concha II, et al. Kinetics, molecular basis, and differentiation of L-lactate transport in spermatogenic cells. Am J Physiol Cell Physiol. 2005;288:C523–34. doi: 10.1152/ajpcell.00448.2003. [DOI] [PubMed] [Google Scholar]
- 4.Halestrap AP, Price NT. The proton-linked monocarboxylate transporter (MCT) family: structure, function and regulation. Biochem J. 1999;343(Pt 2):281–99. [PMC free article] [PubMed] [Google Scholar]
- 5.Garcia CK, Brown MS, Pathak RK, Goldstein JL. cDNA cloning of MCT2, a second monocarboxylate transporter expressed in different cells than MCT1. J Biol Chem. 1995;270:1843–9. doi: 10.1074/jbc.270.4.1843. [DOI] [PubMed] [Google Scholar]
- 6.Koehler-Stec EM, Simpson IA, Vannucci SJ, Landschulz KT, Landschulz WH. Monocarboxylate transporter expression in mouse brain. Am J Physiol. 1998;275:E516–24. doi: 10.1152/ajpendo.1998.275.3.E516. [DOI] [PubMed] [Google Scholar]
- 7.Steffens M, Lamina C, Illig T, Bettecken T, Vogler R, et al. SNP-based analysis of genetic substructure in the German population. Hum Hered. 2006;62:20–9. doi: 10.1159/000095850. [DOI] [PubMed] [Google Scholar]
- 8.Lee HC, Jeong YM, Lee SH, Cha KY, Song SH, et al. Association study of four polymorphisms in three folate-related enzyme genes with non-obstructive male infertility. Hum Reprod. 2006;21:3162–70. doi: 10.1093/humrep/del280. [DOI] [PubMed] [Google Scholar]
- 9.Lee J, Park HS, Kim HH, Yun YJ, Lee DR, et al. Functional polymorphism in H2BFWT-5’ UTR is associated with susceptibility to male infertility. J Cell Mol Med. 2009;13:1942–51. doi: 10.1111/j.1582-4934.2009.00830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yun YJ, Lee HC, Kim JE, Song SH, Lee S. Preliminary analysis of the G178A polymorphism of insulin-like factor 3 in male infertility. Fertil Steril. 2007;88:1706–8. doi: 10.1016/j.fertnstert.2007.01.107. [DOI] [PubMed] [Google Scholar]
- 11.Bongso A, Ng SC, Mok H, Lim MN, Teo HL, et al. Improved sperm concentration, motility, and fertilization rates following Ficoll treatment of sperm in a human in vitro fertilization program. Fertil Steril. 1989;51:850–4. doi: 10.1016/s0015-0282(16)60678-7. [DOI] [PubMed] [Google Scholar]
- 12.Sole X, Guino E, Valls J, Iniesta R, Moreno V. SNPStats: a web tool for the analysis of association studies. Bioinformatics. 2006;22:1928–9. doi: 10.1093/bioinformatics/btl268. [DOI] [PubMed] [Google Scholar]
- 13.Massart A, Lissens W, Tournaye H, Stouffs K. Genetic causes of spermatogenic failure. Asian J Androl. 2012;14:40–8. doi: 10.1038/aja.2011.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang C, Liu W, Ji GX, Gu AH, Qu JH, et al. Genetic variants in TP53 and MDM2 associated with male infertility in Chinese population. Asian J Androl. 2012;14:691–4. doi: 10.1038/aja.2012.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Williams AC, Ford WC. The role of glucose in supporting motility and capacitation in human spermatozoa. J Androl. 2001;22:680–95. [PubMed] [Google Scholar]
- 16.Rees JM, Ford WC, Hull MG. Effect of caffeine and of pentoxifylline on the motility and metabolism of human spermatozoa. J Reprod Fertil. 1990;90:147–56. doi: 10.1530/jrf.0.0900147. [DOI] [PubMed] [Google Scholar]
- 17.Boussouar F, Mauduit C, Tabone E, Pellerin L, Magistretti PJ, et al. Developmental and hormonal regulation of the monocarboxylate transporter 2 (MCT2) expression in the mouse germ cells. Biol Reprod. 2003;69:1069–78. doi: 10.1095/biolreprod.102.010074. [DOI] [PubMed] [Google Scholar]
- 18.Idler RK, Hennig GW, Yan W. Bioinformatic identification of novel elements potentially involved in messenger RNA fate control during spermatogenesis. Biol Reprod. 2012;87:138. doi: 10.1095/biolreprod.112.102434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mishra PJ, Humeniuk R, Longo-Sorbello GS, Banerjee D, Bertino JR. A miR-24 microRNA binding-site polymorphism in dihydrofolate reductase gene leads to methotrexate resistance. Proc Natl Acad Sci U S A. 2007;104:13513–8. doi: 10.1073/pnas.0706217104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Braun RE. Post-transcriptional control of gene expression during spermatogenesis. Semin Cell Dev Biol. 1998;9:483–9. doi: 10.1006/scdb.1998.0226. [DOI] [PubMed] [Google Scholar]


